IPM 5 Fighting Varroa : Biotechnical Tactics – Part 1
Screened Bottom Boards/Open Mesh Floors
A certain percentage of mites drop, or are dislodged, from the bees each day, and fall to the hive floor. About 25% of mites emerging from brood cells also drop (Martin 1998). Many fallen mites are dead or dying, but about half are still alive. Some of those hitch a ride back up to the broodnest by crawling onto a passing bee. Dr. Roger Hoopingarner (2001) suggested that replacing the solid hive floor with screen might prevent fallen healthy varroa mites from remounting a bee for the ride back up into the cluster. Thus the idea of using screened bottoms, or more conveniently, OMFs (Open Mesh Floors) for mite control.
OMFs are hardly a new idea. Wyatt Mangum (2003a) points out that screened bottoms were used 150 years ago for ventilation, trash removal, and as wax moth traps. The concept of using them for varroa control was first formally tested by Pettis and Shimanuki (1999). Pettis found that a ½ inch gap below the screen resulted in a 75% reduction in mite re-entry, while 2 inches resulted in no mites re-entering the colony. Thwarting the mites’ re-entry is a death sentence for them–few mites can survive for longer than two days away from the bees and/or the humidity of the cluster (Garedew 2004; Yoder, et al. 1999). Additionally, I often see ants scavenging fallen mites.
Are OMFs effective at controlling varroa levels? There have been quite a few studies since, and I’ve reviewed the data:
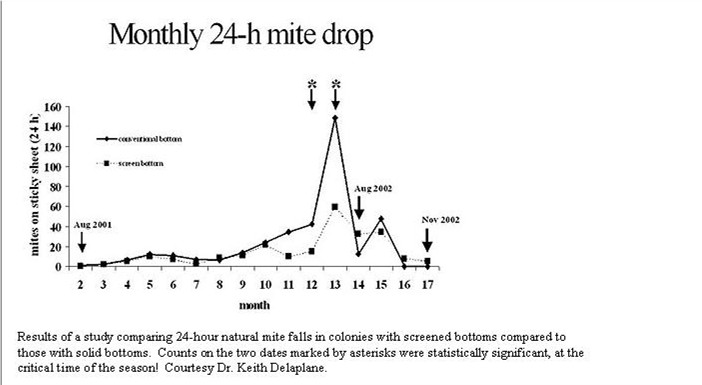
Mangum (2003b) In a spring through late summer test of the efficacy of screened bottoms, recorded a 14% reduction in mite population in one apiary, and 36% in a second. Collecting meaningful data can be tedious–Dr. Mangum has now personally counted over 400,000 mites!
Calderone (http://www.masterbeekeeper.org/B_files/varroa.htm) states that “three years of research at Dyce Laboratory at Cornell University have shown that screen bottom boards have no effect on mite populations.”
Harbo and Harris (2004) found that OMFs did not have any significant effect on food consumption during winter—an important consideration in cold climates.
Delaplane, et al. 2005 Reviewed seven studies. Most found OMFs to be favorable for mite control, and several found increased brood production.
Sammataro, et al. (2004). Found that a combination of hygienic queens and screened bottoms decreased mite populations and increased colony survival.
So, the question is, are OMFs worth the cost? The answer is not a simple yes or no. In the first place, OMFs have several potential benefits in addition to increasing daily mite attrition. These may, or may not, factor into the equation in your particular operation. Screened bottoms also:
1. Allow easy stickyboard insertion and removal (personally, I find this to be a huge plus).
2. Keep mites from returning to the cluster after knockdown by sublethal “flash” treatments or irritants, or from powdered sugar dusting.
3. Increase the efficacy of bee grooming or VSH behavior. This benefit may become more important as bees bred for grooming behavior become available.
4. Increase colony ventilation—I assume (but have not confirmed) drier in winter, cooler in summer.
5. Take advantage of the increased mite drop during hot weather (Sammataro, et al. 2004).
6. In several trials, OMFs apparently increased brood production.
7. Harbo and Harris (2004) state: “ In the colonies with OMFs, there were significantly more varroa on the adult bees than in the brood cells…– this is very good news, because the more time that mites spend out of the cells, the less they breed. In colonies with OMFs mites spent an average of 9.4 days between emerging from a brood cell and entering another cell – for solid floors it was just 4.4 days.”
8. Provide ventilation for moving, or or for temporary restraint of forager flight during pesticide applications.
9. Prevent rainwater from pooling on the hive floor.
10. May decrease the humidity inside the colony, and therefore stress the mite in dry weather.
11. Allow reduced entrances during nectar dearths for prevention of robbing. Robbing bees orient to colony odor, and attempt to enter through the screen, rather than the entrance (pers. obs.).
