First published in: American Bee Journal, October and November 2013
Pesticide Exposure
Oh No, Not Pesticides Again!
Reality Checks
The Two Worlds of Beekeeping
Pesticides and Bee-pocalypse
A Comparison To Some “Control Groups”
The Four Horsemen And The Tip Point
Could Pesticides Cause Colony Mortality And CCD?
Short Memories
The Heart Of The Hive – The Nursery
Industry’s Arguments
But Don’t We Already Know That It’s The Neonicotinoids?
An “Acid Test” Of Neonic Seed Treatment
So Which Pesticides Are Actually To Blame?
The Evidence
Oh Boy, Let’s Do Some Math!
And How About The “Inerts”?
Choosing To Ignore The Obvious
Blinded By Bias
No More Safe Home To Return To
A Historical Artifact
The Beekeeper Contribution To Shifting The Tip Point
Stop Right There!
Undetectable Levels And Hormesis
Wrap Up
Acknowledgements
References
Sick Bees Part 18f7: Colony Collapse Revisited
Pesticide Exposure
Randy Oliver
ScientificBeekeeping.com
Originally published in ABJ Oct and Nov 2013
Oh No, Not Pesticides Again!
Some readers may wonder why I am spending so much time on the issue of pesticides, since to many (if not most) beekeepers, pesticides are a non issue. In answer, the main reason is that the public (and our lawmakers) are being hammered by the twin messages that the honey bee is on the verge of extinction, and that the reason is pesticides. In my writings, I’m attempting to address the validity of both of those claims. Let’s start with the first.
Reality Checks
Honey bees have clearly (and deservedly) become one of today’s most charismatic environmental poster children, and as such are a useful bioindicator that our human activities are having a negative impact upon pollinators, and wildlife in general.
But I also feel that we take care to not overstate or exaggerate our case. One of my greatest concerns is that beekeepers are allowing the media to scare the public with all the hue and cry of an impending bee-pocalypse (and that it is due to a certain type of pesticide). Our complicity in this message (as we enjoy the luxury of basking in the warmth of all the public support) may backfire on us one of these days—putting us into the position of the little boy who cried wolf. Some in the media are starting to notice that the facts don’t support the claim that bees are disappearing (Fig. 1).
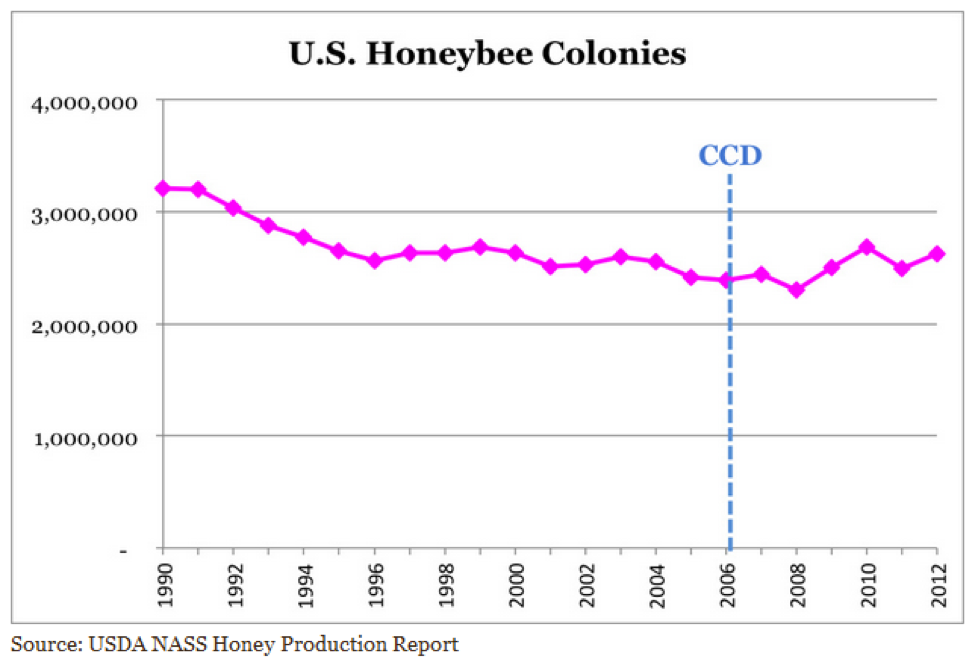
Figure 1. It’s true that it’s more difficult to keep bees healthy these days, but it doesn’t look like bee-pocalypse is imminent (as evidenced by this recently-published chart). Whenever honey and pollination prices are high enough to make beekeeping profitable, resourceful beekeepers somehow manage to recover their colony losses [[i]]. Chart courtesy Shawn Regan [[ii]].
[i] Rucker, RR and WN Thurman (2012) Colony collapse disorder: the market response to bee disease. http://perc.org/sites/default/files/ps50.pdf
[ii] (Broken Link) http://perc.org/articles/everyone-calm-down-there-no-bee-pocalypse
In the rest of the world, the number of managed hives has actually been increasing [3]. And as far as claims that pesticides are driving bees to extinction, Hannah Nordhaus, the author of the excellent book The Beekeeper’s Lament writes:
Reflexively blaming pesticides for all of the honey bee’s problems may in fact slow the search for solutions. Honey bees have enough to do without having to serve as our exoskeletal canaries in a coalmine. Dying bees have become symbols of environmental sin, of faceless corporations out to ransack nature. Such is the story environmental journalism tells all too often. But it’s not always the story that best helps us understand how we live in this world of nearly seven billion hungry people, or how we might square our ecological concerns and commitments with that reality. By engaging in simplistic and sometimes misleading environmental narratives — by exaggerating the stakes and brushing over the inconvenient facts that stand in the way of foregone conclusions — we do our field, and our subjects, a disservice [4].
Further reading: for a detailed and sober analysis of the factors that affect managed bee populations, I highly recommend the review by Drs. vanEngelsdorp and Meixner [5].
The reality is that it is not the honey bee that is being driven to extinction—it is instead the commercial beekeeper who is finding that his traditional business model is becoming less profitable due to today’s greater degree of colony losses and the decreasing availability of good summer forage.
The question is, to what extent are pesticides involved in those problems?
The Two Worlds Of Beekeeping
There are two very different worlds of beekeeping—small scale (hobbyists, who constitute the vast majority of beekeepers by number) and large scale (commercialized professionals, who manage the vast majority of hives), with a small continuum of sideliners bridging the gap.
Hobby beekeeping is currently enjoying a bubble of resurgence, but in the Big Picture in the U.S., hobbyists manage an insignificant number of hives. And those small-scale beekeepers tend to keep their hives close to home, largely avoiding serious exposure to pesticides. But that’s not to say that small-scale beekeepers are immune to pesticide kills; I’ve heard of several this season, and what with all the spraying for West Nile virus and the citrus psyllid, we can expect more of the same. And since there are far more small-scale beekeepers to put pressure on regulators and legislators, I feel that it is a good idea for them to be informed about pesticide issues.
Large-scale beekeepers, on the other hand, typically run migratory operations—moving their hives to almond pollination, and then to other agricultural areas (it’s problematic to keep apiaries of hundreds of hives in the suburbs). The fate of those bees (and their keepers) is largely determined by agricultural land use practices and their degree of exposure to agricultural pesticides.
It is some of those large-scale beekeepers for whom extinction is a valid concern. The reason (as with any other enterprise) is financial—they can only survive so long unless their businesses continue to be profitable [6]. In recent years, they’ve had two things going for them—sky-high honey prices and elevated pollination fees. But all is not rosy—there are reasons for those prices going up; these days it’s simply more costly to produce honey or to provide bees for pollination.
Today’s breathtakingly-high high almond rental rates typically don’t even cover operating costs—even if most of one’s colonies make it through the winter! Today’s 30% average winter loss rate is bleeding profitability from many operations. Not only does the beekeeper need to rebuild his numbers after almonds, but to stay in the black he must also make additional income from paid pollinations or a decent honey crop. And that may no longer be as easy as it used to be for various reasons:
- Formerly bee-friendly farmland has been turned into agri-deserts devoid of any bee forage.
- Honey producers on field crops (such as alfalfa, sunflowers, or cotton) get hammered time and again by pesticide spraying, sometimes watching whole yards of colonies dwindle or go queenless weeks afterwards.
- Paid summer pollination contracts (such as for vine crops) may leave colonies in poor shape for the winter, due to the heavy stocking, the lack of nutritious pollen, and the exposure to multiple pesticides.
These days, the sad fact is that many good beekeepers are barely keeping their heads above water. So the beekeeper’s lament continues—varroa, high winter mortality, and lack of good forage are driving a number of operations into the red.
Practical application: although the “extinction of the honey bee” makes for a good rallying cry, the real concern is the possible extinction of the migratory beekeeper who supplies necessary pollination services to agriculture. So far, the almond industry has been economically propping up the bee industry, but I’m not sure how long that arrangement will be sustainable.
Pestcides And Bee-pocalypse
For some beekeepers, “bee-pocalypse” has already occurred. New York beekeeper Jim Doan, whose case I detailed in a previous article [7], sadly gave it up this year. Here is a beekeeper whose apiaries had been in the same locations for many years without noticeable pesticide problems, but who apparently suffered from devastating spray or dust kills this season and last, as evidenced by piles of fresh dead bees in front of his hives in spring.
Residue analysis of those dead bees clearly showed that they had been exposed to several pesticides, but none of the detects were at levels that would be expected to cause such carnage—so we don’t even know which pesticides or practices to point the finger at! To my scientific mind, this is very frustrating—that our “system” was not able to identify the cause of Jim’s bee kills, to change anything to keep them from recurring, nor compensate an innocent beekeeper for the loss of his livestock and livelihood.
As unlucky as Jim has been, his case is not necessarily the norm. Overall, the issue of environmental toxins is improving. In my own lifetime I’ve seen us clean up our pollution of the air and water, cease atmospheric testing of nuclear weapons, ban DDT, fluorocarbons, and PCB’s, phase out the worst pesticides, and raise the general environmental consciousness. Humans still inflict far too damaging an environmental footprint on Earth, but we are moving in the right direction, and should give ourselves some credit for that!
There is no doubt that pesticides are often involved in bee health issues, but can we blame them for all our problems? That question is best answered by considering the health of those colonies that are not exposed to pesticides:
A Comparison To Some “Control Groups”
There are plenty of beekeepers in non agricultural areas whose apiaries are not exposed to pesticides to any extent. Those hives serve as a “control group,” whose health we can compare to those colonies that do have to deal with pesticides.
For instance, in my own operation of about a thousand hives, their only exposure to pesticides is to the fungicides in the almond orchards (from which they don’t appear to suffer to any serious extent). I haven’t used synthetic miticides in over a decade, rotate my combs, and rarely feed syrup.
Yet, I’ve experienced CCD firsthand, see more queenlessness, unsuccessful supersedure, and experience somewhat higher winter losses than in the old days (meaning before varroa). I hear the same from many others in the pesticide-free control group. The simple fact is that these days it requires better husbandry to maintain productive colonies. Yet we in the “control group” can hardly blame pesticides to be the cause.
And then there are the stationary “treatment free” beekeepers in the middle of intense agriculture who suffer no higher colony loss rates than the norm, despite their apiaries being surrounded by corn and soy [8]. How the heck do we reconcile their success to the problems that the commercial guys experience in the same areas?
Do they owe their success to keeping fewer hives in a yard? To keeping locally-adapted survivor stock? To their placement within flight range of patches of undisturbed forage? To the fact that they don’t move to multiple crops? Or is it because they aren’t contaminating their combs with miticides? Believe me, if I knew the answer, I’d tell you!
As (the very successful) beekeeper Dave Mendes observes, colonies just seem to be more “fragile” these days. It’s no surprise then that the addition of toxins of any sort can help to tip a colony over. The Ericksons [9] put it this way:
Pesticides and their residues in the hive stress bees as do other factors such as weather extremes, food shortages, pests, predators, and disease. Conversely, stress induced by other factors undoubtedly has a significant impact on the level of damage that a pesticide inflicts on a colony.
Note that the above words were written prior to our colonies having to deal with varroa, the varroa-vectored viruses, Nosema ceranae, our evolving brood diseases, GMO’s, neonicotinoids, or Roundup Ready corn.
The Four Horsemen And The Tip Point
Colony growth is a function of the recruitment rate via successful broodrearing vs. the attrition rate of workers due to age, disease, the altruistic departure of sick bees, or the loss of foragers in the field. When recruitment exceeds attrition, colonies grow; when attrition exceeds recruitment, the colony population shrinks. Environmental factors, including toxins, can shift the tip point for colony growth (Fig. 2).
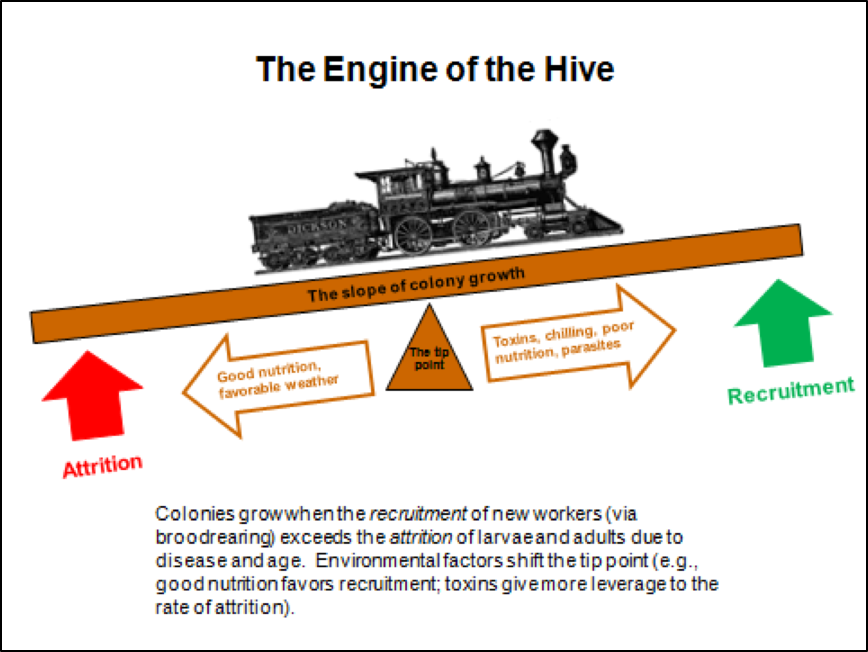
Figure 2. Any colony with a good laying queen has the potential to grow rapidly—the greater the rate of recruitment (successful broodrearing), the steeper the slope of the growth curve. In the real world, such potential growth is often held back by the lack of nutritious pollen, or by the stresses of toxins, chilling, or pathogens (especially the mite-associated viruses, nosema, or EFB). Any of those can strongly shift the tip point, slowing, or even reversing, the rate of colony growth.
In the last decade, something appears to have shifted that tip point—colonies today seem to more readily go into a downhill spiral and queens no longer hold up as well. Could it be due to pesticides?
Could Pesticides Cause Colony Mortality And CCD?
Of course they could! In 2010, after closely observing the progression of experimentally-induced CCD with my collaborator Dr. Eric Mussen, I published the flow chart below (Fig. 3) to detail the interactions and feedback loops involved in the step-by-step collapse of a colony [10]. At the time, I fully intended to further elaborate upon the contribution of toxins, but didn’t get around to it until now.
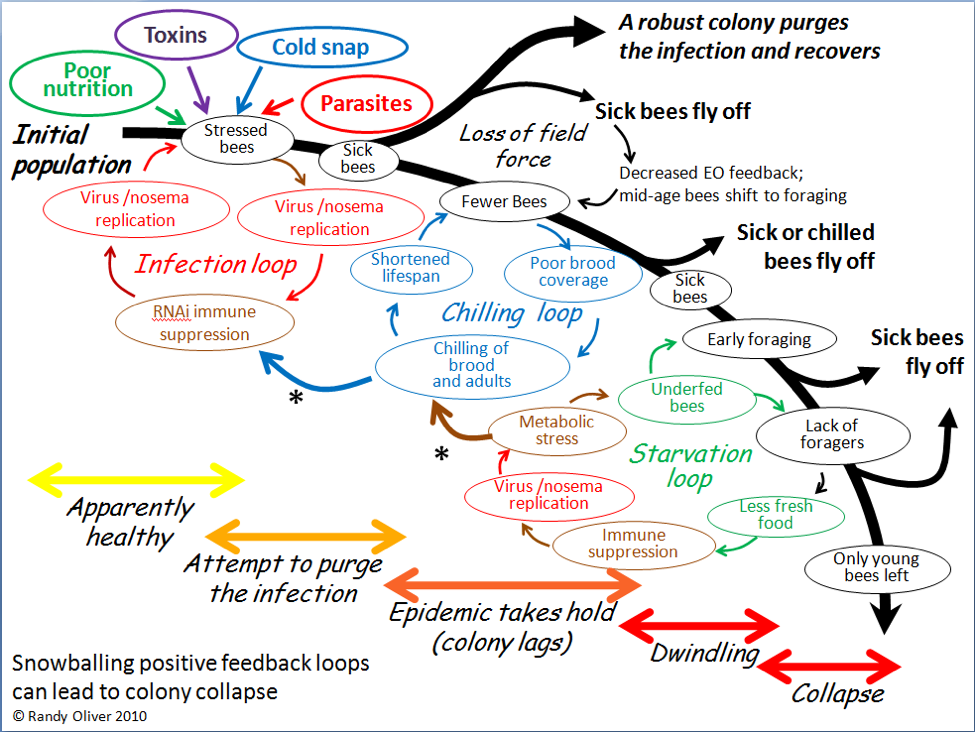
Figure 3. The positive feedback loops that can lead to colony dwindling and/or sudden depopulation. I’ve since observed this process take place in sick colonies time and again.
In the above chart, I called out toxins (which would include pesticides) as one of the “Four Horsemen of Bee Apocolypse” (the four factors at top left). Below I’ve indicated in red those points at which toxins may exacerbate the downhill process (Fig. 4).
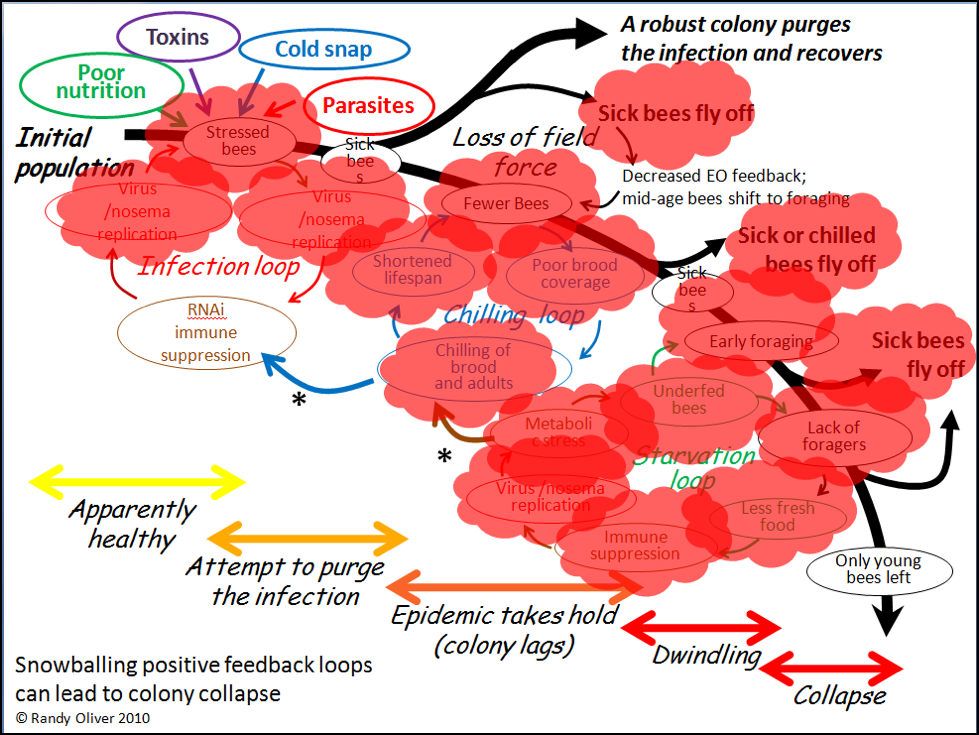
Figure 4. Note that toxins can exert lethal or sublethal effects (red bubbles) at every step in the process of colony dwindling or collapse. Pesticides may in some cases be the prime cause of colony mortality; more frequently they might be “contributory factors,” especially due the prolonged sublethal effects of residues in the beebread or wax.
Please note that in these charts I’m referring to toxins generically, not specifically to manmade pesticides. Such toxins would include natural plant allelochemicals, industrial pollutants, metals such as arsenic or selenium in soil and dust, fungal and bacterial toxins (which may be altered in beebread by the presence of pesticide residues), beekeeper-applied varroacides, HMF in overheated corn syrup, all in addition to any agricultural pesticides. In the words of ecotoxicologist Dr. Helen Thompson, we must pay attention to the total toxin load of the hive, plus any interactions between those chemicals, as well as other contributory factors [11]—a sentiment also echoed by the Fraziers at Penn State [12]. So, back to our original question: Can toxins, including synthetic pesticides, cause colony morbidity or mortality?
Verdict #1: clearly, synthetic pesticides and varroacides may constitute the most serious toxin load for managed bees in agricultural areas, and have the potential kill a colony outright, or to exacerbate positive feedback loops that can result in dwindling, poor overwintering, or collapse.
But does any pesticide specifically cause CCD—“the disappearance of most, if not all, of the adult honey bees in a colony, leaving behind honey and brood but no dead bee bodies” [13] (and no sign of brood diseases or varroa-induced DWV collapse).
Analysis: The most direct way to answer that question is to see whether we can fulfill Koch’s third postulate [14]: can we experimentally create the symptoms of CCD by treating a healthy hive with the pesticide in question?
Verdict #2: to the best of my knowledge, no one has yet duplicated the symptoms of CCD by treating a colony with any pesticide (the most obvious difference being that there are generally plenty of dead bees present in the case of pesticide toxicity). This is notably true for the neonicotinoids, for which any number of researchers have attempted to duplicate CCD symptoms by continually feeding colonies neonic-tainted syrup or pollen.
Hold on—drop those stones! I am not saying that pesticides cannot contributeto CCD or colony morbidity or mortality in general—my chart above clearly illustrates that they have the potential to do so. Yet even those beekeepers who manage to completely avoid pesticides may still experience sudden colony depopulations, dwindling, or excessive winter losses due to some combination the Four Horseman (as in the perfect storm detailed at [15]).
I feel that it is a serious error for us to try to link CCD to pesticides. Pesticides have always been an issue to beekeepers, but CCD-like events have historically come and gone (as in Disappearing Disease—read the description at [16]). Pesticides will remain an issue long after the term “CCD” is forgotten.
Bottom line: Despite the fact that the evidence at hand does not support the case that CCD is directly caused by any pesticide, that fact certainly does not mean that we should ignore pesticide issues. If anything, we beekeepers ourselves have helped to make pesticides even more of an issue these days.
Short Memories
There is a popular myth going around that pesticides only started to become an issue to honey bee colony survival in 2007. In fact, the sublethal effects of pesticides were well known to beekeepers and researchers long before then. If we review the older literature [17], we find that it was already well known that contaminated pollen was a more serious issue to colony health than the in-field kill of foragers. We knew that colonies might collect such tainted pollen from miles away, that dusts were worse than sprays, that young bees may be more susceptible than older bees, and that temperature and humidity had a great deal to do with pesticide toxicity. Pesticide issues were actually far worse in the 1960’s and ‘70’s than they are today, and have generally improved since then (not to say that some new issues haven’t arisen).
On the other hand, the overall contamination of combs with pesticides has increased in recent years due to the direct contribution by we beekeepers ourselves. In virtually any residue analysis of beebread or beeswax these days in any country with varroa, the most prevalent toxins are the beekeeper-applied varroacides [18]–you may wish to refer back to my chart of the “toxicological eras of honey bee evolution [19].
So one question is, To what degree we have shifted the tip point of colony health by contaminating our brood combs with miticides? Let’s explore the broodnest…
The Heart Of The Hive – The Nursery
The insidious, long-term effects of total toxin load (including pesticide and varroacide residues) would be from those that made it into the heart of the hive—the critical stored beebread and the wax of the brood combs (Fig. 5). Note: Dr. David Fischer of Bayer brings to my attention that in the case of imidacloprid, the results of his testing indicates that bees in the hive are more affected by residues in the nectar than by those in the pollen.

Figure 5. Long after a pesticide-sprayed field force has been replaced by newly-recruited foragers, the colony may still need to deal with the lingering effects of pesticide residues in the combs, and especially in the all-important stores of beebread. It is here that such persistent residues can affect colony health and buildup for many months after the initial exposure, and exactly where we should focus our attention.
Here’s some food for thought: a toxin need not actually kill a single bee to mess up a colony. There are many ways in which sublethal levels of toxins can negatively affect the colony population curve. A few examples would be:
- By decreasing the survival rate of larvae (as from residues of varroacides [20, 21], or fungicides [22]) , or by increasing their development time (as effected by various pollutants, plant alleleochemicals, pesticides, or miticides).
- By affecting the proper fermentation of beebread (fungicides).
- By affecting the sensitive nurse bees that must digest that beebread and produce the critical jelly used to feed the brood, queen, and other workers (natural plant toxins, pollutants, or pesticides).
- By affecting the normal behavioral progression of the workers. E.g., if workers initiate foraging prematurely, this greatly reduces their overall longevity, and results in severe depression of colony growth [23] (much more research is crying to be done, but many chemicals would be suspect).
- By requiring bees to allocate precious resources toward the detoxification of the poisons (as per my leaky boat analogy [24]).
- By increasing the virulence of varroa, nosema, or viruses (any number of pesticides and miticides have been implicated [25, 26])
- By affecting normal colony homeostasis, such as thermoregulation of the brood, which is dependent upon the proper assessment of temperature, and the ability to effectively generate heat by the vibration of the wing muscles (neurotoxins would be expected to affect this ability).
- By affecting the longevity of the queen, the viability of spermatozoa, or the ability of a colony to successfully supersede (coumaphos notably had this effect)
- By affecting the production of, or normal communication via pheromones (which include the recognition of brood and the queen) [27] (essential oils, formic acid, other pesticides?)
- By affecting foragers’ ability to communicate by dance, to navigate, to learn (a wide range of pesticides [28]), or to react properly to normal stimuli (neonics can clearly do this [29]; but similar effects could be due to any number of other pesticides).
Bottom line: the toxin load in the broodnest can greatly affect a colony in many ways, generally (but not necessarily always) negatively. The greater the total toxin load with which the colony is forced to deal, the more likely that it will suffer from the combined ill effects.
Industry’s Arguments
In order to present an objective review of pesticide issues, we should also hear Industry’s side of the argument. The industry-funded think tank OPERA [30] takes the position that:
Although, based on the facts outlined above, there does not appear to be any strong evidence that sublethal effects of pesticides play a key role as causative factors behind bee colony mortality (which is likewise supported by the fact that in several monitoring projects no correlation has been found between colony losses and pesticide exposure), sublethal effects are certainly a point where more fundamental research is needed to obtain a clearer picture of the nature of the issue.
The above statements are factually correct in that there is to date no compelling evidence that pesticides are at the root of the elevated rates of colony mortality seen in recent years, and that more fundamental research is clearly needed. But a long history of practical experience by beekeepers with the sublethal (as well as lethal) effects of pesticides leaves no doubt that pesticides certainly have the potential to cause colony health issues.
But Don’t We Already Know That It’s The Neonicotinoids?
The media have already tried and convicted the neonicotinoids as the cause of all bee problems, and it’s currently fashionable to celebrate the restrictions recently imposed on them by the European Union. But it is rational? No one has ever shown convincing evidence that neonics are linked to colony collapse; conversely, there is abundant experimental and on-the-ground evidence that the residues from seed-treated plants do not appear to cause observable harm to colonies [31].
Planting dust, soil drenches, or foliar applications are a different story, but these are generally drift or misapplication issues, hitting individual apiaries, not the bee population as a whole. Our regulators are well aware of these issues, and working to fix the problems.
Regarding the completely unacceptable bee kills due to the dust from corn seeding, of interest is a recent paper by Drs. Chris Cutler and Cynthia Scott-Dupree [32]—environmental toxicologists from Canada’s Dalhousie University—who analyzed the 110 pesticide incident reports received by Canada’s PMRA since 2007. Ranking the reports by the degree of severity of the bee kill, they found that there were over five times as many “major incidents” due to non-neonicotinoid products (including carbofuran, chlorpyrifos, coumaphos, diazinon, dimethoate, fluvalinate, formic acid, permethrin, and phosmet) as there were due to neonics, yet that these incidents are largely ignored by the press and beekeepers, who for some reason single-mindedly focus upon the neonics.
Hey, I’m as concerned about pollinators and pesticides as anyone. A recent review by Goulson [33] points out the excessive use of neonics (actually all pesticides are greatly overused), and details the many environmental questions about this class of chemicals. But here’s the thing—I can read studies all day long, but what I prefer to seek out are actual on-the-ground, real-life observations. Let me share one with you:
An “Acid Test” Of Neonic Seed Treatment
Activists are calling for a ban on clothianidin—the most common neonicotinoid seed treatment. Although honey bees appear to do just fine on seed-treated canola, their species has an advantage over solitary bees and other pollinators, due to their foraging on multiple plant species over a wide area, their social structure, and their processing of the pollen by nurse bees. So honey bees may not be the best indicator of neonic toxicity.
On the other hand, solitary bee species may be a better indicator as to whether neonic residues cause subtle adverse effects. Many solitary bees are “monovoltine,” meaning that they only raise a single generation per year. Because of this, a negative effect on any single female bee could prevent the production of the next generation. It occurred to me that the Alfalfa Leafcutter Bee (Megachile rotundata), which is used to pollinate clothianidin-treated canola (Fig. 6), would provide an excellent “acid test” of clothianidin for several reasons:
- Clothianidin has been shown to be highly toxic to leafcutter bees by topical application [34]. Since neonics are typically an order of magnitude more toxic by oral exposure [35], it is reasonable to expect that the leafcutter bee would be even more susceptible to residues consumed in food.
- Leafcutter bees do all their foraging within a few hundred feet of the nest [36], so those placed in the middle of a canola field would forage solely upon treated canola.
- Each individual female alone forages and provisions her nest, feeding upon the contaminated pollen and nectar as her sole protein and energy sources. If the insecticide negatively affected her behavior, navigational ability, health, or longevity she would be unable to reproduce effectively.
- The male bees use canola nectar as their sole energy source, and if the insecticide residues interfered with their behavior or longevity, the female bees might not get properly mated.
- The larvae consume a diet consisting solely of unprocessed contaminated pollen and nectar (rather than royal jelly), and thus every item in their diet would contain verified concentrations of clothianidin (approximately 1.7 ppb in the pollen; 0.8 ppb in the nectar [37]). Note: as with honey bees, neonicotinoids are virtually nontoxic to the larvae of the leafcutter bee [38].
- The female constructs her nest by cutting (with her mouthparts) leaves from the treated canola plants, which contain even higher residues of clothianidin than the pollen, thus exposing her to even more of the chemical. The larva then develops surrounded by these contaminated leaves, and the pupa overwinters in them.

