The Nosema Problem: Part 7C – The Prevention of Dysentery
Contents
Balancing moisture elimination and heat loss
Broodrearing in the winter cluster
Winter stores ― honey and beebread
The Nosema Problem Part 7c
The Prevention of Dysentery
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in February 2020
Dysentery within the winter cluster may indicate that the colony is suffering from moisture imbalance. Such in-hive defecation can overwhelm the normal colony hygiene that prevents the spread of intestinal parasites, such as nosema and amoeba. Fortunately, the bees ― and the beekeeper ― can take measures to help alleviate moisture imbalance.
Review
Apis mellifera is a tropical insect, which apparently evolved in warm regions of Africa. As the species expanded its range northward, certain races adapted to survive long confinement during the winter by forming a “cluster” that could subsist for months on stored honey. However, the consumption of honey generates water as a byproduct. When it’s warm, bees can fly freely to void that water away from the colony. But that option is not available to bees in a winter cluster.
Trapped in the winter cluster, a bee has four options for dealing with excess water in its system. The first two ― simply trying to “hold it,” or defecation in the cluster ― are problematic. That leaves two other options:
1. Breathing it out (respiratory transpiration), which requires heat generation and ventilation, or
2. Feeding the water (in some form) to other bees.
European races of bees have evolved to use both of the above methods in order to survive long-term clustering when it’s cold. But in order to conserve precious honey stores until the next spring, they have developed behaviors that minimize heat loss (and possibly as a side effect, to offset the loss of “winter bees” to natural mortality).
Balancing moisture elimination and heat loss
Bees have “figured out” ways to vent excess moisture from the cluster with minimal loss of heat (the replacement of which would require more honey consumption).
At cold temperatures, there is apparently scant ventilation in the cluster as evidenced by the observation that there is so little air exchange that oxygen and CO2 concentrations within the cluster can be dramatically different from our ambient air [[1]]. The creation of that hypoxic (low oxygen), reduced-metabolism environment may well have to do with the need for the bees in the core to conserve both energy and moisture in that self-created dry environment, as any air exchange would quickly desiccate them. But as predicted by an elegant model by Omholt, the lower the ambient temperature, the more the bees in the mantle of the cluster accumulate moisture [[2]].
Practical application: the cluster needs to somehow deal with thirsty bees in the core, and water-saturated bees in the mantle ― while minimizing energy consumption and heat loss.
In order to achieve necessary ventilation, bees can create channels within the cluster, as nicely illustrated in a swarm by Bernd Heinrich [[3]]. However, it is not clear whether bees actually use such passive convection currents to any great extent while in winter cluster, as challenged eloquently by Möbus [[4]] ― who questioned whether the bees at the top of the cluster mantle would, or were even able to, vent warm air out the top. Instead, Sachs and Tautz [[5]] concluded that required ventilation is accomplished by the active fanning of only a very few individual bees moving about the cluster:
… the middle section of the hive is rarely ventilated from below. The fanning bees generally move up and down the outer third of the comb alleys. … This behavior actively conveys humidity from the center of the bee cluster to the outer areas of the hive. … In hollow trees, this method of dehumidification has the advantage that virtually no heat is lost. The heat is not fanned outside but rather remains in the hive and rises once more as time goes by.
For the bees to fan ventilation air downward, rather than up, makes sense for two reasons:
· It would minimize water condensation above the cluster, and
· It would maximize heat recovery as that water vapor condensed.
Practical application: Toomema [[6]] placed condensers in the tops and bottoms of hives during winter to determine where moisture actually condenses in the hive. Over 97% was recovered in the lower condensers ― supporting Möbus’ thesis. Control of humidity in the winter cluster is of paramount importance, since it is the main way in which the colony controls water loss. I thank researchers such as Michael Ellis and Kalle Tomemaa for investigating this, and hope that we can eventually incorporate Möbus’s observations into a better understanding of the most conducive hive design for wintering.
But the bees have yet one more trick up their sleeve:
Broodrearing in the winter cluster
This brings up another question for which I’ve long looked for an answer ― why do colonies often engage in broodrearing in the middle of the winter? Indeed, one of Lloyd Harris’ shed-wintered colonies came out of winter confinement stronger than it went in [[7]]. Such stop-and-go broodrearing is energetically expensive, and could result in colony starvation, yet many colonies do it. It doesn’t appear to have anything to do with daylight or the winter solstice, since colonies wintered in pitch-black sheds still initiate broodrearing. Indeed, I’ve found zero evidence to support the claim that cessation or initiation of broodrearing has anything to do with day length. Just ask any Australian beekeeper on the winter solstice as their colonies actively rear brood during the bloom of White Box [[8]], or a keeper of Russian bees that go broodless during the August dearth.
