Sick Bees – Part 4: Immune Response to Viruses
Sick Bees—Part 4
Immune Response to Viruses
Randy Oliver
ScientificBeekeeping.com
First published in American Bee Journal November 2010
Table of Contents
Viruses are obligate intracellular parasites that infect all organisms, from bacteria to humans. Their evolution represents a constant arms race with the host: Viruses need to reprogram host cells in order to produce progeny virus, but this is often successfully limited by the host antiviral defense, which in turn is frequently targeted by the virus, and so forth (Rehwinkel 2010).
A puzzling aspect of CCD is that when bee samples are analyzed, the “normal” immune mechanisms do not appear to be mobilized, despite the fact that the bees are rife with infectious pathogens (Johnson 2009). What could possibly cause such a suppression of the bee immune system?
If you’ll look back at the bee immune system diagram in my last article, you can see that the induced bee immune response—the production of antimicrobial peptides—is dependent upon the upregulation of certain genes. Both this process and the bees’ antiviral RNA response take place at the molecular level of gene expression. Certain pathogens, notably viruses, are able to sabotage this pathway.
Bees vs. Viruses
Viruses are the ultimate parasite—stripped to the absolute minimum. They are nothing more than encapsulated strands of genetic instructions. They are incapable of life on their own, being entirely dependent upon somehow getting into a host cell and hijacking the cellular machinery in order to trick it into producing more copies of the virus. They are so insidious that the line between host and parasite becomes blurred (about a twelfth of the human genome is viral in origin).
Surprisingly, this insinuation of viruses into host genomes appears to often confer evolutionary benefits, such as the introduction of new genes, or the acceleration of evolutionary change. Viruses may cause the extirpation (local extinction) of species, but any host that develops resistance to a strain of virus is then endowed with a competitive advantage over others that do not have such resistance (such as in the case of European human invaders to the New World, whose viral diseases decimated the Native Americans).
Bees are host to at least eighteen viruses, nearly all being single-stranded RNA viruses. Some, such as Sacbrood virus have been with us for some time. Others are “emerging” pathogens—both Deformed Wing Virus (DWV) and Acute Bee Paralysis Virus (ABPV) were once considered to be “economically irrelevant” (Genersch 2010), then, with the arrival of varroa as a vector, they began to devastate colonies, and are still strongly linked to collapsing colonies today (Highfield 2009, Evans 2010, Hunt 2010).
Each time a virus mutates, or shifts hosts between bee or other insect species, it can suddenly cause epidemics as it spreads through a “naïve” population, just as new strains of flu virus can spread through the human population. For instance, Thai (or “Chinese”) Sacbrood Virus has caused massive collapses of colonies of Apis cerana as it spread from Guangdong Province in 1972 to the whole of China and Southeast Asia (Verma 1990). Indeed, as this article goes to press, Dr. Jerry Bromenshenk’s team is on the cusp of announcing that they have found strong indications that there may be a novel virus involved in CCD collapses in the U.S.
Back to School
The genetic blueprints for a bee are carried in its DNA, which are essentially sets of coded instructions, coiled into long strands called chromosomes, which reside in the nucleus of every cell. The coding elements are called “genes,” of which bees have perhaps 20,000—about the same number as humans! Scientists are working to better understand the bee genome—about 50 laboratories worldwide are currently focused on molecular analyses of honey bees, as bees are a perfect experimental organism, since they are relatively simple, well studied animals, yet exhibit complex behaviors and unusual aging aspects.
The “action” of a virus infection takes place largely in the ribosomes—the organelles in the cytoplasm of each cell that “read” the genetic instructions on messenger RNA, and translate them into actual proteins. I’m introducing these terms because I feel that they are important if you wish to understand what is happening in collapsing colonies. If your eyes are starting to glaze over due to the use of “Big Words,” please take a deep breath, and don’t let the jargon scare you—it’ll be worth the effort to get a grasp of what exactly is failing in the bee immune system. I understand that it’s been a while for many of my readers since biology class, so please bear with me, and allow me give you a little refresher. A picture here may be worth a thousand words (Figure 1):
Until the advent of varroa, little attention was paid to bee viruses, and until very recently no one had any idea how the bee immune system fought viruses. The insect immune system is often chauvinistically dismissed as being more primitive than that of humans, since insects do not produce antibodies. However, insects have been surviving the attacks of pathogens since long before humans walked the Earth—they just do it a bit differently.
