What This Site Is About
Firstly, I give permission for use of all material on this website for educational purposes so long as it is attributed to ScientificBeekeeping.com.
This is not a “How You Should Keep Bees” site; rather, I’m a proponent of “Whatever Works for You” beekeeping. I’m a “data over dogma” guy, and I implore my readers to correct me on any information at this website that is out of date or not supported by evidence.
“It’s what you know for sure that keeps you from learning.”
And I’m all about learning. I’d like to make it perfectly clear that I do not consider myself to be the final arbiter on any matter! In investigating many of these controversial subjects, my brain feels like a GPS unit, repeatedly saying, “Recalculating” and sometimes even “Turn around when possible.” This is why I take care to hold no positions, and appreciate being intelligently challenged on any point. If something comes to my attention that makes me rethink or correct anything I’ve written, I am more than happy to rebut myself on these pages.
I’ve visited beekeepers in many countries, and realize that there are as many ways to keep bees as there are beekeepers. The bees don’t care whether you are a commercial or hobby beekeeper, nor whether your personal preference is Langstroth, Warre, top-bar, small cell, foundationless, “natural” or conventional beekeeping–the same biology applies to all. My goal is to provide any and all beekeepers with a resource of readable and straightforward information on how to practice good bee husbandry, and to exercise environmental and community responsibility.
This site is more or less a record of my learning process as I apply my formal training as a biologist to the practice of running my ever-evolving commercial beekeeping operation in California. I have no interest in offering advice (there are plenty of beekeepers more than eager to do that). Rather, what I do offer is evidence-based and scientifically-verified explanations of the biological processes occurring in the hive, as well as the effects of various management options. I then leave it to each beekeeper to use that information in order to make their own better-informed practical management decisions.
In this “post-truth, information overload era” novice beekeepers can be overwhelmed by an internet and popular press chock full of conflicting strong opinions and questionable advice. If you are a beginning beekeeper looking for basic information, or an experienced beekeeper looking for a summary of mite treatment options, I suggest that you go directly to Basic Beekeeping. Otherwise, I suggest that you click on the blue categories to the right of each page to see which articles are available, or go to Articles By Publication Date, or use the Search function at the top of each page to look for topics.
My Background

I started keeping bees as a hobbyist around 1966, and then went on to get university degrees in biological sciences, specializing in entomology. In 1980 I began to build a migratory beekeeping operation in California, and currently run around 1000-1500 hives with my two sons, from which we make our livings (update: Eric and Ian are in the process of taking over the operation–allowing me more time for research).
In 1993, the varroa mite arrived in California, and after it wiped out my operation for the second time in 1999, I decided to “hit the books” and use my scientific background to learn to fight back. I started writing for the American Bee Journal in 2006, and have submitted articles nearly every month since then (see “Articles by Publication Date”).
My writing for the Journal brought me requests to speak at beekeeping conventions, which has also allowed me the chance to visit beekeepers from all over North America and several other continents. I read most every scientific study relating to beekeeping, and regularly correspond with beekeepers and researchers worldwide.
What I try to do in my articles and blogs is to scour scientific papers for practical beekeeping applications, and to sort through the advice, opinion, and conjecture found in the bee magazines and on the Web, taking no positions other than to provide accurate information to Joe Beekeeper, following the suggestion in 1922 by New Zealand beekeeping author Isaac Hopkins:
That scientific accuracy, as opposed to rule of thumb, or guess-work methods, is much needed in commercial production to attain the success we should aim for, will be acceded by all intelligent beekeepers. There are many, however who do not realise this, or at all events, do not sufficiently appreciate the principle in their practice, but are content muddle along in a slipshod fashion to their great loss. From THE BEE WORLD February 1922
I regularly update the articles on this site as new information becomes available, and solicit constructive criticism or comments. Perhaps the best venue for such discussion is at the Informed Discussion of Beekeeping Issues and Bee Biology. Be sure to subscribe to updates, and I’ll email you monthly when I add content to the site https://scientificbeekeeping.com/scientific-beekeeping-newsletter/
Please Donate Here
It is the appreciative feedback that I receive from beekeepers (and researchers) worldwide is what keeps me going (thank you). If you find this website to be of value, please support it (and my independent research projects) with your donations. You can donate via Paypal below. Notice: I will, for tax purposes, treat your donation as a “gift” — given with “detached and disinterested generosity” out of “affection, respect, admiration, charity or like impulses.”