One interesting data set regarding broodrearing during winter comes from an old study by Jeffree in Aberdeen, Scotland. Winters there are cold enough for colonies to go into fairly tight cluster, but it doesn’t get much below freezing (Fig. 1).
Figure 1. Weather averages for Aberdeen, Scotland. Factoring in rain and wind (not shown), bees wouldn’t be expected to do much foraging between late November and early April, nor to break winter cluster. Image from https://weatherspark.com/
But despite the chilly temperatures and lack of incoming pollen, broodrearing appeared to occur intermittently in colonies throughout the winter (Table 1).
|
Table 1. Results of 367 examinations made during September to March from 1945 to 1953. Data from Jeffree [[9]]. |
|||
|
Month |
No. of colonies examined |
Percent with brood |
Square inches of brood (average) |
|
September |
45 |
78% |
76 |
|
October |
106 |
14% |
2 |
|
November |
114 |
25% |
2 |
|
December |
31 |
58% |
10 |
|
January |
18 |
50% |
14 |
|
February |
10 |
100% |
48 |
|
March |
43 |
91% |
50 |
So why would colonies rear brood, even when it was cold outside? As pointed out by Möbus [[10]]:
Even among bees the old saying applies: “Every baby costs its mother a tooth.”
There are two main costs associated with winter broodrearing: Not only must the colony expend precious energy and protein reserves to rear brood, but “winter bees” appear to lose their longevity once they initiate the rearing of brood, as elucidated by Mattila’s analysis of Lloyd Harris’ data [[11]]. Thus, from an evolutionary standpoint, we must assume that the benefit of midwinter brood rearing outweighs the cost. One plausible explanation for the benefit of on-off winter broodrearing was offered independently by two very sharp and experienced observers ― first by Möbus in 1980 [[12]], then by Omholt in 1987 [[13]], and again by Möbus in this very journal in 1998 [[14]].
Practical application: Despite their once-a-decade efforts to bring this explanation to the attention of the beekeeping community, Möbus’ and Omholt’s well-reasoned and observationally-supported papers surprisingly seem to have been largely ignored or forgotten. So I’m trying to bring them back into the light in 2020 ― some 40 years later. As eloquently explained by Omholt:
Möbus suggested that the phenomenon of brood rearing in the winter cluster has a definite survival value for a colony with a water problem, as the production of liquid, glandular brood food will remove some of the individually embarrassing surpluses from the bees, and that the increase in core temperature that follows brood rearing will make efficient evaporation possible.
Establishing a broodnest requires ramping up of the temperature of the center of the cluster (which helps with transpiration loss), and requires the feeding of huge amounts of moisture-rich jelly (67% water) to the larvae. Additionally, there is the need for the bees to then increase the humidity of the core of the cluster enough to prevent the eggs and larvae from desiccating. The three above factors result not only in increased water loss due to respiratory transpiration, but also a massive transfer of water from the nurse bees to the brood.
So let’s do the arithmetic!
According to Alfonsus’ measurements:
Dysentery appears when the fecal accumulations reach 33% of the total body weight of the bees. General defecation does not take place until the accumulation reaches about 45%.
· That 33% accumulation occurs when a bee is holding around 35 mg of water in its rectum.
· A single worker larva at time of pupation weighs around 160 mg, 74% of which consists of water [[15]].
· That works out to at least 118 mg of water required to rear each worker to pupation.
· Thus the rearing of a single larva would easily allow more than three workers to completely dispose of the excess water in their bodies.
· In order for the cluster to transfer the water produced from a weekly consumption of a pound of honey, the colony would need to rear less than half a frame of brood (both sides of the comb) that week.
Omholt’s calculations suggest that at an ambient temperature of 32°F (0°C), a cluster of 15,000 bees (around 8 frames) would be expected to initiate broodrearing at about 43 days after their last cleansing flight ― a figure supported by a number of observations.