Figure 6. Tents covering Alfalfa Leafcutter bee nest boxes in a canola field.
In short, the leafcutter bees would constitute the most severe test case for clothianidin exposure from a seed-treated crop. So I phoned a commercial supplier of leafcutter bees in Ontario (who declined to be named) and asked him whether he had any problems with his bees reproducing or overwintering after being set in clothianidin seed-treated canola. He said that he had been rearing them on such fields for many years and did not observe any problem. I put a good deal of faith into such unbiased field experience by a commercial bee man. You can draw your own conclusions.
So Which Pesticides Are Actually To Blame?
It’s pretty easy to diagnose an acute bee kill, what with piles of twitching bees in front of the hives (see “Signs and symptoms of bee poisoning” at [39]), and in many cases the responsible pesticide can be identified. To sidetrack briefly, remember when I mentioned a few articles back that the residues in Jim Doan’s bee kills did not indicate that the bees contained lethal doses of the chemicals? This made me strongly suspect that we can’t apply the LD50 data (in nanograms per bee) to the values obtained from actual field samples of dead bees. The recent report from Canada [40] confirms this. The highest residue level of clothianidin (from corn planting dust) found in any sample of dead bees from the entrances of a hive was 24 ppb, which works out to about a tenth of the theoretical amount necessary to kill a bee . This finding could be due to the metabolic degradation of the insecticide, but it certainly suggest that the LD50 value should be adjusted lower for samples of dead bees! I am greatly heartened that Canada is moving forward in addressing this issue of bee kills from corn planting dust [41].
Overt bee kills aside, more insidious are the residual effects due to contaminated dust, pollen, or nectar that foragers bring back into the broodnest. I’m told by beekeepers with far more experience with pesticides than I, that after exposure to certain pesticides, colony growth and production come to a standstill, sometimes for months, until the colony clears itself of residues and perhaps eventually recovers (or not). The problem is that few beekeepers (if any) can look inside a hive and diagnose which pesticide (or combination thereof) is causing the problem. He may notice spotty brood, poor buildup, winter dwindling, or queenlessness, but it is very hard to isolate the effect any particular pesticide residue, especially in today’s stew of residues in combs. But that doesn’t mean that we are completely blind…
The Evidence
Due to the rapid turnover of bees in a hive (other than the queen or “winter bees”), if a pesticide were indeed exerting a long-term effect upon colony health, then there would by necessity need to be residues of that pesticide or its degradation products persisting in the combs. With today’s testing equipment, we can detect residues to the parts per billion level, and have quite a large database of residue analyses of beebread samples, which we can perhaps use to either finger or exonerate certain pesticides suspected of being involved in colony health issues.
In a court of law, all evidence would be laid out before the court to determine whether it was substantial enough to make a case against a particular suspect. We can do something similar by reviewing two large publicly-available datasets of actual pesticide analyses of beebread from across the country—one by the Penn State team , the other by the USDA (Tables 1 and 2). I’ve condensed their data to only those pesticide detects that were found in at least 10% (Penn State) or 5% (USDA) of the samples, following this reasoning:
If a pesticide isn’t present in at least 10% of samples, then it isn’t likely to be the cause of widespread problems.
I’ve also color-coded the results as to the type of pesticide, and included the median detection level (to help us to determine whether that dose would be expected to cause colony health problems, or whether it would be insignificant).
| Pesticide |
Present in percent of samples*
|
Median detection if positive for target (ppb)
|
Type of pesticide
|
| Fluvalinate |
88.3
|
40.2
|
Beekeeper-applied miticide
|
| Coumaphos |
75.1
|
13.1
|
Beekeeper-applied miticide
|
| Chlorpyrifos |
43.7
|
4.4
|
Insecticide
|
| Chlorothalonil |
52.9
|
35
|
Fungicide
|
| Pendimethalin |
45.7
|
13.4
|
Herbicide
|
| Endosulfan I |
28
|
4.2
|
Insecticide
|
| Endosulfan sulfate |
26.3
|
2.2
|
Insecticide
|
| DMPF (amitraz) |
31.2
|
75
|
Beekeeper-applied miticide
|
| Atrazine |
20.3
|
8.9
|
Herbicide
|
| Endosulfan II |
20
|
3.8
|
Insecticide
|
| Fenpropathrin |
18
|
7
|
Insecticide
|
| Azoxystrobin |
15.1
|
10.2
|
Fungicide
|
| Metolachlor |
14.9
|
8.1
|
Herbicide
|
| THPI (Captan) |
14.2
|
227
|
Fungicide
|
| Captan |
12.9
|
103
|
Fungicide
|
| Esfenvalerate |
11.7
|
3.3
|
Insecticide
|
| Carbaryl |
10.9
|
36.7
|
Insecticide
|
| Cyhalothrin |
10.9
|
1.7
|
Insecticide
|
Table 1. The 2010 survey by the Penn State team [42], based upon (depending upon the pesticide) either 350 or 247 samples. This study (plus numerous others worldwide) clearly point out that the predominant pesticide residues in brood combs are typically those from the beekeeper-applied miticides (yellow).
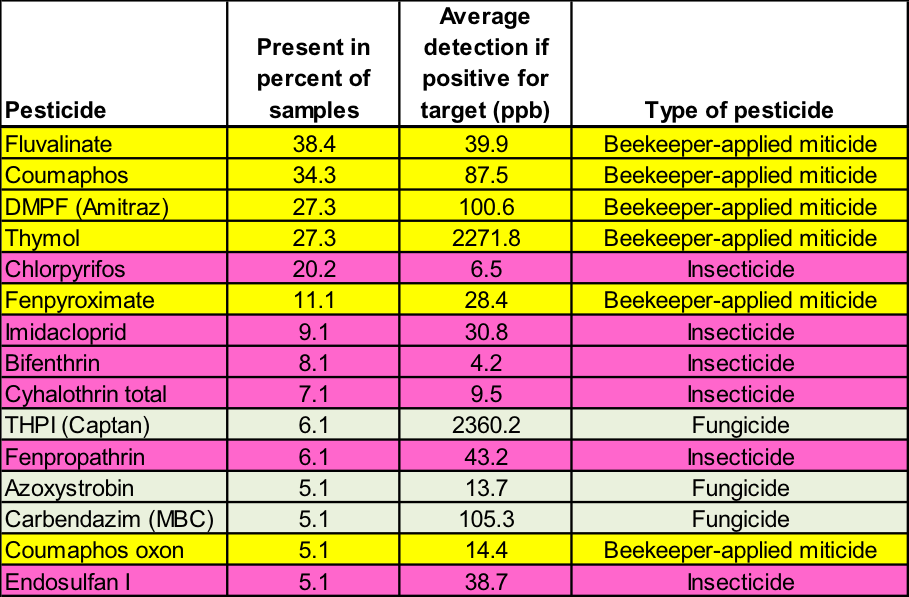 Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.
Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.
Keep in mind that the above surveys screen only for 174 chosen pesticides—compare this number to the roughly 1000 pesticide active ingredients and adjuvants registered for use in California. I’ve discussed the composition of this list with USDA’s Roger Simonds, who runs the tests. It is prohibitively costly to test for every possible pesticide, so one must arbitrarily draw up a limited list of the chemicals of most concern. All are aware that this is a difficult task, since we don’t even know which toxins with which we should be most concerned!
Note that in both surveys, the most common insecticide present was chlorpyrifos– an “old school” (introduced in 1965) organophosphate neurotoxin classified as being “highly toxic” to bees, and marketed as Dursban and Lorsban. Chlorpyrifos was previously widely used by homeowners and residential pest control companies. EPA has since restricted its use due to its toxicity to wildlife and aquatic organisms, and possible links to human health issues [44]—some of the reasons that EPA favors the neonicotinoids as “reduced risk” products.
Oh Boy, Let’s Do Some Math!
But just because a pesticide is present, doesn’t necessarily mean that it is causing measureable harm. A nurse bee may consume about 10 mg of beebread per day [45], so if she consumed that amount of pollen contaminated with chlorpyrifos at 6.5 ppb, then she would have been dosed with 0.065 ng (1 nanogram = 1 billionth of a gram) of the chemical. The question then is, how much chlorpyrifos does it take to actually harm a bee?
One commonly cited figure is that the LD50 for chlorpyrifos given orally is 360 ng/bee. Compare those figures (360 ng for toxicity vs. 0.065 in the daily diet)! Even though chlorpyrifos is a disturbingly common comb contaminant, it is unlikely that the median detected concentration (alone) would be causing colony health problems (not to say that higher doses don’t hurt colonies).
But, you say, some of the neonics are even more toxic than chlorpyrifos. How about the mean 31 ppb found by the USDA in the few samples positive for imidacloprid? The typical nurse bee would consume 0.31 ng, compared to the oral LD50 of about 4-40 ng, so she’d be eating a tenth to a hundredth of the lethal dose. This would be cause for concern, tempered by the fact that a bee can easily metabolize that amount of imidacloprid a day [46]. Such consumption could legitimately be suspected of causing sublethal effects. However, keep in mind that that 31 ppb was an average, which was strongly skewed by a few samples with very high concentrations (which I’d fully expect to cause colony health problems). Plus this is not simply a matter of the average amount of contamination; one must also look at the percentage of positive detects. The Penn State team [47] puts it well:
Our residue results based on 1120 samples which include Mullin et al. (2010) and subsequently more than 230 additional samples do not support sufficient amounts and frequency of imidacloprid in pollen to broadly impact bees.
OK, so how about the varoacides fluvalinate at 40 ppb or the amitraz degradate DMPF at 100 ppb? Surprisingly, I can’t find an oral LD50 for fluvalinate, so the contact toxicity figure (200 ng/bee) will need to suffice. Those residues work out to about 1/500th expected toxicity.
Amitraz scored a bit better, with the nurse bees consuming about 0.01 ng—far below the lethal dose. But a recent study found that an oral dose of 0.2 ng of amitraz causes more than a doubling of the heart rate of a bee [48]—that’s at 1/20th of the average detect! The authors dryly state:
The above responses clearly show that the heart of the honeybee is extremely vulnerable to amitraz, which is nevertheless still used inside beehives, ostensibly to “protect” the honeybees against their main parasite, Varroa destructor.
How vulnerable? Frazier [49] observed that “Dead and dying bees collected around colonies in association with corn had only residues of 2,4-DMPF at 5,160 ppb.” Looks like perhaps the beekeeper inadvertently killed his own bees with an off-label mite treatment that may have overworked their little hearts!
And if those miticide and insecticide residue weren’t enough alone, some of the toxicity of these chemicals is additive or synergistic. The Penn State team again says it well:
[The] pyrethroids… were found in 79.4% of samples at 36-times higher amounts than the neonicotinoids, on average… The mean neonicotinoid residue was 37 ppb (scoring non-detects as 0 ppb), of which only 6.7 ppb was imidacloprid. Pyrethroids, by comparison, were present at a mean residue of 106 ppb and a frequency of 80.3% in pollen samples… Indeed, if a relative hazard to honey bees is calculated as the product of mean residue times frequency detected divided by the LD50, the hazard due to pyrethroid residues is three-times greater than that of neonicotinoids detected in pollen samples [emphasis mine].
The pyrethroids are popular because they are relatively nontoxic to humans. But they can sure kill honey bees. More so, they can cause sublethal effects, such as irreversible inhibition of olfactory learning ability [50].
Hey, we’re only getting rolling! Mussen [51] pointed out a decade ago that fungicides could kill larvae; recent research from the Tucson lab [52] and elsewhere confirm that fungicide residues can mess up the colony (we sometimes observe this in almonds). Of note is that colonies treated with some fungicides were unsuccessful at requeening themselves! And recent research by Zhu [53] found that the relative toxicity of larvae to the commonly-detected fungicide chlorothalanil was almost 40 times higher than that of chlorpyrifos. Fungicides are frequently found at high concentrations in beebread.
I cannot help from returning to the refrain that instead of limiting our concern to any single pesticide, that we should be looking at the total toxin load that the colony is forced to deal with.
And How About The “Inerts”?
The pesticide detection analyses above do not look at the “inert” adjuvants in the pesticide “formulation.” These chemicals not only help to disperse the pesticide over the waxy leaf surface, but also aid in its penetration through the insect cuticle, thus making the pesticide relatively more toxic to the bee!
Mullin and Ciarlo [54, 55] found that:
Formulations usually contain inerts at higher amounts than active ingredients, and these penetrating enhancers, surfactants and adjuvants can be more toxic on non-targets than the active ingredients. For example, we found that the miticide formulation Taktic® was four time more orally toxic to adult honey bees than the respective active ingredient amitraz. Impacts of ‘inerts’ in pollen and nectar alone or in combination with coincident pesticide residues on honey bee survival and behavior are unknown.
The researchers also found that:
Learning was [rapidly] impaired after ingestion of 20 µg of any of the four tested organosilicone adjuvants, indicating harmful effects on honey bees caused by agrochemicals previously believed to be innocuous.
One of the common adjuvants is a solvent NMP, described by BASF [56]:
NMP can be used as a solvent or co-solvent for the formulation of insecticides, fungicides, herbicides, seed treatment products and bioregulators where highly polar compounds are required. NMP is given preference over other highly polar solvents because it is exempt from the requirement of a tolerance when used as a solvent or co-solvent in pesticide formulations applied to growing crops, and it possesses a favorable toxicological and environmental profile.
The key words above are that these toxic solvents are “exempt from tolerance” [57], so they are sprayed all over crops along with the active ingredients of pesticides (including imidacloprid). Yet Zhu [58] recently reported that NMP can rapidly kill bee larvae. The authors conclude that:
Our study suggests that fungicide, the inert ingredient and pesticide interaction should be of high concern to honey bee larvae and overall colony health. None of these factors can be neglected in the pesticide risk assessment for honey bees.
Choosing To Ignore The Obvious
There is no doubt that neonics have the potential to harm bees, but the question is, do they really cause as much problem in the real world as we’ve been led to believe? This is not a matter of convincing the masses; this is an investigation of fact and evidence. For a pesticide to cause harm to a colony of bees, two necessary elements must occur:
- The bees must be exposed to the pesticide. Evidence for this is best determined by chemical analysis of the pollen in the combs, since residues in the bodies of dead bees may be degraded, and because water-soluble insecticides such as the neonics are not absorbed into the wax (residues in the wax do document the history of exposure to lipophilic pesticides).
- The pesticide must be present at a concentration above a trivial level.
When we take the time to determine which pesticides bees are actually found in the combs of hives, neonicotinoids are seldom present, or if detected are often at biologically irrelevant concentrations. Imidacloprid was detected in fewer than 3% of Mullin’s 350 samples, and clothianidin not at all! Similarly, there were zero detects for clothianidin in the 99 USDA samples; imidacloprid was only present in 9%. Likewise, a number of European studies have shown similar results (reviewed in [59]).
In a recent study, the Fraziers [60] looked at hives placed in cotton, corn, alfalfa, apples, pumpkins, almonds, melons, blueberries, or wild flowers, and identified the residues in collected pollen, in returning foragers, and in dead or dying bees near the hives. Again, the only neonic noted was thiamethoxam in alfalfa (in which dying bees contained residues of ten different pesticides). However, there were alarmingly high detects of fungicides, the insecticide acephate, and the metabolite of the beekeeper-applied miticide amitraz.
The latest data comes from Dr. Jeff Pettis [61], whose group determined the pesticides in bee-collected pollen from six crops: apple, blueberry, cranberry, cucumber, pumpkin, and watermelon. Of the 35 pesticides detected, beekeeper-applied miticides and ag fungicides predominated (sometimes at alarming levels), followed by common organophosphate, pyrethroid, and cyclodiene insecticides (again sometimes at alarming levels). In the 17 samples tested, residues of neonics were only found in the samples from the apple orchards, and only one was found at a biologically-relevant concentration.
So my question is why the heck are so many activists pursuing the single-minded focus upon the neonics, when the clear evidence is that neonics are not commonly found in bee-collected pollen, and if present, are generally at levels that do not appear to negatively affect colony health [62]?
There is a lot more to pesticide issues than the neonics alone, and by focusing our attention solely upon them, we ignore the often far more serious effects of other pesticides.
Blinded By Bias
During the intense focus upon neonicotinoids the past few years, we’ve learned that exposure of bees to these insecticides can result in all sorts of sublethal effects. Unfortunately, many researchers appear to be wearing blinders as to the effects of other pesticides. The resulting narrowness of these studies skews our perspective—if we only look for effects from the neonics, we don’t know how to rank the biological relevance of those effects relative to the effects of all the other toxins to which bees are exposed, generally to greater extent.
A practical complaint to researchers: if you are going to look for sublethal effects of neonics, please include positive controls of some other pesticides, so that we can learn whether the neonics are better or worse than the alternatives!
I commend one group that recently decided to take a look at the effects of a common herbicide upon the development of bee larvae [63]. The results of this straightforward and meticulous study are an eye opener!
The researchers found that exposing bee larvae to even infinitesimal amounts of the herbicide paraquat prevented them from fully developing their critical oenocyte cells (see box).
| Oenocyte cells are not only involved in the production of lipids and lipoproteins, but they also appear to play a role in the constitution of external cuticle in both larvae and adults. In addition, they are involved in intermediary metabolism and synthesize hydrocarbons to waterproof cuticle or to make beeswax. Furthermore, oenocytes secrete hormones, especially those involved in larval and adult development. They are also described as the major cells expressing cytochrome P450 reductase, which is involved in detoxification of toxins [information paraphrased from the cited paper]. |
Exposure to even a part per trillion of paraquat suppressed the development of these extremely important cells. The authors conclude:
This study is the first which reports an effect of a pesticide at the very low concentration of 1 ng/kg, a concentration below the detection limits of the most efficient analytic methods. It shows that chemicals, including pesticides, are likely to have a potential impact at such exposure levels.
Who woulda thunk? Paraquat isn’t included in the standard screening for pesticide residues, so we don’t even know how prevalent it is in hives! The above findings should make it clear that we need to go back to the beginning if we are to understand the sublethal effects of pesticides (and adjuvants), even at perhaps undetectable levels.
We do know that here were 812,000 lbs of paraquat applied in California in 2010, as opposed to only 266,000 lbs of imidacloprid. Paraquat shows strong adverse effects upon bee larvae at a part per trillion, as compared to imidacloprid, which is so minimally toxic to bee larvae that no one has even been able to determine an LD50! So the amount of paraquat applied has far greater potential to cause problems to bees in agricultural areas (Fig. 7).
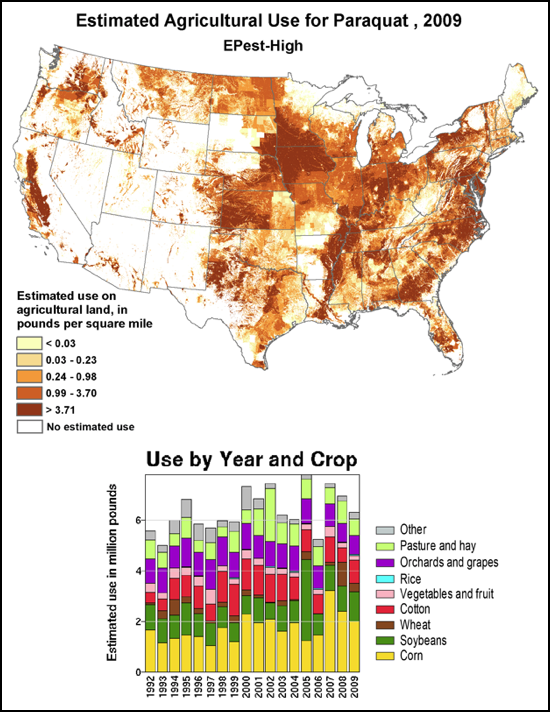
Figure 7. The herbicide paraquat appears to be harmful to bee larvae at levels as low as 1 part per trillion. Note the wide variety of crops, and the extensive areas to which it is applied.
So here we have clear scientific data from a well-designed laboratory experiment that a commonly-applied pesticide has the ability to cause immune suppression and other adverse effects in developing bees, yet these results have been virtually ignored by beekeepers and environmental groups. I just don’t understand it!
No More Safe Home To Return To
Out of their protective hive, honey bees live in a hostile world, full of predators, deadly weather, and toxic agents (both natural and manmade). But the bees of old could generally return to a “safe” home, in which the transmission of natural toxins was largely minimized by the behavior of foragers, and by the processes of the conversion of nectar to honey, and of pollen into jelly (via the digestion of beebread by nurse bees). Both of these processes help to prevent the transmission of toxins from the foragers to the queen and the brood.
With the advent manmade pesticides, bees may no longer have that “safe” home to return to. Beebread and the wax combs nowadays are often contaminated with any number of pesticides (in addition to natural plant toxins and industrial pollutants). But this is not a “new” problem:
A Historical Artifact
Even before we had the ability to detect pesticide residues in combs to the parts per billion level, pesticide analyses often found easily-detectable levels of insecticides in bee hives. As a frame of reference, I sought out a historical artifact—the residues in the beeswax that had been rendered by beekeepers and reprocessed into a sheet of “clean” foundation. I was lucky enough to find that such a sample had recently been analyzed by the Tucson Bee Lab. Dr. Diana Sammataro forwarded me the results of the analysis of an undated “very old” piece of wax foundation from the Northeast (Table 3).
THIS IS THE TABLE !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
|
Pesticides in an old piece of beeswax foundation.
|
|
Positive residue detect
|
ppb
|
| Pendimethalin |
13.1
|
| Endrin |
156
|
| Dieldrin |
160
|
| Trifluralin |
3.6
|
| DDT p,p’ |
32.7
|
| Heptachlor |
35.1
|
| Malathion |
4.3
|
| Chlorpyrifos |
4.6
|
| Dicofol |
6.8
|
| PCB’s |
8190
|
| Chlorothalonil |
84.6
|
Table 3. We can narrow down the foundation’s date of manufacture by the residues present. Pendimethalin was first registered in 1972 (the same year that DDT was banned), and since there were no residues of fluvalinate, the foundation was clearly produced prior to the arrival of varroa around 1990. Thanks to Dr. Diana Sammataro and the Tucson Bee Lab.
Clearly, pesticide-contaminated combs are hardly a new phenomenon. In the above example, the beeswax batch used to produce the foundation came not from a single hive, but rather from the combined wax from many hives, likely from many beekeepers, and thus would represent an average sample of the degree of contamination somewhere in that 1972-1990 time frame. And that doesn’t take into account whether the raw wax came mainly from cappings (which would have been minimally contaminated), or whether it went through the common practice of being filtered through activated carbon. But any colony started on such foundation purchased from a beekeeping supply house would clearly have had to deal with at least the residues of these lipophilic toxins from the get go!
An aside: perhaps of interest is something that I noticed years ago when I switched from dipping my own wax queen cell cups to using plastic cups. My “take” rate became better and more consistent. Was that because the beeswax at the time was contaminated with residues?
The Beekeeper Contribution To Shifting The Tip Point
One thing that is “new” is that since the arrival of varroa, we’ve upped the ante—all commercial beeswax is now contaminated with residues of beekeeper-applied synthetic miticides. The three most prevalent synthetic chemicals found in combs today all get there by being applied by beekeepers for mite control.
Practical note: And although there is no reason to be concerned about the tainting of honey by the legal use of these miticides, the beekeeper/applicator should be aware that both amitraz and tau-fluvalinate make California’s list of “chemicals known to the State to cause reproductive toxicity,” and coumaphos is of concern because it is a “cholinesterase-inhibiting pesticide.” No varroacide is harmless to bees [64]—but the benefits of mite control generally (but not always) outweigh the adverse effects due to the miticide residues.
We beekeepers have clearly shifted the baseline for pesticide contamination of combs, which increases the total toxic load even before the contribution by agricultural pesticides.
Stop Right There!
Although it is a very attractive hypothesis to blame our problems on miticide or pesticide residues, let’s do a reality check. On good forage in good weather, plenty of beekeepers see their colonies thrive even on old, dark, seriously-contaminated combs; but under stressful conditions those same residues might contribute to poor colony performance or even mortality.
No study has yet found support for the hypothesis that miticide residues are the cause of our current bee problems (although one would have every reason to suspect that they may contribute). In fact, vanEngelsdorp [65] found that surprisingly, higher levels of coumaphos residues negatively correlated with colony survival. How could this be? One possible explanation is that those beekeepers who used it experienced better mite control. But there is also another intriguing possibility—hormetic effects.
Undetectable Levels And Hormesis
Is your head spinning yet? I’ve presented evidence that undetectable levels of some pesticides could harm bees, that “inert” adjuvants can do the same, and that combs are often chock full of all sorts of pesticide and varroacides residues. Criminy, it’s a wonder that bees survive at all! Or is it?
Bees have long been exposed to all sorts of natural, and recently, manmade toxins, and survived. Toxicity is a complicated subject. The only thing that separates a medicine from a poison is the dose. In general, if a pesticide has been tested upon adult and larval bees and found to have no observable adverse effects at a certain concentration, we would not expect to see adverse effects at lower concentrations. However, there are exceptions to this general rule—toxicity may vary up or down depending upon the dose [66]!
I’ve previously mentioned the term hormesis [67]— the paradoxical effect of toxins at low concentrations. The paradox is that although most chemicals are toxic at high concentrations, the majority are likely beneficial at low concentrations. For those interested in this fascinating phenomenon, I suggest Dr. Chris Cutler’s excellent and thought-provoking review [68]. It is not only possible, but actually probable that lose doses of pesticides may exert a beneficial effect upon a colony! (Don’t be ridiculous—I’m not suggesting that bees are better off for the presence of pesticides!).
Wrap Up
Toxins, whether natural or manmade, are clearly a potential issue in colony health. To what degree pesticides contribute to colony morbidity or mortality is dependent upon exposure, the dose, and a host of associated factors. Beekeepers have long noticed that their bees often do better if allowed to forage on pesticide-free land. But many beekeepers today tell me that their bees do just fine in the middle of intense agricultural areas—so this is not a black or white situation.
In recent years beekeepers themselves have greatly added to the degree of contamination of their combs. Introductions of novel pesticides and adjuvants keep changing the picture. And now we’re finding that pesticides that we formerly assumed were harmless to bees (fungicides and herbicides) may actually be quite toxic to larvae! Then there is the scary finding that undetectable levels of some pesticides might cause health issues, countered by the fascinating subject of hormesis.
I certainly do not profess to understand all this, but I have come to the following conclusions:
- That bees have had to deal with toxins for a long time,
- That pesticides will be with us for the foreseeable future,
- That varroacides have likely added to the problem,
- That pesticides can cause lethal and long-term sublethal effects in the hive, but
- That many beekeepers in agricultural areas no longer consider pesticides to be a serious issue, whereas,
- That colonies may go downhill after being exposed to some agricultural chemicals, or combinations thereof,
- That toxicology in the hive is complex, and that there are few simple answers,
- That it is unlikely that any single pesticide is to blame for our current colony health issues,
- That we still have a lot to learn!
Next month I will look at the distribution of both managed colonies and of pesticide applications in the United States, and their relationship to bee health problems.
Acknowledgements
As always, I could not research these articles without the assistance of my longtime collaborator Peter Loring Borst, to whom I am greatly indebted. I also wish to thank Drs. Jim and Maryann Frazier, Chris Mullin, David Fischer, Eric Mussen, Thomas Steeger, and Roger Simonds for their generosity in taking the time to discuss pesticide issues with me.
References
[2](Broken Link) http://perc.org/articles/everyone-calm-down-there-no-bee-pocalypse
[4] http://thebreakthrough.org/index.php/journal/past-issues/issue-1/an-environmental-journalists-lament/
[5] vanEngelsdorp and Meixner (2010) op. cit.
[6] Rucker (2012) op. cit.
[16] Wilson, WT and DM Menapace (1979) Disappearing disease of honey bees: A survey of the United States. ABJ March 1979: 185-186.
“Certainly with both pesticide-related and [Disappearing Disease]-caused bee losses, the adult population of a colony may be reduced rapidly to a “handful” of bees or, in some cases, the entire population may be lost.
“However, in the case of pesticide poisoning, there is usually evidence of pesticide application…the worker bees either die in the field or in or near the hive depending on the type of pesticide. When the field force is killed and they “disappear,” many dead or dying bees may be seen on the ground in the field or on the ground between the treated field and the apiary…If the foraging bees bring poison into the hive, then the nurse bees either die in the hive or at the entrance so one can see many crawling and tumbling adults and large amounts of neglected brood. Exposure to pesticides over an extended period results in very weak colonies, and some die out.
“In the case of [Disappearing Disease], the situation is quite different. The colonies frequently have gone through a period o nectar and pollen collection with active brood rearing [as in typical CCD]. Then the weather has turned unseasonably cool and damp and remained adverse for from about 3 to 14 days…During the inclement weather, the bee populations dwindle because the worker bees disappear from the hive leaving a “handful” of bees and the queen. Often these small populations recover and increase in size during hot weather and a long nectar flow or, or occasionally, the entire population absconds…”
[17] Johansen CA and DF Mayer (1990) Pollinator Protection: A Bee & Pesticide Handbook. Wicwas Press.
[21] Medici SK, Castro A, Sarlo EG, Marioli JM, Eguaras MJ (2012) The concentration effect of selected acaricides present in beeswax foundation on the survival of Apis mellifera colonies. J Apic Res 51: 164–168
[22] Eric C. Mussen, Julio E. Lopez, and Christine Y. S. Peng (2004) effects of selected fungicides on growth and development of larval honey bees, Apis mellifera L. (Hymenoptera: Apidae). Environmental Entomology 33(5):1151-1154.
[23] Frazier, J.L., M.T. Frazier, C.A. Mullin & W. Zhu – Does the reproductive ground plan hypothesis offer a mechanistic basis for understanding declining honey bee health? http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[25] Wu JY, Smart MD, Anelli CM, Sheppard WS (2012) Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. J Invert Path 109: 326–329
[27] Maisonnasse A, et al (2010) E-β-Ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS ONE 5(10): e13531. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0013531 These researchers studied (E)-b-ocimene, a volatile terpene commonly produced by plants to attract predatory mites, but also a critical pheromone produced by the brood and the queen.
[34] Scott-Dupree, CD, et al (2009) Impact of currently used or potentially useful insecticides for canola agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae). J Econ Entomol 102(1):177-82.
[36] Hobbs, GA (1967) Domestication of Alfalfa Leaf-cutter Bees. Canada Dept. of Agriculture. Ottawa: Queen’s Printer and Controller of stationary.
[37] Dr. Jerry Bromenshenk, pers. com.
[38] Abbott, VA, et al (2008) Lethal and sublethal effects of imidacloprid on Osmia lignaria and clothianidin on Megachile rotundata (Hymenoptera: Megachilidae). J Econ Entomol 101(3):784-96.
[40] PMRA (2013) Evaluation of Canadian Bee Mortalities Coinciding with Corn Planting in Spring 2012.
[42] Mullin CA, et al. (2010) op. cit.
[44] Christensen, K.; Harper, B.; Luukinen, B.; Buhl, K.; Stone, D. 2009. Chlorpyrifos Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. http://npic.orst.edu/factsheets/chlorptech.pdf.
[45] Rortais, A (2005) Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 36: 71–83.
[46] Cresswell, JE, et al (2012) Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115: 365– 371.
[47] Frazier (2011) op. cit.
[49] Frazier, et al (2011) Assessing the reduction of field populations in honey bee colonies pollinating nine different crops. ABRC 2011
[51] Mussen, et al (2004) op. cit.
[53] Zhu, W., D. Schmehl & J. Frazier (2011) Measuring and predicting honey bee larval survival after chronic pesticide exposure http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[54] Mullin, C.A., J. Chen, W. Zhu, M.T. Frazier & J.L. Frazier – The formulation makes the bee poison. ABRC 2013
[55] Ciarlo TJ, Mullin CA, Frazier JL, Schmehl DR (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7(7): e40848. doi:10.1371/journal.pone.0040848
[56] (Broken Link!) http://www2.basf.us/diols/bcdiolsnmp.html
[58] Zhu, et al (2011) op. cit.
[60] Frazier, M.T., S. Ashcraft, W. Zhu & J. Frazier – Assessing the reduction of field populations in honey bee colonies pollinating nine different crops http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[61] Pettis, et al (2013) op. cit.
[62] A recent study confirm that the neonic residues in corn, soy, and canola pollen are at very low concentrations. Henderson, C.B. a, J.J. Bromenshenka, D.L. Fischerb. Clothianidin exposure levels from bee-collected pollen and nectar in seed-treated corn and canola plantings. ABRC 2013 http://bees.msu.edu/wp-content/uploads/2013/01/ABRC-abstracts-2013.pdf
[64] Boncristiani, H., et. al. (2011) Direct effect of acaricides on pathogen loads and gene expression levels of honey bee Apis mellifera. Journal of Insect Physiology. 58:613-620.
[65] vanEngelsdorp, D, et al () Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J. Econ. Entomol. 103(5): 1517-1523. (Broken Link!) http://www.eclecticparrot.com.au/research_papers/VanEngelsdorp%202010%20Weighing%20risk%20factors%20in%20Bee%20CCD.pdf
[66] Cutler GC, Ramanaidu K, Astatkie T, and Isman MB. (2009) Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag Sci 65:205-209
[68] Cutler, GC (2013) Insects, insecticides and hormesis: evidence and considerations for study. Dose-Response 11:154–177 (Broken Link!) http://dose-response.metapress.com/app/home/contribution.asp?referrer=parent&backto=issue,2,11;journal,3,34;linkingpublicationresults,1:119866,1
First published in: American Bee Journal, March 2014
CONTENTS
We Are Two Adaptive Species
Honey Bees Are Designed For Rapid Adaptation
The Human-Facilitated Realized Niche Of The Bee
The Natural Limiting Factors Of The Honey Bee Population
Limiting Factor: The Weather
Limiting Factor: Predation
Limiting Factor: Competition For Nest Cavities
Limiting Factor: Carrying Capacity Of The Habitat
Limiting Factor: Reproductive Success Rate
Limiting Factor: Competition And Intercolony Parasitism
Limiting Factor: Pathogen Transmission
A Special Case: The Viruses
Conclusion
Footnotes and Citations
What’s Happening to the Bees?
Part 2
Originally published in ABJ March 2014
Randy Oliver
ScientificBeekeeping.com
Over the past few decades, as a beekeeper/biologist I’ve had the opportunity to watch evolution in action. I’ve observed the catastrophic effects upon colony health due to the introduction of new parasites, periodic pathogen epidemics, and the more subtle effects of changing land use practices and climate change. I’ve also witnessed the evolution of both recreational beekeeping and the bee industry, as we’ve been forced to change our management practices and income streams due to the aforementioned biological and environmental factors, plus changes in markets and the impacts of regulatory decisions.
My point is that things change, each change altering the realized niche of both bees and beekeepers. Neither Nature nor the Market are the least bit sentimental. Those who don’t adapt to change die.
The adaptive process may make headlines, or it may go largely unnoticed. But one thing’s for sure—the more that we understand the changes in the parameters of the bee and beekeeper niches, the better we can successfully engage in the adaptive process.
We Are Two Adaptive Species
Both Apis mellifera, and those Homo sapiens categorized as “beekeepers” have proven to be incredibly adaptive species (ecologists would say that we exhibit a high degree of plasticity). At times, each of our populations face limiting factors that weed out the least fit. And the higher the failure rate of individual colonies or beekeeping operations, the stronger the selective pressure to adapt.
So what sort of failure rate is “normal” for bees under optimal natural conditions (the fundamental niche)? Let’s do the math! Given: an established and stable local population of bees under optimal conditions. In the spring each colony will produce at least one swarm. At that point, the colony population will have temporarily at least doubled. But such a rate of reproductive increase is obviously unsustainable, since by definition a “stable” population ends with the same number of colonies each year. So simple arithmetic tells us that in nature, on average, at least half of all colonies will succumb each season, even under the best of conditions.
Although half the colonies will fail on average, it is not the average colony that fails. The key point is that it is the least fit that tend to fail, and a greater proportion of the most fit survive. This considerable amount of selective pressure is what drives adaptation and evolution in each natural population of bees.
It’s the same with beekeepers. The failure rate for beginning beekeepers is often even higher than the above. Commercial operations also fail; despite record high prices for honey and pollination services, our recent elevated colony mortality rate is eliminating the profit margin for some operators, who, sadly, will go out of business. But, again, the hard fact is that it is the less fit (or less adaptable) operations that are failing—I speak with plenty of operators who aren’t experiencing egregiously high winter losses, and whose businesses are firmly in the black. It is these operations that are successfully adapting to their realized niche.
Honey Bees Are Designed For Rapid Adaptation
As I study the honey bee reproductive strategy, one principle jumps out:
Everything about the honey bee reproductive process is designed for rapid adaptation to changes in its realized niche, and for the recovery of the bee population from decimation events [1].
Apis mellifera has the highest known rate of genetic recombination of any animal. And those “experimental” recombinations are then filtered for success through the haploid drones, who have only one set of chromosomes, meaning that only the best novel combinations of genes (alleles) have any chance of being passed to the next generation.
The genetic combinations of the fittest drones then predominate in the matings that occur in drone congregation areas, thus ensuring that each virgin queen has the best chance of loading up with the best genetics that the overall bee population in that area has to offer. And she further ensures the maximum diversity of her offspring by mating with multiple drones. Add to this the incredible epigenetic plasticity of the honey bee [2], and we have an organism able to quickly adapt to whatever Nature throws at it!
I observe something similar with beekeepers. Those who are consistently trying new things and adapting to the changing biological and business environments are those who tend to be the most successful in the long run.
The Human-Facilitated Realized Niche Of The Bee
OK, so in nature, half the colonies fail each year. But provided facilitation by human beekeepers, that rate can drop to around 10% for well-managed operations. However, in recent years, U.S. beekeepers have been reporting distressingly high rates of colony failure. Clearly, something in the realized niche of our honey bees has changed in the last decade (one or more limiting factors). In order to attempt to figure out exactly which factors are responsible, let’s first determine what the primary limiting factors were for honey bee populations prior to humans. Perhaps then we can better understand how our actions (and the Earth’s burgeoning human population) are affecting honey bee survival. And maybe then we can take steps to make life easier for our beloved bees (and improve the bottom line of those of us who make our living at keeping ‘em).
The Natural Limiting Factors Of The Honey Bee Population
Limiting Factor: The Weather
The weather would be an obvious limiting factor—colonies are stressed by extreme cold, unfavorable flight weather during the spring or summer, or by lack of forage and water during droughts. Such intermittent weather events may sporadically cull the bee population, but would only affect long-term adaptation if they occurred regularly. So let’s focus upon which factors limited bee populations in favorable years, during which they have the intrinsic ability to increase exponentially.
Limiting Factor: Predation
Honey bees are herbivores. The populations of many herbivores are controlled by predators (Fig. 1). If the population of the prey species becomes dense, predator species ramp up their numbers to take advantage of the food source. The result is typically an oscillating predator/prey population dynamic (this is one of the bases for integrated pest management in agriculture).