The bee antiviral response is based upon an ancient mechanism first discovered in plants (for an excellent history see Matzke 2004), but now known be common to virtually all forms of life—RNA interference (RNAi). RNAi “silences” the expression of genes between the transcription of the genetic code and its translation into functional proteins.
| There are many “Big Words” related to this subject. Scientists therefore us a lot of acronyms, which can be confusing to the first-time reader. Below are definitions of the acronyms used in this article. | |
| CCD | Colony Collapse Disorder—the definitive “symptom” being the often sudden disappearance of the adult bees, relative to the amount of brood present. |
| DNA | The double-stranded genetic blueprints (genes) for the function of an organism; carried in the chromosomes. |
| RNA | The single-stranded transcription product of DNA. |
| mRNA | Messenger RNA– carries genetic code from DNA to the ribosomes for translation into proteins. |
| miRNA | Micro-RNA. Regulate the expression of genes, and thereby cell function. |
| RNAi | RNA interference. Used in “gene silencing” and antiviral immune response. |
| dsRNA | Double-stranded RNA. A temporary step necessary for the replication of RNA viruses. |
| siRNA | Short (or small)-interfering RNA. Short strands of RNA formed by cleavage by the Dicer enzyme. The critical component of the bee antiviral response. |
| DWV | Deformed Wing Virus |
| KBV | Kashmir Bee Virus |
| IAPV | Israeli Acute Paralysis Virus |
| ABPV | Adult Bee Paralysis Virus |
| I am also taking liberties with some terms. The term “symptoms” properly applies only to subjective sensations reported by a patient, whereas researchers observe “signs” of a disease in animals. The term “epidemic” only applies to human diseases; however, I’m using it anyway for bees, rather the proper term, “epizootic.” | |
The bee immune system exploits a quirk of typical viruses—that they are single-stranded RNA viruses (similar to human cold viruses), whose RNA strand can be directly translated into proteins by the ribosomes. First we must better understand exactly how an RNA virus infects a cell (please refer back to Figure 1):
- The typical bee virion “recognizes” a specific type of cell (typically gut cells, then later brain or salivary gland cells) by its specific “receptor” proteins on the cell membrane surface, to which they bind.
- Once bound to the membrane, the virion releases its RNA strand into the cell cytoplasm (cellular fluid).
- The cell’s ribosomes mistakenly recognize the virus RNA strand as normal messenger RNA, and translate it into a long “polyprotein,” which is then cleaved into functional virus proteins and micro-RNA’s. These in turn suppress the bee immune system, and hijack the ribosomal translation functions to produce (or direct the formation of) the components necessary to form new virions (the protein coat, etc). At this stage, there is little direct antiviral response to the virus, and it is not yet replicating (yet it can still greatly harm the cell).
- Finally, the virus needs to have its RNA strand replicated in full, in order to have it packaged into new virions (the assembly of which is directed by translated viral enzymes). To do this, the viral proteins produced by the cell use the original viral “sense” RNA strand as a template, and produce a mirror image copy (“antisense” strand) along it. At this point, there now exists for the first time a double-stranded virus RNA.
- If not suppressed by the bee immune system, the cell will then produce thousands of copies of the virus within hours! (For further reading, I recommend Roizman 1996).
Critical to the bee immune suppression of viruses is that the bee cell immediately recognizes the temporary double-stranded RNA’s as being foreign, since they are not normally produced in normal cellular translation processes. At this point, a picture is again worth a thousand words (Figure 2).