Or Personal checks can be mailed directly to me at:
Randy Oliver
14744 Meadow Dr.
Grass Valley, CA 95945
Be sure to specify whether the check is a “Gift” or whether you are going to claim it as a deductible “Expense.”
Thank you!
News and Blogs
In order to be notified by email of updates and additions to this website, please sign up at ScientificBeekeeping Updates (I will not share your personal info or email with anyone, nor clog your inbox; I update once every few months at best).
Extended-release oxalic acid
I get a lot of questions about my research into extended-release oxalic acid (“OAE”). This treatment is not yet approved for use in the U.S., but I suspect that it will be a game-changer for managing varroa. Researchers can find details on how I created the test sponges for my 2020 trials at the end of Mite Control While Honey is on the Hive: Part 4
For instructions for preparation and use of OAE under permit, see How to Use OAE.
I’ve created a very useful varroa control model for all to use–check it out here. It is designed to run in Excel, and can be used to run simulations for mite management in your own operation.
Nicole from Heritage Acres interviewed me about the state of bees, breeding for mite resistance, and extended-release oxalic acid, treatment-free beekeeping, mite drift, and my recent research. You can listen to it at this podcast
My assistant Brooke Molina shot a quick video of me demonstrating how to use my home-made plastic cups to perform a swirl-type mite wash–showing how it takes less than 4 minutes per hive. Randy’s Mite Wash Video
Extended-release oxalic acid
There is a crying need for a safe and effective varroa treatment for use during hot weather, when there are honey supers on the hive. I am working with USDA-ARS to get this application method approved by EPA. My latest update is at: Extended-Release Oxalic Acid Progress Report
An objective assessment of the neonics
I was recently asked to write an assessment of the neonics targeted for the nursery trade group–the University of California Nursery and Floriculture Alliance. It’s brief and simple.
You can read it here:
linkMy colony age distribution chart
I get a lot of requests for the colony age distribution chart that I created from Lloyd Harris’ data from Manitoba hives. Thanks to beekeeper Kat Satnik for pointed out a typo in previous versions. You can download a copy here.
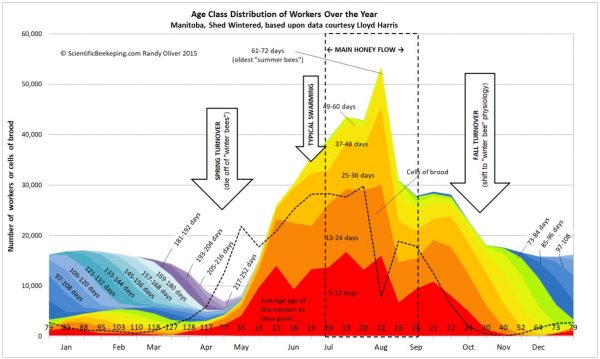
Mite control updates 25 January 2018
Many of you have noticed the recent discovery that lithium salts may be of use in varroa control. I’ve gotten some lithium citrate and will be testing this season. It is currently legal to feed colonies a lithium salt as a nutritional supplement, but I cannot recommend putting it into your hives prior to further formal testing.
Re oxalic acid in glycerin (oxalic shop towels), I’ve made big strides in developing protocols for incubator trials this winter to test various formulations for best efficacy against the mites, coupled with minimal adverse effects to the bees.

These cup cages in my home incubator allow me to place precise amounts of various oxalic acid solutions on a measured square of cellulose fabric (note the blue piece of towel in the left hand cage) sized to be proportional to the surface area of the combs in a hive (using a piece of beeswax-coated plastic foundation as proxy). The screens at the bottoms of the cups allow me to measure the percentage of mites killed by the treatment.

