Sick Bees – Part 18F7: Colony Collapse Revisited – Pesticide Exposure
A Comparison To Some “Control Groups”
The Four Horsemen And The Tip Point
Could Pesticides Cause Colony Mortality And CCD?
The Heart Of The Hive – The Nursery
But Don’t We Already Know That It’s The Neonicotinoids?
An “Acid Test” Of Neonic Seed Treatment
So Which Pesticides Are Actually To Blame?
Choosing To Ignore The Obvious
No More Safe Home To Return To
The Beekeeper Contribution To Shifting The Tip Point
Undetectable Levels And Hormesis
Sick Bees Part 18f7: Colony Collapse Revisited
Pesticide Exposure
Randy Oliver
ScientificBeekeeping.com
Originally published in ABJ Oct and Nov 2013
Oh No, Not Pesticides Again!
Some readers may wonder why I am spending so much time on the issue of pesticides, since to many (if not most) beekeepers, pesticides are a non issue. In answer, the main reason is that the public (and our lawmakers) are being hammered by the twin messages that the honey bee is on the verge of extinction, and that the reason is pesticides. In my writings, I’m attempting to address the validity of both of those claims. Let’s start with the first.
Reality Checks
Honey bees have clearly (and deservedly) become one of today’s most charismatic environmental poster children, and as such are a useful bioindicator that our human activities are having a negative impact upon pollinators, and wildlife in general.
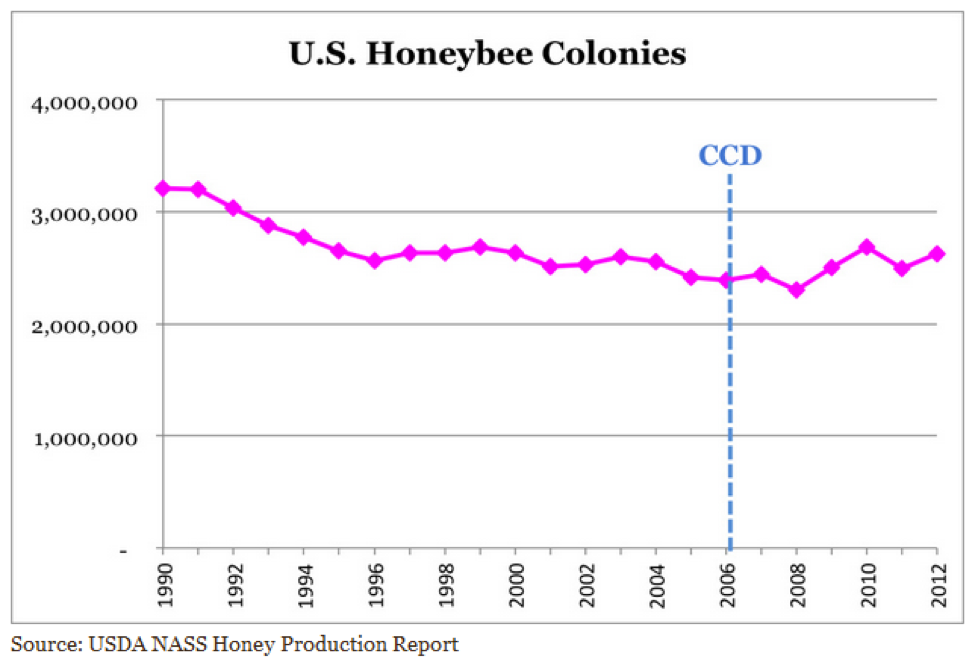
But I also feel that we take care to not overstate or exaggerate our case. One of my greatest concerns is that beekeepers are allowing the media to scare the public with all the hue and cry of an impending bee-pocalypse (and that it is due to a certain type of pesticide). Our complicity in this message (as we enjoy the luxury of basking in the warmth of all the public support) may backfire on us one of these days—putting us into the position of the little boy who cried wolf. Some in the media are starting to notice that the facts don’t support the claim that bees are disappearing (Fig. 1).
Figure 1. It’s true that it’s more difficult to keep bees healthy these days, but it doesn’t look like bee-pocalypse is imminent (as evidenced by this recently-published chart). Whenever honey and pollination prices are high enough to make beekeeping profitable, resourceful beekeepers somehow manage to recover their colony losses [[i]]. Chart courtesy Shawn Regan [[ii]].
[i] Rucker, RR and WN Thurman (2012) Colony collapse disorder: the market response to bee disease. http://perc.org/sites/default/files/ps50.pdf
[ii] (Broken Link) http://perc.org/articles/everyone-calm-down-there-no-bee-pocalypse
In the rest of the world, the number of managed hives has actually been increasing [3]. And as far as claims that pesticides are driving bees to extinction, Hannah Nordhaus, the author of the excellent book The Beekeeper’s Lament writes:
Reflexively blaming pesticides for all of the honey bee’s problems may in fact slow the search for solutions. Honey bees have enough to do without having to serve as our exoskeletal canaries in a coalmine. Dying bees have become symbols of environmental sin, of faceless corporations out to ransack nature. Such is the story environmental journalism tells all too often. But it’s not always the story that best helps us understand how we live in this world of nearly seven billion hungry people, or how we might square our ecological concerns and commitments with that reality. By engaging in simplistic and sometimes misleading environmental narratives — by exaggerating the stakes and brushing over the inconvenient facts that stand in the way of foregone conclusions — we do our field, and our subjects, a disservice [4].
Further reading: for a detailed and sober analysis of the factors that affect managed bee populations, I highly recommend the review by Drs. vanEngelsdorp and Meixner [5].
The reality is that it is not the honey bee that is being driven to extinction—it is instead the commercial beekeeper who is finding that his traditional business model is becoming less profitable due to today’s greater degree of colony losses and the decreasing availability of good summer forage.
The question is, to what extent are pesticides involved in those problems?
The Two Worlds Of Beekeeping
There are two very different worlds of beekeeping—small scale (hobbyists, who constitute the vast majority of beekeepers by number) and large scale (commercialized professionals, who manage the vast majority of hives), with a small continuum of sideliners bridging the gap.
Hobby beekeeping is currently enjoying a bubble of resurgence, but in the Big Picture in the U.S., hobbyists manage an insignificant number of hives. And those small-scale beekeepers tend to keep their hives close to home, largely avoiding serious exposure to pesticides. But that’s not to say that small-scale beekeepers are immune to pesticide kills; I’ve heard of several this season, and what with all the spraying for West Nile virus and the citrus psyllid, we can expect more of the same. And since there are far more small-scale beekeepers to put pressure on regulators and legislators, I feel that it is a good idea for them to be informed about pesticide issues.
Large-scale beekeepers, on the other hand, typically run migratory operations—moving their hives to almond pollination, and then to other agricultural areas (it’s problematic to keep apiaries of hundreds of hives in the suburbs). The fate of those bees (and their keepers) is largely determined by agricultural land use practices and their degree of exposure to agricultural pesticides.
It is some of those large-scale beekeepers for whom extinction is a valid concern. The reason (as with any other enterprise) is financial—they can only survive so long unless their businesses continue to be profitable [6]. In recent years, they’ve had two things going for them—sky-high honey prices and elevated pollination fees. But all is not rosy—there are reasons for those prices going up; these days it’s simply more costly to produce honey or to provide bees for pollination.
Today’s breathtakingly-high high almond rental rates typically don’t even cover operating costs—even if most of one’s colonies make it through the winter! Today’s 30% average winter loss rate is bleeding profitability from many operations. Not only does the beekeeper need to rebuild his numbers after almonds, but to stay in the black he must also make additional income from paid pollinations or a decent honey crop. And that may no longer be as easy as it used to be for various reasons:
- Formerly bee-friendly farmland has been turned into agri-deserts devoid of any bee forage.
- Honey producers on field crops (such as alfalfa, sunflowers, or cotton) get hammered time and again by pesticide spraying, sometimes watching whole yards of colonies dwindle or go queenless weeks afterwards.
- Paid summer pollination contracts (such as for vine crops) may leave colonies in poor shape for the winter, due to the heavy stocking, the lack of nutritious pollen, and the exposure to multiple pesticides.
These days, the sad fact is that many good beekeepers are barely keeping their heads above water. So the beekeeper’s lament continues—varroa, high winter mortality, and lack of good forage are driving a number of operations into the red.
Practical application: although the “extinction of the honey bee” makes for a good rallying cry, the real concern is the possible extinction of the migratory beekeeper who supplies necessary pollination services to agriculture. So far, the almond industry has been economically propping up the bee industry, but I’m not sure how long that arrangement will be sustainable.