Möbus tested the hypothesis experimentally by caging queens in their winter cluster (to prevent broodrearing) ― most colonies then developed dysentery within 3-4 weeks and grew weaker.
Practical application: Dysentery may result from the failure of a colony to initiate winter broodrearing (perhaps due to an aged queen). This is a beautiful and elegant explanatory hypothesis. If true, the practical application is that it would perhaps help to explain why entering winter with a young queen and abundant stores of beebread helps a colony to survive the winter. More research on this subject is clearly needed!
Practical applications
I’ve now covered at least some of the ways that honey bees naturally deal with moisture balance in the winter cluster. This understanding then suggests ways that beekeepers could manage their colonies for optimal wintering success.
Optimal Cluster size
Again we can look at nicely-aged studies by old-school bee researchers, including those of Jeffree [[16]], performed when I was only a child. Jeffree sought to determine the optimal size for the winter cluster, hypothesizing that:
[Small colonies] will have more bees in the cold, outer shell, all making great efforts to stay alive by converting honey into heat ― and accumulating more and more ‘waste water’ within the totality of the cluster. The center being small, no movements in or out of the cluster center can cope with the situation and, only cleansing flights can ― theoretically ― bring relief. When these are not possible, it seems that dysenteric conditions must come about, forcing bees to defecate in the hive, on combs.
… but very large clusters are at a disadvantage in that they either tend to cool too much at the outside or else overheat in the centre. Somewhere between these two extremes would presumably lie an optimal wintering size.[[17]]
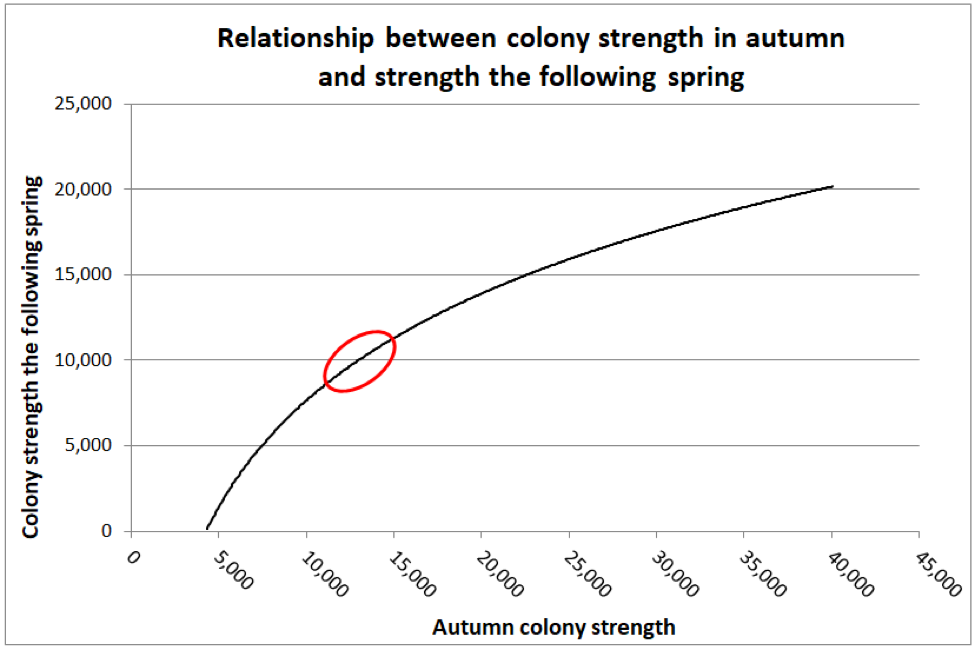
In one study, Jeffree and Allen intermittently measured the strengths of 153 colonies in total, divided over the course of two winters in Scotland, in order to see how late-season starting strength correlated with colony strength the following spring ― for colonies with or without nosema infection. They concluded that:
The percentage loss of bees over winter was found to be significantly greater for very large and for very small colonies than for colonies of medium size. In this sense, the fact of a theoretically optimum size for wintering was thus established.
I found the manner in which their data was presented a bit difficult to interpret, so I graphed it differently below (Fig. 2).
Figure 2. Jeffree and Allen found that as far as predicting colony strength in April, that there was a diminishing return from increased strength going into winter, as indicated by the diminishing slope of the curve. I’ve circled the authors’ suggested “sweet spot” for optimal autumn strength (at least for what I’m assuming were Apis mellifera mellifera in Aberdeen’s moderately-cold winters). The researchers also found that if a colony was infected with nosema, that there was a benefit to starting with a few thousand extra bees, in order to account for the reduced survivorship of the infected workers.