I’ve also recently perfected a protocol that allows me to quickly titrate the amount of oxalic acid that actually gets transferred to the bees’ bodies (note the pink indicator solution in the cup with bees). I’ve only run one formal trial using this method so far, but it shows great promise for me to be able to quickly screen for the optimal application method for distributing oxalic acid within the hive.
I thank you all for your donations in support of this research. I will continue to publish and post updates.
The 2017 Eclipse
Thanks to Idaho beekeepers Steve Sweet and Kevin Duesman for inviting Stephanie and I to join the Treasure Valley Beekeepers Association in camping out and viewing the eclipse directly under the path of totality!

We also shared the experience with some other bee researchers–Annette Bruun Jensen (from Denmark), Dennis vanEngelsdorp, and Steve Sheppard.
Update 10 March 2017 to the OA shop towel link below
There has been huge interest in my article from the Jan ABJ on extended-release oxalic acid dissolved in glycerin, and applied on shop towels. Please go to this link for updates: https://scientificbeekeeping.com/oxalic-shop-towel-updates/
Updates: Jan 29, 2016
California almond season is upon us! We’ve had it easy the past few seasons in almonds, since the lack of rain during our drought kept the orchards relatively dry. Not so this year! The orchards are a mess, and many are impassable.

My sons Eric and Ian, and I spent the past week welding up our new boom loader (original design, on the truck to the far right, largely constructed while working under a tent during the rain). We got a 3-day break in the rain this week, and used the window of opportunity to start moving our hives into the most problematic orchards. I took the photo of Eric and Ian with our three trucks as we arrived in the morning for offloading. It was relatively dry upon arrival, but it started raining shortly thereafter, and was a sloppy mess by the time we had finished unloading two hours later (after having to replace one loader motor, and swap a battery between the trucks–the usual almond problems).
The drought made beekeeping really tough last season, and we had to feed a record amount of pollen sub and syrup to our colonies in late summer and fall. Varroa only added to our problems. But we poured TLC (and dollars) into our hives, with the result that our colonies are looking OK for almonds (knock on wood).
I’ve added several new articles to the website, continuing on Colony Buildup and Decline, as well as investigating the fermentation of beebread (see https://scientificbeekeeping.com/articles-by-publication-date/).
I also updated my ppt on oxalic acid.
Beekeeper Jeff Anderson (and coplaintiffs) have recently filed a lawsuit against EPA to remove the current loophole that allows growers to plant pesticide-treated seed without the normal restrictions regarding pesticide application. EPA interpreted existing law as such:
Treated seed (and any resulting dust-off from treated seed) may be exempted from registration under FIFRA as a treated article and as such its planting is not considered a “pesticide use.”
The above loophole has allowed serious problems with planting dust to remain unresolved. The lawsuit explains that the current EPA guidance document:
states there will not be investigation or enforcement against any of their bee kills or other harm caused by neonicotinoid-coated seeds or resulting contaminated dust because the kills and other harm incidents are “not considered a ‘pesticide use.'”
Although I am not of the anti-neonic camp, and feel that seed treatments are perhaps the best use of neonics, they are indeed potent insecticides, and anyone (including the guy pulling the seeder) should have training in pesticide application, and follow restrictions to reduce pesticide drift. Thus I feel that beekeepers should support Jeff in this important lawsuit. Read more at: (Broken Link!) http://pollinatorstewardship.org/?p=3903
On the subject of pesticides, Dr. May Berenbaum has recently published the most succinct summary of the history of insecticide use that I’ve had the pleasure to read. Read it at: (Broken Link!) Does the Honey Bee “Risk Cup” Runneth Over?
We’re now pushing 30 years with varroa, and from the look of it, in many operations varroa is winning. Lately I’ve been giving presentations on “A New Era in Mite Management,” which I plan to spin into a series of articles.