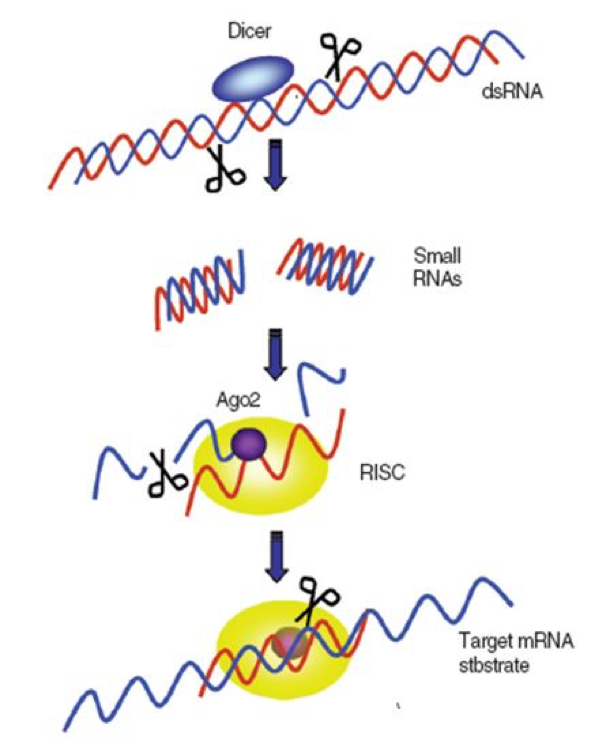 |
| Newly-forming virus strand sticks to the mirror strand and is destroyed |
Figure 2. A greatly simplified illustration of how the RNA-silencing-based antiviral response works. In order to replicate, the single-stranded RNA viruses must rely upon the host cell to make copies of them. This process requires the temporary formation of a double strand of the virus–the “sense” strand acting as a template for its mirror image, the “antisense” strand). The bee cell contains enzymes called “Dicers” that immediately recognize the double-stranded RNA and cleave it into short pieces (siRNA–“small-interfering RNA”). These small pieces are then transferred to Argonaute (Ago) proteins, which bind to the single antisense strand of the chopped up virus RNA, thus forming the “RNA-induced Silencing Complex” (RISC). The bound strand acts as a template that binds to other newly-forming virus strands, which Argonaute then destroys. This action prevents the virus from being able to replicate. Illustration courtesy of Seokyoung Kang (2008) by permission.
The above process is amazingly effective and quick. It is remarkably clever in that the “diced” siRNA’s, by being only about 25 nucleotides long, contain just enough code to be specifically identified as a foreign gene, yet are short enough to make it difficult for viruses to evolve resistance by slightly tweaking their genetic code (see MacRae 2006, which has some stunning graphics). Also of note is that RNAi only works on viruses that are attempting to replicate—if the virus simply “hides” without trying to copy itself, Dicer simply ignores it.
Even more important, is that for the RNAi response to be effective at the whole bee and colony level, the siRNA products must spread from an infected cell to other cells, and then to other bees. This appears to be exactly what happens in the bee colony. For the first step, bees produce a protein that ferries the siRNA products across cell membranes, so the immunity can spread to the whole bee (Hunter 2010). It has not yet been confirmed, but the guess is that they make their way to the jelly produced by nurse bees. Once in the jelly, they have been demonstrated to confer resistance to larvae that consume them (Liu 2010).
It was widely reported that honey bees possess fewer immune sequences than were found in other insects (actually, compared only to the mosquito and the fruit fly), but what is seldom mentioned is that bees “possess more RNAi pathway components relative to flies…, and because bees appear to more readily mount a systemic RNAi response than do flies…it follows that bees should be quite capable of battling viruses and arguably other pathogens through knockdowns based on double-stranded RNAs of pathogen expressed genes” (Evans/Spivak 2009). Notably, this form of response to viral attack is actually quicker than that of humans (Li 2004), and provides a long-term memory similar to that resulting from the antibodies produced in mammals.
Now let’s cut to the chase! In one of the most intriguing CCD papers to date (Johnson/Evans/Robinson/Berenbaum 2009), the authors compared gene expression between bees from CCD colonies originating on both the east and west coasts to that of bees from healthy colonies sampled before the emergence of CCD. They found that:
“Overall, elevated expression of pesticide response genes was not observed.
“Genes involved in immune response showed no clear trend in expression pattern despite the increased prevalence of viruses and other pathogens in CCD colonies.
“Microarray analysis revealed unusual ribosomal RNA fragments that were conspicuously more abundant in the guts of CCD bees. The presence of these fragments may be a possible consequence of … viral infection.”
