Reevaluating Beebread: Part 3 – For Preservation or Digestion?
Reevaluating Beebread: Part 3
For Preservation or Digestion?
Randy Oliver
ScientificBeekeeping.com
First published in ABJ Dec 2015
CONTENTS
Anderson’s Investigatory Approach
Previous Research
Identification Of The Microbes Involved (via DNA Sequencing)
Bacterial Abundance During The Fermentation Process
Nurse Bee Feeding Preference By Age Of Beebread
The Pollen To Microbe Biomass Ratio
The Degree Of Digestion Of Hive-Stored Pollen
Dr. Anderson’s Conclusions
Summary
Next
References And Notes
We’ve now identified the bacterial players. So let’s see what Dr. Kirk Anderson’s lab has found to date about why bees turn raw pollen into beebread.
In this article, I’m going to briefly summarize Dr. Anderson’s well-designed investigations [1]. My readers know that I can be critical of sloppy science; conversely, I feel that truly exemplary scientific research should receive kudos. The investigation by Kirk and his associates meets this standard, and is a textbook example of good scientific research and writing. Kirk laid out his investigation methodically, questioning each premise of the “predigestion hypothesis,” and seeing whether there was actual evidence either supporting or refuting the claim. I will quote from Kirk’s papers extensively in italics [2].
Anderson’s Investigatory Approach
Scientists typically specify the hypothesis to be tested and lay out their game plan:
It was hypothesized that co-evolved microbes orchestrate the long-term conversion of stored pollen into a more nutritious food source, a process involving microbial succession, anaerobic breakdown of materials, the release of pollen cell contents and/or predigestion by moulds. Here, we use a multifaceted approach to determine whether hive-stored pollen of honey bees involves significant nutrient conversion or ‘pre-digestion’ by microbes. To this end, we explore [reordered]
- whether the differences in bacterial richness and diversity between newly collected and hive-stored pollen are consistent with a preservation or nutrient conversion environment
- the absolute number of bacteria in stored pollen,
- the association between bacterial abundance and pollen storage time,
- the time period associated with pollen storage prior to ingestion by nurse bees,
- the pollen to microbe biomass ratio, and
- the degree of digestion of hive-stored pollen
Previous Research
Hypothesis to be tested: whether the fermentation of beebread requires inoculation of the pollen with symbiotic “core” gut bacteria via the crop as proposed by Vásquez and Olofsson [3]: “Our research has identified the bacteria involved and revealed that bees, in producing bee bread, add all the beneficial [lactic acid bacteria] to the pollen when they collect it at the site of the flower.”
Vásquez and Olofsson’s early research was very exciting, but they apparently overstated the case in claiming that bees added all the beneficial lactic acid bacteria to pollen, and especially that those bacteria came from the crop. Scientists already knew that pollen, and especially nectar, typically contains lactic acid and other bacteria prior to being visited by bees [4]. And subsequent research by others found that there were very few bacteria in the bee crop [5]. The question then is whether the pollen loads of returning foragers showed signs of being spiked with the core gut bacteria.
What Anderson found: In previous work, we determined that freshly-collected pollen from returning foragers or beebread contains incidental amounts of core hind-gut bacteria, suggesting that this core gut community does not contribute substantially to the conversion or preservation of pollen stores… This new finding contrasts markedly with the previous culture-dependent view…
Note: Dr. Anderson writes in a meticulous and precise scientific manner, appropriate for the journals in which he publishes (I again commend him for his exemplary backing up of each of his claims with accurate citations and supplemental data). It is polite scientific convention to say that a finding “contrasts markedly” with a previous view; a layman might say “man, did those guys get it wrong.”
Identification Of The Microbes Involved (via DNA Sequencing)
Hypotheses to test: if pollen is indeed inoculated by the bees with core gut bacteria in order to ferment it into beebread, then the bacterial species composition in beebread would shift towards core bacteria during the fermentation process.
Anderson found that: “The bacterial communities found in hive-stored pollen did not differ from those of newly collected pollen, but both sample types varied significantly by season.” He found that only a small percentage of the bacteria found in freshly-gathered pollen loads were core hind-gut bacteria (in some cases as little as 3/10ths of a percent). Even more telling was that fermented beebread contained an even lower proportion of core gut bacteria than did newly-collected pollen.