Figure 1. Foraging bees face plenty of predators, such as wasps, assassin bugs, birds, spiders, and dragonflies. One of “my” bees (above) became a meal for a robber fly.
Although predators of foraging bees may take a bite out of the population of older bees in the hive, they do not appear to be a primary limiting factor of the overall bee population. An exception to this rule might be the Asian Hornet (Vespa velutina) (now introduced in Europe), which can decimate small colonies of bees by picking off returning foragers [3].
A more serious form of predation is direct invasion of the hive. A healthy colony can generally repel or otherwise deal with small invaders such as ants, wasps, and Small Hive Beetles. More problematic are those large mammalian predators willing to ignore the bees’ defensive stinging, such as bears, skunks, honey badgers, and humans. The bees’ main defense against such predators is to nest in inaccessible fortifications. And that leads us to…
Limiting Factor: Competition For Nest Cavities
Bees are pretty picky about the nest cavities that they choose, strongly preferring elevated tree cavities having small, defendable entrances [4] (Fig. 2). In treeless areas, the lack of suitable nest sites could well have been a limiting factor for the honey bee population. But this is unlikely to have been the primary limiting factor anywhere that patches of ancient forest were within range.

Figure 2. This hollow black oak provides a protected nest cavity impenetrable by our local black bears.
Although a lack of suitable cavities may not the main limiting factor of the bee population, it does bring to mind another fascinating aspect of bee behavior. Please allow me to digress for a bit. Not all beekeepers are aware that intra- and inter-species parasitism is common among bees, wasps, and ants. For example, queens of a number of bumblebee species parasititically invade and take over other bumblebee colonies. And the Cape Bee (Apis mellifera capensis) is famous for its ability to parasitically take over colonies of the Savannah Bee (Apis mellifera scutellata) [5]. This sort of deplorable behavior appears to be ingrained in the bee genome– bees covet the fruits of their neighbors’ hard work.
Part of the Africanized honey bees’ ability to rapidly expand its range in the Americas was likely its ability to invade and usurp the established nests of European bees [6]. Lately, Dr. Wyatt Mangum has been reporting on his observations of similar behavior by ostensibly non-Africanized bees in Virginia [7].
Such takeovers give the usurping swarm a profound advantage. Rather than needing to establish a nest and provision it with stores from scratch, it can simply take over an established colony, essentially hijacking its combs, stores, and entire workforce. Although Mangum, so far as I know, is the first to report such behavior for European honey bees, his observation may answer a question that has been bugging me for years: why do European bees send out what appear to be doomed swarms in late summer?
Bees typically swarm in spring, for the obvious reason that that timing allows the swarm colony to establish and provision a nest in time for winter. But in actuality, there are two peaks of swarming during a season (Fig. 3). I’d long noticed this, and wondered why in the heck a colony would bother to swarm in the late summer—natural selection should have eliminated such suicidal behavior. It just didn’t make sense!

Figure 3. Seasonal timing of swarm emergence dates in New York, showing the main late-spring peak, followed by a lesser peak around the first of September. Why the heck would colonies swarm in late September? After Seeley [[i]].
[i] I transcribed and plotted the data from Fig. 1 in Seeley, TD, et al (1989) Bait hives for honey bees. Cornell Coop Ext Inf. Bull. No. 187. http://ecommons.cornell.edu/bitstream/1813/2653/2/Bait%20Hives%20for%20Honey%20Bees.pdf
I assumed that the data was for New York and from the following paper, but I was unable to obtain a copy:
Fell, R. D., et al (1977) The seasonal cycle of swarming in honeybees. J. Apic. Res. 16:170-173.
But Mangum’s observations reminded me that I’ve also seen in past years late-summer swarms landing on hives, and also seen balled queens in hives in late summer. Perhaps I simply never put it all together! Could it be that this is an innate, but previously unrecognized, behavior in European bees?
It is certainly biologically plausible that under certain circumstances European colonies behave like their Africanized brethren (they are, after all, the same species), and send out older queens who have already successfully built up a colony (and perhaps even a second swarm colony), but still have enough vigor left to do an invasive usurpation of a nearby nest. If the colony were already superseding that queen, then it would be an inexpensive gamble to send her out, accompanied by a hit team of experienced workers, to try to take over an unsuspecting colony, its stores, and its workforce shortly before winter.
Pardon my digression—let’s return to our search for the main limiting factor of the natural bee population.
Limiting Factor: Carrying Capacity Of The Habitat
The maximum population density for the realized niche of a population is set by the carrying capacity of that particular environment, typically limited by resources such as food. However, the honey bee is a special case; similar to the bear, the colony can gorge when food is plentiful, and store “fat” (honey and beebread) as reserves for lean times (as during overwintering).
There are indeed areas in which colonies can barely put on enough honey during the main flow in order to make it through the winter. But by definition, such areas would not meet a primary requirement of the fundamental (optimum) niche of the honey bee, so we can disregard such areas from this discussion. What we are interested in is the limiting factor of the bee population in areas that normally produce a good honey flow.
Limiting Factor: The Timing Of The Bloom
Honey bees are defined by their ability to store food reserves—honey and beebread—to see them through lean times. But there are times other than the main honey flow during which the availability of nectar, and especially pollen, are of critical importance to the ability of colonies not only to survive, but also to reproduce. Colonies must build up and produce a swarm early enough in the season that both parent and swarm colony have fighting chances to store enough honey to make it through the following winter [9]. Such buildup requires the initiation of broodrearing in the middle of winter, which is in turn dependent upon having stored a large supply of beebread during the fall pollen flow [10].
What we must keep in mind is that a colony of bees is only effective at putting away a honey surplus if it has grown a large enough population to efficiently forage upon and store the available nectar. Timing is everything. Too large a population at the wrong time of the year would be counterproductive, since those hungry mouths would consume more honey than they were able to store. A locally-adapted population of bees times its buildup to coincide with the main flow, and then quickly shrinks back to survival mode.
Many of us tend to base our idea of typical bee behavior upon that of commercially-selected Italian stock. Such stock, originally adapted to Mediterranean climates, and bred for continuous broodrearing and high honey production, can hardly be expected to be representative of wild type bees adapted to cold-winter areas (I am not dissing Italian-type bees—they are well adapted to build up early for almond pollination).
Bees adapted to colder winters, such as the Carniolans or Russians, are far more responsive to the environment, especially to the availability of pollen. As soon as plants start producing pollen in spring, bees of these races explode into action —working even in cool and wet weather, and madly brood up.
The reason for their frenzy is that they must build up their population early enough to produce a swarm in time for it to have a decent chance at establishing a nest and putting away adequate winter honey stores during the brief main honeyflow (typically May through June; in temperate climates, colonies may only gain weight for a few weeks a year). I’ve previously graphed the bee colony’s amazing ability to quickly build up [11]. An absolute requisite for such a rapid rate of growth is a monster supply of pollen in the early spring.
After the main flow comes the summer dearth. When pollen is unavailable, wild-type bees sit tight in survival mode [12], cooling their heels and conserving their energy. I’ve seen Russian bees cease broodrearing in August in the arid California foothills, appearing as though they were queenless. In the adjacent yards, my Italian stock just keep on rearing brood, and required supplemental feeding of protein in order to keep them in decent shape for going into winter..
So I suspect that the limiting factor for bees in a natural realized niche is not the amount of food available during a brief period of food abundance (the main honey flow), but rather the quality and quantity of forage available during spring and late summer/fall.
Practical application: the realized niche of the honey bee is likely largely defined not by the amount of nectar available during the main honey flow, but rather by the quantity and quality of pollen available prior to and after that period. A successful colony requires a dependable abundance of quality pollen and nectar for early spring buildup; and then adequate late-season pollen to ensure its ability to produce a cluster of protein-rich “winter bees” and to store beebread for midwinter broodrearing.
Nevertheless, prior to man, biologically productive areas typically supported a diversity of native vegetation that produced exactly such an extended season-long buffet of nutrition. Thus, I doubt that during favorable years, a lack of available spring or fall food resources was the limiting factor of the bee population prior to man.
Limiting Factor: Competition For Those Food Resources
Competition for food resources, either against other species or one’s own species, is a common limiting factor of the realized niche. So what sort of competition do bees face?
Anywhere that there are flowering plants, there are pollinators that have coevolved with them. Most are insects (although in some areas, birds, bats, or other mammals may be important). Does competition with other pollinators limit the honey bee population?
Let’s think about it. Since one can place a hive of bees into most any favorable habitat and still make honey despite the presence of established populations of native pollinators, I suspect that competition with other species is not normally the limiting factor of the honey bee population. On the other hand, as any beekeeper quickly recognizes, honey bee colonies certainly compete with one another!
Beekeepers tend to focus upon the amount of nectar available during the main honey flow, and understand that one can overload an area with managed hives. But how about the density of a natural population of bees—does the amount of nectar available during the main flow limit that population?
Again, let’s check easily-verifiable observations. In areas with well-established populations of feral bees (Australia, Hawaii, formerly in the U.S), one can bring in additional managed colonies, yet still produce a substantial honey crop during the main flow—clearly, nectar is produced in excess of what an established natural population of bees can harvest. It follows then that at a “normal” population density of unmanaged colonies, competition for nectar during the main flow was not the limiting factor for colony survival.
On the other hand, competition for pollen during spring or fall could well be a limiting factor. And along with that competition comes…
Limiting Factor: Intercolony Parasitism
Competition between colonies does not occur only for nest sites or at the flower; it can also happen directly at the hive. All’s fair in love, war, and in Nature. If you can save yourself effort by stealing the fruits of another’s labor, so be it. The term for this is kleptoparasitism (kleptoparasite: a bird, insect, or other animal that habitually robs animals of other species of food).
What we call “robbing” is a form of kleptoparasitism, and during early spring or the summer dearth, the robbing pressure between colonies can brutal. There would be a clear competitive advantage to those colonies that successfully robbed honey from others; conversely, there would be an advantage to those colonies best able to defend their hard-won stores.
Robbing behavior may also be more insidious than the overt invasion of a weak colony by a strong colony. Dr. Wyatt Mangum detailed the sneaky “progressive robbing” of one colony by another [13]. Such robbing would constitute an insidious drain upon the victim colony. In areas of high colony population density, I suspect that robbing pressure–at times other than during major honey flows–is a limiting factor in colony density.
And this very robbing behavior brings us to our last suspect factor– that famous Horseman of the Apocolypse, Pestilence.
Limiting Factor: Pathogen Transmission
As most beekeepers soon find out, honey bees are host to a number of parasites and pathogens. To a biologist, a pathogen is a parasite (such as a virus, fungus, or bacterium) that can cause disease. These parasites can cause the bees to suffer from either endemic or epidemic infections. A well-adapted parasite typically does not, under normal circumstances, cause serious disease, but rather smolders in the bee population of each individual colony as an endemic infection; sacbrood virus or Nosema apis follow this model. These well-adapted parasites are typically vertically transmitted, that is, from parent to offspring, or in the case of bees, from mother colony to daughter colony. They can generally be found in a colony, but so long as the colony is not stressed, there are no symptoms of disease.
Other parasites tend to go epidemic, sometimes in recurring cycles; chalkbrood, Chronic Bee Paralysis Virus, American Foulbrood, and European foulbrood fall into this category. Individual colonies are able to “clear” themselves of these parasites; the parasites maintain a presence in the local bee population, but may not be found in every colony. Epidemic parasites are largely dependent upon horizontal transmission from colony to colony, and unlike the well-adapted endemic parasites, actually benefit by the weakening or death of an infected colony. As such, they would be considered to be density dependent infectious diseases. As the density of the host (honey bee colonies) in the environment increases, the opportunities for transmission of the parasite from colony to colony directly increases [14]. The pathogen causing the disease can only persist if the host density exceeds a certain threshold (if bee colonies were scattered beyond flight distance, there would be slim possibility for an infectious pathogen to transmit from one colony to another).
Conversely, not being regularly exposed to a pathogen removes the selective pressure for the bees to maintain genetic (or epigenetic) resistance. Thus, in nature, epidemics of certain pathogens ebb and flow, often decimating a host population one year, at which point the host density is decreased to the extent that the pathogen nearly disappears, struggling to maintain a foothold in the few remaining, and most resistant, hosts. As the host population then recovers over the years, an epidemic may then recur (higher host densities favor virulent mutations of the pathogen). This is a common cyclic pattern in insect species with large populations, and bees are no exception [15].
Practical application: After a plague it may take years for a particular pathogen to again recover its hold in the bee population. And it may take a special combination of environmental circumstances, and perhaps coinfection with other parasites, in order for it to do so.
For most species of wildlife, natural populations tend to reach some sort of dynamic equilibrium, with pestilence being the ultimate limiting factor if all other conditions are optimal. Anderson [16] explains:
It is likely that interplay between the pathogenicity of viral, bacterial, [or] protozoan infections and the nutritional state of the host contributes importantly to the density-dependent regulation of natural populations, with the parasites greatly amplifying the effects of low levels of nutrition.
Such pestilence typically occurs in the form of epidemics, during which the pathogen(s) efficiently spread through a stressed and overcrowded population, the key word being overcrowded. Too many colonies of bees in an area is a recipe for disaster—a ticking time bomb just waiting for the right combination of environmental circumstances and the presence of a virulent pathogen (or combination thereof).
A Special Case: The Viruses
But it’s a bit more complicated when reservoir hosts are involved. That is, when a pathogen is not limited to honey bees as its sole host. And this is the case with the “bee” viruses, most or all of which appear to actually be generic insect viruses that bees pick up when they visit flowers. So it is likely that the density of the bee population is not limited merely by diseases specific to honey bees, but also by the entire pool of viruses that infect pollinating insects [17] (Fig. 4).
Breaking news: As I type these words, a collaboration of researchers associated with the USDA ARS labs is about to release a stunning paper [18], in which they detail how a plant virus is now infecting both honey bees and varroa, and appears to be associated with collapsing colonies. Their findings suggest that varroa may be a key player in the cross-kingdom jump of this virus. The complexity of the bee, mite, plant, and virus web of infection continues to astound us!