I’ve also got a backlog of research trials that we’ve done (funded by the donors to ScientificBeekeeping), but have not yet had time to publish. There just haven’t been enough hours in my days, due to building our operation and my many speaking engagements.
The good news is that we’ve finally reached the point that my sons are getting ready to take over the reins of our business (now at around 1200 hives), which I hope will free up time for me to catch up on the home front and concentrate on beekeeping research (as well as to improve the website).
Updates: Jan 9, 2016
A recently-filed lawsuit by beekeeper Jeff Anderson deserves our support, in order to close a huge loophole in pesticide regulation. Currently, the EPA does not classify pesticides applied on treated seed as pesticide “applications,” and are thus exempt from the restrictions and liability due to drift or misuse as are other pesticide applications. The registration of seed treatments as pesticide applications will allow better monitoring of the overall environmental impact and fate of seed-applied pesticides (not only the neonics). For more information, see: (Broken Link!) Pollinator Stewardship News.
Also, see my updates on oxalic acid at Varroa treatments
Updates: Nov 2
There have been a couple of excellent and objective reviews of our state of knowledge on the effects of neonicotinoids on bees. Both are open access. The lay reader may wish to simply read the summaries in the second link.
A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators
A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators
I’ve updated my analysis of the recent paper Neonicotinoid pesticides severely affect honey bee queens.
I also suggest the reading of an excellent Master’s Thesis by Julia Goss of the Swedish University of Agricultural Sciences: Neonicotinoids and Honeybee Health. Julia tracked varroa, nosema, and virus levels in 96 colonies, equally divided between 16 fields of oilseed rape, half seed treated with clothianidin, half as untreated controls. She measured parasite levels before (June) and after flowering of the crop (late July-early August). Results: despite the confirmed exposure of the Test colonies to clothianidin at much higher rates than we ever see in North America, there were no differences in any of the parasite levels following exposure to the insecticide.
Update: August 23: I was asked to comment on Harvard Medical School’s Dr. Lu’s recent paper on neonics in Massachusetts. This may be of interest to those re a general discussion of the issue of good science vs. poor science. Read it at A Review of Dr. Lu’s Latest.
Update June 26: I added a post that I made to Bee-L on monitoring varroa at Monitoring Varroa.
Update May 9: I’ve updated First Year Beekeeping, an added Oxalic acid dribble tips.
April 29: I’ve been derelict in updating the website, and have about 12 articles to post. A number of ABJ readers have asked me to post the following graphic from one of my recent articles.
 You can view a full-sized version at Colony Demography.
You can view a full-sized version at Colony Demography.
I occasionally comment on bee issues or the news, or link to interesting blogs by others on beekeeping, bee biology, or the environment.
The “Flow Hive”
In recent months there has been a great deal of buzz about the “Flow Hive,” developed by a father/son team of Australian beekeepers. The device consists of an arrangement of molded plastic parts that act as foundation upon which the bees build honey combs, but which can then be shifted by the turn of a handle to break open the cells of ripe honey and allow it to drain out of the hive through tubes. Although innovative, it is similar to a patent from 1939 (http://www.freepatentsonline.com/2223561.pdf).
The Flow Hive is likely the most well-funded beekeeping device ever brought to market, due to its inventors incredible media-savvy marketing via crowd sourcing on the internet. By means of producing brilliant and compelling fundraising videos, they have raised enough money to bring their product to market. Kudos to them!
I suspect that much of their funding has come from non beekeepers, who have always been fascinated by the promise of hive from which liquid honey could be directly taken without the need for actually handling bees.
The question regarding the Flow Hive is whether it will turn out to be practical, especially with regard to cost and whether it will stand up to repeated use. Longtime beekeepers tend to be skeptical, since we’ve seen so many beekeeping inventions come and go over the years.
But who knows? I’m as eager as anyone to see whether the Flow Hive proves to be a revolution in beekeeping. We’ll see once the completed hives get delivered to buyers. I wish the developers the best of luck. Only time will tell whether the device actually flies or flops.