Practical application: Jeffree’s sweet spot works out to be the equivalent of around 6 deep Langstroth frames fully covered tightly with bees [[18]] ― what we’d call an 8-frame (or better) cluster. But “optimal” is likely relative to how cold it gets, as well as one’s goal in spring (having large colonies for almond pollination, as opposed to reducing swarming before the honey flow).
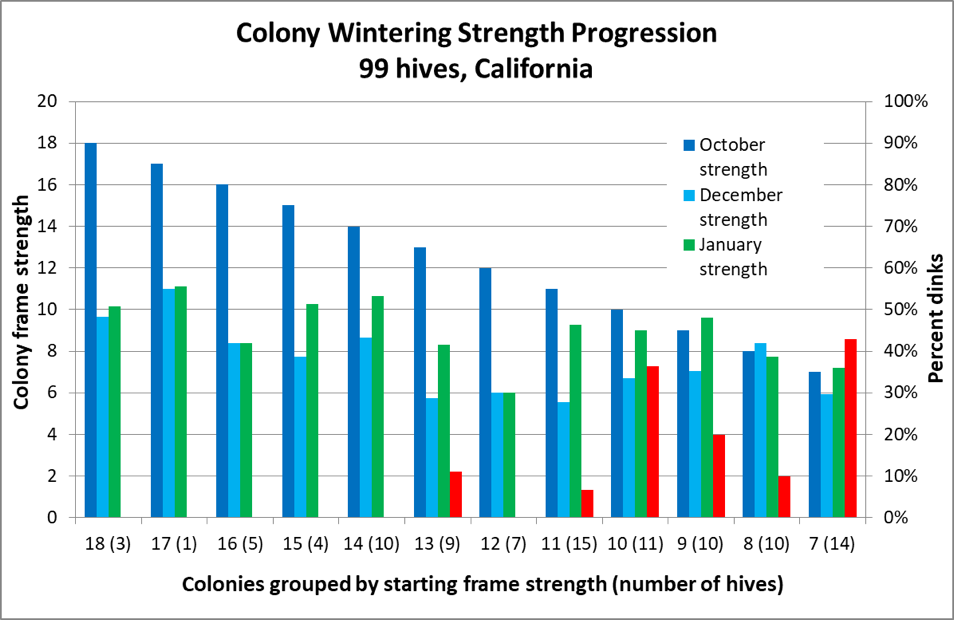
But based upon some data that I published some years ago [[19]], it appeared that my own Italians tended to shoot for an 8-frame cluster in December, with those that started much larger in October exhibiting an apparent shedding of excess bees during November (Fig. 3).
Figure 3. The dark blue columns represent colony strength in October; the turquoise columns strength in December ― note how the difference between the two diminishes with starting strength. This data appears to confirm Jeffree’s conclusion that there is a diminishing return for very large autumn strength ― note the amount of growth by weaker colonies between December and late January (green columns). However, also note for hives going into winter at less than 14-frame strength in October, there was an increased occurrence of them later being too small to take to almonds (the red columns indicating the percentage that turned out to be “dinks”) ― perhaps due to existing nosema or viral issues (which I did not measure).
So why would those strong colonies reduce their winter cluster size to such a great extent? Möbus ran an experiment by combining two colonies into one ― in order create “super colonies.” He found that when there are too many bees in the winter cluster, or if the hive is too well insulated in a mild climate, that the cluster couldn’t deal with its own generated heat, which resulted in both the overheating of the cluster core, as well as forcing “thirst-crazy” bees to fly out to seek water, resulting in them quickly chilling and dying outside of the hive.
Practical applications: The optimal cluster size for wintering is likely dependent upon the ambient temperature. At temperatures around freezing, undersize clusters may be more prone toward dysentery (this assumption needs to be confirmed by research), whereas oversized clusters may suffer from overheating and thirst. This situation may be reversed if the bees are forced to deal with subzero (°F) temperatures ― Omholt’s model suggests that large clusters under those conditions would accumulate moisture.