Note that the results did not indicate that pesticides were the problem. More surprisingly, the bees’ induced immune response was not upregulated, despite their sick bodies being rife with pathogens! What could cause such a suppression of the normal immune response? The answer likely is linked to their finding of those “unusual ribosomal RNA fragments.”
The paper cautiously offered various hypotheses to explain the presence of the fragments, but one of the authors, May Berenbaum, was more candid in an interview (Yates 2009):
“The one consistent indicator of CCD across samples collected at multiple times and in multiple places was the overabundance of ribosomal fragments. … viruses ‘hijack the ribosome,’ taking over the cellular machinery to manufacture only viral proteins.”
What does she mean by this? Most bee viruses are classified as “picorna-like” viruses (pico = tiny, so “like the tiny RNA viruses” of vertebrates). Picorna viruses have an unusual way of hijacking ribosomal function. The ribosomes normally only translate messenger RNA that is marked with a “password” at one end. But picorna viruses have figured out a way to sneak into the middle of the ribosome without a password (Ongus 2006). So what they then do is to produce enzymes that remove the password from the normal bee messenger RNA’s, so that they can no longer be read by the ribosomes! By doing so, they hijack the cell’s ribosomes to produce virus proteins at the expense of bee proteins!
Berenbaum continues:
“The loss of ribosomal function would explain many of the phenomena associated with CCD. If your ribosome is compromised, then you can’t respond to pesticides, you can’t respond to fungal infections or bacteria or inadequate nutrition because the ribosome is central to the survival of any organism. “
Take a moment to grasp the implications of the above. Bees infected by viruses can lose most immune function, as well as the ability to perform other metabolic functions, as a result of the viral infection!
Practical Application
Beekeepers will soon have at their disposal, from Beeologics, an antiviral medication that mimics the natural bee RNAi response. The product, RemebeeTM, is made by creating two discrete dsRNA components that are identical to “conserved” regions of the IAPV genome (Maori 2009; “conserved” means that all known strains of the virus have almost exactly the same sequence; one of the conserved regions is also found in the closely-related KBV and perhaps ABPV). This is the product with which I treated the colonies in the California trial that I described in “Sick Bees 2.” I can now share with you some of the yet unpublished results (the results of the 2008 trials will soon be published).
Prior to the feeding of Remebee, some colonies already exhibited siRNA’s for IAPV prior to us inoculating them with the virus (it had been previously confirmed that IAPV existed in some of my colonies). Of note, is that in the Florida trial, the non-treated hive with the highest natural siRNA levels before and after infection had the highest bee population (of the control group) at the end-point analysis. In contrast, the two control hives that had no siRNAs either before or after infection were either dead or were extremely weak at the end point. This finding indicates that colonies that are able to naturally ramp up an siRNA response to viruses are better able to survive.
When fed to bees in syrup, enough of the product is absorbed into their gut cells so as to initiate the antiviral response: Bee Dicer proteins recognize the dsRNA as being foreign, and chop it up to create siRNA’s, which then confer resistance to IAPV and KBV. It is noteworthy that in colonies fed Remebee, the diced siRNA’s are not merely absorbed, but actually amplified by the bees, and still found to be present four weeks after the last treatment, which is a much longer-lasting effect than I expected!
We fed the test hives Remebee prior to inoculating them with the virus cocktail, and then took samples two weeks after inoculation. After being infected, the siRNA levels increased dramatically in the hives that had been pretreated with Remebee, much more so than in the unmedicated control group. It appears that treating hives with Remebee prior to virus exposure primes them to initiate a stronger antiviral response should they subsequently be exposed to the virus.
I will show the graphs for colony survivability in an upcoming article, but would like to make an announcement at this time: Beeologics has gotten FDA permission to experimentally release Remebee to beekeepers. They are looking for some commercial beekeepers who would like to test the product in their operations this winter. You can contact Nitzan Paldi directly at nitzan@beeologics.com.
Viruses Fight Back
Viruses are unthinking strands of genetic code, so how do they deal with the bees’ powerful RNA silencing immune response? The answer is that the viruses launch a preemptive strike by suppressing that immune response before it is initiated, and by further tweaking the ribosomal machinery to their benefit. This is akin to defeating an army by simply infiltrating its command headquarters and then rewriting the orders going out to the manufacturing sector, supply chain, and the troops.