Figure 4. These Russian bees near the hive entrance are in the process of cooking a too-bold bald-faced hornet to death [[i]]. Hornets and yellowjackets eat bees, and are thus exposed to bee pathogens. Exposure also goes the other way as the wasps contaminate nectar with virus particles when they visit flowers, or, as in this case, if the dying wasp exudes any body fluids.
[i] Ugajin A, et al. (2012) Detection of neural activity in the brains of Japanese honeybee workers during the formation of a “hot defensive bee ball”. PLoS ONE 7(3): e32902.
Historically (meaning prior to varroa), these viruses were sporadically present in bee colonies, but generally as inapparent (free of noticeable symptoms) infections [20]. It was only under certain circumstances that they went epidemic and caused noticeable morbidity or mortality of colonies:
Taken together, these data indicate that bee virus infections occur persistently in bee populations despite the lack of clinical signs, suggesting that colony disease outbreaks might result from environmental factors that lead to activation of viral replication in bees [21].
In any case, in some years in some localities, such “perfect storms” of environmental factors, virulent mutations of one or more pollinator viruses, and coinfections with other parasites have historically led to serious colony collapse events [22]. This is not a new thing!
Conclusion
My original question was what were the primary limiting factors in the realized niches of the honey bee prior to human influence? I hope that I have adequately covered these factors, since I feel that our understanding of them is critical for us to be better beekeepers, and to make good management decisions.
As always, I prefer to let the reader draw his/her own conclusions, but to me it appears that that the primary limiting factors in colony survival in favorable areas were most likely the density-dependent competition for pollen during spring and fall, coupled with the associated transmission of certain pathogens.
The above factors have long been associated with epidemics in the bee population. It appears to me that our bees today are in the midst of an ongoing and complex multi-pathogen epidemic largely precipitated by the actions of mankind. In the next installment of this article I will explore how this situation came about, examining how changes in world trade, agriculture, the environment, and in beekeeping practices have affected the realized niche of the bee, its parasites and pathogens, and the business models of beekeepers. My hope is that by fully understanding how we inadvertently helped to create the problem, that perhaps we can better take steps to help our poor bees deal with the problem, and for ourselves to stay in business in the process.
Footnotes and Citations
1 “Decimation events” being plagues, droughts, wildfires, extreme weather events, etc.
2 http://www.nature.com/news/job-swapping-makes-its-mark-on-honeybee-dna-1.11418#/b1
3 Tan, K, et al (2007) Bee-hawking by the wasp, Vespa velutina, on the honeybees Apis cerana and A. mellifera. Naturwissenschaften 94(6): 469-472. Open access.
4 Seeley TD and RA Morse (1978) Nest site selection by the honey bee, Apis mellifera. Insectes Sociaux 25: 323–337.
5 Martin, S, et al (2002) Usurpation of African Apis mellifera scutellata colonies by parasitic Apis mellifera capensis workers. Apidologie 33: 215-232.
Danka, RG, RL Hellmich, TE Rinderer (1992) Nest usurpation, supersedure and
colony failure contribute to Africanization of commercially managed European honey
bees in Venezuela. Journal of Apicultural Research 31 (3/4): 119-123.
6 Schneider, SS, et al (2004) Seasonal nest usurpation of European colonies by African swarms in Arizona, USA. Insectes Sociaux 51(4):359-364.
7 Mangum, W (2010) The usurpation (takeover) of established colonies by summer swarms in Virginia. ABJ 150(12): 1139-1144.
Mangum, W (2012) Colony takeovers (usurpations) by summer swarms: they chose poorly. ABJ 153(1): 73-75.
Mangum, W (2013) Summer swarms with queen balling. ABJ 153(2): 163-165.
8 I transcribed and plotted the data from Fig. 1 in Seeley, TD, et al (1989) Bait hives for honey bees. Cornell Coop Ext Inf. Bull. No. 187. http://ecommons.cornell.edu/bitstream/1813/2653/2/Bait%20Hives%20for%20Honey%20Bees.pdf
I assumed that the data was for New York and from the following paper, but I was unable to obtain a copy:
Fell, R. D., et al (1977) The seasonal cycle of swarming in honeybees. J. Apic. Res. 16:170-173.
9 Otis, GW and JM Wearing-Wilde (1992) Net reproductive rate of unmanaged honeybee colonies, (Apis mellifera L.). Ins. Soc. 39:157-165.
10 Seeley, TD and PK Visscher (1985) Survival of honeybees in cold climates: the critical timing of colony growth and reproduction. Ecological Entomology 10: 81-88.
11 https://scientificbeekeeping.com/sick-bees-part-17-nosema-the-smoldering-epidemic/
12 This physiological change to diutinus bees occurs when newly-emerged workers sense queen pheromone, but no young brood pheromone, which tells them that the colony is in survival mode. This typically occurs in fall, but can also occur during summer dearth. See https://scientificbeekeeping.com/an-adaptable-workforce/
13 Mangum, W (2012) Robbing: Part 2: Progressive robbing. ABJ 152(8): 761-764.
14 Hudson PJ, et al (2001) The Ecology of Wildlife Diseases. Oxford University Press, Oxford.
15 https://scientificbeekeeping.com/sick-bees-part-9-pathogens-and-plagues/
16 Anderson, RM and RM May (1979) Population biology of infectious diseases: Part I. Nature 280(2): 361-367.
17 Singh R, et al (2010) RNA viruses in hymenopteran pollinators: evidence of inter-taxa virus transmission via pollen and potential impact on non-Apis hymenopteran species. PLoS ONE 5(12):e14357.
18 Lian JL, Cornman RS, Evans JD, Pettis JS, Zhao Y, Murphy C, Peng WJ, Wu J, Hamilton M, Boncristiani HF, Jr., Zhou L, Hammond J, Chen YP. 2014. Systemic spread and propagation of a plant-pathogenic virus in European honeybees, Apis mellifera. mBio 5(1):e00898-13. doi:10.1128/mBio.00898-13. Open access.
19 Ugajin A, et al. (2012) Detection of neural activity in the brains of Japanese honeybee workers during the formation of a “hot defensive bee ball”. PLoS ONE 7(3): e32902.
20 Hornitzky, M (1987) Prevalence of virus infections of honeybees in eastern Australia. Journal of Apicultural Research 26(3) : 181-185.
Anderson, DL and AJ Gibbs (1988) Inapparent virus infections and their interactions in pupae of the honey bee (Apis mellifera Linnaeus) in Australia. J. Gen. Virol. 69: 1617-1625.
Mouret, C, et al (2013) Prevalence of 12 infectious agents in field colonies of 18 apiaries in western France. Revue Méd. Vét. 164(12): 577-582. http://www.revmedvet.com/2013/RMV164_577_582.pdf
21 Tentcheva, D, et al (2004) Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol 70(12): 7185-7191. http://aem.asm.org/content/70/12/7185.full
22 Underwood, R and D vanEngelsdorp (2007) Colony collapse disorder: have we seen this before? Bee Culture 135(7): 13-18. http://ento.psu.edu/pollinators/publications/underwood
First published in: American Bee Journal, February 2014
What’s Happening to the Bees?
Originally published in ABJ Feb 2014
Randy Oliver
ScientificBeekeeping.com
I’m realizing that what I thought was going to be a quick review of CCD has turned into a very long series of detailed articles, and I’m not even near reaching the conclusion. So on this 20th anniversary of my first seeing the parasite that changed beekeeping worldwide, I thought that I’d interrupt the Sick Bees series and attempt to explain, more briefly (hah!), and from an ecological perspective, what’s happening to the bees these days, and how beekeepers are being forced to adapt.
One Wild Ride!
In 1994 I saw my first varroa mite—on a stickyboard that had been placed by the county bee inspector under one of my hives. Little did I know how much that little maroon speck was about to change my life!
Varroa clobbered me (or more precisely my bees). And then did it again; and then again. I finally got pissed off and decided that I should either give up beekeeping altogether, or dust off my scientific training and really learn about bee and mite biology, and how to apply it to practical beekeeping.
What’s interesting is that my very first article (on almond pollination), in the fall of 2006, was published just as Dave Hackenberg’s colonies began to collapse from CCD. The occurrence of CCD put the bee research community into high gear to try to figure out what the heck was happening. And that created a “niche” for a practical beekeeper with a biological background, willing to act as a translator of the scientific findings to an alarmed beekeeping community. I unwittingly stepped into that niche, and it swallowed me up. A mere seven years later, and to my utter surprise, I’ve gone from being an obscure sideline beekeeper to a globetrotting speaker on bee health and management.
I’ve now been fortunate enough to visit both professional and recreational beekeepers in every part of North America and in several other countries. I’ve seen many styles of beekeeping, from the tropics to the Arctic, heard the local problems and concerns, and had the chance to learn from some very smart and successful beekeepers. I’ve attended scores of conferences, read countless scientific papers, and picked the brains of the world’s best apicultural researchers. Then I’ve done my best to share what I’ve learned with others. I’ve met scores of wonderful people and made a lot of new friends, and I’d like to take this opportunity to thank you all for the appreciative and effusive support!
How We’ve Benefitted From CCD
CCD has been a mixed blessing to beekeepers. It brought grown men and women to tears (see the film The Last Beekeeper [1]), and the elevated rate of colony mortality in recent years has made it difficult to keep our operations in the black. But it also pushed our scientific community to learn more about the biology of the honey bee than they had in a great many years. And many of us are much the better beekeepers for it.
Unlike that of other livestock, the true contribution of pollinators to U.S. agricultural production is not reflected by farm gate sales figures, so bees have traditionally not received their fair share of USDA research funding, nor does the beekeeping industry have the lobbying clout of the cattle, poultry, or pork producers. But we’ve benefitted from the public awareness of the plight of pollinators, which has resulted in the shifting of some funding our way [2] (although bees still only get about a tenth the amount of money set aside for research on beef production). In addition, universities, grantors, and other governments have recently supported a great deal of research into honey bee and pollinator health (I only wish that the millions who signed the internet petitions to “save the bees” had instead each donated a single dollar toward bee research).
Misunderstanding And Misinformation
Although CCD refers to a specific set of symptoms [3], the media soon began to use the term for any sort of honey bee mortality (as did many beekeepers). And although the epizootic appears to have largely run its course, speculation ran rampant as to the cause(s) of “CCD,” and continues to do so with every new “documentary” and press release. Although “CCD” remains the poster child of colony losses, a blue-ribbon group of bee researchers cautions:
During the winter of 2008/2009, ∼10% of the 2.3million managed honey bee colonies in the US died with “CCD-like symptoms”, and US beekeepers self-diagnosed CCD as only the 8th most important contributor to colony mortality, behind starvation, queen-related issues, and parasites. The point is, honey bees die from many things. We must be careful to not synonymize CCD with all honey bee losses [4] (emphasis mine).
I’m typing these words as I fly over the beautiful jigsaw-puzzle-like frozen Manitoba landscape on my return from a speaking engagement in Sweden, where the film More than Honey had been recently shown. To my considerable surprise, the Swedish beekeepers (Fig. 1), after viewing the movie, were under the very strong impression that the bee problems in the U.S. were due to our brutal commercial beekeeping practices, and the moving of hives to the deadly almond orchards in California.

Figure 1. Beekeeper Göran Sundström at one of his apiaries in Sweden. Göran typically keeps 12 hives in an apiary, and goes to considerable trouble to comply with some rather arbitrary rules to have his honey certified as “organic.” The red paint is a traditional color for rural buildings in this area.
As it happens, the director of that film had stayed with me during his initial scouting visit to the U.S., and I was responsible for introducing him to my friend John Miller, who was unfortunately (and I’m sure unknowingly) to be cast in the role of the evil bee abuser, so I felt some responsibility to dispel those misconceptions to the concerned audience. And this brings me to my next subject…
Bees are currently enjoying a great deal of attention from a fearful public eager to do something, anything to help them. This could be a really good thing for the bees, for beekeepers, and for the environment as a whole if such public concern and activism could be guided into meaningful actions. I can’t really blame the public for being confused, since the entertaining and sensational docu-dramas about the impending extinction of the honey bee resonate more emotionally than do the dry and qualified explanations by scientists as to the “multifactorial” causes of colony mortality.
As a result of all the misinformation and hysteria out there, an unsure and distrustful public puts pressure their representatives to pass this or that new regulation to “save the bees.” This scares me. I feel that we should heed the sage advice of Thomas Jefferson:
People are inherently capable of making proper judgments when they are properly informed.
And therein lies the problem: due to the complexity of what’s happening with bees these days from the biological, environmental, agricultural, and economic standpoints, it’s danged hard to be “properly informed.” My gosh, just look at me trying to do that “informing”—I first thought that the “Sick Bee” series was only going to be two or three articles long! So what to do?
Challenging One’s Beliefs
One should be careful about embracing the popular stories about why “the bees are dying.” Some of the myths resonate so emotionally that they win uncritical acceptance by the mainstream, despite the fact that they cannot be reconciled with obvious facts (e.g., that bees can indeed thrive surrounded by GMO corn and soy, or on neonic-treated canola). As the popular scientific author Stephen Jay Gould pointed out:
The most erroneous stories are those we think we know best – and therefore never scrutinize or question.
Anyone who knows me (or has had the misfortune of trying to promote an unsubstantiated argument in my presence) can tell you that I’m a challenging and provocative person by nature. I’ve found that the best way to get to the truth is to learn how to argue your opponent’s side of a debate as well as you can argue your own. Therefore, I am more than willing to play Devil’s Advocate any time that I see one side of a legitimate argument being ineffectually presented. And I’m ruthlessly skeptical of any claims that do not jibe with what I see with my own eyes.
As you can imagine, this has earned me my share of vitriol from those who “know the truth” (read [5] for an enlightening discussion). Luckily, my mailbox runs about a hundred to one with thank you letters from beekeepers who appreciate my evaluation of the issues. I take this responsibility seriously; in order to remain objective and unbiased, I go out of my way to constantly question every one of my opinions (I avoid making “conclusions”). I eschew holding any “beliefs,” but rather adhere to the following principles:
- That I should respect Nature and all forms of life (my ethical environmentalist side),
- That I should thoroughly investigate all research and explanations of any subject, and avoid cherry picking data that suits my ideological convictions (my curious open mindedness and willingness to do my homework).
- That I should base my opinions upon information and experimental results which stand up to scrutiny and questioning (my scientific side),
- That I should then truth-check those opinions against on-the-ground evidence and observations (my practical side).
Unfortunately, many crusaders allow their commendable environmental consciousness (and innate fear of technology) to override the last three principles, which is understandable, since doing the homework is really hard, and our understanding of the biology involved is as yet incomplete. But I have some suggestions as to where to start…
A Homework Assignment
Allow me to first assign you some required reading. Put down this article and read Berndt Heinrich’s fascinating book Bumblebee Economics [6]. Heinrich studied the minute details of exactly how bees make a living in their ecological niche, focusing upon the economics of energy utilization. His revelationary insights changed my understanding of bee life completely. Then for something entirely different read Ron Miksha’s Bad Beekeeping [7] for a perspective on the economic trials and tribulations faced by professional beekeepers (his comments on p. 243 are especially relevant). I’ll wait ‘til you’re done…
OK, I hope those books were as thought-provoking to you as they were to me! Now let’s take a look at the health of bees and beekeeping from ecological and economic perspectives.
It’s All About Economics
Again and again, I find that everything boils down to economics and finding the right niche. This applies to both honey bees and to the business of beekeeping—either thrives in its ideal niche, and either must either adapt or die if the parameters of the niche change. And boy howdy, how we have changed the parameters of both of our niches in recent years!
Practical application: In this real world, each species, and each business, strives to exploit a niche to which it is particularly well adapted. A change in any of the parameters that define a particular niche may affect the profitability and survival of that species or business. If that species or business is efficient and profitable in its particular niche, then it thrives; if not, if must either adapt or go extinct.
For the remainder of this article, I will view the situation of both bees and beekeepers through the lenses of ecology and economics, and the changes that have occurred in the parameters that define our niches.
Let’s Define Some Terms
Pollinators are in decline over much of the world, and have been for some time [8]. We beekeepers are mainly concerned with our favorite pollinator, the European honey bee, Apis mellifera, native to Europe and Africa, but now introduced worldwide. Unless I specify otherwise, henceforth I will be referring to this species.
It occurs to me that if pollinators have long been in decline worldwide, then that would imply that something has changed in their ecological niches (and that it started before the introductions of cell phones, neonics, or GMO’s). It also occurred to me that the niches occupied by beekeepers have changed substantially (mine sure has; indeed several times). I’ll try not to burden you with too many new terms:
Habitat—where the bee species lives (or could live). The bees in the U.S. are mongrel hybrids of various European or African races [9], each originally adapted to specific microhabitats in their home countries.
Ecological niche—a description of the bees’ “occupation” in its specific microhabitat, including all environmental parameters and interactions with other species.
Fundamental niche— the potential full range of environmental conditions and resources that the honey bee as a species could possibly occupy and use, without the limitations of predation, competition, or other factors.
Realized niche—the less-than-optimal niche that each subspecies of bee actually occupies; constrained by weather, resources, parasites, etc. In its home range, various subspecies of honey bee adapted to narrow realized niches occurring in the warm Mediterranean, the cold Alps, the British heathland, the Egyptian desert, the African savannah, etc. In each of those niches, the bees adapted to the seasonality of local nectar flows, the local plant toxins, temperature, predators, and parasite pressure. Conditions may not have been optimal, but each subspecies was economically successful at “making a living” within those parameters.
So let’s list the most important parameters of the fundamental niche of the European honey bee:
- It is a colonial species, existing as a superorganism with generally a single reproductive queen, supported by multiple patrilines of sterile workers, each exhibiting slightly differing genetics, behaviors, and resistance to parasites, toxins, and diseases (this within-hive diversity is extremely important, but often ignored by beekeepers).
- It is a generalist species, able to gather food resources from a wide variety of plants. As such, it is adapted to metabolizing a wide range of toxic plant alleleochemicals (and by extension, synthetic pesticides).
- It is primarily a pollinator; its diet normally consists solely of nectar and pollen, although those raw foodstuffs are processed into other products (honey, beebread, and jelly) for the actual consumption by the majority of the members of the superorganism.
- The bee cannot forage unless the ambient temperature is above roughly 55°F (12°C). This limiting factor constrains its range to those areas that have adequate bloom available when the temperature exceeds that value; any colony with hungry brood when daytime temperatures do not exceed 55°F will soon become stressed due to an inadequate supply of protein (this is a huge management tip).
- Unlike other insects, the European honey bee stores vast quantities of processed food for later consumption when resources are scarce.
- This allows the colony to do something that no other species of temperate insect can do—maintain an elevated body temperature, and rear brood, throughout the winter.
- In order to maintain that colonial body temperature, the European honey bee requires a protective insulated cavity within which to nest (Fig. 2).
- Somehow, the European honey bee evolved with remarkably few parasites—the only significant ones being Nosema apis, the bacteria causing the two foulbrood diseases, the fly Braula, rare infection by two opportunistic fungi in pollen (chalkbrood or stonebrood), and what were normally “economically unimportant” infections by any of several insect viruses.

Figure 2. These bees are at the top of the winter cluster at the interface between empty cells and sealed honey. Despite the air temperature being below freezing, the temperature of the bees beneath the surface was 67°F (19°C). This remarkable technique of using stored sugars from the previous summer as an energy source during winter allows the honey bee to overwinter as a populous colony, ready to exploit early spring pollen and nectar sources.
In summary, the honey bee requires a dry cavity, in which it maintains a tropical environment throughout the year, allowing it to exploit food sources any time that the temperature is above 55°F, it is adapted to metabolize a wide variety of plant toxins, it only requires a brief honeyflow to provide it with food stores for the remainder of the year, and it evolved under low parasite pressure.
Practical application: this last point is of huge import when we attempt to understand the biological changes that have occurred in European honey bee populations worldwide in the last decades. To wit, virus infections have become serious “emerging diseases” [10].
Add The Beekeeper
OK, now let’s add one more term:
Facilitation—Optimal conditions in the fundamental niche occur infrequently, if ever. The job of the beekeeper is to optimize the conditions for his bees as best he can, such as by supplemental feeding, medicating for parasites, protecting from predators, providing a larger nest cavity, or providing water or winter insulation. Such “facilitation” may allow bees to survive outside of their fundamental niche.
Practical application: I recently enjoyed a lunchtime conversation with a couple of professional beekeepers who moved their hives from almonds, to the tallow bloom in Texas, to the Dakotas for summer, and then to a mild area in California for wintering (their problem is having too many bees each spring). What they are doing is facilitating the optimal fundamental niche for their bees for the entire year (there are many other ways of doing so).
Practical application flip side: If a beekeeper is keeping colonies alive outside of their fundamental niche, such as in densely-packed apiaries, in areas of crop monoculture or high exposure to toxic chemicals, in flowerless forest or dry grassland, or by the chemical suppression of parasites, should that beekeeper falter in his constant facilitation, his bees may not be able to continue to survive without such help.
Limiting Factors
The realized niche of the honey bee is constrained by limiting factors, which may change from season to season. Common limiting factors for populations of honey bees are:
Climate—bees have very wide “tolerance limits” for cold, heat, rain, and length of seasons. But at the edges of their tolerance limits, colonies will be stressed, and may not be able to deal with other limiting factors.
Competition for food—in some areas there is such an abundance of nectar during the main flow that there is little competition (an important point when speaking with native pollinator advocates). The main competition for food resources occurs at other times of the year; assume that there is serious competition happening if robbing behavior is evident.
Suitability of available food—not all plants produce honey-bee-friendly nectar or pollen, especially outside of the honey bees’ native range. This is clearly evident in America and Australia, where some pollen sources are notably nutritionally inadequate for honey bees (think corn, blueberry, watermelon, pumpkin, some eucalypts). And in some areas or under dearth conditions, bees will unwittingly collect naturally toxic pollen or nectar. And of course, some human-applied pesticides make the available food unsuitable.
Competition for nest sites—without hollow trees or other natural cavities, honey bees cannot survive the winter in temperate climates (Fig. 3).

Figure 3. One of my colonies swarmed late this spring and built open-air combs in a nearby hawthorn tree. We noticed it when the leaves fell in early December, following a week of subfreezing temperatures and a foot of snow. You can’t see it, but there is still a cluster of live bees (which I hope to rescue when I’m done writing this article). The population had obviously grown large enough to build and completely cover all the combs, and could easily have survived the winter had it only found an appropriate cavity in which to build its nest.
Predation—Such as birds, bears, wasps, and ants. The main predator of bees, of course, are humans, who often rob too much honey from the hive, resulting in winter starvation.
Parasitism—Again, natural populations of European honey bees appear to historically have been minimally affected by parasites under normal conditions. We will return to how this has changed.
Transmission of parasites—This is very density dependent—the more colonies within flight range, and the more competition for resources, the greater the transmission of parasites. The swapping of combs by beekeepers also changes this dynamic.
Toxins—Natural plant allelochemicals, soil metals, industrial pollution, agrochemicals, and recently, a huge influx of beekeeper-applied miticides.
Bees have a wide range of tolerance for some limiting factors, and more narrow ranges for others. Usually, several factors interact (sometimes synergistically) to limit bee populations.
Practical application: there is often a single limiting factor that is the determinant for colony survival. A concept used in ecology is “Liebig’s law of the Minimum” (Fig. 4). A beekeeper can work hard all season long to do everything he can for his bees, but should he overlook any single critical limiting factor, Liebig’s Law may come into play, and he may lose his colonies.
Figure 4. An illustration of Liebig’s Law of the Minimum as it applies to the practice of beekeeping. Despite everything else that you do to fill the barrel, the most unfavorable limiting factor(s) (or some combination thereof) at any critical period of time will limit the bees’ (and your) success. Adapted from [[i]].
[i] Barrel illustration after Dobenecks, taken from Wikipedia http://en.wikipedia.org/wiki/Liebig%27s_law_of_the_minimum
Practical application: Each race of honey bee in Europe specialized by adapting to certain combinations of limiting factors, and thus gained a fitness advantage in its particular habitat. Since the arrival of varroa, which wiped out much of the feral population, the overall genetics of the U.S. bee population have likely shifted toward those propagated by commercial queen producers [12].
These “all-purpose” bee stocks are typically bred for color, temperament, and honey production, and maintained with a high degree of facilitation by the queen producer (feeding, treatments). There is no reason to expect those stocks to be well adapted for survival without constant facilitation. This is why I strongly support regional queen breeding for locally-adapted stock.
What Are The Limiting Factors For Honey Bees Today?
It would have been so simple had CCD actually been caused by cell phones! We could have banned the danged things, and wouldn’t have to listen to people walking around loudly and obliviously talking to themselves. But alas, it appears to be more complex than that.
So as a biologist, it occurs to me to go back before CCD, in fact, to go back even further in time, and ask the question, “Which factor(s) limited honey bee populations in Europe prior to modern management by humans”? And then we can work forward in time to see what’s changed since then. To be continued…
Footnotes and Citations
1 Directed by Jeremy Simmons (2009), and recommended for those who didn’t experience the horror of CCD personally. Unfortunately, I can’t suggest anywhere to purchase a copy of this well-done and heart wrenching film.
2 Pollinator Research.–The Committee is aware that pollinators are responsible for the production of one-third of the Nation’s food supply, but the number of managed honeybee colonies in the United States has dropped in half since 1940. Because of the importance of pollinators in the production of the Nation’s food supply and their impact on the stability of our agricultural economy, the Committee encourages [the Agricultural Research Service] to continue to dedicate resources to protecting the health of both honeybees and other native bees, including continued research into colony collapse disorder. http://www.gpo.gov/fdsys/pkg/CRPT-112srpt73/html/CRPT-112srpt73.htm
3 Symptoms of CCD:
1) In collapsed colonies
a) The complete absence of adult bees in colonies, with no or little build up of dead bees in the colonies or in front of those colonies.
b) The presence of capped brood in colonies.
c) The presence of food stores, both honey and bee bread
i) which is not immediately robbed by other bees
ii) when attacked by hive pests such as wax moth and small hive beetle, the attack is noticeably delayed.
2) In cases where the colony appear to be actively collapsing
a) An insufficient workforce to maintain the brood that is present
b) The workforce seems to be made up of young adult bees
c) The queen is present
d) The cluster is reluctant to consume provided feed, such as sugar syrup and protein supplement
From vanEngelsdorp, D, et al (2006, revised Jan 5, 2007) Investigations into the causes of sudden and alarming colony losses experienced by beekeepers in the fall of 2006. Preliminary Report: First Revision.
4 Williams, GR, et al (2010) Colony Collapse Disorder in context. Bioessays 32(10): 845–846. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3034041/
5 Janabi, F (2013) From anti-GMO to pro-science: ‘A Layman’s Guide to GMOs’. http://www.geneticliteracyproject.org/2013/12/03/from-anti-gmo-to-pro-science-a-laymans-guide-to-gmos/#.UqkDXdJ_dyI
6 Heinrich, B (2004) Bumblebee Economics. Harvard University Press. I highly recommend all of Heinrich’s books—he’s a brilliant scientist and an engaging writer whose passion it is to understand the details of how organisms survive in their niches.
7 Miksha, R (2004) Bad Beekeeping. Trafford. For a more data-based analysis, see:
Laate, EA (2013) Economics of Beekeeping in Alberta 2011. http://www1.agric.gov.ab.ca/$Department/deptdocs.nsf/all/agdex14472/$FILE/821-62.pdf
8 CSPNA &NRC (2007) Status of Pollinators in North America. https://download.nap.edu/login.php?record_id=11761&page=%2Fdownload.php%3Frecord_id%3D11761
9 The evolutionary origin of the European honey bee is currently under debate by scientists. See the following:
Han, F, et al (2012) From where did the Western honeybee (Apis mellifera) originate? Ecol. Evol. 2(8): 1949–1957. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3433997/
Kotthoff, U, et al (2013), Greater past disparity and diversity hints at ancient migrations of European honey bee lineages into Africa and Asia. Journal of Biogeography 40: 1832–1838. http://onlinelibrary.wiley.com/doi/10.1111/jbi.12151/pdf
10 Genersch, E & M Aubert (2010) Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet Res. 41(6): 54. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2883145/
11 Barrel illustration after Dobenecks, taken from Wikipedia http://en.wikipedia.org/wiki/Liebig%27s_law_of_the_minimum
12 Delaney, DA, et al (2009) Genetic characterization of commercial honey bee (Hymenoptera: Apidae) populations in the United States by using mitochondrial and microsatellite markers. Ann. Entomol. Soc. Am. 102(4): 666-673.
First published in: American Bee Journal, September 2013
The Slaughter of the Innocents
Why the Current Hoopla on Pesticides?
Ecosystems and Agriculture
Our Creation of “Pests”
The Slaughter of the Innocents
Pesticide (Mis)use Today
The Nitty-Gritty
The Killing Fields
Chronic Exposure
Hitting the Colony Where it Really Hurts
Progress in Reducing Pesticide Use
A Brighter Future
References
Sick Bees Part 18f6: Colony Collapse Revisited
the Slaughter of the Innocents
First published in ABJ September 2013
Randy Oliver
ScientificBeekeeping.com
The Slaughter of the Innocents
I’ve just learned something that helps me to understand what drives me to write these articles. Scientists have recently discovered that some people’s brains are wired for wanting to share useful information with others [1]. I’ve always been this way—I love learning about things and then sharing what I’ve learned with others. Learning about pesticide issues with bees has certainly been an educational experience for me, taking me down roads that I’d never imagined, and forcing me to rethink many of my previous assumptions. So please allow me to continue into my investigation of pesticides and their relationship to colony mortality.
Why the Current Hoopla on Pesticides?
The pesticide situation for bees has clearly improved since the “bad old days” of the 1960’s and ‘70’s [2], and doesn’t seem to be much different than when the Ericksons wrote about the subject in ABJ back in the 1980’s (a “must read” for anyone interested in this subject; copies at my website [3]). Yet today a media circus has convinced the public that certain pesticides are suddenly driving bees to extinction, and our industry has come together to bring a test case against the EPA to see whether we can finally get more bee-friendly restrictions in the labeling of new pesticide registrations. So why the current renewed interest in pesticide issues?
The simple answer is that beekeepers are hurting! Winter colony losses are averaging an excessive 30 percent, which makes beekeeping less profitable. It is simply more difficult and expensive to keep bees these days. Everyone is looking for something simple to blame, and pesticides have a long history of causing problems for bees. The mass media love to spin public fears of into doomsday stories, and they know that this story has all the necessary elements to sell to a largely urbanized population far removed from the realities of agriculture, nature, and beekeeping, with an innate distrust of any sort of chemicals or the companies that make them, and a renewed environmental consciousness (a good thing).
Then add the circumstantial widespread adoption of a new class of insecticides (Fig. 1), with scary-sounding words like “CCD,” “neonicotinoid,” “neurotoxin,” “lethal dose,” and “systemic,” and activists who have adopted our beloved honey bee their cause célèbre. Anyone can go to the internet to enjoy the current theatre of the absurd in which bloggers, advocates, and petitioners spin legitimate science, imaginative speculation, and outright misinformation into a frenzied free for all. Yet the question still remains, to what extent are pesticides truly to blame?
 1994 |
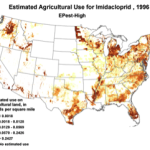 1996 |
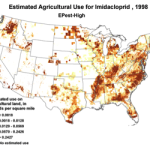 1998 |
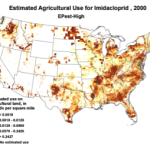 2000 |
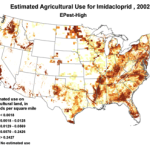 2002 |
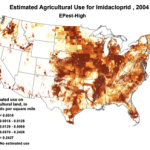 2004 |
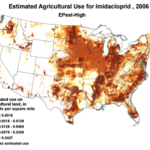 2006 |
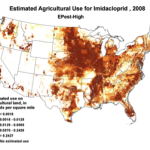 2008 |
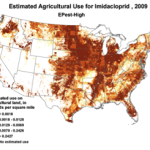 2009 |
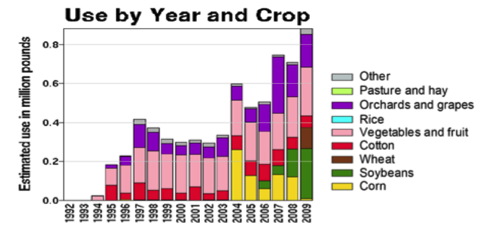 Use of the neonicotinoid insecticide imidacloprid over time. USGS data |
Figure 1. Use of the older classes of insecticides is being supplanted by newer (and ostensibly more eco-friendly) compounds such as the neonicotinoids. The widespread adoption of one of them has garnered the most attention—there were 284 published scientific studies with the word “imidacloprid” in the title in 2012 through the first half of 2013 alone! Yet we are still trying to determine whether any adverse sublethal effects of these insecticides are related to the generally increased rate of winter colony losses since the winter of 2004/2005. Source of maps [4].
Yet for all this interest in pesticides and bees, no one has yet produced a smoking gun that conclusively shows that pesticides are actually the major problem. I’ve previously listed studies from every continent that failed to make any clear link between pesticides and increased colony mortality [5]. But that doesn’t necessarily mean that they aren’t contributing to colony morbidity—a “weakening” or state of unhealthiness. Beekeepers and toxicologists have long known that pesticide residues can cause slower colony build up, poor brood patterns, decreased honey production, increased queen supersedure or failure, small populations in fall, or poor wintering.
But it’s silly to blame all beekeeping problems on one thing. I’ve no doubt that pesticides are a serious issue for some beekeepers (especially the large operators with thousands of hives in the middle of intense agriculture), but I also have seen that they are also a non issue for the majority of beekeepers elsewhere. What I’m trying to figure out is exactly where pesticides fit into the larger picture of the elevated rate of colony mortality of late. Since most exposure of honey bees to pesticides is in either agricultural or landscape settings, let me start my investigation into this subject from the ecological perspective of agricultural systems.
Ecosystems and Agriculture
Every form of life on Earth is part of a local ecosystem, in which the plants, animals, and microbes exist in some sort of “dynamic equilibrium”—commonly referred to as the “balance of Nature” (which in fact can tip wildly one way or the other). The more mature an ecosystem, the greater the diversity of species, the more complex the “food web,” and the more stable and resilient that balance.
One of the main factors that keeps any single plant species from dominating the landscape are the herbivorous insects that can quickly adjust their populations to exploit such a food resource. In response, through the process of “coadaptation,” the plants fight back against those insects with constantly-evolving chemical warfare. As beekeepers, we should keep in mind how insects (including honey bees) perceive the environment very differently than the way in which we humans do—insects are far more attuned to the chemical attractants, deterrents, and toxic metabolites of plants.
When we humans practice agriculture, we greatly disrupt or destroy the preexisting natural ecosystem and replace it with an artificial system designed to favor certain domesticated plants—“high-yielding” cultivars which generally possess less innate resistance to herbivorous insects.
In traditional agricultural systems, farmers grew locally-adapted (“heirloom”) cultivars of crops that often displayed some degree of resistance to the local insects. In addition, farms used to be small family affairs, and tended to be biologically diverse, containing a wide variety of domesticated and wild plants, grazing livestock, insect-eating fowl, cropland rotated with pasture, and a scattering of woodlots and hedgerows. This diversity helped to maintain a balance of food sources and natural predators which helped to keep any single insect species from taking too large a bite out of the farm’s total production (not to say that there weren’t problems with pests—especially with generic feeders such as locusts). But diversified farms are resilient in that they tend to limit pest populations. This traditional model of farming would be considered to be “sustainable,” since prior to synthetic fertilizers and pesticides, a farm’s viability from one generation to the next depended upon maintaining soil fertility and functional ecosystems.
On the other hand, in today’s “get big or get out” conventional agricultural model (in the U.S., 85% of farm sales are produced by less than 10 percent of farms, which control 44 percent of farm acreage [6), farmers now plant hundreds or thousands of acres to a single cultivar of domesticated plant. Such a system is ecologically unstable, since it essentially offers an unlimited buffet to whichever species of insects are adapted (or adapt) to consume that particular variety of plant. This sort of agricultural hubris then creates a demand for poisons to kill those plant-eating insects.
Our Creation of Pests
Keep in mind that every plant and insect that we now consider to be a “pest” was originally a part of some natural ecosystem. It is only if such an insect has the audacity to feed on our favored plant that we deem it a “pest.” But remember that such a value judgment is a human construct—that herbivorous insect is innocent in intent and certainly feels no malice towards us! Take the case of the Western Corn Rootworm (WCR), a close relative of the familiar 12-Spotted Cucumber Beetle (also called the Southern Corn Rootworm). The beetle, in its original ecosystem (which did not include corn until Native American farmers brought it north from Mexico) lived unnoticed on native gourds and prairie grasses [7]. But being an opportunistic species, it adapted to feeding on cultivated corn when that crop began being grown in vast monocultures.
In 1909 the WCR was first noticed to be damaging corn in Colorado, but wasn’t a problem in the Midwestern Corn Belt until the 1940’s [8]. Since then it has become one of the most economically-important pests of corn, accounting for about a billion dollars worth of lost revenue a year. It’s important to remember that for a hundred years, farmers minimized WCR damage by managing their farm ecosystem to disfavor the pest. This was done by the simple practice of rotating corn with pasture or legumes—non-host plants for the WCR—and by providing habitat for the parasites and predators of the rootworm—tachinid flies, parasitic wasps, nematodes, lacewings, etc.
But when government price supports made the growing of corn more and more profitable, farmers began to plant “corn on corn.” In doing so, they also grew huge populations of corn rootworms—an insect blindly fulfilling its “role” of bringing an artificial ecosystem back into balance by consuming an overabundant plant species. By working against Nature, farmers create their own “pest” problems.
Every pest also has natural enemies—predators, parasitic wasps, viruses, etc.—that tend to bring excessive populations back into “balance.” Relying upon pesticides rather than cultural practices often messes up this dynamic system, resulting in an even greater resurgence of the population of the pest. As Dr. Patricia Muir explains in her excellent syllabus on pesticides [9]:
One clear example of this effect involves corn in the US. In the 1940’s, little or no insecticide was applied to corn, and losses to insects were 3.5% of production. Since then, insecticide use on corn has increased more than 1000-fold, whereas losses due to insects (especially to the corn rootworm complex) have increased to 12%. This is largely attributed to reductions in crop rotation. When corn is rotated with another crop on which corn pests (such as corn rootworms) cannot survive, the pest population declines during the non-corn years. However, when corn is grown continuously (as it is today on 20 – 40% of US acreages depending on the year and on who you read!), populations of these pests are well fed every year and can increase immensely.
The Slaughter of the Innocents
Rather than reevaluating how to better farm their land in an ecologically-friendly and sustainable model, conventional farmers are pushed by market forces and wooed by pesticide salesmen to “control” these newly-created pests by the extensive application of synthetic insecticides (Fig. 2). In the process of applying these insecticides, vast numbers of “innocent” off-target species (such as honey bees and other “beneficials”) are often slaughtered (see a visual account of this at [10]).
Figure 2. If you add up all the blue dots (each representing 10,000 acres treated with insecticides), it’s easy to see why in some areas it’s hard for beekeepers to find “safe” places for their hives. Source USDA.
Pesticide (Mis)use Today
In today’s factory farming model, one prepares the soil bed, plants the seeds, and controls pests with poisons. It does not have to be this way. Over 50 years ago, entomologists at the University of California laid the foundation for many of the most important concepts in Integrated Pest Management (IPM) to reduce our dependence upon pesticides. Integrated pest management can be defined as the practice of preventing or suppressing damaging populations of insect pests by the application of comprehensive and coordinated control tactics. IPM emphasizes the least possible disruption to agro-ecosystems and encourages natural pest control mechanisms.
IPM has time and again been demonstrated to be both practical and cost effective—greatly reducing the need for pesticide applications by helping to maintain a more natural balance between herbivorous insects (what we call “pests”) and their natural predators.
Despite the long and successful history of IPM [11], it is unfortunate that chemically-based pest control still predominates in U.S. agriculture (the reader may note that IPM also applies to beekeeping, yet is practiced by only by a minority of beekeepers). A recent lament by prominent bumblebee researcher Dr. David Goulson (in his excellent review of neonic issues) describes the situation well [12]:
When I was at University in the 1980s, I read Rachel Carson’s Silent Spring, and was taught about the terrible mistakes made in agriculture in the 1950s and 1960s when indiscriminate use of persistent, broad-spectrum insecticides, and the abandonment of traditional cropping practices such as rotations, led to huge pest outbreaks. The pest insects had all become resistant, while their natural enemies had largely been eradicated. As a result, an approach called integrated pest management (IPM) had been developed, and we were taught that this was the future of pest control. IPM is predicated on minimizing pesticide use: farmers monitor their crop pests, and only take action when necessary; they encourage natural enemies as far as possible, use crop rotations and other cultural controls to suppress pests, and only use the insecticides as a last resort. Even then, they avoid those that persist in the environment.
Whatever happened to this philosophy? Why are we now applying pesticides prophylactically to more or less all crops? Did we learn nothing from our past mistakes?
It is disheartening to hear that Agriculture is not embracing IPM to the extent that it could. But who is to blame? As one researcher wryly notes:
It seems that a new form of IPM has become commonplace across many commercial corn and soybean fields–insurance pest management. The increased use of crop production inputs without scouting or use of economic thresholds is being exacerbated by high commodity prices, and it will probably continue under the current crop production parameters. However, there are likely to be unintended and unforeseen long-term negative consequences, such as insecticide resistance and reductions in natural enemy populations–the same potential undesirable outcomes mentioned more than 50 years ago by the University of California entomologists–when fundamental ecological principles of pest management are largely ignored [13].
And those long-term consequences are exactly what will make overdependence upon pesticides unsustainable. At some point, all of agriculture will be forced into sustainable IPM. Farmers are willing to go along—they certainly don’t like spraying dangerous pesticides on their land and around their families. But in the current economic climate, the consumer clearly votes with their pocketbook by rewarding farmers for producing cheap, cosmetically-perfect food. That apple in the supermarket may have come from orchard sprayed as many as 20 times in a season! Pesticide salesmen are very effective at marketing their products, giving lip service to IPM, but winkingly suggesting that it might be a good idea to minimize one’s risks with just one more chemical application.
On the other hand, university researchers and publicly-funded extension agents are trying to educate growers on more eco-friendly methods, while at the same time the EPA applies pressure for farmers to phase out the more ecologically-damaging pesticides. And like it or not, the adoption of genetically-engineered Bt cultivars has led to decreased pesticide use in some commodity crops. Overall, pesticide use appears to have been declining a bit since the late 1990’s, and beekeepers nationwide tell me that things are better than they used to be (Fig. 3).
Figure 3. The EPA’s data show that insecticides (which generally exhibit the most direct impact to bees) constitute about a tenth of total pesticide application. However, recent research indicates that some herbicides, fungicides, and surfactants may also harm bees. But judging insecticide use by pounds applied does not account for the higher toxicity of some of the newer insecticides, which although used at lower rates, may have equal or greater environmental impact. Source of chart [[i]].
[i] http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf
Some of the newer classes of insecticides are more targeted, having less impact on bees and other beneficial insects. Keep in mind, though, that all pesticides are mere Band-Aids, with a limited effective life until the insects evolve resistance. Nature always bats last, and resorting solely to the development and application of new classes of synthetic insecticides is a fool’s errand in the long run. But for now, pesticides are the name of the game, and we beekeepers should make an effort to truly understand how they fit into the big picture of bee health.
The Nitty-Gritty
There is nothing new about pesticide problems with honey bees. Reading the Erickson’s 1983 articles on pesticides in this magazine is like déjà vu all over again! In their section with the above title, they explain:
Whether we like it or not, pesticides are an important part of world agriculture and they are here to stay for the foreseeable future. Our task is to learn to live with them and keep bees in their presence. Our success will be largely measured in economic terms… [we] must deal within the real world of dollars and cents, business survival and compromise. As Dr. Eva Crane…has pointed out “the best that beekeepers can hope for, in the light of the great need to kill pest insects, is an “acceptable level of mortality among their bees.”
Beekeepers realize that in order to get locations, that they gotta get along with the landowners, who are often farmers (or friends of the farmers). If the beekeeper raises a stink, he may lose his welcome. So in general, commercial beekeepers accept the occasional bee kill as a normal cost of doing business.
The Killing Fields
We must acknowledge that the EPA and the agricultural community have made great strides in cleaning up the environmental insult due to pesticides by phasing out the worst offenders, yet we still seem to be stuck in the same place—beekeepers still live in fear of having an apiary wiped out by some pesticide application (or misapplication) (Fig. 4).