Pestcides And Bee-pocalypse
For some beekeepers, “bee-pocalypse” has already occurred. New York beekeeper Jim Doan, whose case I detailed in a previous article [7], sadly gave it up this year. Here is a beekeeper whose apiaries had been in the same locations for many years without noticeable pesticide problems, but who apparently suffered from devastating spray or dust kills this season and last, as evidenced by piles of fresh dead bees in front of his hives in spring.
Residue analysis of those dead bees clearly showed that they had been exposed to several pesticides, but none of the detects were at levels that would be expected to cause such carnage—so we don’t even know which pesticides or practices to point the finger at! To my scientific mind, this is very frustrating—that our “system” was not able to identify the cause of Jim’s bee kills, to change anything to keep them from recurring, nor compensate an innocent beekeeper for the loss of his livestock and livelihood.
As unlucky as Jim has been, his case is not necessarily the norm. Overall, the issue of environmental toxins is improving. In my own lifetime I’ve seen us clean up our pollution of the air and water, cease atmospheric testing of nuclear weapons, ban DDT, fluorocarbons, and PCB’s, phase out the worst pesticides, and raise the general environmental consciousness. Humans still inflict far too damaging an environmental footprint on Earth, but we are moving in the right direction, and should give ourselves some credit for that!
There is no doubt that pesticides are often involved in bee health issues, but can we blame them for all our problems? That question is best answered by considering the health of those colonies that are not exposed to pesticides:
A Comparison To Some “Control Groups”
There are plenty of beekeepers in non agricultural areas whose apiaries are not exposed to pesticides to any extent. Those hives serve as a “control group,” whose health we can compare to those colonies that do have to deal with pesticides.
For instance, in my own operation of about a thousand hives, their only exposure to pesticides is to the fungicides in the almond orchards (from which they don’t appear to suffer to any serious extent). I haven’t used synthetic miticides in over a decade, rotate my combs, and rarely feed syrup.
Yet, I’ve experienced CCD firsthand, see more queenlessness, unsuccessful supersedure, and experience somewhat higher winter losses than in the old days (meaning before varroa). I hear the same from many others in the pesticide-free control group. The simple fact is that these days it requires better husbandry to maintain productive colonies. Yet we in the “control group” can hardly blame pesticides to be the cause.
And then there are the stationary “treatment free” beekeepers in the middle of intense agriculture who suffer no higher colony loss rates than the norm, despite their apiaries being surrounded by corn and soy [8]. How the heck do we reconcile their success to the problems that the commercial guys experience in the same areas?
Do they owe their success to keeping fewer hives in a yard? To keeping locally-adapted survivor stock? To their placement within flight range of patches of undisturbed forage? To the fact that they don’t move to multiple crops? Or is it because they aren’t contaminating their combs with miticides? Believe me, if I knew the answer, I’d tell you!
As (the very successful) beekeeper Dave Mendes observes, colonies just seem to be more “fragile” these days. It’s no surprise then that the addition of toxins of any sort can help to tip a colony over. The Ericksons [9] put it this way:
Pesticides and their residues in the hive stress bees as do other factors such as weather extremes, food shortages, pests, predators, and disease. Conversely, stress induced by other factors undoubtedly has a significant impact on the level of damage that a pesticide inflicts on a colony.
Note that the above words were written prior to our colonies having to deal with varroa, the varroa-vectored viruses, Nosema ceranae, our evolving brood diseases, GMO’s, neonicotinoids, or Roundup Ready corn.
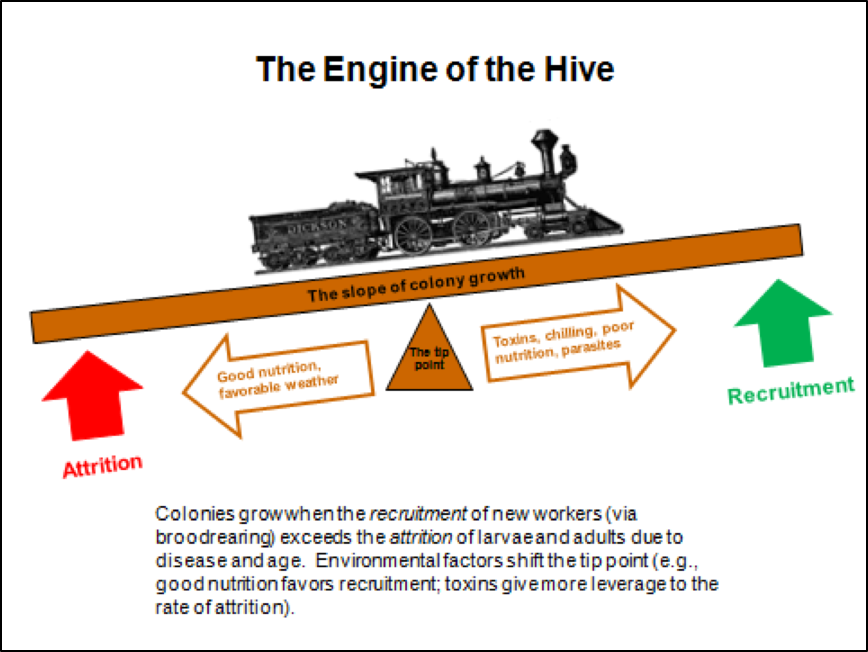
The Four Horsemen And The Tip Point
Colony growth is a function of the recruitment rate via successful broodrearing vs. the attrition rate of workers due to age, disease, the altruistic departure of sick bees, or the loss of foragers in the field. When recruitment exceeds attrition, colonies grow; when attrition exceeds recruitment, the colony population shrinks. Environmental factors, including toxins, can shift the tip point for colony growth (Fig. 2).
Figure 2. Any colony with a good laying queen has the potential to grow rapidly—the greater the rate of recruitment (successful broodrearing), the steeper the slope of the growth curve. In the real world, such potential growth is often held back by the lack of nutritious pollen, or by the stresses of toxins, chilling, or pathogens (especially the mite-associated viruses, nosema, or EFB). Any of those can strongly shift the tip point, slowing, or even reversing, the rate of colony growth.
In the last decade, something appears to have shifted that tip point—colonies today seem to more readily go into a downhill spiral and queens no longer hold up as well. Could it be due to pesticides?
Could Pesticides Cause Colony Mortality And CCD?
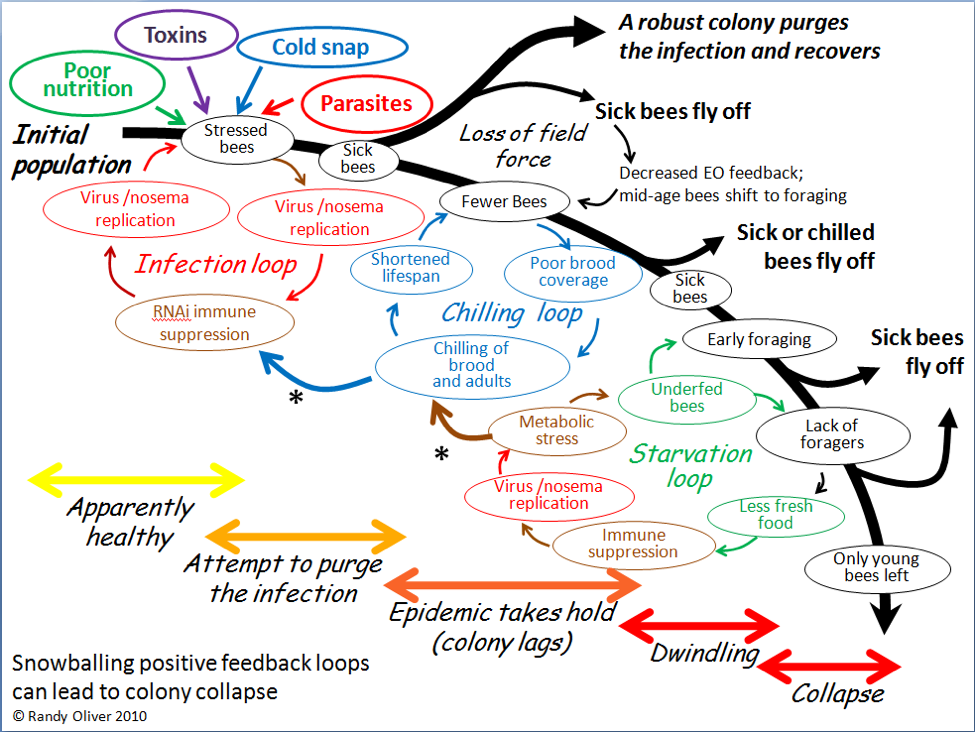
Of course they could! In 2010, after closely observing the progression of experimentally-induced CCD with my collaborator Dr. Eric Mussen, I published the flow chart below (Fig. 3) to detail the interactions and feedback loops involved in the step-by-step collapse of a colony [10]. At the time, I fully intended to further elaborate upon the contribution of toxins, but didn’t get around to it until now.