And then there is the question of bee genetic stock. Cold-adapted races (e.g., Carniolans or Russians) may winter well in a 6-frame or smaller cluster, yet still build up rapidly once pollen becomes available in the spring [[20]].
Optimal cluster size may also be different for a beekeeper intending to go to almond pollination with strong colonies to be split immediately after bloom, as opposed to one who aims for minimal honey consumption during long confinement, and lack of swarming before a later-occurring honey flow.
Winter stores ― honey and beebread
I’ll quote from Farrar’s timeless advice [[21]]:
Whether a colony survives the winter in good condition is determined more by its make-up than by the kind or amount of protection. A good colony normally requires 60 or more pounds of well-ripened honey and the equivalent of 3 to 6 frames of pollen.
Unripened honey contains an excess of moisture. And with honey that crystallizes in the comb, a high-moisture supernatant may form as the glucose precipitates out. Bees feeding upon unripened honey or dilute supernatant may develop dysentery. Beekeepers in areas in which their colonies go into winter with crystallization-prone honey reserves (such as canola or ivy) report that their colonies winter better on sugar syrup (perhaps containing inverted sugar). I’m not at all clear on the effect of local honeydews. As far as stored pollen (beebread), this is necessary to provide the protein necessary for broodrearing.
Practical application: A few natural honeys are tough for colonies to winter on; colonies sometimes do better on stored sugar syrup converted to “honey.” But keep in mind that it’s important to give the bees time to ripen any late-fed sugar syrup to remove excess moisture. Feeding syrup during the winter can overload a colony with moisture, thus the recommendation to feed fondant, granulated sugar, or sugar bricks.
Clarification: I am not suggesting that converted sugar syrup is “better” for the bees than most natural honeys, which contain trace amounts of protein and minerals, which may have some benefit to “winter bees” that do not have access to stored beebread.
Many beekeepers around the world harvest most of their colonies’ honey for sale, and then feed back less-valuable sugar syrup, and their colonies seem to winter well.
Disclaimer: I have a bias towards “naturalness,” in my own life, as well as for my bees. For the first 25 years of my beekeeping career I eschewed any sort of artificial feeding. But then one day a friend who was a professional beekeeper said “I don’t know whether feeding sugar syrup is good or bad biologically, but I can tell you that it’s often like waving a magic wand over a hive.” This observation forced me to rethink whether I was letting my own bias prevent me from practicing optimal bee husbandry.
There is no lack of strong opinions among beekeepers, and one reader questioned my suggestion that the feeding of sugar syrup can be beneficial to a colony, especially with regard to winter stores. So I recently reviewed the literature.
Back in 1977, Dr. Floyd Moeller of USDA summarized the results of their studies on winter prep. His recommendations indicated that honey bees winter well on cured sugar syrup [i].
Guler [ii] more recently found that in their study, the feeding of sucrose or sucrose/invert blend resulted in better wintering than in control colonies feeding upon their honey (I would not extrapolate this finding to suggest the syrup is necessarily better than honey).
Szawarski [iii] found no apparent differences in winter performance of colonies consuming honey or sucrose syrup.
Wang’s findings [iv] suggest that sucrose maintains adequate gut microbial community over winter.
Lastly, Papežíková [v] observed no substantial effect of sugar type upon nosema infection.
Conclusion: The scientific evidence confirms beekeeper experience around the world – bees (similar to hummingbirds) are well-adapted to making use of refined sugar.
I now have many years of experience in feeding sucrose syrup and sucrose/HFCS blends (as well as various sugar fondants and dry forms). We feed judiciously (since it is labor intensive), but don’t hesitate to use sugar to benefit our colonies when indicated. We still winter our colonies mainly on natural honey.
After learning to use sugar syrup, I agree with my friend’s observation that sugar (in either syrup or solid form) is one of the most useful tools available to the beekeeper.
[i] Moeller, F (1977) Overwintering of honey bee colonies. USDA Production Research Report #169.
[ii] Guler, A, et al (2018) Effects of Feeding Honey Bees (Hymenoptera: Apidae) With Industrial Sugars Produced by Plants Using Different Photosynthetic Cycles (Carbon C3 and C4) on the Colony Wintering Ability, Lifespan, and Forage Behavior. Journal of Economic Entomology11(5): 2003–2010.
[iii] Szawarski, N, et al (2019). Effect of abscisic acid (ABA) combined with two different beekeeping nutritional strategies to confront overwintering: Studies on honey bees’ population dynamics and nosemosis. Insects 10(10): 329.