Figure 4. A bee kill in an almond orchard this spring. Surprisingly, no insecticides were involved! These bees were killed by a tank mix of herbicides, spray oil, and liquid fertilizer. A number of colonies were killed outright and others were weakened. Fortunately the beekeeper was present when the spraying took place and was able to stop the spray rig before more colonies were damaged.
Some pesticides are more acutely toxic to bees than others (for a ranked list, see [15]). And there are more pesticide problems associated with some crops than with others—a recent survey of beekeepers indicated that cotton, corn, alfalfa, vine crops, and berries were most problematic [16]. A pesticide kill can be less predictable than an Oklahoma tornado—timing, temperature, humidity, stage of bloom, the repellency of the product to bees, where the bees are foraging that day, or a shift of the wind can make all the difference in the world!
One thing to keep in mind is that a pesticide that is acutely toxic to individual bees may actually be safer to the colony as a whole. A product that kills foragers before they return to the hive may decimate the field force, but have relatively little long-term effect on the hive so long as it isn’t repeated.
Practical application: a number of beekeepers have found that it is necessary to give their bees a recovery period on pesticide-free forage between pollinations. Good forage or the feeding of a protein supplement may also help the colony to rebuild.
I am not discounting the impact of serious acute bee kills—I know plenty of beekeepers who have gotten hit hard time and again, losing entire yards of strong colonies when the crop that they are working is sprayed in full bloom.
However, in some cases even a dramatic kill with piles of dead bees in front of the hive may not be the end of the world. For example, the seed planting dust from corn (containing fungicides and a neonic) readily adheres to foragers, but is relatively non toxic to them unless the humidity is quite high. Unfortunately, this property then allows them to bring the toxic dust back into the interior of the hive, where it may even kill the queen. More insidiously, the poison gets incorporated into their pollen loads and packed into the beebread, where it subsequently kills pollen-hungry young workers and drones.
Luckily, since neonics are water soluble, after that thin layer of pollen is consumed, the colony generally recovers (provided that the queen wasn’t killed). If there is then abundant “clean” pollen available, the colony may quickly rebuild to full strength.
Acute pesticide kills are ugly, but obvious, since there are generally piles of dead bees at the entrance. But how about the impact upon colony health from those pesticides that don’t kill the foragers outright, but that are then inadvertently carried back into the hive?
Chronic Exposure
Once in the hive, there are several routes of exposure by which pesticide residues can be transferred from the returning foragers to other members of the colony:
- External transfer of dust or oily residues from bee to bee in the crowded cluster.
- Transfer via nectar shared between bees.
- Storage of tainted honey.
- Transfer of residues in pollen to nurse bees and drones.
- Contamination of the beeswax combs.
The first two modes of exposure would likely be short term. We should be more concerned with chronic exposure via the honey and pollen.
Via the honey
Only pesticides with some degree of water solubility can dissolve into honey. The most common residues found in honey in the U.S. are those from the beekeeper-applied miticides (coumaphos, fluvalinate, and amitraz), although I suspect that any effects from such residues would normally be insignificant due to their very low concentration. It may surprise some that as of 2007 (the last year of USDA testing), the neonicotinoids, which are far more water soluble, were not detectable in any tested honey sample at the 20 ppb lower limit [17].
Via the pollen and combs
A more significant problem is due to the unusual nest of honey bees—it is built of beeswax and then reused year after year. The combs of a hive have the property of readily absorbing environmental contaminants, and then shielding them from any water of sunlight that might wash them away or degrade them. So unlike other insects, honey bees return each night to a nest that may become more and more toxic over time!
This is especially a problem since most insecticides, some herbicides, and common adjuvants [18] are fat-soluble (“lipophilic”)–a property that allows them to better penetrate the waxy cuticle of insects and leaves. When chemically-stable lipophilic toxins are brought back with pollen, they readily diffuse into the combs, from which they can then transfer back into the fatty acids of subsequent pollen loads, into worker jelly, or directly through the skin of larvae, pupae, or adult bees–for years afterwards. The beekeeper-applied miticides fluvalinate and coumaphos are notable for falling into this category.
Hitting the Colony Where it Really Hurts
The “legacy effects” of these residues in the combs are a vexing problem, since they hit the colony precisely where they have the potential to do the most harm—in the nursery. Various researchers have developed models of colony population dynamics [19], which invariably find that anything that negatively effects either larval survival or the age of initiation of foraging will suppress the normal rate of colony population growth. This concept should be self evident, since the intrinsic rate of growth (r) of a colony is the result of the simple equation r = birth rate – death rate. If either larval survival or worker longevity is decreased to any extent, the colony simply cannot grow, or may dwindle or collapse.
The problem is that the beekeeper would have a hard time pinning the cause of the poor performance or slow death of his colonies after a particular pesticide exposure (or combination thereof), due to the stew of residues found in combs these days, the main ingredients usually being the beekeeper-applied miticides. Please be clear that I’m not saying that those miticide residues are the sole problem, but they are likely contributory factors. Some circumstantial credence is lent to this hypothesis by the common observation that “treatment free” beekeepers in areas of intense agricultural exposure often do not appear to suffer from the sorts of fall and winter losses as do beekeepers who routinely apply synthetic miticides. I am not saying that foregoing mite treatments will reduce winter losses; nor am I saying that miticide residues are the cause of losses.
A few years ago I was reviewing the data from a trial in which a group of Dave Hackenberg’s hives had been tracked for the course of nearly a year. At one point, Dave had split the hives between several apple orchards, and then brought them back together for other pollinations. What struck me was the legacy effect of one of the apple orchards, which appeared to be the eventual kiss of death for any colony that had been placed there during bloom—not immediately, but later in the season. This legacy effect haunts beekeepers who plan on taking strong colonies to almonds in February—those hives that had foraged in some agricultural areas the previous summer simply dwindle or die later in the season.
This dismaying observation has led a number of beekeepers to take a pass on summer pollination contracts, since the rental income does not cover their later colony losses. The situation is reaching the point that growers who continue to create killing fields for bees may be forced to pay a “rental rate” equal to the replacement cost for what would essentially be a “disposable pollination unit” (similar to the cardboard bumblebee nests supplied to greenhouse growers). These would be one-time-use colonies sacrificed to pollinate the crop, with full knowledge that they would be expected to succumb afterward. To me, this would be a sad commentary on our agricultural system—rather than fixing the problem, that growers would be throwing money at it instead, showing no concern for the survival of pollinators. I truly hope that it never comes to this!
Progress in Reducing Pesticide Use
We must acknowledge that great strides have been made in recent years towards cleaning up our act with regard to the environmental insult of pesticides. Unfortunately, it’s often difficult to get accurate and relevant data on actual pesticide use. The EPA and the USGS do some tracking of sales and application, but run several years behind in reporting. However, in recent years, the trend has been to phase out the most human-toxic and environmentally destructive insecticides, as evidenced by these figures from California (Fig. X).
Figure X. Reduction in use of organophosphate and carbamate insecticides in California over time—this is a good thing for bees, wildlife, and humans! Unfortunately, the top three insecticides (chlorpyrifos, aldicarb, and acephate) applied to farmland nationwide in 2007 (the most recent data) were still all in this group. Sources [[i]].
[i] http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf http://www.cdpr.ca.gov/docs/pur/pur11rep/chmrpt11.pdf
A Brighter Future
On the negative side, commodity prices are high, encouraging farmers to indiscriminately apply pesticides without regard to actual need. My heart goes out to those beekeepers (and their bees) who experience devastating pesticide kills or delayed late-season losses due to pesticides, and feel that it is completely unfair that they are not compensated for those losses. Even more so, it is a sad commentary on our agricultural system that pollinators cannot survive in some areas.
On the brighter side, public sentiment for the environment and pollinators is growing, and our congressional representatives are well aware of this. The agricultural industry and the chemical companies are under pressure from the public and the EPA, and are diligently working to develop “reduced risk” insecticides and biopesticides (microorganisms, pheromones, or compounds essentially identical to naturally occurring compounds). New technologies and IPM advocacy by extension agents offer great promise for reducing the need for pesticide applications. Beekeepers, through the National Pollinator Defense Fund, are pushing for better pesticide labeling and enforcement of existing law (please donate). And of course Mother Nature will eventually force farmers to embrace integrated pest management and sustainable agroecological practices [21].
For the large commercial beekeepers in areas of intense agriculture, pesticides are clearly often still a problem. But when I speak to beekeepers all over the country, for most, pesticides are no longer a major issue. The system is not perfect by any means, but we appear to be heading in the right direction.
I need to wrap up this article (at this moment I’m pounding away at the keyboard 35,000 feet above the surreal checkerboard of Midwestern cornfields, in which it’s hard to imagine how any honey bee colony could find enough to eat). I’ll continue next month with an assessment of our current understanding of the contribution of various pesticides to colony health problems.
References
[1] Wolpert, S (2013) How the brain creates the ‘buzz’ that helps ideas spread. http://newsroom.ucla.edu/portal/ucla/how-the-brain-creates-buzz-247204.aspx
[2] https://scientificbeekeeping.com/sick-bees-part-18f-colony-collapse-revisited-pesticides/
[3] https://scientificbeekeeping.com/historical-pesticide-overview/
[4] http://water.usgs.gov/nawqa/pnsp/usage/maps/compound_listing.php
[5] https://scientificbeekeeping.com/sick-bees-part-18f-colony-collapse-revisited-pesticides/
[6] 2007 figures http://www.census.gov/compendia/statab/2012/tables/12s0835.pdf
[7] Oyediran, IO, et al (2004) Prairie grasses as hosts of the Western Corn Rootworm (Coleoptera: Chrysomelidae). Environ. Entomol. 33(3): 740-747. (Broken Link!) http://web.missouri.edu/~hibbardb/CV_files/prairie%20grasses%20as%20hosts%20of%20the%20western%20corn%20rootworm.pdf
[8] Meinke, L, et al (2009) “Western corn rootworm ( Diabrotica virgifera virgifera LeConte) population dynamics” . Faculty Publications: Department of Entomology. Paper 244. http://digitalcommons.unl.edu/entomologyfacpub/244
[9] http://people.oregonstate.edu/~muirp/whynot.htm A great online pesticide syllabus by Dr. Patricia Muir.
[10] http://gardenbees.com/cotton%20spray/cottonspray.htm
[11] http://ipmworld.umn.edu/ipmchap.htm
[12] Goulson, D (2013) Neonicotinoids and bees. Significance. 10(3): 6–11. One of the best objective reviews on neonic issues.
[13] Gray, M (2012) Insurance pest management the norm in many corn and soybean fields of the Midwest. http://bulletin.ipm.illinois.edu/article.php?id=1597
[14] http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf
[15] Protecting Honey Bees from Pesticides http://extension.entm.purdue.edu/publications/E-53.pdf
[16] http://pesticideresearch.com/site/?page_id=24
[17] USDA (2008) Pesticide Data Program Annual Summary, Calendar Year 2007. http://www.ams.usda.gov/AMSv1.0/getfile?dDocName=STELPRDC5074338
[18] Mullin, CA, et al (2012) Pesticide formulation adjuvants may impact honey bee health. http://www.americanbeejournal.com/site/files/828/79344/436207/598079/2012_Proceedings_ABJ.pdf
[19] E.g., Zhu, WT, et al (2012) A stage-structured model of honey bee colony population dynamics assessing impacts of pesticides and other stressors. Ibid.
[20] http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf http://www.cdpr.ca.gov/docs/pur/pur11rep/chmrpt11.pdf
[21] De Schutter, O (2010) Report submitted by the Special Rapporteur on the right to food, http://www2.ohchr.org/english/issues/food/docs/A-HRC-16-49.pdf
First published in: ABJ December 2012
Genetically Modified Plants
What Is Genetic Modification?
There’s Nothing New About Transgenics
GMOs
An Odd Series of Connections
The Vilifying of Monsanto
What Are They Up To?
Practicality Overrides Principle
Hold the Hate Mail
The Changing Face of Agriculture
Bt Crops
Roundup Ready
Direct Effects of Roundup Use
Indirect Effects of Roundup Use
The Future of Roundup
Reality Check
Looking Ahead: The Chemical Treadmill & Pest Resistance
Additional Discussion
The Back Story on Plant Breeding and GM Crops
The Profit Motive
Enter GM Crops
The Second “Green Revolution”
Cautions About GM
Perspectives on GM
So What’s The Problem?
Acknowledgements
References
Sick Bees – Part 18E:
Colony Collapse Revisited – Genetically Modified Plants
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in Dec 2012
Genetically modified (or GM) plants have attracted a large amount of media attention in recent years and continue to do so. Despite this, the general public remains largely unaware of what a GM plant actually is or what advantages and disadvantages the technology has to offer, particularly with regard to the range of applications for which they can be used [1].
The above quote is certainly an understatement! Genetically Modified Organisms (GMO’s) are a highly contentious topic these days, and blamed by some for the demise of bees. In researching the subject, I found the public discussion to be highly polarized—plant breeders and farmers are largely enthusiastic (with appropriate reservations) about the benefits of genetic engineering, whereas health and environmental advocacy groups tend to be fearful of the new technology [2]. I will largely save my review of the history and pros and cons of GM crops for my website, and focus this article upon how GMO’s relate to honey bee health.
What is genetic modification?
The knowledge of genetics was not applied to plant breeding until the 1920’s; up ‘til then breeders would blindly cross promising cultivars and hope for the best. With today’s genetic engineering, breeders can now take a gene from one plant (or animal, fungus, or bacterium) and splice it into the DNA of another plant. If they get it just right, the new gene can confer resistance to frost, drought, pests, salinity, or disease. Or it could make the crop more nutritious, more flavorful, etc. Such genetically modified crops are also called “transgenic,” “recombinant,” “genetically engineered,” or “bioengineered.”
There’s nothing new about transgenics
There is nothing new about transgenic organisms, in fact you (yes you) are one. Viruses regularly swap genes among unrelated organisms via a process called “horizontal gene transfer” [3]. For example, the gene which is responsible for the formation of the mammalian placenta was not originally a mammal gene—it was inserted into our distant ancestors by a virus. If a gene introduced by a virus confers a fitness advantage to the recipient, then that gene may eventually be propagated throughout that species’ population. Until recently, we didn’t even know that this process has occurred throughout the evolution of life, and didn’t know or care whether a crop was “naturally” transgenic!
GMO’s
Both the scientific community and industry have done a terrible job at explaining genetic engineering to a distrustful public. There are clearly potential issues with genetic engineering, but they are being carefully addressed by independent scientists [4] and regulatory agencies, especially in Europe:
From the first generation of GM crops, two main areas of concern have emerged, namely risk to the environment and risk to human health.… Although it is now commonplace for the press to adopt ‘health campaigns’, the information they publish is often unreliable and unrepresentative of the available scientific evidence [5].
Jeffrey Smith, in his book “Seeds of Deception” [6] details a number of legitimate issues and early missteps in bioengineering, as well as pointing out the substantial political influence firms such as Monsanto have upon researchers, regulators, and legislators. We should be cautious to take their assurances with a grain of salt. On the other hand, I’ve checked the claims of other anti-GMO crusaders for factual accuracy, and found that many simply don’t hold water. For example, two headlined studies of late, one on rats fed GE corn and Roundup herbicide, and another on the purported increased use of herbicides due to GE crops simply do not stand up to objective scrutiny [7]. It bothers me that the public is being misled by myths and exaggeration from both sides.
From my point of view, GE holds incredible promise and should be pursued in earnest, yet must also be very carefully monitored and regulated. In any case, GE crops have been widely adopted in U.S. agriculture (Table 1), and thus are now a part of beekeeping.
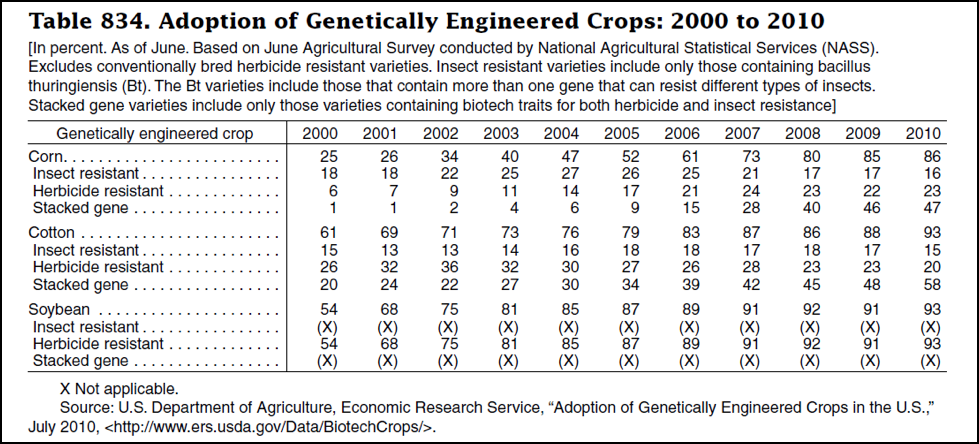
Table 1. The genetically engineered traits available to farmers have evolved rapidly as technology improves and as such crops become more widely adopted. Table from http://www.census.gov/compendia/statab/2012/tables/12s0834.pdf.
An Odd Series of Connections
In 1972, the dean of biological sciences at my university hired me to set up a “world class insectary” (which I did). I raised mass quantities of insects for hormone extraction, in the hope that we might develop a new generation of eco-friendly insecticides [8]. Several years later I was shocked when Monsanto–a widely-despised chemical company with a sordid history– then hired him to create “a world-class molecular biology company” (which he apparently did). In 2002, Monsanto was spun off as an independent agricultural company.
Jump forward to 2010, when I had the good fortune to work with an Israeli startup—Beeologics—and witnessed the efficacy of their eco-friendly dsRNA antiviral product for honey bees. But to bring the product to market, they needed more backing. To my utter astonishment, they recently sold themselves to Monsanto!
The Vilifying of Monsanto
These days one can simply mention the name “Monsanto” in many circles, and immediately hear a kneejerk chorus of hisses and boos. Sure, it had been easy for me to enjoy the camaraderie of riding the anti-Monsanto bandwagon; but I realized that that I shouldn’t allow that sort of fun to substitute for the responsibility of doing my homework and getting to the actual facts of the matter! When I did so, I found that some of Monsanto’s actions did indeed deserve opprobrium; but that much of the criticism directed at the current company is undeserved (Monsanto suffers from an ingenerate inability to practice effective PR). Concurrent with the purchase of Beeologics, Monsanto hired well-respected apiarist (and columnist) Jerry Hayes to head up a new honey bee health division, and appointed some prominent beekeepers (not me) to its advisory board. It dismays me that some beekeepers then immediately jump to the erroneous conclusion that Jerry has sold his soul to the Devil—nothing could be further from the truth!
What are they up to?
Some beekeepers imaginatively feared that Monsanto was about to create a GM bee or was up to some other nefarious plot. But in reality, Monsanto’s vision of its future direction is anything but evil—I suggest that you peruse their website for your own edification [9], [10]. Of course I was curious as to why they had purchased Beeologics, since the market for bee medicine is far too tiny to draw the interest of a giant corporation. But one needn’t be some sort of psychic in order to figure out a corporation’s plans—all you need do is to read its recent patents, which are a virtual crystal ball for seeing ten years into the future. So I searched out any patents containing the words “Monsanto” and “RNAi.”
To my great relief, I found that Monsanto was not up to some evil plot—far from it! I suggest you read two of the patents yourself [11]:
Chemical pesticidal agents are not selective and exert their effects on non-target fauna as well…Some chemical pesticidal agents have been shown to accumulate in food, and to exhibit adverse effects on workers that manufacture and apply such chemical agents. Thus there has been a long felt need for methods for controlling or eradicating… pest infestation on or in plants, i.e., methods which are selective, environmentally inert, non-persistent, biodegradable, and that fit well into pest resistance management schemes. Plant biotechnology provides a means to control pest infestations by providing plants that express one or more pest control agents. Recombinant pest control agents have generally been reported to be proteins selectively toxic to a target pest that are expressed by the cells of a recombinant plant.
What the patents tell us is that Monsanto clearly sees that the public is sick of pesticides. Genetic RNAi technology would allow plant breeders to develop crop cultivars that control insect pests in the same manner that the plants naturally control viruses. All that the breeder need do would be to identify a unique target protein in a particular pest, and then splice a gene into the plant to produce a “blocking” dsRNA molecule that would prevent the pest from building that specific protein. The beauty is that dsRNA molecules are already naturally found in plant tissues, the blocking molecule would be entirely specific for that pest alone, completely nontoxic to humans or other non target species, and be rapidly biodegradable. It would be a win all around (except for the pest)—crop protection, no toxic pesticides, and a sustainable farming technology (as well as a market for Monsanto’s products, since they would need to continually develop slightly different cultivars in order to avoid pest resistance). Who’d have guessed that Monsanto would be leading the way toward developing eco-friendly pest control? Life is full of surprises!
Practicality overrides principle
Some folk make GM crops out to be some sort of abomination of nature, and shun them with religious fervor. I’m not sure that this is the best course for environmentalists to take, and that perhaps, in the face of an expanding human population and a warming climate, we should leave all the possible plant breeding solutions on the table. The organic farming community wholeheartedly endorses the biotechnology of “marker assisted selection” [12], yet arbitrarily draws the line at the directed insertion of desirable genes. This may sound like heresy, but as an environmentalist, I suggest that GE holds great promise for developing more nutritious plants that don’t require pesticides, fertilizer, or irrigation—all of which would be wins for organic farming.
From a biological standpoint, I simply don’t see GM crops as being any more inherently dangerous than conventionally bred crops. Our domestic plants today are often far from “natural”—you wouldn’t recognize the ancestors of many. Be aware that even conventionally bred cultivars of several crops (beans, potatoes, celery, etc.) often turn out to be too toxic for humans.
This is not by any means a fluff piece for Monsanto or agribusiness. Farming is not what it used to be. In the U.S., 85% of farm sales are produced by less than 10% of farms, which hold 44% of farm acreage [13]. A mere six companies collectively control around half of the proprietary seed market, and three quarters of the global agrochemical market [14]. I abhor such corporate domination; neither do I see today’s high-input agricultural practices as being either sustainable or ecologically wise.
That said, human demands upon the Earth’s finite ecosystem are growing. There are only about 4.5 acres of biologically productive land on the surface of the Earth available for each current human inhabitant. Depending upon the culture’s lifestyle, we use anywhere from 25 acres (U.S.) to as little as 1 acre (Bangladesh) to feed and clothe each person. Unfortunately for the bee (and many other species), due to human population growth there are over 200,000 additional human mouths to feed every single day—each requiring the conversion of another couple of acres of natural habitat into farmland!
It doesn’t take a mathematician to figure out that if we wish to conserve natural ecosystems that we need to get more yield out of existing cropland! And one of the best ways to do that is to breed crops that are more productive and pest-resistant. The plant scientists in the corporate labs are making huge strides in developing such cultivars, both by GM and conventional breeding. If they manage to file a patent [15], so what?—other breeders can easily “steal” the germplasm away from the patented genes, and in any case, the patents expire after 20 years!
Monsanto has seen the writing on the wall—farmers and consumers are demanding not only more food production, but also more eco-friendly agricultural practices. Monsanto research is heading in that direction with their conventional breeding programs, the development of “biological” insecticides [16], and the goal of producing pesticide-free dsRNA crops. Add to that that the company could actually bring to market dsRNA medications against bee viruses, nosema, and perhaps varroa. All would be huge wins for the honey bee and beekeepers!
Hold the hate mail
Full disclosure: so despite my innate aversion to corporate dominance and corporate agriculture, I feel that we beekeepers should work with Monsanto to develop products for the beekeeping industry, as well as bee-friendly cultivars of crop plants, and have thus personally decided to be a cooperator in their initial bee research trial. Is this some sort of Faustian bargain? I don’t know, but as a condition of my cooperation, I asked, and Monsanto agreed, to allow me to share the data collected with the beekeeping community—which could be a big win for us, since Monsanto has some of the best analytic labs in the world! I feel that it is far better to have Monsanto working on the side of beekeepers, rather than perhaps against us. At this point, I’d like to leave the GM debate behind, and address the facts of the matter as to any relationship between GM crops and CCD.
The Changing face of agriculture
Genetic engineering has clearly changed the face of agriculture in the U.S. (Fig. 1).
Figure 1. These three crops account for over half of all U.S. acreage planted to principal crops, and all are worked to some extent by bees. Data from http://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us.aspx
As can be seen from the figure above, any bees near corn, soy, or cotton are going to be exposed to pollen and nectar from GM plants, as well as to indirect effects due to the technology. So could GM crops be the cause of CCD?
Bt Crops
Biological plausibility: the insecticidal Bt toxins in GM corn and cotton pollen could harm adult or larval bees.
Organic farmers have long used the spores of the bacterium Bacillus thuringiensis (Bt) to kill caterpillars. Bt spores germinate in the caterpillar gut, and the bacterium produces insecticidal crystalline proteins (Cry proteins) that bind to specific receptors on the insect intestinal wall. Since different insect species have different receptors on their gut cells, different strains of Bt have evolved to specifically kill various caterpillars, beetles, mosquitoes, etc. [17]. The proteins are so species specific that wax moths can be controlled on combs by Bt aizawai, which produces Cry proteins that are toxic to moth larvae, but not to bees.
Molecular biologists tweak these Cry proteins to make them even more species specific, and then insert them into plant DNA, so that the plant then produces the proteins itself, thus making its tissues toxic to the target species. In order to delay the inevitable evolution of Cry-resistant pests, growers plant a percentage of “refuge” crop not containing the Cry genes. Even so, any particular Cry gene will only be effective for some number of years until resistant pests show up.
People have expressed concern about a poisonous substance being introduced into plant tissues, and to them I highly recommend the paper “Misconceptions about the Causes of Cancer” [18]. The reality is that plant tissues are naturally awash in poisonous substances. Plants have needed to repel herbivores throughout their evolution, and since plants can’t run, hide, or bite back, they do it chemically. Many of our most popular fruits, nuts, grains, and vegetables (and especially herbs and spices) contain powerful phytotoxins. Their wild ancestors required cooking or leaching before the plant was edible to humans. Plant breeders systematically select for cultivars with lower levels of (the often strongly flavored) toxins.
Plants that are naturally resistant to pests contain more phytotoxins, often produced in response to damage from insects. For example, the sprouts of wheat, corn, and rye produce potent mutagens (enjoy that cup of wheatgrass juice!) [19]. And some plants naturally contain symbiotic bacteria and fungi in their tissues, which produce non-plant chemicals [20]. There is absolutely nothing biologically novel about insecticidal toxins in plant tissues.
The toxicity (or lack thereof) of Cry proteins to non-target organisms, especially upon two “charismatic” species—the honey bee and the monarch butterfly—has been well studied [21], [22], [23]. A recent and very well-designed experiment on the effect of GM Bt corn pollen upon the growth and survival of honey bee larvae was recently performed by a team of independently-funded German researchers [24]. They added pollen from four different sources to a standard semi-artificial larval diet.
Results: surprisingly, the larvae fed the pollen from the “stacked” GM corn containing a combination of three different Cry proteins exhibited a higher survival rate (100%), than those fed non-GM corn pollen! To me, a big plus for this study was that they also included a positive control of pollen from a wild plant said to be harmful to bees—only about 30% of those larvae survived! This finding confirmed that even some natural pollens are quite toxic, and that we should compare any toxicity trials of pesticides with those of the natural phytotoxins in nature.
Analysis: CCD and colony mortality occur even in the absence of GM Bt crops; feeding GM Bt pollen to adult bees or larvae does not cause observable adverse effects.
Verdict on Bt crops: The specific Bt cry proteins used in GM crops were intentionally chosen to not cause harm to bees. There is no evidence to date that they do. On the other hand, Bt crops require less use of insecticides that are clearly toxic to bees [25].
Roundup Ready
Monsanto’s pitch is that Roundup Ready® (RR) crops allow farmers to practice weed-free “no till” farming, which saves both topsoil and money. The catch is that farmers must then douse their fields with Monsanto’s flagship product, Roundup (ensuring sales of that herbicide—a great marketing strategy). Bayer CropScience has followed suit by introducing crops resistant to its Liberty herbicide, which has a different mode of action.
Herbicide-resistant crops do indeed address several major environmental problems:
- No till farming does in fact require less labor and reduces soil compaction.
- Farmers get greater production due to less competition from weeds.
- No till also reduces the amount of petrochemical fuel involved in tillage.
- No till greatly reduces soil erosion, which has long been a major environmental concern.
- No till may help to sequester carbon in the soil, and to rebuild soil.
So what’s not to love about Roundup Ready? There are a few main complaints—(1) the massive spraying of the active ingredient, glyphosate, for which there is questionable evidence that it may be an endocrine disruptor [26], (2) claims of intimidation by Monsanto of farmers who choose not to plant RR seed, and (3) the environmental impact and sustainability of the sort of weed-free monoculture possible with RR crops.
So how do Roundup and RR crops relate to honey bees?
Direct Effects of Roundup Use
Biological plausibility: either the active ingredient (glyphosate), or the adjuvants could cause bee toxicity.
The EPA has thoroughly reviewed the research and found glyphosate to be practically nontoxic to bees (and humans). They have found the same for Roundup’s adjuvant polyoxyethylene-alkylamine. However, some beekeepers tell me that they see increased bee mortality following the spraying of glyphosate (Fig. 3), but are not sure whether it was a generic product, or perhaps contained additional ingredients (surfactants, fungicides, or insecticides) added to the tank mix.