Viruses are so good at this, that some species of wasps actually inject a specific virus into the caterpillars that they parasitize, in order to suppress the caterpillar’s immune response against the wasps’ larvae (Pruijssers 2006). Some insect viruses have even figured out how to prevent the last-resort immune defense of an infected cell—programmed sacrificial suicide (apoptosis)—allowing long-lasting “latent” infections! (Narayanan 1998).
This is cutting edge science, not yet thoroughly understood, but great strides are being made. Any papers or texts more than ten years old are likely out of date! The deeper I’ve looked into it, the more fascinating and complex it becomes, as we begin to grasp the tactics in the virus/host never ending “game” of suppression of the suppressors of the suppressors. Allow me to quote Scaria (2006):
The exclusive dependence of viruses on the host cellular machinery for their propagation and survival also make them highly susceptible to the vagaries of the cellular environment like short RNA mediated interference. It also gives the virus an opportunity to fight and/or modulate the host to suit its needs. Thus the range of interactions possible through miRNA-mRNA cross-talk at the host-pathogen interface is large. These interactions can be further fine-tuned in the host by changes in gene expression, mutations and polymorphisms. In the pathogen, the high rate of mutations adds to the complexity of the interaction network.
The last point of the above quote, about the high rate of viral mutations is of great import. The RNA viruses are notable for their high mutation rate. Even the change of a single base molecule on the RNA strand can have a dramatic effect upon the virulence of the virus! (Shiboleth 2007).
What we beekeepers observe in the field is the year-by-year evolutionary process in action, as some colonies fall sick with odd symptoms, then see the population rebound as resistant survivors supplant the less fortunate. The bee/virus interaction becomes a sort of interactive game, played at the genetic and ribosomal level, but unfortunately observable only with specialized laboratory equipment. It is only recently that scientists even knew what to look for!
Sick pupae, typical of a virus epidemic as varroa levels peak in September. Best I can tell is that they are dying from DWV or perhaps other viruses. I see this generally happening if the mite infestation reaches about 10% (30 mites in an alcohol wash of ½ cup of bees from the broodnest). I started noticing these sort of symptoms several years ago, and am seeing more this year than ever! This photo is of a small patch of intense infection in one brood frame; in the rest of the hive, sick pupae and larvae were more scattered.
Sick pupae, typical of a virus epidemic as varroa levels peak in September. Best I can tell is that they are dying from DWV or perhaps other viruses. I see this generally happening if the mite infestation reaches about 10% (30 mites in an alcohol wash of ½ cup of bees from the broodnest). I started noticing these sort of symptoms several years ago, and am seeing more this year than ever! This photo is of a small patch of intense infection in one brood frame; in the rest of the hive, sick pupae and larvae were more scattered.
MicroRNA’s
In the past decade, researchers have discovered that there are a whole set of genetic instructions whose functions had been previously overlooked. These are the genes that code for micro-RNA’s, which like messenger RNA’s are shuttled from the nucleus to the ribosomes, but there, instead of coding for proteins, act as regulatory instructions for genetic expression (microRNA’s are an extremely hot topic in biology, and well reviewed in Wikipedia).
Scientists have only recently discovered that bacteria (Navarro 2008) and especially viruses (Scaria 2006) either produce micro-RNA’s or target host micro-RNA’s essential to the host immune system. Realize that every type of cell (gut, brain, hemocyte) contains fine-tuned mechanisms to regulate the expression of each of the thousands of specific proteins needed for it to function. Viruses can muck up these mechanisms to their own advantage (Pacheco 2010). The end result can be that the bulk of normal cellular products become the sort of ribosomal “trash” found in the CCD study cited earlier.