12. Remove 2 lbs of wood from each cedar bottom board—a surprisingly noticeable difference!
That’s quite a list, huh? Allow me to stray from the research results, and share my personal thoughts with regard to my own operation. Making a change to OMFs would represent a substantial investment for me. I wondered if they would be a waste of money if indeed I were to find completely mite tolerant bees. The deciding factor for me was the list above, especially the first four items. I built and installed OMFs for 300 of my colonies last year. I’ve been very happy with them. Besides potentially reducing mite counts, and making hygienic behavior and flash treatments more effective, the main upside is that (in my design at least) they make taking stickyboard samples a breeze. The only downside I’ve noticed is that you have to be a little careful in tossing them around, or when using a hand truck, to avoid bending the screen.

Changing to screened bottoms is a definite expense. We first converted our existing bottoms by cutting the centers out with a saw, then began building standardized new ones from scratch. Our equipment is all hot-dipped in a paraffin/rosin tank, and left unpainted.

Our current design, with the stickyboard partially inserted. Most of the time the stickyboard is removed, to allow hive trash and mites to fall to the ground.

We left a “reveal” of wood wide enough for a hive tool between the beeways and the screen.

We experimented with closing off some screens during winter. This is a photo of the stickyboard (without grease) placed above the screen to seal off ventilation. It looked like this at first inspection in the spring. Some of the hive trash is bits of pollen supplement.
In my Nevada desert locations, where vicious ants are a problem, OMFs don’t appear to cause problems. Indeed, I see ants foraging beneath the screens to grab fallen mites. My colonies on OMFs appeared to winter well in both subfreezing Nevada conditions, and the wet California winter.
I performed one informal test to see whether mite levels were lower after a season in colonies with OMFs—as much as I’d like to tell you that there was a big difference, there wasn’t. However, one thing that will really impress you about OMFs is to watch the mites drop through them when you dust a colony with powdered sugar (to be covered in my next article)!
Photos of various screened bottom boards and pallets can be found at: www.wvu.edu/~agexten/varroa/Screnbotbrdppt.pdf
OMFs are available from the major bee suppliers, or you can build your own. Here are some tips:
1. Use 8 mesh (3mm) screen—larger screens permit pollen pellets or bees to pass through.
2. Construct with (expensive) stainless steel screen if you’re going to use formic acid.
3. Do not extend the screen beyond the entrance, or bees will drop pollen loads through it.
4. You cannot leave stickyboards under the screen for extended periods, or hive trash will build up and make a breeding ground for wax moths and small hive beetles.
There are a number of OMFs on the market. However most either lack features I wanted, or didn’t fall within my budget, so I designed my own (see photos). Since few mites fall in the back or at the sides of a hive, I left those areas of the floor solid for strength, and just wide enough to scrape with a hive tool. However, I have since learned that Sammataro, et al. (2004) found that the proportion of mitefall that is alive is higher at the rear and sides of the hive compared with that falling from center frames near the hive entrance. Also, when the cluster moves to the south side during winter, more mites might fall on the wood. So perhaps the screen should cover the entire bottom—it’s a question of strength vs. efficacy . I really like my side insertion of the stickyboards (made from corrugated plastic signboard with a finger hole). I cut the stickyboard narrow enough to fit between the beeways, so that it can also be used above the screen to seal off the bottom in winter (if desired), or for fumigation.
Bottom line: OMFs generally decrease mite buildup, especially with mite-tolerant bees, but will not control varroa alone. They may increase the amount of brood. They have a number of other potential benefits—especially for stickyboard insertion, and nonlethal mite knockdown treatments.
Update October 2010:
Screened bottoms handy for hobby beekeepers if they are using stickyboards to monitor mite levels, and/or using powdered sugar dusting for mite control. However, if you are using essential oils or formic acid for mite control, I’m not clear whether there is an advantage to having a solid bottom, or covering the screen. For large-scale beekeepers, it may be that the various problems with screened bottoms likely outweigh their benefits.
Recommendations
Don’t jump into anything wholesale without trying it out first on a smaller scale! In your test yards, give some alternative tactics a trial, saving chemical miticides as backups. My first attempts at nonchemical mite management were financial disasters—I would recommend that one exercise caution in implementing change in any successful system that you currently practice. But I definitely think it would be wise for every beekeeper to familiarize themselves with proven alternative methods of mite management before chemical control fails. You may be surprised at how profitable it is to keep robust colonies that are not stressed by miticide treatments and residues, or plagued by unhealthy mite levels between treatments!