Figure 3. The positive feedback loops that can lead to colony dwindling and/or sudden depopulation. I’ve since observed this process take place in sick colonies time and again.
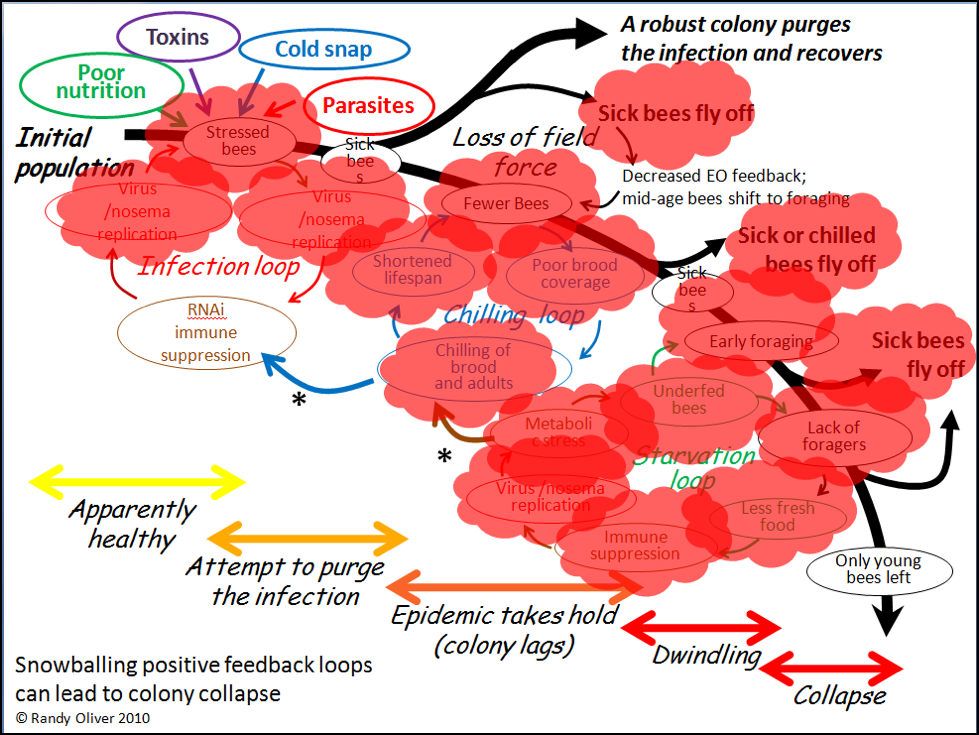
In the above chart, I called out toxins (which would include pesticides) as one of the “Four Horsemen of Bee Apocolypse” (the four factors at top left). Below I’ve indicated in red those points at which toxins may exacerbate the downhill process (Fig. 4).
Figure 4. Note that toxins can exert lethal or sublethal effects (red bubbles) at every step in the process of colony dwindling or collapse. Pesticides may in some cases be the prime cause of colony mortality; more frequently they might be “contributory factors,” especially due the prolonged sublethal effects of residues in the beebread or wax.
Please note that in these charts I’m referring to toxins generically, not specifically to manmade pesticides. Such toxins would include natural plant allelochemicals, industrial pollutants, metals such as arsenic or selenium in soil and dust, fungal and bacterial toxins (which may be altered in beebread by the presence of pesticide residues), beekeeper-applied varroacides, HMF in overheated corn syrup, all in addition to any agricultural pesticides. In the words of ecotoxicologist Dr. Helen Thompson, we must pay attention to the total toxin load of the hive, plus any interactions between those chemicals, as well as other contributory factors [11]—a sentiment also echoed by the Fraziers at Penn State [12]. So, back to our original question: Can toxins, including synthetic pesticides, cause colony morbidity or mortality?
Verdict #1: clearly, synthetic pesticides and varroacides may constitute the most serious toxin load for managed bees in agricultural areas, and have the potential kill a colony outright, or to exacerbate positive feedback loops that can result in dwindling, poor overwintering, or collapse.
But does any pesticide specifically cause CCD—“the disappearance of most, if not all, of the adult honey bees in a colony, leaving behind honey and brood but no dead bee bodies” [13] (and no sign of brood diseases or varroa-induced DWV collapse).
Analysis: The most direct way to answer that question is to see whether we can fulfill Koch’s third postulate [14]: can we experimentally create the symptoms of CCD by treating a healthy hive with the pesticide in question?
Verdict #2: to the best of my knowledge, no one has yet duplicated the symptoms of CCD by treating a colony with any pesticide (the most obvious difference being that there are generally plenty of dead bees present in the case of pesticide toxicity). This is notably true for the neonicotinoids, for which any number of researchers have attempted to duplicate CCD symptoms by continually feeding colonies neonic-tainted syrup or pollen.
Hold on—drop those stones! I am not saying that pesticides cannot contributeto CCD or colony morbidity or mortality in general—my chart above clearly illustrates that they have the potential to do so. Yet even those beekeepers who manage to completely avoid pesticides may still experience sudden colony depopulations, dwindling, or excessive winter losses due to some combination the Four Horseman (as in the perfect storm detailed at [15]).
I feel that it is a serious error for us to try to link CCD to pesticides. Pesticides have always been an issue to beekeepers, but CCD-like events have historically come and gone (as in Disappearing Disease—read the description at [16]). Pesticides will remain an issue long after the term “CCD” is forgotten.
Bottom line: Despite the fact that the evidence at hand does not support the case that CCD is directly caused by any pesticide, that fact certainly does not mean that we should ignore pesticide issues. If anything, we beekeepers ourselves have helped to make pesticides even more of an issue these days.
Short Memories
There is a popular myth going around that pesticides only started to become an issue to honey bee colony survival in 2007. In fact, the sublethal effects of pesticides were well known to beekeepers and researchers long before then. If we review the older literature [17], we find that it was already well known that contaminated pollen was a more serious issue to colony health than the in-field kill of foragers. We knew that colonies might collect such tainted pollen from miles away, that dusts were worse than sprays, that young bees may be more susceptible than older bees, and that temperature and humidity had a great deal to do with pesticide toxicity. Pesticide issues were actually far worse in the 1960’s and ‘70’s than they are today, and have generally improved since then (not to say that some new issues haven’t arisen).
On the other hand, the overall contamination of combs with pesticides has increased in recent years due to the direct contribution by we beekeepers ourselves. In virtually any residue analysis of beebread or beeswax these days in any country with varroa, the most prevalent toxins are the beekeeper-applied varroacides [18]–you may wish to refer back to my chart of the “toxicological eras of honey bee evolution [19].
So one question is, To what degree we have shifted the tip point of colony health by contaminating our brood combs with miticides? Let’s explore the broodnest…
The Heart Of The Hive – The Nursery
The insidious, long-term effects of total toxin load (including pesticide and varroacide residues) would be from those that made it into the heart of the hive—the critical stored beebread and the wax of the brood combs (Fig. 5). Note: Dr. David Fischer of Bayer brings to my attention that in the case of imidacloprid, the results of his testing indicates that bees in the hive are more affected by residues in the nectar than by those in the pollen.
Figure 5. Long after a pesticide-sprayed field force has been replaced by newly-recruited foragers, the colony may still need to deal with the lingering effects of pesticide residues in the combs, and especially in the all-important stores of beebread. It is here that such persistent residues can affect colony health and buildup for many months after the initial exposure, and exactly where we should focus our attention.
Here’s some food for thought: a toxin need not actually kill a single bee to mess up a colony. There are many ways in which sublethal levels of toxins can negatively affect the colony population curve. A few examples would be:
- By decreasing the survival rate of larvae (as from residues of varroacides [20, 21], or fungicides [22]) , or by increasing their development time (as effected by various pollutants, plant alleleochemicals, pesticides, or miticides).
- By affecting the proper fermentation of beebread (fungicides).
- By affecting the sensitive nurse bees that must digest that beebread and produce the critical jelly used to feed the brood, queen, and other workers (natural plant toxins, pollutants, or pesticides).
- By affecting the normal behavioral progression of the workers. E.g., if workers initiate foraging prematurely, this greatly reduces their overall longevity, and results in severe depression of colony growth [23] (much more research is crying to be done, but many chemicals would be suspect).
- By requiring bees to allocate precious resources toward the detoxification of the poisons (as per my leaky boat analogy [24]).
- By increasing the virulence of varroa, nosema, or viruses (any number of pesticides and miticides have been implicated [25, 26])
- By affecting normal colony homeostasis, such as thermoregulation of the brood, which is dependent upon the proper assessment of temperature, and the ability to effectively generate heat by the vibration of the wing muscles (neurotoxins would be expected to affect this ability).