Instead, the most common bacteria found in beebread were strains of Lactobacillus kunkeei. This floral/fruit bacterium thrives in fructose-rich aerobic environments, producing lactic acid as a metabolic byproduct, which has the benefit of making its environment inhospitable to competing yeasts and bacteria. Bees create an ideal environment for L. kunkeei by enriching pollen with fructose-rich nectar–thus its rapid preservation of beebread by the process of acidic “pickling.” L. kunkeei is one of the few bacteria that can survive (perhaps in a dormant state) in honey and beebread, likely quickly rejuvenating when exposed to diluted nectar or honey in the crop.
Kirk and others [6]) have found that the bacterial community in bees may differ a bit from hive to hive, and by season or forage, but there is always a “core” hindgut biota (apparently passed via a fecal/oral route of some sort) [7]. The story is as yet far from complete, but it appears that honey bees have a commensal or mutualistic relationship with these core species, but also utilize free-living flower bacteria, such as L. kunkeei, to their advantage. An interesting recent study by Blum [8], working with the fruit fly Drosophila, points out that a host does not necessarily need to maintain a population of endosymbionts, but may instead continually replenish it via its food:
The Drosophila system may represent an alternative mutualism strategy that we term “quotidian replenishment,” which is intended to indicate the need for daily replenishment to obtain a consistent [bacterial] community in the animal. In this model, the symbiotic community in Drosophila is maintained through frequent ingestion from an external reservoir of bacteria [its food]… Furthermore, our study shows that one member of the microbiome, Lactobacillus plantarum, protects the fly from intestinal pathogens. These results suggest that, although not always present, the microbiota can promote salubrious [9] effects for the host.
It appears that the honey bee may use a combination of vertical transmission (colony to daughter colony) of the core endosymbionts, coupled with the advantageous use of naturally-occurring floral bacteria (similar to the way that humans make sauerkraut or silage). Of interest is that some free-living bacteria can exert salubrious [10] effects on host immunity (which opens the market for bee probiotics) [11].
Bacterial Abundance During The Fermentation Process
Hypothesis tested: if pollen is indeed digested by core bacteria during the fermentation of beebread, then the number of those bacteria would increase during the process.
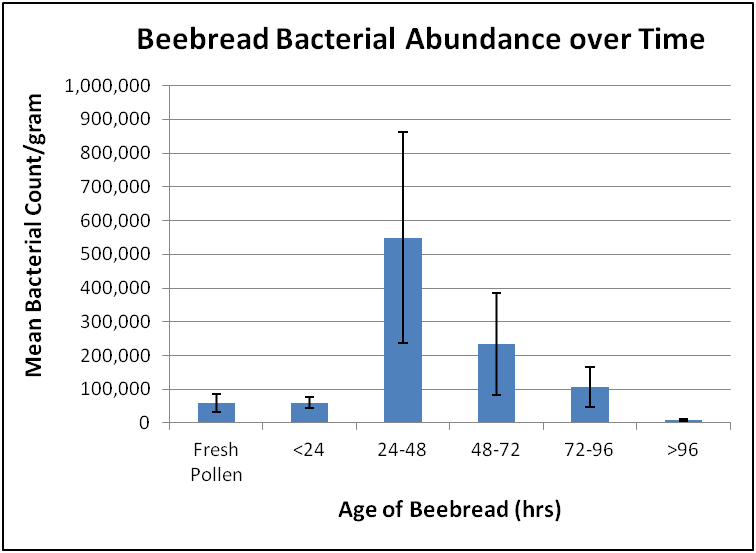
What Anderson found: that bacterial abundance increased only during the first two days of fermentation, and then decreased thereafter (Fig. 1). This finding does not support the digestion hypothesis, instead indicating that the formation of lactic acid acts to protect the pollen in beebread from microbial degradation.
Figure 1. After a day’s fermentation, the bacterial count of stored pollen increased greatly from that of fresh incoming pollen, and then dropped sharply as the beebread became too acidic for further microbial growth. After Anderson (2014) [[i]].