It may appear that I’ve made a strong circumstantial case pointing at one or more viruses, perhaps in synergy with nosema, as being the agents of colony collapse. The strongest smoking gun is that we were able to duplicate the symptoms of CCD in test yards by inoculating colonies with a virus cocktail from another beekeeper’s collapsing hives, and that suppression of those viruses appeared to cause some protection. However, I already had at least some strains of those viruses (plus N. ceranae) in my (otherwise healthy) operation prior to the start of the trial, and one of the control colonies did not seem to suffer. So I must be cautious about solely blaming the viruses as the initiating causal agents of colony collapse. I also, want to be clear that in this article I have extrapolated the current state of knowledge of similar viruses to the bee model, and that further work needs to be done before we can say that we definitively understand what is happening.
Inapparent Virus Infections
There is some good news, in that it is generally not in the interest of a virus to actually kill the bee, as the main method of transmission for the most virulent viruses appears to be via live bees either by oral/fecal transmission, or as a result of being vectored by varroa mites. The exception to this live-bee rule is when the mid-aged hygienic bees transfer virions from virus-killed brood to other bees (common with sacbrood and DWV, but not necessary for the transmission of either). (Less virulent strains of virus may also “vertically” transmit in semen or through a queen’s infected eggs).
Of interest is that the most virulent bee viruses tend to exist in an “inapparent” infection—a term coined by Australian virologist Denis Anderson (1988)–meaning that one can detect the presence of the virus in bees, but that there are no noticeable negative effects due to the infection. Another Australian (Benecke 2007) explains: “It seems likely that bees carry the virus at all times but only show symptoms when they are stressed in some way. Thus bees may not so much ‘catch’ a viral disease but for some reason fail to suppress a virus they are already carrying” [emphasis mine}.
So go back to my discussion of viral replication. The bee cell may ignore the virus so long as the virus doesn’t attempt to actually replicate. The question then is what exactly triggers the sort of multi-virus epidemics typical in collapsing colonies? What are the causal agents, and which are mere opportunistic pathogens? I’ve already discussed some of the triggers–poor nutrition, chilling, environmental toxins, and parasite infection. Believe me, many researchers are working long, painstaking hours to try to be the first to figure out the specific cause or causes (the “etiology”) of CCD (just read the “Methods” section in the following paper)!
In the most descriptive CCD paper to date, vanEngelsdorp, Evans, et al (2009), state that:
“While no single pathogen or parasite was found with sufficient frequency to conclude a single organism was involved in CCD, pathogens seem likely to play a critical (albeit secondary) role. CCD colonies generally had higher virus loads [higher titers across the board; KBV was especially prevalent in sick colonies] and were co-infected with a greater number of disease agents than control colonies” (55% of CCD colonies were infected with 3 or more viruses as compared to 28% of control colonies).
So what happens when there are multiple parasites suppressing the bee immune system at the same time, and screwing with their ribosomal functions? I will continue on that subject in my next article.
Acknowledgements
RNAi is cutting edge science, the explanations of which are often buried in very arcane scientific journals; I thank Peter Borst for his tireless ferreting out of related research. I greatly appreciate the assistance of several scientists who have given their time to help clarify things for me. First, the Israeli virologists Ilan Sela, who identified and named Israeli Acute Paralysis Virus, and his associate Eyal Maori. Through Prof. Sela, I was introduced to RNAi, and to Nitzan Paldi, the inventor of the Remebee concept, with whom it has been a great pleasure to collaborate. I also wish to thank virologist Wayne Hunter, who brought his expertise with insect viruses to the USDA bee lab, and was a co investigator with me for the Remebee trials. Finally, Jay Evans of the USDA lab, who has been a workhorse for CCD research and bee immune function, has always been there for me to bounce ideas against, and to whom we beekeepers all owe a debt of gratitude for his tireless efforts.
I greatly appreciate the helpful reviews of the manuscript by Nitzan Paldi and Michelle Flenniken.
References
Agudelo-Romero P, Carbonell P, Perez-Amador MA, Elena SF (2008) Virus adaptation by manipulation of host’s gene expression. PLoS ONE 3(6): e2397.
Anderson, DL and AJ Gibbs (1988) Inapparent virus Infections and their interactions in pupae of the honey bee (Apis mellifera Linnaeus) in Australia. J. Gen. Virol. 69: 1617-1625.
Benecke, FS (2007) Commercial Beekeeping in Australia. RIRCD Publication 07-059.