- By affecting the longevity of the queen, the viability of spermatozoa, or the ability of a colony to successfully supersede (coumaphos notably had this effect)
- By affecting the production of, or normal communication via pheromones (which include the recognition of brood and the queen) [27] (essential oils, formic acid, other pesticides?)
- By affecting foragers’ ability to communicate by dance, to navigate, to learn (a wide range of pesticides [28]), or to react properly to normal stimuli (neonics can clearly do this [29]; but similar effects could be due to any number of other pesticides).
Bottom line: the toxin load in the broodnest can greatly affect a colony in many ways, generally (but not necessarily always) negatively. The greater the total toxin load with which the colony is forced to deal, the more likely that it will suffer from the combined ill effects.
Industry’s Arguments
In order to present an objective review of pesticide issues, we should also hear Industry’s side of the argument. The industry-funded think tank OPERA [30] takes the position that:
Although, based on the facts outlined above, there does not appear to be any strong evidence that sublethal effects of pesticides play a key role as causative factors behind bee colony mortality (which is likewise supported by the fact that in several monitoring projects no correlation has been found between colony losses and pesticide exposure), sublethal effects are certainly a point where more fundamental research is needed to obtain a clearer picture of the nature of the issue.
The above statements are factually correct in that there is to date no compelling evidence that pesticides are at the root of the elevated rates of colony mortality seen in recent years, and that more fundamental research is clearly needed. But a long history of practical experience by beekeepers with the sublethal (as well as lethal) effects of pesticides leaves no doubt that pesticides certainly have the potential to cause colony health issues.
But Don’t We Already Know That It’s The Neonicotinoids?
The media have already tried and convicted the neonicotinoids as the cause of all bee problems, and it’s currently fashionable to celebrate the restrictions recently imposed on them by the European Union. But it is rational? No one has ever shown convincing evidence that neonics are linked to colony collapse; conversely, there is abundant experimental and on-the-ground evidence that the residues from seed-treated plants do not appear to cause observable harm to colonies [31].
Planting dust, soil drenches, or foliar applications are a different story, but these are generally drift or misapplication issues, hitting individual apiaries, not the bee population as a whole. Our regulators are well aware of these issues, and working to fix the problems.
Regarding the completely unacceptable bee kills due to the dust from corn seeding, of interest is a recent paper by Drs. Chris Cutler and Cynthia Scott-Dupree [32]—environmental toxicologists from Canada’s Dalhousie University—who analyzed the 110 pesticide incident reports received by Canada’s PMRA since 2007. Ranking the reports by the degree of severity of the bee kill, they found that there were over five times as many “major incidents” due to non-neonicotinoid products (including carbofuran, chlorpyrifos, coumaphos, diazinon, dimethoate, fluvalinate, formic acid, permethrin, and phosmet) as there were due to neonics, yet that these incidents are largely ignored by the press and beekeepers, who for some reason single-mindedly focus upon the neonics.
Hey, I’m as concerned about pollinators and pesticides as anyone. A recent review by Goulson [33] points out the excessive use of neonics (actually all pesticides are greatly overused), and details the many environmental questions about this class of chemicals. But here’s the thing—I can read studies all day long, but what I prefer to seek out are actual on-the-ground, real-life observations. Let me share one with you:
An “Acid Test” Of Neonic Seed Treatment
Activists are calling for a ban on clothianidin—the most common neonicotinoid seed treatment. Although honey bees appear to do just fine on seed-treated canola, their species has an advantage over solitary bees and other pollinators, due to their foraging on multiple plant species over a wide area, their social structure, and their processing of the pollen by nurse bees. So honey bees may not be the best indicator of neonic toxicity.
On the other hand, solitary bee species may be a better indicator as to whether neonic residues cause subtle adverse effects. Many solitary bees are “monovoltine,” meaning that they only raise a single generation per year. Because of this, a negative effect on any single female bee could prevent the production of the next generation. It occurred to me that the Alfalfa Leafcutter Bee (Megachile rotundata), which is used to pollinate clothianidin-treated canola (Fig. 6), would provide an excellent “acid test” of clothianidin for several reasons:
- Clothianidin has been shown to be highly toxic to leafcutter bees by topical application [34]. Since neonics are typically an order of magnitude more toxic by oral exposure [35], it is reasonable to expect that the leafcutter bee would be even more susceptible to residues consumed in food.
- Leafcutter bees do all their foraging within a few hundred feet of the nest [36], so those placed in the middle of a canola field would forage solely upon treated canola.
- Each individual female alone forages and provisions her nest, feeding upon the contaminated pollen and nectar as her sole protein and energy sources. If the insecticide negatively affected her behavior, navigational ability, health, or longevity she would be unable to reproduce effectively.
- The male bees use canola nectar as their sole energy source, and if the insecticide residues interfered with their behavior or longevity, the female bees might not get properly mated.
- The larvae consume a diet consisting solely of unprocessed contaminated pollen and nectar (rather than royal jelly), and thus every item in their diet would contain verified concentrations of clothianidin (approximately 1.7 ppb in the pollen; 0.8 ppb in the nectar [37]). Note: as with honey bees, neonicotinoids are virtually nontoxic to the larvae of the leafcutter bee [38].
- The female constructs her nest by cutting (with her mouthparts) leaves from the treated canola plants, which contain even higher residues of clothianidin than the pollen, thus exposing her to even more of the chemical. The larva then develops surrounded by these contaminated leaves, and the pupa overwinters in them.
Figure 6. Tents covering Alfalfa Leafcutter bee nest boxes in a canola field.
In short, the leafcutter bees would constitute the most severe test case for clothianidin exposure from a seed-treated crop. So I phoned a commercial supplier of leafcutter bees in Ontario (who declined to be named) and asked him whether he had any problems with his bees reproducing or overwintering after being set in clothianidin seed-treated canola. He said that he had been rearing them on such fields for many years and did not observe any problem. I put a good deal of faith into such unbiased field experience by a commercial bee man. You can draw your own conclusions.
So Which Pesticides Are Actually To Blame?
It’s pretty easy to diagnose an acute bee kill, what with piles of twitching bees in front of the hives (see “Signs and symptoms of bee poisoning” at [39]), and in many cases the responsible pesticide can be identified. To sidetrack briefly, remember when I mentioned a few articles back that the residues in Jim Doan’s bee kills did not indicate that the bees contained lethal doses of the chemicals? This made me strongly suspect that we can’t apply the LD50 data (in nanograms per bee) to the values obtained from actual field samples of dead bees. The recent report from Canada [40] confirms this. The highest residue level of clothianidin (from corn planting dust) found in any sample of dead bees from the entrances of a hive was 24 ppb, which works out to about a tenth of the theoretical amount necessary to kill a bee . This finding could be due to the metabolic degradation of the insecticide, but it certainly suggest that the LD50 value should be adjusted lower for samples of dead bees! I am greatly heartened that Canada is moving forward in addressing this issue of bee kills from corn planting dust [41].
Overt bee kills aside, more insidious are the residual effects due to contaminated dust, pollen, or nectar that foragers bring back into the broodnest. I’m told by beekeepers with far more experience with pesticides than I, that after exposure to certain pesticides, colony growth and production come to a standstill, sometimes for months, until the colony clears itself of residues and perhaps eventually recovers (or not). The problem is that few beekeepers (if any) can look inside a hive and diagnose which pesticide (or combination thereof) is causing the problem. He may notice spotty brood, poor buildup, winter dwindling, or queenlessness, but it is very hard to isolate the effect any particular pesticide residue, especially in today’s stew of residues in combs. But that doesn’t mean that we are completely blind…
The Evidence
Due to the rapid turnover of bees in a hive (other than the queen or “winter bees”), if a pesticide were indeed exerting a long-term effect upon colony health, then there would by necessity need to be residues of that pesticide or its degradation products persisting in the combs. With today’s testing equipment, we can detect residues to the parts per billion level, and have quite a large database of residue analyses of beebread samples, which we can perhaps use to either finger or exonerate certain pesticides suspected of being involved in colony health issues.
In a court of law, all evidence would be laid out before the court to determine whether it was substantial enough to make a case against a particular suspect. We can do something similar by reviewing two large publicly-available datasets of actual pesticide analyses of beebread from across the country—one by the Penn State team , the other by the USDA (Tables 1 and 2). I’ve condensed their data to only those pesticide detects that were found in at least 10% (Penn State) or 5% (USDA) of the samples, following this reasoning:
If a pesticide isn’t present in at least 10% of samples, then it isn’t likely to be the cause of widespread problems.