Figure 3. A farmer spraying glyphosate herbicide over Roundup Ready corn seedlings. Photo courtesy of beekeeper Larry Garrett.
Analysis: there is no strong evidence that the spraying of Roundup or generic glyphosate herbicide is directly causing significant bee mortality. However, Drs. Jim and Maryann Frazier have legitimate concerns about the effect of some adjuvants—especially the organosilicones [27], [28].
Indirect Effects of Roundup Use
Biological plausibility: the elimination of weeds reduces bee forage.
The success of Roundup Ready technology has allowed farmers to largely eliminate weeds from their fields (at least until the inevitable resistant weeds take over). But they don’t stop there—nowadays they practice “clean farming” and use herbicides to burn off every weed along the fencerows and in the ditches—the very places that bees formerly had their best foraging. This elimination of flowering weeds severely reduces the amount of available of bee forage, plus kills off the host plants of native pollinators (such as monarch butterflies) and beneficial insects.
European honey bees evolved in Europe (hence the name), and are adapted to the nutrition provided by Old World flowering plants. Many of the weeds in North America are old friends of the honey bee. On the other hand, honey bees were never exposed to corn, soybeans, sunflowers, or squashes until recently; neither corn nor sunflowers supply complete amino acid profiles in their respective pollens. Until the advent of Roundup Ready, the weeds in an around crops provided alternative nectar and pollen sources for bees; today there is often nary a bee-nutritious weed to be seen in or around a field of corn or soybeans (Fig. 4).

Figure 4. I took this photo of a no-till herbicide-resistant corn field, prior to the shading canopy of the crop closing over. Note the total lack of any sort of bee forage (or any species of anything other than corn). The soil surface is a far cry from the original densely vegetated prairie sod. Prior to RR, there was more weedy forage for bees, and especially from the traditional weed-controlling crop rotation into legumes or pasture.
Update: there’s a great deal of debate about the safely of Roundup (the formulated product with it’s surfactants) and its active ingredient, glyphosate. From http://npic.orst.edu/factsheets/archive/glyphotech.html
“In plants, glyphosate disrupts the shikimic acid pathway through inhibition of the enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase. The resulting deficiency in EPSP production leads to reductions in aromatic amino acids that are vital for protein synthesis and plant growth.
As far as the claims that glyphosate causes cancer (notably Non-Hodgkin’s Lymphoma), I agree with the regulatory agencies that the case against glyphosate is very weak. As far as glyphosate being an endocrine disruptor, I’ll leave it to the researchers and regulatory agencies to figure it out. As I type these words, I’ve actually got caged bees to which I’m feeding glyphosate (at a field-realistic dose) for a trial, and not seeing increased mortality. But some research indicates that it may be harmful to the gut microbiota. This sort of research takes time, and eventually we’ll figure out just how safe or harmful glyphosate is to bees, humans, or the environment.
But nothing in nature is simple. Eliminating the competition of weeds and insects may allow plants to hold back from the production of natural toxins. And a surprising piece of research found corn kernels from plants sprayed with either of two different herbicides actually contain more of the healthful carotenoids [30]!
The Future of Roundup
It took Monsanto several years to genetically engineer Roundup-resistant crops, yet took farmers only slightly longer to inadvertently produce Roundup-resistant weeds by the conventional breeding technique of applying a strong selective pressure–the continuous application of Roundup!
Weed management scientists consider glyphosate to be a once-in-a-100-year discovery—it works on 140 species of weeds, and is relatively environmentally friendly. However, its overuse has led to the creation of several “driver weeds” that could soon lead to its redundancy in corn, soy, and cotton acreage [31]. This will drive farmers to turn to other herbicides (which will also in time fail). We can only hope that someday they will be forced back into practicing crop rotation into legumes and pasture.
Reality Check
In order to clarify cause and effect, I often seek out extreme cases. Such would be the situation in the Corn Belt, where I could compare the USDA’s hive and honey data from the old days to those under today’s intense planting of GM crops (Fig. 5)!
Figure 5. The most intense planting of GM crops is in Iowa and Illinois (the dark green areas of the map above). U.S. farmers planted nearly 100 million acres of corn this year, and 76 million of soy. That is enough acreage to cover the entire state of Texas with GM crops!. Source: http://www.nass.usda.gov/Charts_and_Maps/Crops_County/pdf/CR-PL10-RGBChor.pdf
So I went through the tedious process of downloading and transcribing the NASS agricultural census figures for Iowa. I entered the amount of corn acreage, the total number of colonies in the state, and what I consider to be the best measure of colony health—honey yield per hive (which of course is largely weather dependent, but should show any trends). I plotted the data below (Fig. 6):

Figure 6. Bee and corn data from Iowa, and the dates of introduction of corn pest control technologies. The dotted line is median honey yield per colony. No factor appears to have affected honey production, but colony numbers have decreased since the arrival of varroa. Gaps are missing data. Source NASS.
Note: for non beekeepers, varroa is a parasitic mite that arrived in the U.S. around 1990 and quickly became, and still remains, the Number One problem in bee health–far more than any other factor.
Over the years, corn acreage increased by 18%. Other than the prodigious crop of 1988, honey production has averaged around 67 lbs per hive. The thing that stands out is the plot of number of colonies. Hive numbers jumped up in the late 1980’s, likely due to federal honey price support payments, which peaked in 1988, and were cut off in 1994 [[i]]. Colony numbers peaked in 1990, the same year that varroa arrived in Ohio, and went down from there, leveling off to about half the number of hives present in the 1970’s.
I fully expected honey yields to decrease concurrent with the adoption of Roundup Ready varieties, but they didn’t! Colonies still produce as much honey today as they did in the past, but this might be partially due to having fewer bees working the same amount of land, or to increased soybean nectar (which saved a number of Midwestern beekeepers from disaster during this year’s droughts).
Perhaps even more surprising is the fact that in a state covered in corn and soy, colony productivity did not appear to be affected by the introductions of either Bt or Roundup Ready corn, nor by the universal use of neonicotinoid seed treatments (between corn and soy, on over roughly two thirds of the entire state land area). Note that honey yields actually increased for a few years following the introduction of clothianidin seed treatment!
Tellingly, hive numbers started to decrease after the arrival of varroa, and plummeted in the late 1990’s as fluvalinate failed as a miticide, and many beekeepers simply threw up their hands and quit the business.
Verdict on herbicide tolerant crops: from a nutritional standpoint, the increased use of herbicides, and the associated weed free “clean farming” has certainly not helped the bees in corn/soy areas, but it is hard to make a case for them causing colony collapse.
Verdict on GM crops in general: Allow me to quote from the USDA:
…there is no correlation between where GM crops are planted and the pattern of CCD incidents. Also, GM crops have been widely planted since the late 1990s, but CCD did not appear until 2006. In addition, CCD has been reported in countries that do not allow GM crops to be planted, such as
Switzerland [33].
Looking Ahead: The Chemical Treadmill & Pest Resistance
It is interesting to observe the evolution of agriculture from the perspective of a biologist. Simple systems in nature are inherently less stable than complex systems. The current agricultural model in the U.S. exemplifies simplicity to the extreme—plant a single species into bare soil year after year, killing any competitive weeds or insects with pesticides (either sprayed, systemic, or engineered into the plants), and attempt to maintain fertility by adding energy-costly fertilizer. From a biological perspective, such a strategy is little more than an intense selective breeding program for the most resistant pests, and doomed to escalating chemical and energy inputs until the system collapses under its own weight.
I’m anything but a salesman for either Bt nor RR crops. Both are mere short-term solutions—resistant bugs and weeds are already starting to spread. I also have questions about the benefits of herbicide-intense no till planting [34], and hope that farmers return to alternative methods of weed control [35]. Luckily, the system will likely be self correcting, eventually forcing humanity to practice more sustainable methods of farming the land. However, I suggest that those methods may well include the wise use of biotechnology.
Additional Discussion
The Back Story on Plant Breeding and GM Crops
Traditionally, farmers simply replanted with the seeds from the most desirable individual plants year after year; this is the simplest form of “selective breeding.” For example, all the various cole crops (cabbage, kale, broccoli, cauliflower, kohlrabi, Brussels sprouts) were developed by intentionally selecting for unusual forms of the species (resulting from random recombination of the natural allelic diversity, spontaneous mutants, or natural hybrids). This sort of selective breeding tends to result in a diverse assembly of locally-adapted cultivars. In Oaxaca, Mexico– the birthplace of corn–some 150 traditional varieties of maize are grown without pesticides or herbicides, thereby maintaining an invaluable reservoir of genetically-diversity “germplasm,” which breeders can then cross and backcross in order to develop new cultivars (e.g., for pest or drought resistance).
In the early years of the U.S., seeds from desirable cultivars were distributed to farmers by the government, and plant breeding was performed at universities and at the USDA [36]. But since every strain breeds true, a farmer could save the seed and replant, leaving little opportunity for seed companies to make a buck. So in 1883, they formed the American Seed Trade Association and began to lobby for the cessation of the government programs.
The Profit Motive
In the early part of the 20th century, the companies began to promote hybrids— crosses of two (or more) different strains or species that exhibited some sort of “hybrid vigor”—offering greater production, tastier fruit, or some other desirable characteristic. Hybrids were a godsend to the companies, since they are often sterile or don’t breed true, meaning that farmers needed to purchase (rather than save) seed each season.
The seed lobby eventually shifted public funding away from the free distribution of selected seedstocks to instead encouraging the USDA and universities to develop inbred parental lines and breeding stock that the seed trade could then use to create proprietary hybrid varieties. By 1960, farmers planted less than 5% of corn from saved seed; and less than 10% of soybeans by 2001. As on-farm familiarity with the saving of seed was forgotten, farmers became willing consumers of produced seed.
Enter GM Crops
Then in 1980, the Supreme Court decided that seed companies could patent new varieties if they contained distinct and novel genetic markers. This meant that farmers (in some countries) could now be required to sign licensing agreements to allow them to use the patented seed each season [37] (there is a hodge-podge of international patent laws in this regard [38]).
The Second “Green Revolution”
The first “green revolution” was based upon fertilizer, pesticides, and hybrid seed (and also resulted in forcing farmers onto “agricultural treadmills”–making them less self sufficient and sustainable, and more reliant upon purchased seed, pesticide and fertilizer use, and upon borrowed money).
In 1950 the Secretary of Agriculture Ezra Benson said to farmers, “Get big or get out.” His 1970s successor, Earl Butts, repeated that message, and exhorted farmers to “plant fence row to fence row” and to “adapt or die.” Politicians who understood that a well-fed electorate is a happy electorate promoted policies that resulted in the destruction of the small family farm. Our policy of price supports and favorable treatment of agribusiness has changed the face of the American farm and the composition of the American diet [39].
Today’s “second green revolution” is based upon technological advances in plant genetics (including GM) and the (at least partial) replacement of nasty pesticides with “biologicals.” As an environmentalist, I find the new revolution to be more promising for ecological sustainability, but it is not without its downside—the current consolidation of agribusiness. As I mentioned before, farms, seed companies, and chemical companies are all being bought up by a few main players. Philip Howard details this consolidation in a free download [40], from which I quote:
This consolidation is associated with a number of impacts that constrain the opportunities for renewable agriculture. Some of these include declining rates of saving and replanting seeds, as firms successfully convince a growing percentage of farmers to purchase their products year after year; a shift in both public and private research toward the most profitable proprietary crops and varieties, but away from the improvement of varieties that farmers can easily replant; and a reduction in seed diversity, as remaining firms eliminate less profitable lines from newly acquired subsidiaries.
He then speaks of the concept of the “treadmill”:
For the majority of farmers, however, the result is that they must constantly increase yields in order to simply maintain the same revenue. [Monsanto’s sales pitch is that economic success in farming is driven by yield per acre [41]. Those that are unable to keep up with this treadmill will “fall off,” or exit farming altogether. Their land ends up being “cannibalized” by remaining farmers who seek to increase scale of production as another means of keeping up with the treadmill, leading to the increasing centralization of agriculture. Farmers who have managed to stay in business have adapted to this process, and are typically on the leading edge of the adoption of new technologies. As a result, they have a high degree of confidence in science and technological innovations.
However, this problem has nothing to do with GMO’s, but is rather due to the public’s unknowing acceptance of the practice. Capitalism inevitably leads to consolidation unless consumers stop supporting corporate agribusiness with their pocketbooks and their votes, and start demanding that their government enforce antitrust efforts and better support small farmers.
But we are allowing economics and politics to distract us from the topic at hand—the technology of genetic engineering in plant breeding.
Cautions About GM
The most vocal critic of genetic modification is Jeffrey Smith, fear-mongering author of Seeds of Deception, producer of the film Genetic Roulette, and executive director of the inappropriately-named Institute for Responsible Technology. Smith is a gifted and effective communicator, as well as being a practitioner of “yogic flying” [42]. I will be the first to say that Smith’s anti-GMO claims [43] would scare the pants off of anyone, and make for compelling story! The problem is that he plays loose with the facts—most of his claims simply do not stand up to any sort of scientific scrutiny. I suggest that for an objective analysis of the facts, that you visit AcademicsReviewed.org, a website that tests popular claims against peer-reviewed science. They address each of Smith’s alarming “facts” one by one [44]. It is a thrilling ride to open the two web pages side by side, first being shocked by Smith’s wild and scary claims, and then reading the factual rebuttal to each! The thing that most bothers me about Smith’s writing is that he treats GM cultivars generically, rather than specifically addressing the merits or concerns for them individually. This makes little sense, since any conventional crop has cultivars that cause human allergy or contain excessive levels of natural toxins, yet no one calls for the testing of each of them!
Perspectives On GM Crops
As you may have guessed by now, to me, the GM debate should not be about being pro or con, rather it should be about the intelligent discussion of reconciling its promise with its problems. The GE genie is out of the bottle, and I can’t see that anyone is going to put it back in–so we might as well work with it! So let’s cut through the hype and hysteria, the fears and judgments, and try to objectively look at the facts of the matter:
- From a plant breeder standpoint, genetic engineering holds incredible promise for the development of crops that could be tremendously beneficial to humans or the environment. For example, “Transgenic cotton has reduced the need for conventional insecticides used against lepidopteran [pests] an average in the USA about 59.4% [and] Texas 74.7%…an average number of pesticide applications in conventional cotton has fallen from 4.3 in 1995 to 2.1 in the USA… with benefits to human health and the environment” [45].
- GM is only a part of plant breeding—most advances continue to be in conventional breeding, now assisted by “marker assisted selection,” which is embraced by environmentalists [46].
- However, someone needs to pay for the research, and the taxpayer is not doing it! For a thoughtful discussion of the benefits of gene patents, see [47].
- Novel genetic markers can be patented, and a licensing fee can be charged, despite the fact that they are not GM!
- From a consumer standpoint, advanced breeding techniques can result in cheaper and more nutritious food, and less environmental impact from farming.
- Consumers have erroneously been led to believe that GM crops are dangerous to their health, and call for application of the precautionary principle. My gosh, please read “Misconceptions about the causes of cancer” [48]. Few foods are entirely “safe”! And “safety” can never be proven—it can only be disproven. And no studies have ever disproven the safety of GM crops, nor have doctors noticed anyone ever getting sick from them, despite our eating them for 15 years!
- In truth, some scientists argue that plants produced by classical breeding methods should undergo the same safety testing regime as genetically modified plants. There have been plenty of instances where plants bred using classical techniques have been unsuitable for human consumption, causing toxicity or allergic reactions.
- Those that speak of applying the “precautionary principle” should read Jon Entine’s trenchant analysis of the fallacy of overapplication of that principle [49]. In truth, our regulators (EPA and FDA) vigorously apply the precautionary principle in the form “reasonable certainty of no harm.”
- The benefits of seed biotechnology cannot be realized without good seed germplasm to start with. So a few large seed companies started buying up their competitors to acquire the most productive and desirable varieties.
- The downside of the above practice is that by 2008, 85% of GM maize patents and 70% of non-maize GM plant patents in the U.S. were owned by the top three seed companies: Monsanto, DuPont, and Syngenta [50]. Note that economists figure that when four firms control 40% of a market, it is no longer competitive; in the case of GE crops, the top four seed firms control 56% of the global proprietary seed market!
- On the flip side, these profits are an incentive for the large corporations to invest in innovative plant breeding research—Monsanto spends about $2 million a day on this. This is important to keep in mind in an increasingly hungry world.
- On the dark side, Monsanto’s nearly $12 billion in annual sales allows the company to lobby regulators, influence universities, and spin the news. These are standard business practices for any large corporation, but hardly make Monsanto uniquely evil.
- Be aware that patented genes are of use only if inserted into high-producing cultivars–which are developed by conventional breeding (which constitutes nearly half of Monsanto’s plant breeding budget). These desirable cultivars have no patent protection. Monsanto uses a non GE technology called SMART = Selection with Markers and Advanced Reproductive Technologies. SMART technology is warmly embraced by environmental groups [51].
- Adding a genetic marker allows a company to identify its proprietary strains, like putting a nametag on a dog. But clever breeders can back engineer the desirable germplasm out from patent protection.
- And remember that patents expire after 20 years. The patents for Roundup Ready soybeans expire in 2014—at which time farmers, universities, and seed companies will then be free to propagate and sell the variety [52]. Patents are granted in order to spur innovation; by filing for patent protection, a company must make its discoveries public knowledge. This is a good thing.
- Monsanto invests 44% of its R&D on conventional (as opposed to GM breeding).
- Monsanto has also given rights to some of their patented crops to poorer countries, and recently donated a database of some 4000 genetic markers from cotton to Texas A&M [53]. The university plant breeders are excited in that the information will assist them in their conventional (non-GM) breeding of cotton, to the benefit of the environment [54 ].
- From the farmer’s standpoint, he has the choice of purchasing GE varieties that may be more productive, reduce insecticide use, or reduce tillage costs [55]. Keep in mind that there is nothing keeping him from purchasing “conventional” non-GM seed—it is available (I checked, and it sells at about half the cost of GM seed). In our free enterprise system there is nothing to keep non-GM seed companies from selling an alternative product if there is a demand. Farmers who are unimpressed by GM varieties freely switch back to conventional seed.
- From an agricultural standpoint, the widespread adoption of a few favored crop varieties (GM or not) can result in the irreplaceable loss of crop genetic diversity—this is of great concern to plant breeders. If you haven’t yet seen the graphic of our loss of crop genetic diversity from National Geographic magazine, you should! [56]. Luckily, this does not appear to be occurring yet with maize in Oaxaca [57], but there is a legitimate concern that economics will force traditional farmers out of business, leading to the loss of heirloom varieties. However, this is not a GM issue, but rather an effect of consolidation.
- From a sustainability standpoint, there is nothing to prevent constant breeding innovation to keep pace with pest evolution. Genetically engineered crops can play a role in sustainable farming as our agricultural practices begin to shift to more ecologically sustainable methods.
- One should keep in mind how the simple splicing of a virus gene into the papaya saved the Hawaiian papaya growers from the ravages of ringspot virus—the GE papaya is the mainstay of the industry, and by virtue of keeping the virus in check actually allows nearby organic papayas to thrive. Yet ecoterrorists recently hacked down thousands of GM trees [58]. It’s interesting to read the history of “Golden Rice” [59] to see how the anti-GMO lobby is specifically scared that the success of such a lifesaving crop might open the door for acceptance of other GM plants!
Update Jan 2013
News item: Leading Environmental Activist’s Blunt Confession: I Was Completely Wrong To Oppose GMOs. Blog in Slate Magazine
“If you fear genetically modified food, you may have Mark Lynas to thank. By his own reckoning, British environmentalist helped spur the anti-GMO movement in the mid-‘90s, arguing as recently at 2008 that big corporations’ selfish greed would threaten the health of both people and the Earth. Thanks to the efforts of Lynas and people like him, governments around the world—especially in Western Europe, Asia, and Africa—have hobbled GM research, and NGOs like Greenpeace have spurned donations of genetically modified foods.
So What’s The Problem?
The problem is that anti-GMO advocacy groups are determined to put a stop to all GE technology. They targeted California with Prop 37, which applied only to packaged foods and produce. A more cynical take on Prop 37 was that it was all about marketing: “If your produce is no different in terms of taste, safety and nutrition from a competitor, and costs more, apparently the only marketing option is to create a negative image of your competitor’s product” [60].
If Prop 37 had been successful, the promoters would then have targeted restaurants, the meat and dairy industry, and the beverage industry. I personally feel that this is an extreme position, what with the human population growing hungrier every day, and climate change threatening agriculture worldwide with heat, drought, pestilence, and salinity problems. Not only that, but GM crops hold promise for cheap omega-3 fatty acids (so that we don’t have to harvest fish for them), cost-effective biofuels, and less expensive pharmaceuticals.
A good blog on the problem with the anti-GMO fear campaign can be found at [61], from which I quote:
It would be bad enough if something like the Seralini study simply contributed to the unnecessary angst amongst consumers around the world. It also has very real political, economic and practical effects. For instance brand conscious food companies have used their leverage to prevent the development of GMO versions of potatoes, bananas, coffee and other crops because they fear controversy. Apple growers worried about the market response are opposing the introduction of a non-browning apple even though it was developed by one of their own fruit companies. French activists destroyed a government-run field trial of a virus-resistant root stock which could have made it possible to produce good wine on sites that have become useless because of contamination with sting nematodes and the virus they vector. California voters have the potential to pass a seriously flawed “GMO labeling” initiative next month that could only serve the purposes of the lawyers and “natural products” marketers who created it. More importantly, European and Japanese importers of wheat essentially blackmailed the North American wheat producers into blocking biotech wheat development because those companies were nervous about consumer response in countries where GMO angst is so high. This has delayed by decades not only specific desirable trait development, but also what might have been an enormous private investment in a crop that is critically important for feeding a lot more people than just those in those rich countries. There is a huge cost of “precaution” based on poor science.
I believe that people should be well informed before taking a stance on important issues. I’d like to suggest one last excellent blog by an independent U.C. Berkeley evolutionary biologist and medical researcher:“How Bt Corn and Roundup Ready Soy Work – And Why They Should Not Scare You [62].
Acknowledgements
As always, thanks to my friend and collaborator in research Peter Loring Borst, and to anyone who still reads my articles after finding out that I’ve collaborated with Monsanto!
References
[1] Key S, et al (2008) Genetically modified plants and human health. J R Soc Med.101(6):290-298. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2408621/
[2] For example: Antoniou, M, et al (2012) GMO myths and truths.
(Broken Link!) http://earthopensource.org/files/pdfs/GMO_Myths_and_Truths/GMO_Myths_and_Truths_1.3.pdf
[3] Chiba S, et al. (2011) Widespread endogenization of genome sequences of non-retroviral RNA viruses into plant genomes. PLoS Pathog 7(7): e1002146.
[4] Domingo, JL and JG Bordonaba (2011) A literature review on the safety assessment of genetically modified plants. Environment International 37: 734–742.
[5] Key (2008) op. cit.
[6] Smith, JM (2003) Seeds of Deception. Yes! Books
[7] Séralini, GE, et al (2012) Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chemical Toxicology (2012) http://foodpoisoningbulletin.com/wp-content/uploads/Toxicity-of-Roundup-Ready-Maize.pdf;
Reviews
http://www.forbes.com/sites/henrymiller/2012/09/25/scientists-smell-a-rat-in-fraudulent-genetic-engineering-study/2/
http://www.efsa.europa.eu/en/faqs/faqseralini.htm#9, http://www.emilywillinghamphd.com/2012/09/was-it-gmos-or-bpa-that-did-in-those.html,
(Broken Link!) http://www.ask-force.org/web/Seralini/Anonymous-Rat-List-Spaying-2003.pdfs, http://storify.com/vJayByrne/was-seralini-gmo-study-designed-to-generate-negati;
Benbrook, CM (2012) Impacts of genetically engineered crops on pesticide use in the U.S. — the first sixteen years. Environmental Sciences Europe 24:24 http://www.enveurope.com/content/pdf/2190-4715-24-24.pdf,
Review http://weedcontrolfreaks.com/2012/10/do-genetically-engineered-crops-really-increase-herbicide-use/#more-432
[8] http://journals.tubitak.gov.tr/agriculture/issues/tar-04-28-6/tar-28-6-1-0309-5.pdf
[9] http://www.monsanto.com/whoweare/Pages/monsanto-history.aspx
[10] http://www.businessweek.com/stories/2010-01-10/monsanto-v-dot-food-inc-dot-over-how-to-feed-the-world
[11] Methods for genetic control of plant pest infestation and compositions thereof
http://www.freepatentsonline.com/8088976.html
http://www.freepatentsonline.com/7943819.html
[12] http://www.greenpeace.org/australia/PageFiles/348427/smart-breeding.pdf
[13] 2007 figures http://www.census.gov/compendia/statab/2012/tables/12s0835.pdf
[14] ETC Group (2008) Who owns nature? Corporate power and the final frontier in the commodification of life. http://www.etcgroup.org/sites/www.etcgroup.org/files/publication/707/01/etc_won_report_final_color.pdf
[15] Blakeney, M (2011) Trends in intellectual property rights relating to genetic resources for food and agriculture. http://www.fao.org/docrep/meeting/022/mb684e.pdf This document covers the hodge-podge of international patent laws regarding plants and animals.
[16] http://www.monsanto.com/products/Pages/biodirect-ag-biologicals.aspx
[17] History of Bt http://www.bt.ucsd.edu/bt_history.html
Mode of action http://www.bt.ucsd.edu/how_bt_work.html
[18] Gold 2002 Misconceptions about the causes of cancer http://potency.berkeley.edu/pdfs/Gold_Misconceptions.pdf A “MUST READ”!
[19] Buchmann CA, et al (2007) Dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) and 2,4-dihydroxy-1,4-benzoxazin-3-one (DIBOA), two naturally occurring benzoxazinones contained in sprouts of Gramineae are potent aneugens in human-derived liver cells (HepG2). Cancer Lett. 246 (1-2):290-9.
[20] http://en.wikipedia.org/wiki/Endophyte
[21] Duan JJ, et al (2008) A meta-analysis of effects of Bt crops on honey bees (Hymenoptera: Apidae). PLoS ONE 3(1): e1415.
[22] Center for Environmental Risk Assessment (2011) A review of the environmental safety of the Cry1Ab protein. http://cera-gmc.org/docs/cera_publications/cry1ab_en.pdf
[23] Han, P, et al (2012) Does transgenic Cry1Ac + CpTI cotton pollen affect hypopharyngeal gland development and midgut proteolytic enzyme activity in the honey bee Apis mellifera L. (Hymenoptera, Apidae)? Ecotoxicology. 2012 Aug 7. [Epub ahead of print]
[24] Hendriksma HP, et al (2011) Testing pollen of single and stacked insect-resistant bt-maize on in vitro reared honey bee larvae. PLoS ONE 6(12): e28174.
[25] Benbrook, CM (2012) op. cit.
[26] Reviewed in http://www.sourcewatch.org/index.php/Glyphosate
[27] Mullin, C.A., J.L. Frazier, M.T. Frazier & T.J. Ciarlo – A primer on pesticide formulation ‘inerts’ and honey bees. http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011
[28] Ciarlo TJ, CA Mullin, JL Frazier, DR Schmehl (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7(7): e40848.
[29] Johal, GS and DM Huber (2009) Glyphosate effects on diseases of plants. Europ. J. Agronomy 31: 144–152. http://www.organicconsumers.org/documents/huber-glyphosates-2009.pdf
Huber, DM (2010) Ag chemical and crop nutrient interactions – current update. http://www.calciumproducts.com/dealer_resources/Huber.pdf
Reviewed in (Broken Link!) http://www.weeds.iastate.edu/mgmt/2010/glyMndisease.pdf
[30] Kopsell et al. (2009) increase in nutritionally important sweet corn kernel carotenoids following mesotrione and atrazine applications. Journal of Agricultural and Food Chemistry 090619124509017 DOI: 10.1021/jf9013313
[31] Laws, F (2010) http://cornandsoybeandigest.com/issues/will-glyphosate-fall-wayside-resistance-grows
[32] http://www.nationalaglawcenter.org/assets/crs/RS20759.pdf
[33] http://www.ars.usda.gov/is/AR/archive/jul12/July2012.pdf
[34] http://www.misereor.org/fileadmin/redaktion/MISEREOR_no%20till.pdf
[35] http://www.acresusa.com/toolbox/reprints/Organic%20weed%20control_aug02.pdf
[36] http://www.seedalliance.org/Seed_News/SeminisMonsanto/
[37] (Broken Link!) http://earthopensource.org/files/pdfs/GMO_Myths_and_Truths/GMO_Myths_and_Truths_1.3a.pdf
[38] Blakeney, M (2011) Trends in intellectual property rights relating to genetic resources for food and agriculture. http://www.fao.org/docrep/meeting/022/mb684e.pdf This document covers the debate involved in international patent law regarding plants and animals.
[39] Philpott, T (2008) A reflection on the lasting legacy of 1970s USDA Secretary Earl Butz. http://grist.org/article/the-butz-stops-here/; but for a contrary view by an actual corn farmer, read Hurst, B (2010) No Butz About It. (Broken Link!) http://www.american.com/archive/2010/july/no-butz-about-it
[40] Howard, Philip H. 2009. Visualizing consolidation in the global seed industry: 1996–2008. Sustainability, 1(4), 1266-1287. http://www.mdpi.com/2071-1050/1/4/1266/pdf
[41] http://www.monsanto.com/investors/Documents/Whistle%20Stop%20Tour%20VI%20-%20Aug%202012/WST-Fraley_RD_Update.pdf
[42] http://academicsreview.org/reviewed-individuals/jeffrey-smith/
[43] http://responsibletechnology.org/docs/145.pdf
[44] http://academicsreview.org/reviewed-content/genetic-roulette/
[45] Greenberg, S, et al (2012) Economic and Environmental Impact Transgenically Modified Cotton Comparative with Synthetic Chemicals for Insect Control. Journal of Agricultural Science and Technology B 2 750-757.
[46] Greenpeace (2009) Smart Breeding. Marker-Assisted Selection: A non-invasive biotechnology alternative to genetic engineering of plant varieties. http://www.greenpeace.org/australia/PageFiles/348427/smart-breeding.pdf
[47] http://www.genengnews.com/gen-articles/in-defense-of-gene-patenting/2052/
[48] Gold 2002 Misconceptions about the causes of cancer http://potency.berkeley.edu/pdfs/Gold_Misconceptions.pdf
[49] Entine, J (2010) Crop Chemophobia: Will Precaution Kill the Green Revolution? http://www.jonentine.com/pdf/CROPCHEMOPHOBIApre-orderform.pdf
[50] Howard, Philip H. 2009. Visualizing consolidation in the global seed industry: 1996–2008. Sustainability, 1(4), 1266-1287. http://www.mdpi.com/2071-1050/1/4/1266/pdf
[51] http://www.greenpeace.org/australia/PageFiles/348427/smart-breeding.pdf
[52] http://www.monsanto.com/newsviews/Pages/roundup-ready-patent-expiration.aspx;
[53] http://www.cotton247.com/article/3401/monsanto-donates-marker-technology
[54] http://www.youtube.com/watch?v=dcZyFH_eITQ
[55] http://www.biofortified.org/2012/05/the-frustrating-lot-of-the-american-sweet-corn-grower/#more-8670
[56] http://ngm.nationalgeographic.com/2011/07/food-ark/food-variety-graphic If you didn’t see this graphic in National Geographic, you should!
[57] (Broken Link!) http://researchnews.osu.edu/archive/mexmaize.htm
[58] http://www.huffingtonpost.com/2011/08/20/genetically-modified-papayas-attacked_n_932152.html
[59] http://en.wikipedia.org/wiki/Golden_rice
[60] http://westernfarmpress.com/blog/proposition-37-gone-probably-not-forgotten?
[61] http://appliedmythology.blogspot.com/2012/10/can-damage-from-agenda-driven-junk.html?utm_source=feedburner&utm_medium=email&utm_campaign=Feed%3A+AppliedMythology+%28Applied+Mythology%29
[62] http://www.science20.com/michael_eisen/how_bt_corn_and_roundup_ready_soy_work_and_why_they_should_not_scare_you
First published in: ABJ November 2012
Updates
Geomagnetic Flux
Driftwatch
Back to the Suspects for CCD
Ag Exposure
A Pesticide-Free Control Group
CRP Lands
Good News
Acknowledgements
References
Sick Bees Part 18D: Colony Collapse Revisited
Agricultural Exposure
Randy Oliver
ScientificBeekeeping.com
Originally published in ABJ November 2012
“It’s what you know for sure that keeps you from learning.”
And I’m all about learning. I’d like to make it perfectly clear that I do not consider myself to be the final arbiter on any matter! In investigating many of these controversial subjects, my brain feels like a GPS unit, repeatedly saying, “Recalculating” and sometimes even “Turn around when possible.” This is why I take care to hold no positions, and appreciate being intelligently challenged on any point. If something comes to my attention that makes me rethink or correct anything I’ve written, I am more than happy to rebut myself on these pages.
Updates
Geomagnetic Flux
In my last article I dismissed geomagnetic flux as the cause of CCD, but also said that I was corresponding with proponent Dr. Tom Ferrari. A point that he recently made is that the timing of a flux event is critical—it must occur during, or immediately before flight hours. Although I still have a healthy skepticism about solar flares causing colony collapse, I am keeping an open mind that they may indeed affect bee homing ability, and could plausibly contribute to forager loss.
Driftwatch
In a recent article, I put in a good word for Purdue’s Driftwatch program [1], based upon the positive feedback that I had gotten from some beekeepers. However, I wish to thank beekeeper Jeff Anderson for bringing to my attention legitimate concerns about its uncritical promotion by state agencies.
First, some background on pesticide regulation. Pesticides are registered and labeled at the federal level by the EPA. States must follow those labels–they may impose further restrictions, but not fewer. In general, the state has primary authority for monitoring pesticide applicators to ensure that they comply with label restrictions, and is charged with the responsibility to take enforcement action in the case of violations (as in those resulting in bee kills). The EPA refers to the states as “primacy partners”; each of which may use a “state lead agency” (such as its department of pesticide regulation, agriculture, or environment to enforce the law [2].
A problem may occur when a state writes pesticide use guidelines for the protection of honey bees (and other pollinators). Pesticide applicators may put pressure on the local primacy partner to shift the responsibility of pollinator protection from the EPA and the applicator onto the shoulders of the beekeepers. If guidelines are written to suggest that beekeepers should register their apiary locations, and that applicators about to spray should then notify those beekeepers, the applicators may get the misimpression that such notification absolves them from their responsibility to carefully adhere to label restrictions, especially if there is any wording about the beekeeper moving or covering his hives. Commercial beekeepers strongly object to any suggestion that they be forced to “duck and cover”! And, a beekeeper may be adverse about putting his prime locations into a public database, which might result in some unscrupulous beekeeper moving in right on top of him!
In many places, conscientious applicators do indeed work constructively with beekeepers, and I’ve had them give me courtesy calls to discuss potential spray issues. As much as I appreciate that sort of cooperation, a large commercial beekeeper simply has too many locations, and not enough time to negotiate with every applicator who might be spraying within flight range of every one of his yards. It’s not the beekeeper’s job to be a pesticide expert–that’s the responsibility of the applicator!
The fact is that the EPA label restrictions are designed to protect pollinators, and if the restrictions are carefully followed, the beekeeper theoretically should need not ride herd on every pesticide applicator (Fig.1).