[i] Kirk shared the raw data so that I could chart it out in a manner more understandable to the layman. See the original paper for his graph, which contains more information.
Nurse Bee Feeding Preference By Age Of Beebread
Hypothesis to be tested: if fermentation improved the nutritional quality of pollen, then it would follow that nurse bees would preferentially consume beebread that had fully fermented.
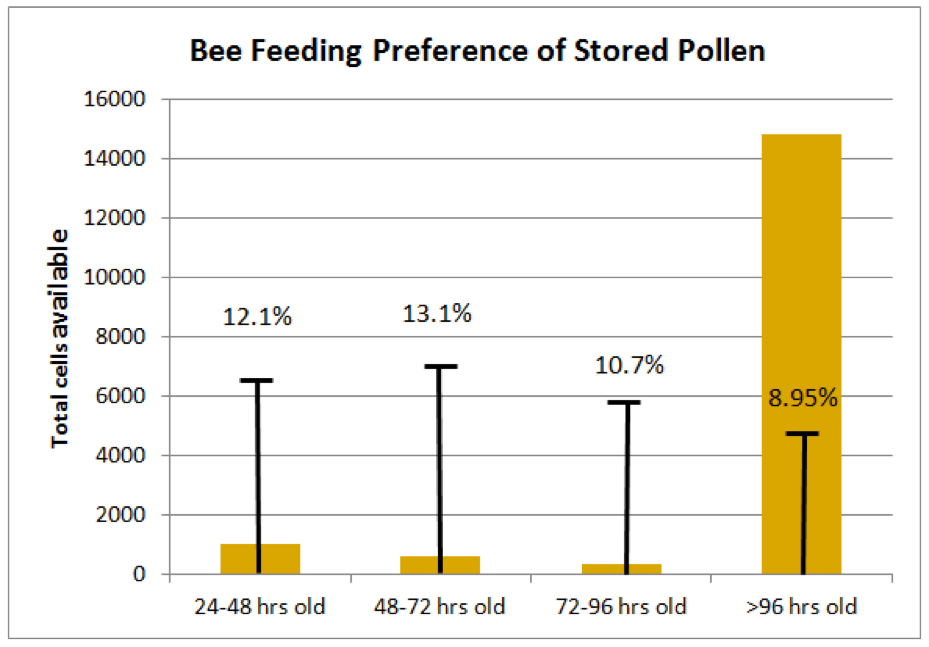
The Method: Kirk’s team meticulously tracked the daily deposition or consumption of pollen in every cell of an active brood frame from each of 8 colonies over 5 days, repeated with different colonies in March, April, and May.
What Anderson found: The results are shown in Fig. 2. Although these measurements were taken during times of pollen surplus (as indicated by the fact that nearly 90% of the beebread cells were over 96 hrs old), the nurse bees appeared to preferentially consume either fresh incoming or slightly fermented pollen to aged beebread. This observation does not support the hypothesis that fermentation improves pollen nutritional quality.
Figure 2. Measurements were taken of the ages of beebread cells in the combs; the orange columns indicate the average number of cells present for each age class of beebread. The capped lines indicate the proportion of that age class of pollen consumed each day. There was apparently an aversion by bees to consume pollen that had undergone extended fermentation [[i]].
[i] Or perhaps a preference for fresh, or slightly fermented, pollen. The overall expected consumption rate for each age class of beebread predicted to be consumed at random based on its abundance on the frames was 9.3%. Compare that to the measured consumption rates shown. Kirk has shown me additional (as yet unpublished) data gathered this spring that shows that the relative consumption rate continues to drop for beebread aged 6, 7, or more days.
Note: despite the observation that bees clearly prefer fresher beebread to older, due to the overwhelming presence of aged beebread in the combs of the colonies he observed, the majority of total beebread consumed (other than the unmeasured consumption of freshly-gathered pollen) was over 96 hrs old, suggesting perhaps that bees make an effort to “rotate the stores.”
The Pollen To Microbe Biomass Ratio
And now Anderson, in a stroke of brilliance, shot right to the crucial question: are there even enough bacteria present in beebread to exert a digestive effect? Bacteria lack teeth, and thus depend upon the enzymes that they produce in order to digest foodstuffs. Pollen does not make it easy for microbes to get to the good stuff inside the protective outer shell.