Note: biotechnical measures are most effective if used concurrently with mite-tolerant bees. Unfortunately, the performance of currently available open-mated mite-tolerant queens may be inconsistent. Keep monitoring mite levels so you don’t get caught by surprise.
If you find this web site to be of value to you, your generous donation will help me to maintain and add to it.
References
Barry, JA, and KS Delaplane (2001) Effects of comb age on honey bee colony growth and brood survivorship. Journal Apic Ress 40: 3-8.
Boecking, O, P. Aumeier, W. Ritter, D. Wittman (2002) Varroatosis-disease complex: is there any interrelation? Apidologie 33: 486
Chen, J. (2006) Presentation at California State Beekeepers Convention.
Delaplane, KS, JA Berry, JA Skinner, JP Parkman and WM Hood (2005) Integrated pest management against Varroa destructor reduces colony mite levels and delays treatment threshold. J. Apic. Res 44(4): 157-162.
Delaplane, K.S. & J.D. Ellis (2006) Varroa IPM: Does it work? Does it pay? Proceedings of the American Bee Research Conference
Garedew, A., E. Schmolz & I. Lamprecht (2004). The energy and nutritional demands of the parasitic life of the mite Varroa destructor. Apidologie 35: 419-430.
Giancarlo and De Jong (2003) The influence of brood comb cell size on the reproductive behavior of the ectoparasitic mite Varroa destructor in Africanized honey bee colonies. Genet. Mol. Res. 2 (1): 36-42.
Goodwin, R M, Taylor, M A, Mcbrydie, H M and Cox, H M. (2006) Drift of Varroa destructor-infested worker honey bees to neighboring colonies. Journal of Apicultural Research 45(3): 155-156.
Harbo, JR and JW Harris (2004) Effect of screen floors on populations of honey bees and parasitic mites (Varroa destructor) J. Apicultural Research 43(3): 114-117.
Harris, J.W., Harbo, J.R., Villa, J.D., Danka, R.G. (2003) Variable population growth of varroa destructor (mesostigmata: varroidae) in colonies of honey bees (hymenoptera: apidae) during a 10-year period. Environmental Entomology/Population Ecology 32(6):1305-1312.
Hoopingarner, R., in Delaplane and Webster, eds. (2001) Biotechnical Control of Varroa Mites. Mites of the Honey Bee. Dadant and Sons, p.197-204.
Kaznowski, Szymas, Jazdzinska, Kazimierczak, Paetz and Mokracka (2005) The effects of probiotic supplementation on the content of intestinal microflora and chemical composition of worker honey bees (Apis mellifera) J. Apic. Res. 44:1, 10-14.
Managing Varroa (Broken Link!) www.csl.gov.uk/science/organ/environ/bee/diseases/documents/managing_varroa_new.pdf
Mangum, W. (2003a) Varroa Mites: Some Historical Perspectives. ABJ 143(7): 555-557.
Mangum, W. (2003b) Experimenting with integrated pest management for varro control. ABJ Oct 2003: 791-793.
Martin, S. J. (1998) A population model of the ectoparasitic mite Varroa jacobsoni in honey bee (Apis mellifera) colonies. Ecological Modeling, 109: 267-281.
Pettis, JS and H Shimanuki (1999) A hive modification to reduce varroa populations. ABJ 139(6): 471-473.
Ringel, J. (2002) Artificial or natural wax? Old or new combs? The influence of the quality of wax on the development of bee colonies. Apidologie 33:498-499.
Rosenkranz, P. (1988) Reproduktion der Bienenmilbe Varroa jacobsoni (Acarina:. Varroidae). Entomologia Generalis 14(2): 111-122.
Sammataro D, G. Degrandi-Hoffman, N Ostiguy, G. Wardell and J. Finley (2004) Testing a combination of control strategies to manage varroa mite (Acari: Varroidae) levels in honey bee (Hymenoptera: Apidae) colonies. International J. Acarology, 30(1): 71-76.
Smith, RK & MM Wilcox (1990) Chemical residues in bees, honey and beeswax. ABJ 130: 188-192.
Waite, RJ, MA Brown, HM Thompson and MH Bew (2003) Controlling European foulbrood with the shook swarm method and oxytetracycline in the UK. Apidologie 34 569-575
Watters, Ethan (2006) DNA Is Not Destiny. Discover Nov. 2006, pp. 32-37, 75.
Yoder, JA, D Sammataro, JA Peteson, GR Needham and WA Bruce (1999) Water requirements of adult females of the honey be parasitic mite, Varroa jacobsoni (Acari: Varroidae) and implications for control. Int. J. Acarol. 25(4): 329-335.