I’ve also color-coded the results as to the type of pesticide, and included the median detection level (to help us to determine whether that dose would be expected to cause colony health problems, or whether it would be insignificant).
| Pesticide |
Present in percent of samples* |
Median detection if positive for target (ppb) |
Type of pesticide |
| Fluvalinate |
88.3 |
40.2 |
Beekeeper-applied miticide |
| Coumaphos |
75.1 |
13.1 |
Beekeeper-applied miticide |
| Chlorpyrifos |
43.7 |
4.4 |
Insecticide |
| Chlorothalonil |
52.9 |
35 |
Fungicide |
| Pendimethalin |
45.7 |
13.4 |
Herbicide |
| Endosulfan I |
28 |
4.2 |
Insecticide |
| Endosulfan sulfate |
26.3 |
2.2 |
Insecticide |
| DMPF (amitraz) |
31.2 |
75 |
Beekeeper-applied miticide |
| Atrazine |
20.3 |
8.9 |
Herbicide |
| Endosulfan II |
20 |
3.8 |
Insecticide |
| Fenpropathrin |
18 |
7 |
Insecticide |
| Azoxystrobin |
15.1 |
10.2 |
Fungicide |
| Metolachlor |
14.9 |
8.1 |
Herbicide |
| THPI (Captan) |
14.2 |
227 |
Fungicide |
| Captan |
12.9 |
103 |
Fungicide |
| Esfenvalerate |
11.7 |
3.3 |
Insecticide |
| Carbaryl |
10.9 |
36.7 |
Insecticide |
| Cyhalothrin |
10.9 |
1.7 |
Insecticide |
Table 1. The 2010 survey by the Penn State team [42], based upon (depending upon the pesticide) either 350 or 247 samples. This study (plus numerous others worldwide) clearly point out that the predominant pesticide residues in brood combs are typically those from the beekeeper-applied miticides (yellow).
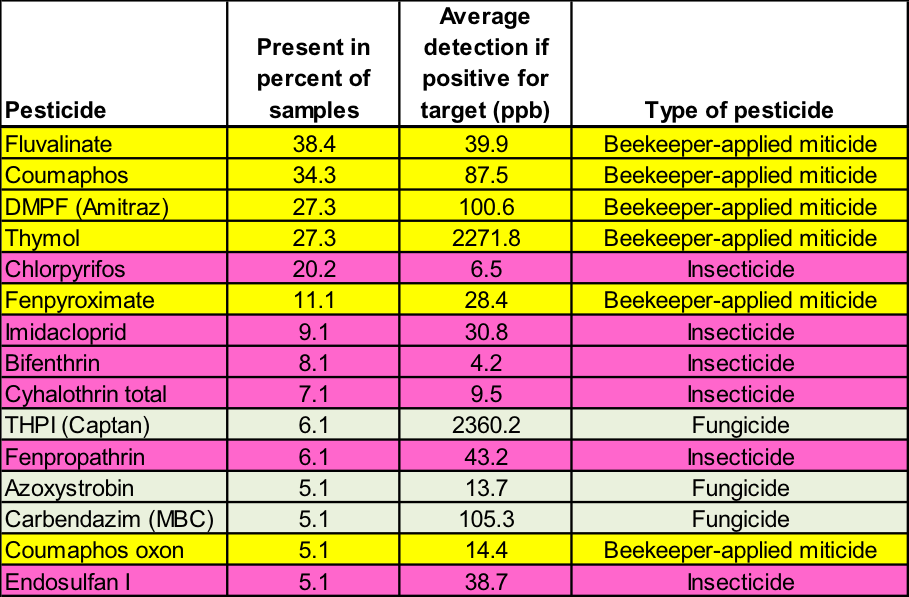 Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.
Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.
Keep in mind that the above surveys screen only for 174 chosen pesticides—compare this number to the roughly 1000 pesticide active ingredients and adjuvants registered for use in California. I’ve discussed the composition of this list with USDA’s Roger Simonds, who runs the tests. It is prohibitively costly to test for every possible pesticide, so one must arbitrarily draw up a limited list of the chemicals of most concern. All are aware that this is a difficult task, since we don’t even know which toxins with which we should be most concerned!
Note that in both surveys, the most common insecticide present was chlorpyrifos– an “old school” (introduced in 1965) organophosphate neurotoxin classified as being “highly toxic” to bees, and marketed as Dursban and Lorsban. Chlorpyrifos was previously widely used by homeowners and residential pest control companies. EPA has since restricted its use due to its toxicity to wildlife and aquatic organisms, and possible links to human health issues [44]—some of the reasons that EPA favors the neonicotinoids as “reduced risk” products.
Oh Boy, Let’s Do Some Math!
But just because a pesticide is present, doesn’t necessarily mean that it is causing measureable harm. A nurse bee may consume about 10 mg of beebread per day [45], so if she consumed that amount of pollen contaminated with chlorpyrifos at 6.5 ppb, then she would have been dosed with 0.065 ng (1 nanogram = 1 billionth of a gram) of the chemical. The question then is, how much chlorpyrifos does it take to actually harm a bee?
One commonly cited figure is that the LD50 for chlorpyrifos given orally is 360 ng/bee. Compare those figures (360 ng for toxicity vs. 0.065 in the daily diet)! Even though chlorpyrifos is a disturbingly common comb contaminant, it is unlikely that the median detected concentration (alone) would be causing colony health problems (not to say that higher doses don’t hurt colonies).
But, you say, some of the neonics are even more toxic than chlorpyrifos. How about the mean 31 ppb found by the USDA in the few samples positive for imidacloprid? The typical nurse bee would consume 0.31 ng, compared to the oral LD50 of about 4-40 ng, so she’d be eating a tenth to a hundredth of the lethal dose. This would be cause for concern, tempered by the fact that a bee can easily metabolize that amount of imidacloprid a day [46]. Such consumption could legitimately be suspected of causing sublethal effects. However, keep in mind that that 31 ppb was an average, which was strongly skewed by a few samples with very high concentrations (which I’d fully expect to cause colony health problems). Plus this is not simply a matter of the average amount of contamination; one must also look at the percentage of positive detects. The Penn State team [47] puts it well:
Our residue results based on 1120 samples which include Mullin et al. (2010) and subsequently more than 230 additional samples do not support sufficient amounts and frequency of imidacloprid in pollen to broadly impact bees.
OK, so how about the varoacides fluvalinate at 40 ppb or the amitraz degradate DMPF at 100 ppb? Surprisingly, I can’t find an oral LD50 for fluvalinate, so the contact toxicity figure (200 ng/bee) will need to suffice. Those residues work out to about 1/500th expected toxicity.
Amitraz scored a bit better, with the nurse bees consuming about 0.01 ng—far below the lethal dose. But a recent study found that an oral dose of 0.2 ng of amitraz causes more than a doubling of the heart rate of a bee [48]—that’s at 1/20th of the average detect! The authors dryly state:
The above responses clearly show that the heart of the honeybee is extremely vulnerable to amitraz, which is nevertheless still used inside beehives, ostensibly to “protect” the honeybees against their main parasite, Varroa destructor.
How vulnerable? Frazier [49] observed that “Dead and dying bees collected around colonies in association with corn had only residues of 2,4-DMPF at 5,160 ppb.” Looks like perhaps the beekeeper inadvertently killed his own bees with an off-label mite treatment that may have overworked their little hearts!
And if those miticide and insecticide residue weren’t enough alone, some of the toxicity of these chemicals is additive or synergistic. The Penn State team again says it well:
[The] pyrethroids… were found in 79.4% of samples at 36-times higher amounts than the neonicotinoids, on average… The mean neonicotinoid residue was 37 ppb (scoring non-detects as 0 ppb), of which only 6.7 ppb was imidacloprid. Pyrethroids, by comparison, were present at a mean residue of 106 ppb and a frequency of 80.3% in pollen samples… Indeed, if a relative hazard to honey bees is calculated as the product of mean residue times frequency detected divided by the LD50, the hazard due to pyrethroid residues is three-times greater than that of neonicotinoids detected in pollen samples [emphasis mine].
The pyrethroids are popular because they are relatively nontoxic to humans. But they can sure kill honey bees. More so, they can cause sublethal effects, such as irreversible inhibition of olfactory learning ability [50].
Hey, we’re only getting rolling! Mussen [51] pointed out a decade ago that fungicides could kill larvae; recent research from the Tucson lab [52] and elsewhere confirm that fungicide residues can mess up the colony (we sometimes observe this in almonds). Of note is that colonies treated with some fungicides were unsuccessful at requeening themselves! And recent research by Zhu [53] found that the relative toxicity of larvae to the commonly-detected fungicide chlorothalanil was almost 40 times higher than that of chlorpyrifos. Fungicides are frequently found at high concentrations in beebread.
I cannot help from returning to the refrain that instead of limiting our concern to any single pesticide, that we should be looking at the total toxin load that the colony is forced to deal with.