[iv] Wang, H, et al (2020) The different dietary sugars modulate the composition of the gut microbiota in honeybee during overwintering. BMC Microbiol 20 : 61.
[v] Papežíková, I,et al (2020). Effect of feeding honey bee (Apis mellifera Hymenoptera: Apidae) colonies with honey, sugar solution, inverted sugar, and wheat starch syrup on nosematosis prevalence and intensity. Journal of Economic Entomology 113(1): 26-33.
It’s difficult for the beekeeper to control how much beebread is stored, but perhaps the feeding of supplemental protein at the right time may be a management tool for helping colonies to deal with moisture balance. Again, this subject is crying for more research.
Hive placement
It helps a colony greatly to be able to take advantage of the occasional warm winter day, which allows it to break cluster, move to honey stores, and for the bees to take cleansing flights (with sick bees being less likely to return).
Practical application: Experience can teach a beekeeper a great deal about locations suitable for wintering. It helps the colony greatly to avoid being in a cold pocket, and to enjoy a warming southern exposure (indeed, insulation on the south side may perhaps be detrimental). Moving hives even a few dozen yards may make all the difference in the world. One may consider providing a dark south-facing surface to absorb sunlight (with minimal insulation on that side), and making sure that the colony enters the winter with the cluster close to a lower entrance (so that workers needing to defecate don’t need to traverse cold abandoned combs on their way out). To get around these (and other) issues, indoor wintering has long been popular in Canada, and is becoming increasingly common in the U.S.
Hive insulation
A number of researchers have run field trials to determine whether there is a benefit to insulating hives during the winter. The results and conclusions drawn are confusing. There may be a benefit to side insulation where winter temperatures are extreme, but top insulation and making sure that the hive is free of air leakage due to wind appears to be more important. Adding a trapped air space or polystyrene insulation under or over the hive cover helps greatly to minimize colony heat loss, and to prevent moisture from condensing under the lid. Top insulation (without top ventilation) also helps to keep the top of the cavity warmer than the lower parts, thus benefitting the colony by helping to recapture the heat of condensation of vented moisture below the cluster.
During a visit to Chile in 2015, where the winter clusters that I observed were very small, beekeeper Vincent Toledo showed me how some beekeepers there swear by placing a plastic “poncho” over the frames containing the cluster, expanding the poncho over adjacent frames once the cluster starts to grow (Fig. 4).
Figure 4. A plastic “poncho” lifted to expose the cluster that it had been draped over. I have not tested ponchos myself, but such a waterproof blanket may help a small cluster to conserve heat and control moisture. Note: this practice was not universally used in Chile.
Boston beekeeper Fatih Uzuner tells me that he is currently experimenting with using inexpensive 65-gallon plastic bags (black or clear) to trap an air space around the hive (of course leaving the hive entrances clear) ― such an arrangement provides a bit of insulation and draft resistance, yet allows solar gain. I’m eager to see his results.
Practical application: Some beekeepers confuse top insulation and moisture absorption. In general, if a filling absorbs moisture, it is unlikely to serve as insulation. I fail to understand the reasoning for absorbing moisture above the cluster ― why not get it to condense below the cluster? It may also be that too much honey or other enclosed space above the cluster could be a problem, since moisture may condense on the cold surfaces [[22]].
Hive ventilation
If you want to get into a fruitless debate, ask some beekeepers their opinions on providing hive ventilation during winter. As noted by Southwick and Moritz [[23]], perhaps we should listen to the “opinion” of the bees:
Many experienced beekeepers know about the value of a top ventilation hole and maintain such an opening throughout the year. However such extra entrances are usually closed with propolis by the bees.
Möbus [[24]] explains:
How often do we read about the need for top ventilation as the answer to all problems? It is supposed to be essential to let the moisture get away from wintering clusters and out of the hive. … Many of the arguments given to back up any recommendations for providing more and more top-ventilation are based on reasoned considerations or anthropomorphic thinking rather than on sharp-eyed observations of bee behaviour. For those who observe behaviour without preconceived ideas it is obvious that the bee has arranged its home to suit its own experiences within the constructed set of combs and the chosen home. … When levels of carbon-dioxide or humidity rise, bees begin to fan and relieve the situation along the line of least resistance: downward and away from the brood nest.