Figure 1. A grower spraying fungicide onto almond trees, and the understory weeds, each in full bloom. This sort of application is permitted by the label, and generally has only minor impact upon bees. However, EPA is closely following recent research on adverse effects of both fungicides and their adjuvants upon colony health.
Practical application: voluntary programs in which beekeepers may register their apiary locations to be notified by applicators can be of benefit (it works for me in my county), and a beekeeper may well wish to negotiate with an applicator about to make a lawful application. But beekeepers must be careful about allowing any “hot button” words involving the moving or covering of hives to be institutionalized in state guidelines, lest applicators get the misimpression that they can then ignore restrictions such as “Do not use on flowering crops or weeds” if they have notified the beekeeper, or that it is then the beekeeper’s responsibility to protect his hives from pesticide misapplication.
Back to the Suspects for CCD
Ag Exposure
Biological plausibility: plausible due to nutritional or pesticide issues.
Honey bees and farming have one major aspect in common—they both prosper on fertile, moist land. Prime bee forage land and prime agricultural land are one and the same. As it is, much of the world’s best acreage for bee forage has been converted to intensive agriculture, often dedicated to the cultivation of a single species of plant.
I’ve had beekeeper after beekeeper tell me how colonies summered on agricultural cropland often go downhill, or don’t make it through the winter. These anecdotal reports are supported by data from at least two studies:
- I mentioned a couple of months ago Dr. Erickson’s demonstration that colonies exposed to permethrin-sprayed corn died during the following winter.
- The Coordinated Action Project’s data for 2009-2012 [3] found that the proportion of land in intensive agriculture within 2 miles of the apiaries correlated with colony mortality. Although pesticides were obvious suspects, the study surprisingly did not find any particular correlations between pesticide levels in trapped pollen and amount of ag exposure, nor any correlations between pesticide exposure and colony mortality!
Curious as to whether recent colony losses (Fig. 2) correlate with the degree of exposure to commercial agriculture (Fig. 3), I checked the National Agricultural Statistics to find which states had the greatest percentages of their land areas in various crops.
Figure 2. Recent colony mortality rates for surveyed states. Compare the apparent correlation between those areas with high loss rates (dark states) with the types of cropland in the following map. Copyright the International Bee Research Association. Reproduced from [[i]] the Journal of Apicultural Research (2011) Issue 50(1): 1-10 by the permission of the Editors.
[[i]] vanEngelsdorp, D, et al (2011) A survey of managed honey bee colony losses in the USA, fall 2009 to winter 2010. Journal of Apicultural Research 50(1): 1-10.
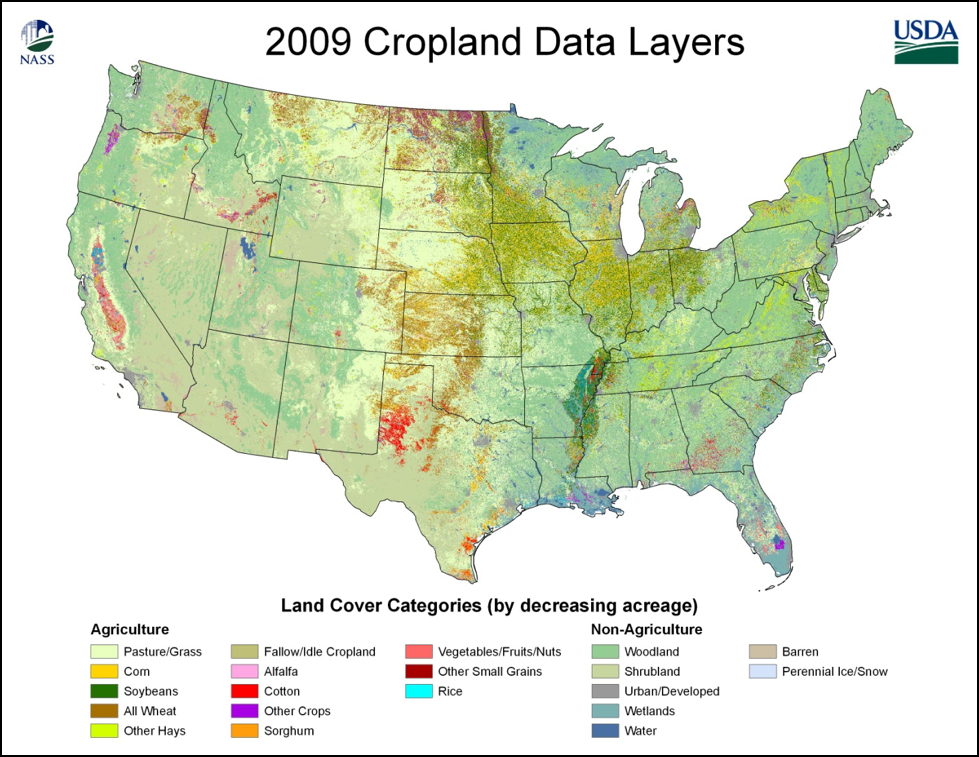
Figure 3. The scope of the impact of farming practices is staggering–roughly 2/3rds of the land area in the entire states of Iowa and Illinois, and half the footage of Indiana and North Dakota, are planted to principal crops (a small amount is pasture). Source http://www.nass.usda.gov/research/Cropland/cdl09_l.jpg
It’s hard to compare the two maps above directly, since beekeepers move hives, and the colony mortality data is very crude by comparison (only to the state level) to the cropland map. However, one can’t help but see that colony mortality appears to be higher in corn, soy, and cotton areas.
As an aside, in researching this subject, I found that even more detailed interactive maps are available from the NASS (Fig. 4):
Figure 4. I created the above map to scout for alfalfa locations (in pink) in a small area of Nevada. The detail of these maps is amazing! Check it out at http://nassgeodata.gmu.edu/CropScape/.
Nutrition
The first thing about agricultural land and bees that generally comes to mind is the impact of pesticides, which I will return to later in this series. However, one must not ignore another important effect of crop monoculture—its impact upon bee nutrition:
“One impact of large-scale agriculture with extended expanses of a single cultivated crop species to honey bees is the availability of pollen, which is the only source of proteins and lipids in the bee diet and thus crucial for their survival and development. Agricultural trends toward larger monoculture farming systems can place pollinating honey bees in situations where they have a restricted choice of dietary pollen” [5].
So what is wrong with a “restricted choice” of pollen? Some “monolectic” species of solitary bees are specialized to feed solely on a single type of pollen (mono = single; lect = to gather). Honey bees, on the other hand, need to collect pollen throughout the season, so must by necessity be “polyletic” since no single plant species blooms for that long. Some pollens (almond, mustards, apples, red gum, etc.) are plentiful and nutritionally complete, but a number (corn, sunflower, blueberry, citrus, pumpkin) are not. If you’ve ever trapped pollen, you’ve noticed that pollen foragers bring home a medley of pollen types, thus increasing their chance of obtaining all necessary nutrients.
In agricultural areas, despite there being vast fields of single species of plants in bloom, bees still go out of their way to collect a diversity of pollens. Dr. Jerry Bromenshenk (in prep) has surveyed pollen loads in agricultural areas for the past few years. In corn country during tassling, he found that on average, corn pollen still constituted less than 25 percent of pollen loads. This is not surprising if you think about it, since if the bees in those areas are producing honey, they sure aren’t getting it from corn, so must have located other forage! When I visited apiaries in the Midwest, I surveyed their surroundings from the ground and via Google satellite maps–it appears that bees are remarkably efficient in finding little patches of good forage scattered among vast seas of corn and soybeans!
Another aspect of commercial crops is that plant breeders select for yield per acre, not for plants that produce nectar or nutritious pollen. Beekeepers report vast differences in bee response to different cultivars of several crops.
Practical application: Colonies may go downhill on certain crops due to poor pollen nutrition; they then need better forage in order to recover. Recent research found that colonies subsisting solely on corn pollen rear less brood, and have shorter worker lifespans [6]. Such colonies cannot be expected to winter well.
However, so long as alternative forage is available, bees may fare well in agricultural areas, provided that they don’t take a hit from pesticides.
A Pesticide-Free Control Group
I will return in a subsequent article to the impact of pesticides, but for now let me say that the few studies that have looked at pesticide levels in beebread do not clinch the case for pesticides being the only problem for bees in ag areas [7].
My apiaries often serve as a “control group” with regard to pesticides, since I avoid (other than in almonds) areas in which pesticides are used. Yet I still experience, in some locations, poor buildup, late summer dwindling, and poor winter survival. A case in point is a pumpkin pollination contract that I had for several recent years in an area of Nevada surrounded by desert (Fig. 5).
Figure 5. I pollinated 40 acres of pumpkins grown in this irrigated oasis in the middle of the desert. The only other “green” is a sod farm (most of the rectangular checks) and some center pivot alfalfa. No pesticides were used in this valley, yet colonies still fared poorly. Imagery from Google maps, ©DigitalGlobe, GeoEye, USDA Farm Service Agency, TerraMetrics.
The only forage available was 40 weedy acres of pumpkins, quite a bit of irrigated alfalfa, and natural Rabbitbrush in fall. Pesticides were not used anywhere in the valley. I’d move strong colonies in each July, heavy with stores, treated for mites, and with a 3-lb chunk of pollen supplement. The poor bees experienced boom and bust situations (mostly bust). The colonies simply starved on the forage provided by the pumpkins and weeds between alfalfa blooms (typically two blooms), and I generally had to resort to emergency open drum feeding and pollen supplement to keep them alive. Depending upon how much alfalfa was under irrigation that year, they might be able to rally and fill the combs with honey, or not. Some years they would rebound somewhat when Rabbitbrush came into bloom, but generally not enough to build up for winter.
In the above example, my 40 colonies were the only ones on about two square miles of irrigated green cropland. But there is no way that they could have survived on their own. There simply wasn’t enough consistent mixed forage to support them.
Living at the Edge and on the Edge
Unlike in natural areas, in which pollen and nectar flows transition fairly gradually, on agricultural lands they can be cut off in a matter of hours (just watch how fast a modern swather takes down a field of alfalfa in full bloom—breaks your heart). A flow can suddenly end when fields are tilled, when flowering weeds are mowed or burned off with an herbicide, or when every plant in a crop finishes blooming all at the same time. The bees are then forced to forage at the edges of the fields (Fig. 6).
Such a sudden cessation of food intake can also quickly bring a colony to the edge of serious protein deficit (not to mention the lack of nectar). The nurse bees in such a stressed colony must immediately deal with all the protein-hungry brood and foragers, and the colony must shift to survival mode.
The more I study bees, the more it appears to me that colonies are often living right on the edge of disaster. As I pointed out with my growth rate graphs [8], normal colony growth requires phenomenal production of brood, with a complete turnover of the summer population about every 5 weeks. Colonies can go from boom to bust in a matter of days if nutritious pollen and nectar become unavailable. Colony immunocompetence falters and broodrearing is curtailed, setting up conditions for varroa, nosema, or viruses to explode.
Practical tip: Here at Samemistaketwiceagain Apiaries, we find that as with many management issues involving bees, being proactive is much more cost effective than being reactive—it’s easier to maintain colony momentum than to restart them after they’ve come to a halt! A little supplemental feeding during dearth can go a long way towards healthier colonies.