A pollen grain is enclosed in a tough coat called the exine. The exine is composed of a highly decay-resistant biopolymer called sporopollenin. Sporopollenin is so resistant to bacterial enzymes that “Pollen walls…often survive intact and recognizable for millions of years in bog and sediment deposits” [14]. The only place for bacteria to gain access to the nutritious innards of the pollen grain is through the (generally) one or more germination pores in the exine (these also serve as routes for water uptake). These pores are protected by a membrane called the intine, composed of cellulose and pectin. It is this layer that bacteria would need to digest their way through.
Kirk recognized that for bacteria to breach the intine of the pollen pores, that there would need to be a lot of them producing enzymes at those surfaces. So are there enough present? As I’m want to say, let’s do the math! In this case, Kirk did it for us.
Hypothesis: that there are enough enzyme-producing bacteria present in beebread relative to the surface area of the pollen grains to digest their way through the pollen shells.
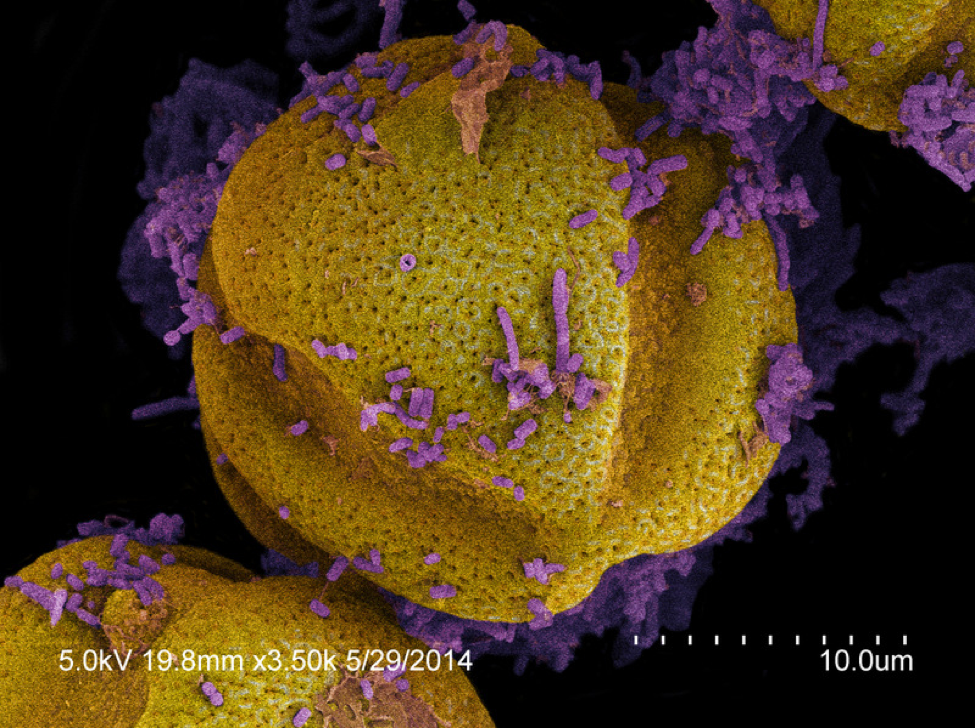
Kirk’s math: He was generous on his estimate of the number of microbes present in mature beebread, quadrupling his actual colony counts to allow for culture bias and fungi, arriving at about 36,900 microbes per gram of pollen. He then counted pollen grains under a scope, estimating about 90 million pollen grains per gram. This works out to about 1 microbe per 2500 pollen grains (Fig. 3). To place this into biological perspective, a single grain of pollen in the bee hindgut is often covered with hundreds or thousands of bacteria (Fig. 4), busily digesting the remnants left over from the digestion that took place in the bee midgut.
As an imaginary visual analogy, I calculated that the proportion of bacteria per surface area of the pollen grains works out to the spatial equivalent of a single BB placed on a football field [15]—hardly enough to produce the enzymes necessary to digest their way through the pollen exine or intine.