And How About The “Inerts”?
The pesticide detection analyses above do not look at the “inert” adjuvants in the pesticide “formulation.” These chemicals not only help to disperse the pesticide over the waxy leaf surface, but also aid in its penetration through the insect cuticle, thus making the pesticide relatively more toxic to the bee!
Mullin and Ciarlo [54, 55] found that:
Formulations usually contain inerts at higher amounts than active ingredients, and these penetrating enhancers, surfactants and adjuvants can be more toxic on non-targets than the active ingredients. For example, we found that the miticide formulation Taktic® was four time more orally toxic to adult honey bees than the respective active ingredient amitraz. Impacts of ‘inerts’ in pollen and nectar alone or in combination with coincident pesticide residues on honey bee survival and behavior are unknown.
The researchers also found that:
Learning was [rapidly] impaired after ingestion of 20 µg of any of the four tested organosilicone adjuvants, indicating harmful effects on honey bees caused by agrochemicals previously believed to be innocuous.
One of the common adjuvants is a solvent NMP, described by BASF [56]:
NMP can be used as a solvent or co-solvent for the formulation of insecticides, fungicides, herbicides, seed treatment products and bioregulators where highly polar compounds are required. NMP is given preference over other highly polar solvents because it is exempt from the requirement of a tolerance when used as a solvent or co-solvent in pesticide formulations applied to growing crops, and it possesses a favorable toxicological and environmental profile.
The key words above are that these toxic solvents are “exempt from tolerance” [57], so they are sprayed all over crops along with the active ingredients of pesticides (including imidacloprid). Yet Zhu [58] recently reported that NMP can rapidly kill bee larvae. The authors conclude that:
Our study suggests that fungicide, the inert ingredient and pesticide interaction should be of high concern to honey bee larvae and overall colony health. None of these factors can be neglected in the pesticide risk assessment for honey bees.
Choosing To Ignore The Obvious
There is no doubt that neonics have the potential to harm bees, but the question is, do they really cause as much problem in the real world as we’ve been led to believe? This is not a matter of convincing the masses; this is an investigation of fact and evidence. For a pesticide to cause harm to a colony of bees, two necessary elements must occur:
- The bees must be exposed to the pesticide. Evidence for this is best determined by chemical analysis of the pollen in the combs, since residues in the bodies of dead bees may be degraded, and because water-soluble insecticides such as the neonics are not absorbed into the wax (residues in the wax do document the history of exposure to lipophilic pesticides).
- The pesticide must be present at a concentration above a trivial level.
When we take the time to determine which pesticides bees are actually found in the combs of hives, neonicotinoids are seldom present, or if detected are often at biologically irrelevant concentrations. Imidacloprid was detected in fewer than 3% of Mullin’s 350 samples, and clothianidin not at all! Similarly, there were zero detects for clothianidin in the 99 USDA samples; imidacloprid was only present in 9%. Likewise, a number of European studies have shown similar results (reviewed in [59]).
In a recent study, the Fraziers [60] looked at hives placed in cotton, corn, alfalfa, apples, pumpkins, almonds, melons, blueberries, or wild flowers, and identified the residues in collected pollen, in returning foragers, and in dead or dying bees near the hives. Again, the only neonic noted was thiamethoxam in alfalfa (in which dying bees contained residues of ten different pesticides). However, there were alarmingly high detects of fungicides, the insecticide acephate, and the metabolite of the beekeeper-applied miticide amitraz.
The latest data comes from Dr. Jeff Pettis [61], whose group determined the pesticides in bee-collected pollen from six crops: apple, blueberry, cranberry, cucumber, pumpkin, and watermelon. Of the 35 pesticides detected, beekeeper-applied miticides and ag fungicides predominated (sometimes at alarming levels), followed by common organophosphate, pyrethroid, and cyclodiene insecticides (again sometimes at alarming levels). In the 17 samples tested, residues of neonics were only found in the samples from the apple orchards, and only one was found at a biologically-relevant concentration.
So my question is why the heck are so many activists pursuing the single-minded focus upon the neonics, when the clear evidence is that neonics are not commonly found in bee-collected pollen, and if present, are generally at levels that do not appear to negatively affect colony health [62]?
There is a lot more to pesticide issues than the neonics alone, and by focusing our attention solely upon them, we ignore the often far more serious effects of other pesticides.
Blinded By Bias
During the intense focus upon neonicotinoids the past few years, we’ve learned that exposure of bees to these insecticides can result in all sorts of sublethal effects. Unfortunately, many researchers appear to be wearing blinders as to the effects of other pesticides. The resulting narrowness of these studies skews our perspective—if we only look for effects from the neonics, we don’t know how to rank the biological relevance of those effects relative to the effects of all the other toxins to which bees are exposed, generally to greater extent.
A practical complaint to researchers: if you are going to look for sublethal effects of neonics, please include positive controls of some other pesticides, so that we can learn whether the neonics are better or worse than the alternatives!
I commend one group that recently decided to take a look at the effects of a common herbicide upon the development of bee larvae [63]. The results of this straightforward and meticulous study are an eye opener!
The researchers found that exposing bee larvae to even infinitesimal amounts of the herbicide paraquat prevented them from fully developing their critical oenocyte cells (see box).
| Oenocyte cells are not only involved in the production of lipids and lipoproteins, but they also appear to play a role in the constitution of external cuticle in both larvae and adults. In addition, they are involved in intermediary metabolism and synthesize hydrocarbons to waterproof cuticle or to make beeswax. Furthermore, oenocytes secrete hormones, especially those involved in larval and adult development. They are also described as the major cells expressing cytochrome P450 reductase, which is involved in detoxification of toxins [information paraphrased from the cited paper]. |
Exposure to even a part per trillion of paraquat suppressed the development of these extremely important cells. The authors conclude:
This study is the first which reports an effect of a pesticide at the very low concentration of 1 ng/kg, a concentration below the detection limits of the most efficient analytic methods. It shows that chemicals, including pesticides, are likely to have a potential impact at such exposure levels.
Who woulda thunk? Paraquat isn’t included in the standard screening for pesticide residues, so we don’t even know how prevalent it is in hives! The above findings should make it clear that we need to go back to the beginning if we are to understand the sublethal effects of pesticides (and adjuvants), even at perhaps undetectable levels.
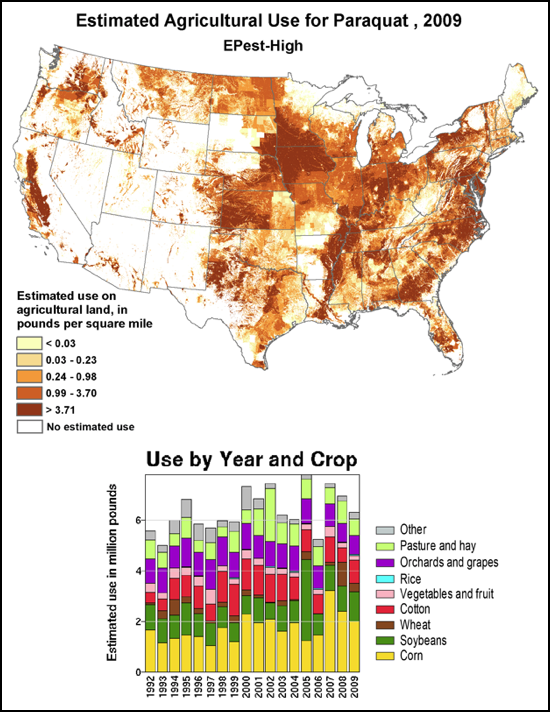
We do know that here were 812,000 lbs of paraquat applied in California in 2010, as opposed to only 266,000 lbs of imidacloprid. Paraquat shows strong adverse effects upon bee larvae at a part per trillion, as compared to imidacloprid, which is so minimally toxic to bee larvae that no one has even been able to determine an LD50! So the amount of paraquat applied has far greater potential to cause problems to bees in agricultural areas (Fig. 7).
Figure 7. The herbicide paraquat appears to be harmful to bee larvae at levels as low as 1 part per trillion. Note the wide variety of crops, and the extensive areas to which it is applied.
So here we have clear scientific data from a well-designed laboratory experiment that a commonly-applied pesticide has the ability to cause immune suppression and other adverse effects in developing bees, yet these results have been virtually ignored by beekeepers and environmental groups. I just don’t understand it!
No More Safe Home To Return To
Out of their protective hive, honey bees live in a hostile world, full of predators, deadly weather, and toxic agents (both natural and manmade). But the bees of old could generally return to a “safe” home, in which the transmission of natural toxins was largely minimized by the behavior of foragers, and by the processes of the conversion of nectar to honey, and of pollen into jelly (via the digestion of beebread by nurse bees). Both of these processes help to prevent the transmission of toxins from the foragers to the queen and the brood.