Neonics in Ontario
A recent hotbed of anti-neonic activism is Ontario, Canada, in which an unlikely coalition of a few beekeepers and some media-savvy NGO’s is pushing the government to ban these insecticides. Let me state very clearly that I myself support organic and sustainable farming, use of Integrated Pest Management, and greatly reduced use of pesticides. That said, I feel that any pesticide regulations, and agricultural recommendations, should be based upon sound science.
An exemplar of this philosophy is Dr. Terry Daynard, formerly a professor and Assistant Dean of the Ontario Agricultural College, and currently a farmer himself. Daynard recently received the “2014 Farm & Food Care Champion” award from Farm and Food Care.org, with the introduction that “Daynard is a champion of agriculture in many ways. He is respected as a farmer, scientist, innovator and agricultural advocate, speaking up and advocating sound science even in the presence of criticism by those that don’t agree with him.”
Dr. Daynard applies a sound and scientific assessment of how misinformation can taint well-intentioned environmental regulation in his blog “Critique of “A Proposal for Enhancing Pollinator Health.””
We all want to minimize agriculture’s negative effects on the environment. This includes greatly reducing our reliance upon pesticides. But such reduction needs to evolve as we learn (or re learn) alternate and more sustainable strategies for growing food. This is best done by rational and sober scientific assessment of current and alternative practices. I commend Dr. Daynard pointing this out.
I’m also impressed by a recent blog by Dr. David Zaruk, who is a Risk Governance Analyst at Risk Perception Management and an Assistant Professor Adjunct in Communications at Vesalius College, VUB, and Facultés universitaires St-Louis in Brussels. He blogs under the name of the “Risk Monger.” He recently posted about the real-life agricultural and ecological consequences of the politically- (as opposed to scientifically-) motivated suspension of neonic seed treatments in the EU. http://risk-monger.blogactiv.eu/2014/09/30/the-save-the-bees-ban-failed-crops-and-another-precautionary-fail-who-is-to-blame/
Read previous blogs here:
https://scientificbeekeeping.com/news-and-blogs-page/
Dec 2, 2013 If you have interest in the recent petitions to ban the neonics, I recommend reading a letter to the respected journal Nature by a British bee researcher, Lynn Dicks, in which she points out the problems with hurried setting of policy based upon political pressure rather than upon careful scientific evaluation of the evidence http://www.nature.com/news/bees-lies-and-evidence-based-policy-1.12443
Such a careful evaluation of all evidence is what I’m all about, even if that is unpopular with those who don’t want to be confused by the facts. I currently feel that the problem with planting dust from corn seeding has finally reached the point where the manufacturers either have to take responsibility for compensating beekeepers who suffer losses due to the application of their products, or EPA and PMRA need to restrict the use of neonic seed treatments to only planters that pass dust emission certification. However, I feel that to date there is not enough evidence to call for a complete ban on the neonics–there are simply too many beekeepers successfully keeping healthy hives in areas of seed-treated crops. Clearly this is a hot issue, and the neonics, along with all pesticides need to be closely watched and regulated. It appears to me that our regulatory agencies are doing a good job at this, even if progress seems to be excruciatingly slow.
The most recent blog of interest is on the real people involved in biotechnology (GMO’s). Steve Savage writes:
“As with any new technology, the development and commercialization of biotech crops is a story about people. Its a story about people with ideas and vision; people who did hard and creative work; people who took career or business risks, and people who integrated this new technology into the complex business of farming… Their story is important, but it tends to get lost in much of the conversation about biotech crops.
Many narratives about “GMOs” leave out the people side, presenting it instead as some faceless, monolithic phenomenon devoid of human inspiration, intention and influence. Thats not how it happened. Other narratives about “GMOs” demonize those who made biotech crops a reality. Such portrayals are neither fair or accurate. The real stories of these people matter, because trust in a technology is greatly influenced by what people believe about those behind it.”
Read the rest at:
http://appliedmythology.blogspot.com/2013/10/the-people-side-of-gmo-crops-part-i.html