Figure 6. The bee in the center of this photo is foraging at the edge of a cornfield that is weed-free after being sprayed with Roundup. The clover on the margins, and whatever grows in the patches of woods, is the only forage between here and the horizon. Modern farming practices greatly reduce the amount and diversity of bee forage. Photo courtesy of beekeeper Larry Garret.
Now add the pesticide component
When the forager force suffers attrition due to pesticides—although perhaps not enough to cause piles of dead bees in front of the hives—this will both reduce incoming nectar and pollen, plus force younger bees to take the places of the poisoned foragers. A strong colony full of sealed brood may be able to rebound from one hit to the foragers, but not from repeated hits, or from a hit late in the season.
Or, pesticide residues in stored pollen or nectar might negatively affect brood survival, harm the nurse bees (due to their eating so much pollen), decrease resistance to parasites, or shorten winter bee longevity. This may be especially true with the cocktail of insecticides, fungicides, and surfactants sometimes found in beebread.
During dearths (and in fall), a colony that shuts down into survival mode due to lack of pollen can generally stick it out until it can rebound when another nectar and pollen flow starts. However, the beekeeper should be aware that when a colony cuts back its population, the relative rate of infestation by varroa can quickly skyrocket! And if a colony in such a condition is then exposed to what would normally be a minor hit by pesticides, the negative effects can be greatly exacerbated.
Practical application: if, due to lack of alternative flora, bees are forced to forage solely on agricultural crops, then they may be exposed to pesticide residues that they would normally avoid, or store larger proportions of nutritionally-incomplete pollens (such as from field corn). Extension apiculturists have long pointed out that feeding colonies pollen supplement may help to mitigate the above problems. This is especially true in late summer as colonies suffer from the triple whammy of normal downsizing, poor nutrition, and rapidly rising varroa infestation rates.
CRP lands
Beekeeper Zac Browning explains that large-scale commercial beekeepers are having a tough time finding safe places to park their hives during the summer: “We’re limited to the fringes of rural America, where we can stay away from pesticides, where we can find wildflowers.”
One of the most popular places to look for locations has been on Conservation Reserve Program lands, for which farmers are paid by the government to convert cropland to long-term vegetative cover for the benefit of the environment. These lands in the northern states are often planted to clover or legumes, thus providing excellent forage for pollinators. As a result, commercial migratory beekeepers flock there during summer (Fig. 7).
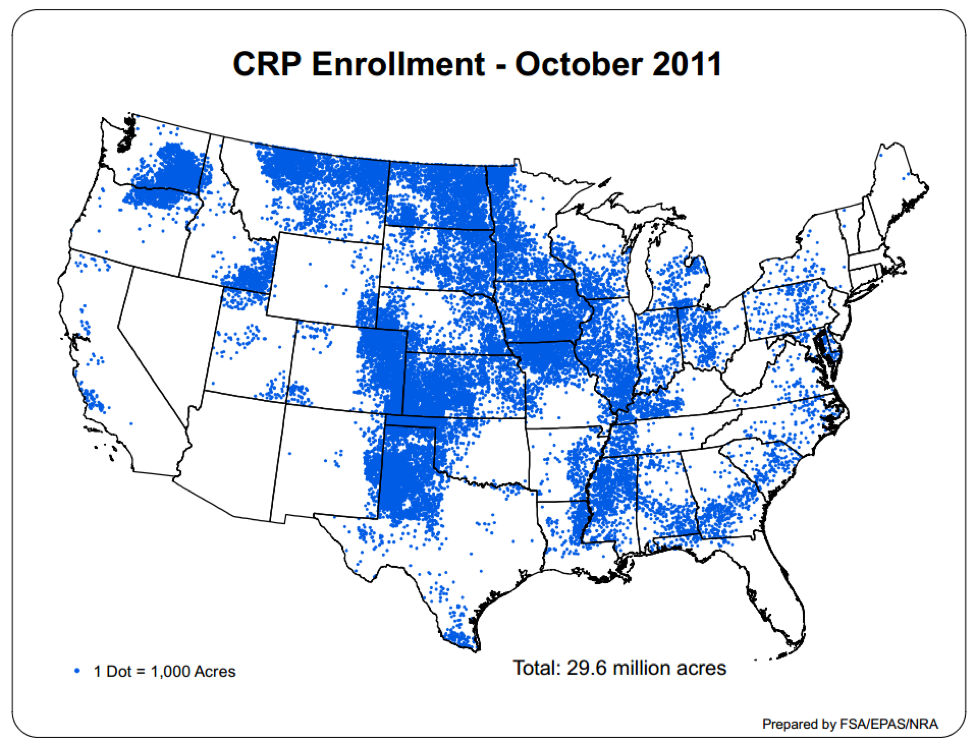
Figure 7. CRP lands often provide good bee forage. Over a third of commercial hives spend the summer in just three states—Montana and North and South Dakota [[i]]. Map from USDA [[ii]].
[[i]] http://usda01.library.cornell.edu/usda/current/Hone/Hone-03-30-2012.pdf
[[ii]] http://www.fsa.usda.gov/Internet/FSA_File/crpenrolloct11dot.pdf
But with the high prices currently being offered for agricultural commodities, farmers are converting bee-friendly CRP land to monoculture cropland, putting the hurt on beekeepers. I don’t expect this situation to improve.
Good news
Press release: August 29, 2012
Portland, Ore.— Last Friday Agriculture Secretary Tom Vilsack announced that the Xerces Society for Invertebrate Conservation, along with collaborating bee researchers, will receive a $997,815 USDA Natural Resource Conservation Service Conservation Innovation Grant to improve pollinator habitat on farms and ranches across the U.S.
Through this project the researchers and conservationists hope to answer questions such as how to best manage wildflower meadows on the edge of farms as long term pollinator habitat, how to control weeds in such pollinator meadows using organic techniques, and how to quantify the effectiveness of various types of flowers in supporting crop-pollinating wild bees and honey bees. Another part of the project will work with native plant nurseries to mass-produce wildflower seed for plants with high pollen and nectar value that are not currently available among the nursery industry.
OK, the above sounds pretty idealistic, but beekeepers can certainly encourage these sorts of efforts to increase pollinator habitat on agricultural lands. Europe has a leg up on us in this direction, and can serve as an example [11]. Many landowners are willing to manage their lands for the benefit of wildlife, including pollinators. There is currently great support for such efforts across the political spectrum; beekeepers should certainly get on board the bandwagon!
Ag Exposure and CCD Conclusion
Colonies in agricultural lands often do not fare as well as those in favorable natural settings. It is not yet clear how much of the problem is due to pesticides or other factors, but the lack of diverse nutritional sources is a prime suspect.
Small-scale beekeepers may have thriving hives in agricultural areas in which large-scale beekeepers report problems. This observation suggests that hobbyists may have better luck in finding good apiary locations, perhaps since they don’t need to unload truckloads of hives.
Agricultural exposure does not fulfill Koch’s postulates as being the cause of CCD, but may well be a contributory factor in colony mortality and collapse.
To be continued…dare I broach the subject of GMO’s?
Acknowledgements
As always, I am immensely indebted to my partner in research, Peter Loring Borst. I wish to thank the hardworking members of our national associations (the National Honey Bee Advisory Board and the AHPA/ABF/EPA beekeeper pollinator protection work group members) who are representing beekeepers’ interests in Washington and at the state level. Darren Cox, Jeff Anderson, Dave Mendes, Steven Coy, and Rick Smith have been especially generous with their time in explaining the politics to me.
References
[1] http://www.driftwatch.org/
[2] http://www.epa.gov/opp00001/enforcement/monitoring.htm
[3] Drummund, F, et al (2012) The First Two Years of the Stationary Hive Project: Abiotic Site Effects. http://www.extension.org/pages/63773/the-first-two-years-of-the-stationary-hive-project:-abiotic-site-effects
[4] vanEngelsdorp, D, et al (2011) A survey of managed honey bee colony losses in the USA, fall 2009 to winter 2010. Journal of Apicultural Research 50(1): 1-10.
[5] Chauzat, M-P, et al (2009) Influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environ. Entomol. 38(3): 514-523.
[6] Höcherl, N, et al (2012) Evaluation of the nutritive value of maize for honey bees. Journal of Insect Physiology 58(2): 278–285.
[7] Thompson, HM (2012) Interaction between pesticides and other factors in effects on bees. http://www.efsa.europa.eu/en/supporting/doc/340e.pdf
[8] https://scientificbeekeeping.com/sick-bees-part-17-nosema-the-smoldering-epidemic/
[9] http://usda01.library.cornell.edu/usda/current/Hone/Hone-03-30-2012.pdf
[10] http://www.fsa.usda.gov/Internet/FSA_File/crpenrolloct11dot.pdf
[11] Wratten, SD, et al (2012) Pollinator habitat enhancement: Benefits to other ecosystem services. Agriculture, Ecosystems & Environment 159: 112–122. Good bibliography of research papers on the subject.
First published in: ABJ October 2012
Sick Bees Part 18c:
Colony Collapse Revisited
Randy Oliver
ScientificBeekeeping.com
Originally published in ABJ October 2012
Beekeeper Management Practices
Environmental Factors
Ozone and Air Pollution
HAARP (The High Frequency Active Auroral Research Program)
Cell Phones/Electromagnetic Radiation (EMR)
Geomagnetic Flux
Chilling
WEATHER AND CLIMATE CHANGE
HABITAT CONVERSION
Summary So Far
Acknowledgements
Further Reading
Citations
Sorry for my recent diversion from this series while I addressed the neonicotinoid insecticides. Please allow me to continue with my retrospective analysis of the suspects suspected or implicated, rightly or wrongly, to be the cause of CCD.
In the previous article of this series, it seems that I hurt the feelers of some beekeepers by irreverently dismissing some of their pet suspects for Colony Collapse. Unlike during the peak of CCD, when researchers were desperately following up every possible lead, I have the advantage of retrospection, as well as now having had the opportunity to observe and study sudden colony depopulation again and again.
However, before I get to the real meat of CCD, I feel that it would be to the benefit of the reader to cut through the muddle of all the various things that have been blamed for being the cause of the disorder by evaluating each of them using scientific logic.
Although most named suspects are biologically plausible, a number can be quickly ruled out by the application of Koch’s postulates—either that suspect isn’t always present during collapse events, or doesn’t always cause the problem when it is present. I’ll address the suspects in the following order (spoiler: I’m saving the best for last).
- Beekeeper management practices
- Environmental factors
- Chemical exposure
- Biological agents
Although my focus will be upon determining the “proximate causative agents” of sudden colony collapse, I’ll also examine how the various suspects may contribute to colony mortality or morbidity in general. In order to pick up the thread of this series, and to review the terms that I’ll be using, you may wish to reread the previous installment (available at my website1.
Beekeeper Management Practices
Due to his being a government employee, Colony Collapse researcher Dr. Jeff Pettis, unlike me, must be careful that his public statements are politically correct. But in his presentations on CCD, he can’t help but candidly note that 25% percent of beekeeping operations accounted for fully 75% of total colony losses. And any apiary inspector will tell you that it was usually the same beekeepers over and over. This fact certainly suggests that beekeeper management practices may be related to the degree of colony mortality in an operation.
Let me be perfectly clear here—CCD can happen to anyone, and there is nothing funny about it. If you’ve never watched the film “The Last Beekeeper,” you should. In it, hard working beekeepers are brought to tears as they watch their operations collapse from CCD just prior to almond bloom—leading to their financial ruin. I’ve experienced CCD myself, and wouldn’t wish it upon anyone!
That said, CCD also quickly became an excuse for absolving oneself of the consequences of PPB (Piss Poor Beekeeping). I’ve heard many a beekeeper who knew damn well that his colonies died from varroa or some other form of neglect, later piously tell a reporter that they were hit by CCD!
Here’s the thing: I’ve visited beekeepers all over the country. Even in areas in which some “noisy” beekeepers blame their elevated colony losses upon pesticides, the weather, the alignment of the stars, or some other factor, there are always other “quiet” beekeepers who experience very low losses under the same conditions. The difference could be luck, but more often appears to be due to better management.
Biological plausibility: Allow me to quote from the original CCD report, back when it was still called “Fall Dwindle Disease”2:
“All [affected operations] experienced some form of extraordinary “Stress” at least 2 months prior to the first incidence of “die off” associated with “Fall dwindle disease”. The nature of this stress was variable but included nutritional stress (apiary overcrowding, pollination of crops with little nutritional value), dramatic pollen and nectar dearth, or varroa mite pressure.”
Honey bee colonies can handle a lot of insults so long as they get enough high-quality forage (Fig. 1) to maintain vigorous broodrearing, and are not hamstrung by parasites. Those beekeepers who make sure that their colonies are always well fed, especially with protein, and never allow varroa infestation to exceed a few percentage points, appear to have far fewer problems than others. In some areas, treatment against nosema also appears to help.

Figure 1. One common denominator for healthy colonies is that they have year ‘round access to high quality forage. A profusion of pollen sources promotes strong broodrearing and a robust immune system. In the arid West, patches of irrigated pasture such as this are precious, and you’d no more tell other beekeepers where you found them than you’d brag about where your favorite fishing hole was.
Analysis:
Transportation stresses from migratory beekeeping—non beekeeper bloggers love to blame CCD on our “unnatural” moving of bees from one location to the next. In truth, bees, due to their innate ability to reorient to a new location after swarming, seem to take being moved in stride. In my own operation, I follow the bloom up the mountains during the season, akin to moving other livestock to better pasture, and my colonies are the better for it. Even colonies moved from the East Coast to California and back do not appear to suffer greatly from transportation.
Multiple pollination contracts—If bees are moved from one pollination contract to another, they may suffer from poor nutrition and exposure to pesticides. This problem can generally be mitigated by supplemental feeding (Fig. 2) or by “resting” them on natural pasture to rebuild.

Figure 2. One management practice that beekeepers learned from the CCD experience is the value of feeding supplemental protein. There are several high quality pollen supplements now on the market. Not only can good nutrition boost the bees’ immunocompetence, but the colony can convert this protein into replacement bees to take the place of those lost due to disease or pesticides.
Feeding of high-fructose corn syrup–HFCS has been blamed with little supportive evidence. Granted, HFCS can become toxic to bees due to the formation of hydroxymethylfurfural if it is overheated or stored for long periods in metal containers, but most commercial beekeepers are now aware of this. No correlation has been found between the feeding of HFCS and CCD.
Overcrowding of locations—just as other livestock cannot thrive if they are overstocked onto insufficient pasture, too many hives in one area compete for limited resources. If a beekeeper places a hundred hives into an apiary that has adequate forage for only two dozen, he can expect those colonies to have problems.
Holding yards—the need to stockpile semi loads of hives at certain times of the year can create serious problems due to:
- The nutritional stress due to inadequate forage as mentioned above.
- The behavioral stress caused by the robbing pressure between overstocked colonies.
- The fact that crowded bees all “share spit” in nearby flowers and at water holes. Need I explain the consequences?
Easy transmission of virulent strains of pathogens (especially the constantly-mutating viruses) that may spontaneously arise in one or more hives (the more hives, the more chance of the evolution of a new strain). Drifting, robbing, and hitchhiking varroa mites can quickly spread that pathogen throughout the entire yard (Fig. 3)! In a number of instances, beekeepers observed CCD spreading from one group of sick colonies to adjacent holding yards.

Figure 3. Perfect conditions for the spread of an epidemic. In this almond orchard, as with many monocultures, there is virtually no bee forage prior to, or immediately after the bloom, resulting in colony nutritional stress. Even in good honey locations, beekeepers must keep in mind the forage conditions at times other than during the flow.
Other commercial beekeeping practices—some “natural beekeeping” advocates have blamed the use of antibiotics, synthetic miticides, or sugar feeding for CCD, but these practices were common prior to CCD, and are used in many operations that have not experienced CCD, so the charges simply don’t stick.
Verdict: I’m not buying the notion that CCD can be blamed on commercial beekeeping practices per se, since no particular practice is always associated with colony collapse, nor does any particular practice always create it. But poorly managed colonies–whether in a large commercial or small organic operation–appear to be more susceptible to mortality or collapse. Good bee husbandry—including proper nutrition and parasite management—goes a long way toward keeping colonies healthy. One need only note how commercial beekeepers were able to ramp up their colony numbers for almond pollination when the growers made it financially worthwhile for them to invest in good management practices!
Environmental Factors
OZONE AND AIR POLLUTION
Biological plausibility: Ozone is highly reactive chemically, and oxidizes organic molecules. “The scent molecules produced by flowers in a less polluted environment, such as in the 1800s, could travel for roughly 1,000 to 1,200 meters; but in today’s polluted environment downwind of major cities, they may travel only 200 to 300 meters…This makes it increasingly difficult for pollinators to locate the flowers.”3
Analysis: Although the negative effect of ozone upon bee foraging success is biologically plausible, neither the timing nor location fit the sporadic occurrence of CCD. The timing is wrong, since ozone levels (and general air pollution) in the U.S. have actually been dropping since the early 1990’s and ozone levels showed a notable decline after 2002.4 Neither does location fit, since CCD occurred in rural areas with little ozone, and conversely is not normally a problem in my area of the Sierra foothills, which often (and unfortunately) has one of the highest ozone levels in the country due to the smog blowing up from Sacramento (easy to confirm, since the ozone quickly destroys anything made of rubber).
Verdict: Although a high ozone levels certainly doesn’t make life any easier for bees (or beekeepers), it does not appear to be the cause of colony collapse.
HAARP (THE HIGH FREQUENCY ACTIVE AURORAL RESEARCH PROGRAM)
HAARP is a favorite of conspiracy theorists, and one website5 presents a convincing case that the high frequency transmissions are the cause of CCD. The hypothesis is that the transmissions are interfering with the bees’ navigational ability. I’m not being frivolous here–some earnest beekeepers implored me to investigate the facts.
Biological plausibility: In brief, the HAARP antenna array in Alaska is a cooperative military/academic experimental station that shoots strong electromagnetic pulses into the ionosphere. Either the resulting wavelengths of light produced in the ionosphere above the station, or the extra low frequency (ELF) radio waves transmitted around the globe could plausibly interfere with the bee navigation system.
Analysis: The emitted electromagnetic energy pulses from HAARP are dwarfed by the natural atmospheric electromagnetic radiation variation from the sun, and their strength drops off according to the inverse square law. At only 150 ft away from the antennas, it already falls within human safety standards. When I did the math, the strength of the signal by the time it finally reaches my apiaries in California would be less than a billionth of the intensity of that typically found near AM broadcast station antennas in many urban areas6.
Similar ELF waves are created by lightning bolts, which strike the Earth some 100 times per second. A single bolt can produce far more electromagnetic radiation that the entire 3600kW output of HAARP.7 And as far as the timing, HAARP started intermittent testing in 1994, but did not actually begin transmitting at full power until 2007, long after CCD started to be reported.
Verdict: The laws of physics and the timing appear to let HAARP off the hook as being the cause of CCD (the math doesn’t support it being the cause of earthquakes either).
The next two factors come under suspicion based upon the hypothesis that CCD is caused by bees being unable to find their way back to the hive, thus leading to sudden colony depopulation.
Cell Phones / Electromagnetic Radiation (EMR)
Biological plausibility: Bees produce tiny molecules of magnetite in their bodies, which they appear to use in navigation.[i] Electromagnetic fields could plausibly disrupt their ability to find their way back to the hive. Alternately, some bee tissues may resonate with certain wavelengths of EMR, leading to biological effects.
Analysis: The intermittent appearance of CCD does not match the steady proliferation of cell phone and other electromagnetic transmissions. More importantly, CCD occurred in areas in which you couldn’t get cell reception; conversely, plenty of apiaries thrived immediately adjacent to cell phone and radio towers, and under electrical transmission lines.
Verdict: Although the cell phone hypothesis certainly resonated with the public (and gave beekeepers fodder for a lot of jokes), there are more cell phone transmissions today than when CCD made the press, yet CCD has largely gone away. One thing to keep in mind with any alleged cause of CCD is that it should also explain the historical appearances of colony depopulations in the older literature—cell phones were not around then.
Geomagnetic Flux
Natural solar flares cause “geomagnetic storms” on Earth. Dr. Tom Ferrari8 has proposed that such storms may be the cause of CCD due to their effect upon bee magnetoreception, causing bees to lose the ability to find their way home.
Biological plausibility: The hypothesis that geomagnetic flux affects bee navigation is biologically plausible, and I have been corresponding with Dr. Ferrari, and have seen his supportive (unpublished) experimental data that forager return takes longer during solar flare events. Solar storms have also occurred as long as the Earth has existed, so could possibly explain historical colony depopulation events.
Analysis: The question boils down to whether CCD is actually caused by the inability of foragers to find their way back to the hive. If solar storms were indeed the cause of CCD, one would expect them to affect all colonies equally over a wide area in which the flux occurred during daylight hours, which does not happen. And since I began correspondence with Dr. Ferrari, I’ve paid particular attention to any news reports of major solar storms to see whether I could observe the resulting geomagnetic flux causing any noticeable depopulations of my apiaries—I haven’t.
Verdict: Although I find Dr. Ferrari’s experimental data to be of great interest, since it appears to indicate that bee navigation is indeed affected by aberrations in the geomagnetic flux, I do not find it to make a compelling case for being the cause of CCD. I do look forward, however, to seeing more research on this aspect of bee navigation.
Chilling
In many recent and historical instances of unusual colony mortality, an unexpected spring or fall chill preceded the event.
Biological plausibility: The unexpected chilling of a colony with brood requires the colony to ramp up its metabolism, which stresses bees already suffering from nosema or virus infections. Such chilling may also suppress the bee antiviral response.9 In addition, should the colony already have a low bee:brood ratio due to a virus or nosema infection, then a cold snap could result in the chilling of the brood, which can greatly shorten the subsequent life spans of those workers.10
Analysis: Dr. Bill Wilson observed in 1979 how “Disappearing Disease” tended to be associated with chill events:
“In the case of [Disappearing Disease]… the colonies frequently have gone through a period of nectar and pollen collection with active brood rearing. Then the weather has turned unseasonably cool and damp and remained adverse for from about 3 to 14 days. Such a situation usually occurs in early spring. During the inclement weather, the bee populations dwindle because the worker bees disappear from the hive leaving a “handful” of bees and the queen.” 11
A similar correlation between chill events and the occurrence of sudden colony depopulation has been noted again and again in the historical record, as it was with the first reports of CCD.12 In my own experience, I have repeatedly observed unseasonable chills to precipitate the sudden collapse of colonies infected with either nosema or viruses. 13
Verdict: There is a strong case to be made for unexpected chilling to contribute to colony collapse. The chilling is not the proximate cause of the exodus of the bees from the hive, but tends to precipitate the chain of events leading to colony collapse (see Sick Bees, Part 2).14
WEATHER AND CLIMATE CHANGE
Naysayers aside, the Earth’s climate appears to be warming, and such change is reflected in shifting weather patterns, which may affect bee forage. Dr. Eric Mussen15 has noted that in some areas of the California foothills, previously common native plants no longer supply fall forage.
Biological plausibility: The weather is well known to be a huge factor in colony survival, due to its indirect effect upon plant production of nectar and pollen, and the ability of bees to forage for them. Temperature extremes (hot or cold) also stress colonies. In addition, the weather appears to affect the levels of nosema and varroa. Climate change affects plant communities, which may then have either positive or negative effects upon pollinator populations—a drier climate may eliminate bee forage, whereas a warming climate would expand favorable bee habitat northward.
Analysis: Dr. Gordon Wardell16 gave an excellent presentation shortly after the first reports of CCD, in which he used weather maps to show how unusual weather patterns appeared to correlate with subsequent increased colony mortality. In certain areas, warm winter weather led to fruitless foraging and the using up of precious stores; in other areas, prolonged spring rains prevented necessary foraging for pollen. On the other hand, this year’s warm January appeared to be very beneficial to bees.
Verdict: Weather and climate change may well be associated with pollinator decline in certain areas, directly affect colony survival, and could well be contributing factors to CCD.
HABITAT CONVERSION
Biological plausibility: The seasonal buildup of colonies, and their health over the rest of the season, is largely a function of the availability of good mixed forage, which is best provided by the diverse plant communities naturally present in areas with fertile soil and ample water.
Analysis: Unfortunately, honey bees are in direct competition with humans for such habitats, as people convert fertile lands into cropland and towns. Habitat loss directly and clearly affects many species, including honey bees. This fact sets it apart from bee-specific factors such as varroa or beekeeper management practices.
It strikes me odd that when people think of the impact of farming upon bees that they focus upon pesticides. In truth, the most destructive annihilator of natural ecosystems is the act of tillage—the mechanical preparation of land for the growing of crops (Fig. 4).

Figure 4. Humans are the bees’ worst competitor, in that we destroy sustentative plant communities, and replace them with artificially-maintained monocultures that are virtual “bee deserts” for most or all of the year. Note the absolute annihilation of the natural plant and animal communities in the tilled cropland behind the tractor (which takes place even in organic agriculture).
I keep bees on several organic farms, and they are lovely to look at. But I choke when someone starts to wax poetic about organic agriculture being in harmony with nature. Try to explain that illusionary harmony to the unfortunate former denizens of the diverse ecosystem that existed previous to the clearing and tillage of that fertile land! In an acre of the former natural ecological community, there may have existed hundreds of species of plants and animals. When converted to farmland, you may be able to count the number of reestablished species on your fingers and toes. And those species of plants that are favorable to bees we generally refer to as weeds!
Habitat conversion to agriculture has changed the face of the most fertile lands on Earth. Unfortunately for the honey bee, the flora of converted lands, rather than being replaced with bee-friendly plants, are largely planted to crops that offer scant nutrition for pollinators (Fig. 5).
Figure 5. Land use in the United States. The yellow pie slices indicate the proportion of each area allocated to cropland–the most biologically productive acreage. Fully two thirds of that cropland is planted to only a handful of crops– corn, soy, hay, wheat, and cotton, which produce forage for bees for only brief periods, if ever. Sources: USDA, Economic Research Service calculations based on data from Major Uses of Land in the United States, 2007; http://www.census.gov/compendia/statab/2012/tables/12s0858.pdf.
Only a tiny proportion of cropland actually requires pollination by bees, but even that fact hardly makes it good bee habitat. Take almond orchards, for instance. Over half of all managed hives in the country are transported to supply the pollination needs of this crop. Why? Because bees can’t survive on land converted to almond orchards when the trees are not in bloom! The almond orchards represent over 1000 square miles of fertile California Central Valley land that becomes a “bee desert” for the 49 weeks of the year that the trees are not in bloom.
Farmers today are also moving away from their previous rotations of legume-rich (and bee friendly) pasture, upon which livestock were formerly put out to graze. The new model is to keep beef and dairy cattle in feedlots, bringing their food—in the form of hay, silage, and corn—to them. Compare the photos below that I took of two dairies in Indiana (Figs. 6 and 7).
 Figure 6. Dairies, such as this one in Indiana, traditionally allowed the cows to graze on legume-rich, bee friendly pasture, often rotated with corn or other crops. Compare the bee forage potential of this ground to that of the dairy below
Figure 6. Dairies, such as this one in Indiana, traditionally allowed the cows to graze on legume-rich, bee friendly pasture, often rotated with corn or other crops. Compare the bee forage potential of this ground to that of the dairy below

Figure 7. At this “modern” dairy corn will be grown for silage, and brought to confined animals in the name of “efficiency.” Note the distinct lack of bee forage in the foreground.
Newer beekeepers may not notice the effects of land use change due to the “shifting baseline syndrome”—in which we take for granted the current state of affairs, not knowing or remembering how it used to be. In this matter, the old timers (once you get past the “the older I get, the better I was” part) are a valuable resource of historical knowledge to which we can compare the situation of today. For example, I ran my hives to irrigated alfalfa for some 25 years, until the demand for high protein “dairy hay” caused the farmers to start cutting it at the slightest hint of bloom, greatly reducing the honey crop. Even so, since summer bee pasture is at such a premium in the West, it got to the point that I could throw a rock and hit another beekeeper’s hives at any of my long-held locations. So even though one would not see any particular change in land use in the area, those fields went from being my most productive locations to not being worth the effort to move bees to.
Verdict: Although habitat conversion is not likely the proximate cause of colony mortality, colonies stressed by lack of good forage are less able to cope with parasites, pesticides, overcrowding, and other insults. The conversion of meadows and other biologically productive lands to monocultures, the practice of fencepost-to-fencepost tillage and the elimination of hedgerows, “clean farming” requirements by food processors to remove extraneous animal habitat, the shift away from pasturing livestock, and the placing of fallow lands into cultivation, have all resulted in loss of bee forage. Such habitat change is the scientific community’s number one suspect for pollinator decline in general.18 It doesn’t directly cause CCD, but colonies that suffered from CCD often came from areas of poor forage. This physical elimination of food sources is likely a major cause in increased colony mortality worldwide, since malnourished colonies cannot thrive.
It will be difficult to reverse the trend, but land management practices can make farmland more pollinator friendly. A number of organizations worldwide are promoting such practices, and public pressure will greatly help to promote the conservation of biodiversity. See References for more information.
Summary So Far
None of the above discussion is revelatory, since this series is largely retrospective. However, I felt it necessary to grant some myths a dignified death. Next I’ll move onto some more contentious issues, such as agricultural exposure, GMO’s, and pesticides.
Acknowledgements
Thanks as ever to my friend and collaborator Peter Loring Borst for his untiring help in literature review. And thanks to all the researchers who perform the tedious hard work of investigating colony mortality—it is only through their efforts and helpful correspondence that I could attempt my methodical analysis of this subject.
All would be academic if it were not for the smart and hardworking professional beekeepers who keep me informed. My sons and I are continually learning how to better manage our own hives. My articles are simply a reflection of what goes through my head each day as I try to digest all the scientific research, and then apply it in a practical manner to our own operation.
Most importantly, thanks for the appreciation and support that I get from beekeepers large and small worldwide. We are all in this together.
Further Reading
OPERA (2011) Bee health in Europe – Facts and Figures http://www.pollinator.org/PDFs/OPERAReport.pdf One of the best overall objective reports, from a European think tank called OPERA. I highly recommend.
AFSSA (2009) Mortalités, effondrements et affaiblissements des colonies d’abeilles (Weakening, collapse and mortality of bee colonies). http://www.afssa.fr/Documents/SANT-Ra-MortaliteAbeilles.pdf This free download, translated into English, is an excellent overall review of colony mortality in Europe by the French Food Safety Agency.
Landscape enhancement for bees
Support beekeeper Kathy Kellison’s nonprofit Partners for Sustainable Pollination http://pfspbees.org/
Project Apism is working to get growers to plant bee forage in California http://projectapism.org/content/view/142/61/
Decourtye, A, E Mader, N Desneux (2010) Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 41: 264–277. Free download
Wrattena, SD, et al (2012) Pollinator habitat enhancement: Benefits to other ecosystem services. Agriculture, Ecosystems and Environment 159: 112– 122.
An excellent download for increasing pollinator habitat on farmland can be found at ftp://ftp-fc.sc.egov.usda.gov/NH/WWW/New%20England_NRCS_Pollinator_Tech_Note_FINAL.pdf
And to their credit, Syngenta has a program! http://operationpollinator.com
Citations
1 https://scientificbeekeeping.com/sick-bees-part-18b-colony-collapse-revisited/
2 vanEngelsdorp, D, et al (2006) “Fall-Dwindle Disease”: Investigations into the causes of sudden and alarming colony losses experienced by beekeepers in the fall of 2006. http://www.freshfromflorida.com/pi/plantinsp/apiary/fall_dwindle_report.pdf
3 McFrederick, Q.S., J.C. Kathilankal, and J.D. Fuentes (2008) Air pollution modifies floral scent trails. Atmospheric Environment 42:2336.
4 http://www.epa.gov/airtrends/aqtrends.html
5 (Broken Link!) http://d1027732.mydomainwebhost.com/articles/articles/HAARP%20Jamming%20Bees%20= %20CCD.htm
6 http://www.haarp.alaska.edu/haarp/faq.html
7 Bianchi, C and A Meloni (n.d.) Terrestrial natural and man-made electromagnetic noise. http://www.progettomem.it/doc/MEM_Noise.pdf
8 Reviewed by Wajnberg, E, et al (2010) Magnetoreception in eusocial insects: an update. http://rsif.royalsocietypublishing.org/content/7/Suppl_2/S207.full.pdf+html?sid=4cea0921-cf0e-48df-80f8-fe2206db6976
9 Ferrari, T.E. & A.B. Cobb (2011) Correlations between geomagnetic storms and colony collapse disorder. And Honey bees, magnetoreception and colony collapse disorder. http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011
10 Bailey, L (1969) The multiplication and spread of sacbrood virus of bees. Ann. App. Biol. 63: 483-491.
11 Medrzycki, F, et al (2010) Influence of brood rearing temperature on honey bee development and susceptibility to poisoning by pesticides. Journal of Apicultural Research 49 (1): 52-59.
12 Wilson, WT, and DM Menapace (1979) Disappearing disease of honey bees: A survey of the United States. ABJ March 1979: 184-186.
13 Dr. Jerry Bromenshenk, pers comm.
14 Oliver, R (2012) Sick Bees 18a—Colony Collapse Revisited https://scientificbeekeeping.com/sick-bees-part-18a-colony-collaspse-revisited/
15 Oliver, R (2010) Sick Bees – Part 2: A Model of Colony Collapse. https://scientificbeekeeping.com/sick-bees-part-2-a-model-of-colony-collapse/
16 Dr. Eric Mussen, pers comm.
17 Presentation to Western Apicultural Society, Tucson, AZ, 2007.
18 Blacquière, T (2010) Care for bees: for many reasons and in many ways. Proc. Neth. Entomol. Soc. Meet. 21: 35-41. http://edepot.wur.nl/185843
 Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.
Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.



















