Practical application: bees have been wintering in cavities far longer than humans have been building hives. If they want to close the door to prevent drafts, perhaps it’s for a reason.
I’ve reviewed a ton of studies, and am not convinced that under most circumstances top ventilation is of benefit to the colony. Toomemaa [[25]] says it well:
However, provision of too much ventilation also removes heat vital for bees and forces them to increase their metabolic rate to compensate for the heat loss. Increased food consumption results in increased production of metabolic water and increased moisture in the hive.
He also cites studies (in Russian) that found that decreased ventilation in hives during the winter resulted in less honey consumption, less bee mortality, and better brood rearing in the spring.
Practical application: If indeed the natural instinct of bees is to fan moisture-laden air out of the bottom of the cluster in order to recycle heat, then the beekeeper creating an air convection current via top ventilation may be counterproductive. Again, we need more good practical research on the subject of moisture regulation in the winter cluster.
Literature cited
[1] van Nerum, K, and H Buelens (1997) Hypoxia-controlled winter metabolism in honeybees (Apis mellifera). Comparative Biochemistry and Physiology 117(4):445-455
[2] Omholt, SW (1987) Why honeybees rear brood in winter. A theoretical study of the water conditions in the winter cluster of the honeybee, Apis mellifera J. Theor. Biol. 128: 329-337.
[3] Heinrich, B (1981) The mechanisms and energies of honeybee swarm temperature regulation. J. Exp. Biol. 91: 25 ― 55. Heinrich’s fascinating papers and books are must reads for those wishing to gain a deeper understanding of bee biology.
[4] Möbus, B (1990?) Damp, condensation and ventilation. Scottish Beekeepers Annual. Available at
https://poly-hive.co.uk/damp-condensation-and-ventilation-brood-rearing-in-the-winter-cluster/
[5] Sachs & Tautz (2017) Op cit.
[6] Toomemaa, K, et al (2013) Determining the amount of water condensed above and below the winter cluster of honey bees in a North – European Climate. Journal of Apicultural Research 52(2): 81-87.
[7] Harris, JL (1980) A population model and its application to the study of honey bee colonies. MS Thesis, Univ of Manitoba. http://mspace.lib.umanitoba.ca/bitstream/1993/14743/1/Harris_A_population.pdf
[8] Thanks to Trevor Weatherhead.
[9] Jeffree, EP (1956) Winter brood and pollen in honeybee colonies. Insectes Sociaux 3(3): 417―422.
[10] Möbus, B (1990?) op cit.
[11] Mattila H, et al (2001) Timing of production of winter bees in honey bee (Apis mellifera) colonies. Insectes Sociaux. 48: 88―93.
[12] Möbus, R (1980) Proceeds 27th International Congress of Apiculture
[13] Omholt, SW (1987) Op cit.
[14] Möbus, B (1998b) Brood rearing in the winter cluster. ABJ July 1998: 511-514.
[15] Rembold, H & J Kremer (1980) Characterization of postembryonic developmental stages of the female castes of the honey bee, Apis mellifera L.. Apidologie 11(1): 29-38.
Ghosh, S, et al (2016) Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. Journal of Asia-Pacific Entomology 19: 487―495.
[16] Jeffree, E & D Allen (1956) The influence of colony size and of nosema disease on the rate of population loss in honey bee colonies in winter. J. Economic Entomology 49(6): 831-834.
Jeffree, EP (1956) Op cit.
[17] Jeffree, EP (1959) The size of honey-bee colonies throughout the year and the best size to winter. Central Association of Bee-Keepers, Essex.
[18] Burgett, M. & I. Burikam. 1985. Number of adult honey bees (Hymenoptera: Apidae) occupying a comb: a standard for estimating colony populations. J. Econ. Entomol. 78: 1154-1156.
[20] Thomas Rinderer, pers. comm.
[21] Farrar, CL (1944) Productive management of honeybee colonies in the Northern States. USDA Circular No. 702. Free download at Google Books.
[22] Toomemaa, K, et al (2013) Determining the amount of water condensed above and below the winter cluster of honey bees in a North – European Climate. Journal of Apicultural Research 52(2): 81-87.
[23] Southwick, E & R. Moritz (1987) Op cit.
[24] Möbus, B (1990?) Op cit.
[25] Toomemaa, K, et al (2013) Op cit.