Bonning, BC and WA Miller (2010) Dicistroviruses. Annu. Rev. Entomol. 55:129–50.
Evans, J (2010) The role of multiple pathogens in colony collapse disorder. 110th General Meeting of the American Society for Microbiology.
Genersch E and M Aubert (2010) Emerging and re-emerging viruses of the honey bee (Apis mellifera L.) Vet. Res. 41(6): 54.
Highfield, AC, A El Nagar, L Mackinder, ML Laure, J Noël, MJ Hall, SJ. Martin, and DC. Schroeder (2009) Deformed wing virus implicated in over-wintering honeybee colony losses. Appl. Environ. Microbiol 75(2): 7212-7220.
Hunt, G (2010) Breeding bees for resistance to parasites and diseases. Bee Culture 138(7): 34-36.
Hunter, W, J Ellis, D vanEngelsdorp, J Hayes, D Westervelt, E Glick, M Williams, I Sela, E Maori, J Pettis, D Cox-Foster, N Paldi (2010) Large-scale field application of RNA interference (RNAi) technology to reduce impact of Israeli Acute Paralysis Virus (IAPV) induced disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathogens, in press.
Kang, S and YS Hong (2008) RNA Interference in Infectious Tropical Diseases Korean J Parasitol Vol. 46, No. 1: 1-15.
Kennerdell, J.R. and RW Carthew (2000) Heritable gene silencing in Drosophila using double-stranded RNA. Nat Biotechnol 18: 896-898.
Lai, MMC (1992) RNA recombination in animal and plant viruses. Microbiological Reviews 56(1): 61-79.
Li, WX, et al (2004) Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. PNAS 101(5): 1350–1355.
Liu X, Zhang Y, Yan X, Han R (2010) Prevention of Chinese sacbrood virus infection in Apis cerana using RNA interference. Curr Microbiol (online) DOI 10.1007/s00284-010-9633-2.
MacRae, IJ, et al (2006) Structure of Dicer and Mechanistic Implications for RNAi. Cold Spring Harbor Symposia on Quantitative Biology, Volume LXXI. rna.berkeley.edu/Publications/cshsqb-71-73.pdf
Maori, E, N Paldi, S Shafir, H Kalev, E Tsur, E Glick, I Sela (2010) IAPV, a bee-affecting virus associated with Colony Collapse Disorder can be silenced by dsRNA ingestion. Insect Molecular Biology 18(1): 55–60.
Matzke MA, Matzke AJM (2004) Planting the seeds of a new paradigm. PLoS Biol 2(5): e133. doi:10.1371/journal.pbio.0020133 http://www.plosbiology.org/article/info:doi/10.1371/journal.pbio.0020133
Narayanan, K (1998) Apoptosis: its role in microbial control of insect pests. Current Science 75(2): 114-122.
Navarro, L, et al (2008) Suppression of the MicroRNA pathway by bacterial effector proteins. Science 321(5891): 964 – 967.
Ongus, JR (2006) The non-translated region of Varroa destructor virus 1 (genus Iflavirus): structure prediction and IRES activity in Lymantria dispar cells. Journal of General Virology 87: 3397–3407.
Pacheco, A and E Martinez-Salas (2010) Insights into the biology of IRES elements through riboproteomic approaches. Journal of Biomedicine and Biotechnology 2010, Article ID 458927, 12 pages.
Roizman , B (1996) [Virus] Multiplication. In Medical Microbiology, The University of Texas Medical Branch at Galveston. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mmed&part=A2284
Scaria, V, et al. (2006) Host-virus interaction: a new role for microRNAs. www.retrovirology.com/content/3/1/68
Shiboleth, YM, et al (2007) The conserved FRNK Box in HC-Pro, a plant viral suppressor of gene silencing, is required for small RNA binding and mediates symptom development. J Virol. 81(23): 13135–13148.
vanEngelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, et al. (2009) Colony Collapse Disorder: A Descriptive Study. PLoS ONE 4(8): e6481. doi:10.1371/journal.pone.0006481
Verma, LR, BS Rana, S Verma (1990) Observations on Apis cerana colonies surviving from Thai Sacbrood Virus infestation. Apidologie 21: 169-174.