With the advent manmade pesticides, bees may no longer have that “safe” home to return to. Beebread and the wax combs nowadays are often contaminated with any number of pesticides (in addition to natural plant toxins and industrial pollutants). But this is not a “new” problem:
A Historical Artifact
Even before we had the ability to detect pesticide residues in combs to the parts per billion level, pesticide analyses often found easily-detectable levels of insecticides in bee hives. As a frame of reference, I sought out a historical artifact—the residues in the beeswax that had been rendered by beekeepers and reprocessed into a sheet of “clean” foundation. I was lucky enough to find that such a sample had recently been analyzed by the Tucson Bee Lab. Dr. Diana Sammataro forwarded me the results of the analysis of an undated “very old” piece of wax foundation from the Northeast (Table 3).
THIS IS THE TABLE !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
|
Pesticides in an old piece of beeswax foundation. |
|
|
Positive residue detect |
ppb |
| Pendimethalin |
13.1 |
| Endrin |
156 |
| Dieldrin |
160 |
| Trifluralin |
3.6 |
| DDT p,p’ |
32.7 |
| Heptachlor |
35.1 |
| Malathion |
4.3 |
| Chlorpyrifos |
4.6 |
| Dicofol |
6.8 |
| PCB’s |
8190 |
| Chlorothalonil |
84.6 |
Table 3. We can narrow down the foundation’s date of manufacture by the residues present. Pendimethalin was first registered in 1972 (the same year that DDT was banned), and since there were no residues of fluvalinate, the foundation was clearly produced prior to the arrival of varroa around 1990. Thanks to Dr. Diana Sammataro and the Tucson Bee Lab.
Clearly, pesticide-contaminated combs are hardly a new phenomenon. In the above example, the beeswax batch used to produce the foundation came not from a single hive, but rather from the combined wax from many hives, likely from many beekeepers, and thus would represent an average sample of the degree of contamination somewhere in that 1972-1990 time frame. And that doesn’t take into account whether the raw wax came mainly from cappings (which would have been minimally contaminated), or whether it went through the common practice of being filtered through activated carbon. But any colony started on such foundation purchased from a beekeeping supply house would clearly have had to deal with at least the residues of these lipophilic toxins from the get go!
An aside: perhaps of interest is something that I noticed years ago when I switched from dipping my own wax queen cell cups to using plastic cups. My “take” rate became better and more consistent. Was that because the beeswax at the time was contaminated with residues?
The Beekeeper Contribution To Shifting The Tip Point
One thing that is “new” is that since the arrival of varroa, we’ve upped the ante—all commercial beeswax is now contaminated with residues of beekeeper-applied synthetic miticides. The three most prevalent synthetic chemicals found in combs today all get there by being applied by beekeepers for mite control.
Practical note: And although there is no reason to be concerned about the tainting of honey by the legal use of these miticides, the beekeeper/applicator should be aware that both amitraz and tau-fluvalinate make California’s list of “chemicals known to the State to cause reproductive toxicity,” and coumaphos is of concern because it is a “cholinesterase-inhibiting pesticide.” No varroacide is harmless to bees [64]—but the benefits of mite control generally (but not always) outweigh the adverse effects due to the miticide residues.
We beekeepers have clearly shifted the baseline for pesticide contamination of combs, which increases the total toxic load even before the contribution by agricultural pesticides.
Stop Right There!
Although it is a very attractive hypothesis to blame our problems on miticide or pesticide residues, let’s do a reality check. On good forage in good weather, plenty of beekeepers see their colonies thrive even on old, dark, seriously-contaminated combs; but under stressful conditions those same residues might contribute to poor colony performance or even mortality.
No study has yet found support for the hypothesis that miticide residues are the cause of our current bee problems (although one would have every reason to suspect that they may contribute). In fact, vanEngelsdorp [65] found that surprisingly, higher levels of coumaphos residues negatively correlated with colony survival. How could this be? One possible explanation is that those beekeepers who used it experienced better mite control. But there is also another intriguing possibility—hormetic effects.
Undetectable Levels And Hormesis
Is your head spinning yet? I’ve presented evidence that undetectable levels of some pesticides could harm bees, that “inert” adjuvants can do the same, and that combs are often chock full of all sorts of pesticide and varroacides residues. Criminy, it’s a wonder that bees survive at all! Or is it?
Bees have long been exposed to all sorts of natural, and recently, manmade toxins, and survived. Toxicity is a complicated subject. The only thing that separates a medicine from a poison is the dose. In general, if a pesticide has been tested upon adult and larval bees and found to have no observable adverse effects at a certain concentration, we would not expect to see adverse effects at lower concentrations. However, there are exceptions to this general rule—toxicity may vary up or down depending upon the dose [66]!
I’ve previously mentioned the term hormesis [67]— the paradoxical effect of toxins at low concentrations. The paradox is that although most chemicals are toxic at high concentrations, the majority are likely beneficial at low concentrations. For those interested in this fascinating phenomenon, I suggest Dr. Chris Cutler’s excellent and thought-provoking review [68]. It is not only possible, but actually probable that lose doses of pesticides may exert a beneficial effect upon a colony! (Don’t be ridiculous—I’m not suggesting that bees are better off for the presence of pesticides!).
Wrap Up
Toxins, whether natural or manmade, are clearly a potential issue in colony health. To what degree pesticides contribute to colony morbidity or mortality is dependent upon exposure, the dose, and a host of associated factors. Beekeepers have long noticed that their bees often do better if allowed to forage on pesticide-free land. But many beekeepers today tell me that their bees do just fine in the middle of intense agricultural areas—so this is not a black or white situation.
In recent years beekeepers themselves have greatly added to the degree of contamination of their combs. Introductions of novel pesticides and adjuvants keep changing the picture. And now we’re finding that pesticides that we formerly assumed were harmless to bees (fungicides and herbicides) may actually be quite toxic to larvae! Then there is the scary finding that undetectable levels of some pesticides might cause health issues, countered by the fascinating subject of hormesis.
I certainly do not profess to understand all this, but I have come to the following conclusions:
- That bees have had to deal with toxins for a long time,
- That pesticides will be with us for the foreseeable future,
- That varroacides have likely added to the problem,
- That pesticides can cause lethal and long-term sublethal effects in the hive, but
- That many beekeepers in agricultural areas no longer consider pesticides to be a serious issue, whereas,
- That colonies may go downhill after being exposed to some agricultural chemicals, or combinations thereof,
- That toxicology in the hive is complex, and that there are few simple answers,
- That it is unlikely that any single pesticide is to blame for our current colony health issues,
- That we still have a lot to learn!
Next month I will look at the distribution of both managed colonies and of pesticide applications in the United States, and their relationship to bee health problems.
Acknowledgements
As always, I could not research these articles without the assistance of my longtime collaborator Peter Loring Borst, to whom I am greatly indebted. I also wish to thank Drs. Jim and Maryann Frazier, Chris Mullin, David Fischer, Eric Mussen, Thomas Steeger, and Roger Simonds for their generosity in taking the time to discuss pesticide issues with me.
References
[1] Rucker, RR and WN Thurman (2012) Colony collapse disorder: the market response to bee disease. http://perc.org/sites/default/files/ps50.pdf
[2](Broken Link) http://perc.org/articles/everyone-calm-down-there-no-bee-pocalypse
[3] vanEngelsdorp, D and MD Meixner (2010) A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. Journal of Invertebrate Pathology 103: S80–S95. http://www.sciencedirect.com/science/article/pii/S0022201109001827
[4] http://thebreakthrough.org/index.php/journal/past-issues/issue-1/an-environmental-journalists-lament/
[5] vanEngelsdorp and Meixner (2010) op. cit.
[6] Rucker (2012) op. cit.
[11] Thompson, HM (2012) Interaction between pesticides and other factors in effects on bees. http://www.efsa.europa.eu/en/supporting/doc/340e.pdf
[12] Frazier, J, et al (2011) Pesticides and their involvement in colony collapse disorder. http://www.extension.org/pages/60318/pesticides-and-their-involvement-in-colony-collapse-disorder#.UgO3zKyaucw A must read!
[14] For an explanation refer to https://scientificbeekeeping.com/sick-bees-part-18b-colony-collapse-revisited/
[16] Wilson, WT and DM Menapace (1979) Disappearing disease of honey bees: A survey of the United States. ABJ March 1979: 185-186.
“Certainly with both pesticide-related and [Disappearing Disease]-caused bee losses, the adult population of a colony may be reduced rapidly to a “handful” of bees or, in some cases, the entire population may be lost.
“However, in the case of pesticide poisoning, there is usually evidence of pesticide application…the worker bees either die in the field or in or near the hive depending on the type of pesticide. When the field force is killed and they “disappear,” many dead or dying bees may be seen on the ground in the field or on the ground between the treated field and the apiary…If the foraging bees bring poison into the hive, then the nurse bees either die in the hive or at the entrance so one can see many crawling and tumbling adults and large amounts of neglected brood. Exposure to pesticides over an extended period results in very weak colonies, and some die out.
“In the case of [Disappearing Disease], the situation is quite different. The colonies frequently have gone through a period o nectar and pollen collection with active brood rearing [as in typical CCD]. Then the weather has turned unseasonably cool and damp and remained adverse for from about 3 to 14 days…During the inclement weather, the bee populations dwindle because the worker bees disappear from the hive leaving a “handful” of bees and the queen. Often these small populations recover and increase in size during hot weather and a long nectar flow or, or occasionally, the entire population absconds…”
[17] Johansen CA and DF Mayer (1990) Pollinator Protection: A Bee & Pesticide Handbook. Wicwas Press.
[18] Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, et al. (2010) high levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 5(3): e9754. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0009754
[20] Wu JY, CM Anelli, WS Sheppard (2011) Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 6: e14720 http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0014720
[21] Medici SK, Castro A, Sarlo EG, Marioli JM, Eguaras MJ (2012) The concentration effect of selected acaricides present in beeswax foundation on the survival of Apis mellifera colonies. J Apic Res 51: 164–168
[22] Eric C. Mussen, Julio E. Lopez, and Christine Y. S. Peng (2004) effects of selected fungicides on growth and development of larval honey bees, Apis mellifera L. (Hymenoptera: Apidae). Environmental Entomology 33(5):1151-1154.
[23] Frazier, J.L., M.T. Frazier, C.A. Mullin & W. Zhu – Does the reproductive ground plan hypothesis offer a mechanistic basis for understanding declining honey bee health? http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[25] Wu JY, Smart MD, Anelli CM, Sheppard WS (2012) Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. J Invert Path 109: 326–329
[26] Pettis JS, Lichtenberg EM, Andree M, Stitzinger J, Rose R, et al. (2013) Crop Pollination Exposes Honey Bees to Pesticides Which Alters Their Susceptibility to the Gut Pathogen Nosema ceranae. PLoS ONE 8(7): e70182. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0070182#pone.0070182-Chaimanee1
[27] Maisonnasse A, et al (2010) E-β-Ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS ONE 5(10): e13531. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0013531 These researchers studied (E)-b-ocimene, a volatile terpene commonly produced by plants to attract predatory mites, but also a critical pheromone produced by the brood and the queen.
[28] Decourtye A, et al. (2005) Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Archives of Environmental Contamination and Toxicology 48: 242–250. http://www.environmental-expert.com/Files/6063/articles/4909/QM245Q254G1T6X0R.pdf
[20] Yang E-C, Chang H-C, Wu W-Y, Chen Y-W (2012) Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 7(11): e49472. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0049472
[30] Bee health in Europe -Facts & figures 2013. OPERA http://operaresearch.eu/files/repository/20130122162456_BEEHEALTHINEUROPE-Facts&Figures2013.pdf
[31] The study by Drs. Scott-Dupree and Cutler is yet unpublished, but a summary can be found at http://www.producer.com/daily/ontario-field-study-finds-no-link-between-seed-treatments-bee-deaths/
[32] Cutler, GC, CD Scott-Dupree, DM Drexler (2013) Honey bees, neonicotinoids, and bee incident reports: the Canadian situation. Pest Management Science http://onlinelibrary.wiley.com/doi/10.1002/ps.3613/abstract
[33] Goulson, Dave (2013) An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology 50: 977–987. https://www.sussex.ac.uk/webteam/gateway/file.php?name=goulson-2013-jae.pdf&site=411
[34] Scott-Dupree, CD, et al (2009) Impact of currently used or potentially useful insecticides for canola agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae). J Econ Entomol 102(1):177-82.
[35] Blacquière, et al (2012) Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment http://www.gesundebiene.at/wp-content/uploads/2012/02/Neonicotinoide-in-bees.pdf
[36] Hobbs, GA (1967) Domestication of Alfalfa Leaf-cutter Bees. Canada Dept. of Agriculture. Ottawa: Queen’s Printer and Controller of stationary.
[37] Dr. Jerry Bromenshenk, pers. com.
[38] Abbott, VA, et al (2008) Lethal and sublethal effects of imidacloprid on Osmia lignaria and clothianidin on Megachile rotundata (Hymenoptera: Megachilidae). J Econ Entomol 101(3):784-96.
[40] PMRA (2013) Evaluation of Canadian Bee Mortalities Coinciding with Corn Planting in Spring 2012.
[41] PMRA (2013) Action to Protect Bees from Exposure to Neonicotinoid Pesticides http://www.hc-sc.gc.ca/cps-spc/alt_formats/pdf/pest/part/consultations/_noi2013-01/noi2013-01-eng.pdf
[42] Mullin CA, et al. (2010) op. cit.
[43] Rennich, K, et. al (2012) 2011-2012 National Honey Bee Pests and Diseases Survey Report. http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2011_National_Survey_Report.pdf
[44] Christensen, K.; Harper, B.; Luukinen, B.; Buhl, K.; Stone, D. 2009. Chlorpyrifos Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. http://npic.orst.edu/factsheets/chlorptech.pdf.
[45] Rortais, A (2005) Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 36: 71–83.
[46] Cresswell, JE, et al (2012) Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115: 365– 371.
[47] Frazier (2011) op. cit.
[48] Papaefthimiou, C, et al (2013) Biphasic responses of the honeybee heart to nanomolar concentrations of amitraz. Pesticide Biochemistry and Physiology 107(1): 132–137. http://www.sciencedirect.com/science/article/pii/S0048357513001120
[49] Frazier, et al (2011) Assessing the reduction of field populations in honey bee colonies pollinating nine different crops. ABRC 2011
[50] Tan K, Yang S, Wang Z, Menzel R (2013) Effect of flumethrin on survival and olfactory learning in honeybees. PLoS ONE 8(6): e66295. doi:10.1371/journal.pone.0066295. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0066295
[51] Mussen, et al (2004) op. cit.
[53] Zhu, W., D. Schmehl & J. Frazier (2011) Measuring and predicting honey bee larval survival after chronic pesticide exposure http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[54] Mullin, C.A., J. Chen, W. Zhu, M.T. Frazier & J.L. Frazier – The formulation makes the bee poison. ABRC 2013
[55] Ciarlo TJ, Mullin CA, Frazier JL, Schmehl DR (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7(7): e40848. doi:10.1371/journal.pone.0040848
[56] (Broken Link!) http://www2.basf.us/diols/bcdiolsnmp.html
[58] Zhu, et al (2011) op. cit.
[59] Blacquière, T, et al (2012) Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21(4): 973–992. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3338325/
[60] Frazier, M.T., S. Ashcraft, W. Zhu & J. Frazier – Assessing the reduction of field populations in honey bee colonies pollinating nine different crops http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[61] Pettis, et al (2013) op. cit.
[62] A recent study confirm that the neonic residues in corn, soy, and canola pollen are at very low concentrations. Henderson, C.B. a, J.J. Bromenshenka, D.L. Fischerb. Clothianidin exposure levels from bee-collected pollen and nectar in seed-treated corn and canola plantings. ABRC 2013 http://bees.msu.edu/wp-content/uploads/2013/01/ABRC-abstracts-2013.pdf
[63] Cousin M, Silva-Zacarin E, Kretzschmar A, El Maataoui M, Brunet J-L, et al. (2013) Size changes in honey bee larvae oenocytes induced by exposure to paraquat at very low concentrations. PLoS ONE 8(5): e65693. doi:10.1371/journal.pone.0065693 http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0065693
[64] Boncristiani, H., et. al. (2011) Direct effect of acaricides on pathogen loads and gene expression levels of honey bee Apis mellifera. Journal of Insect Physiology. 58:613-620.
[65] vanEngelsdorp, D, et al () Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J. Econ. Entomol. 103(5): 1517-1523. (Broken Link!) http://www.eclecticparrot.com.au/research_papers/VanEngelsdorp%202010%20Weighing%20risk%20factors%20in%20Bee%20CCD.pdf
[66] Cutler GC, Ramanaidu K, Astatkie T, and Isman MB. (2009) Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag Sci 65:205-209
[68] Cutler, GC (2013) Insects, insecticides and hormesis: evidence and considerations for study. Dose-Response 11:154–177 (Broken Link!) http://dose-response.metapress.com/app/home/contribution.asp?referrer=parent&backto=issue,2,11;journal,3,34;linkingpublicationresults,1:119866,1