Figure 3. A scanning electron micrograph of dried beebread. The pink substance is dried simple sugars, which constitute about 40-50% by weight. Note the distinct lack of bacteria. Electron micrographs courtesy Kirk Anderson.
Figure 4. For comparison, these pollen grains from a nurse bee hindgut are covered with live bacteria (stained purple).
Conclusion: there simply aren’t enough microbes present in beebread to cause any significant degree of digestion of the pollen grain contents. Kirk succinctly concludes that: “Hive-stored pollen lacks the microbial biomass needed to alter pollen nutrition.”
The Degree Of Digestion Of Hive-Stored Pollen
Kirk is hardly the first bee researcher to point out that beebread contains few microbes. Back in 1983 Klungess and Peng [15], using microscopy, found few microorganisms in fresh beebread (but more in dark, old beebread). They concluded that:
The substances, presumably nectar or diluted honey and enzymes from the ventriculus and salivary gland, that bees add to pollen during packing on to the corbiculae [pollen baskets] and into cells, and the bee bread ripening process, do not break down the pollen contents. Nor do the microorganisms associated with fresh and old bee bread appear to cause destruction of pollen intine or the cytoplasm. .. It is, therefore, proposed that the substances added to pollen by bees during storage function as a preservative. We found no visible evidence that these substances pre-digest the pollen so as to make the nutrients more available to bees during subsequent digestion and absorption.
Compare this to Peng’s previous finding that most (but not all) pollen grains are rapidly digested during their passage through the nurse bee midgut, which contains few microbes [16].
Hypothesis: if bacteria indeed digest the pollen grains in beebread, this should be easily observed through microscopy.
Anderson’s finding: “There were no discernible morphological differences between newly collected and hive-stored pollen.”
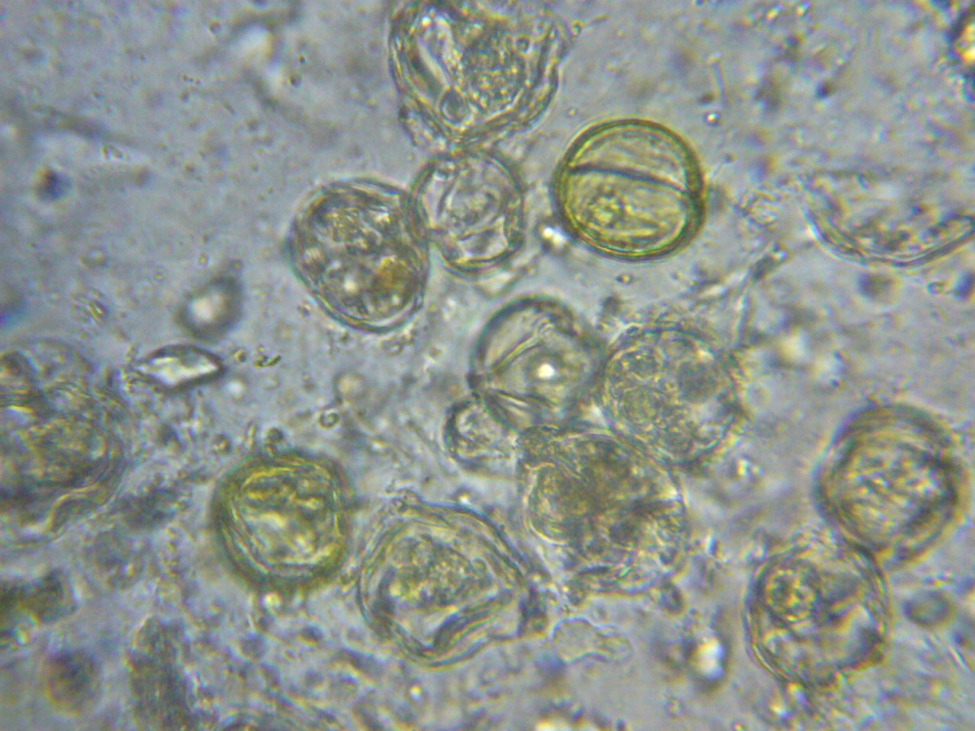
This should be something that any beekeeper could easily verify for him/herself. So I looked at aged beebread from my own hives under the scope (at 400 and 1000x), and also found very few microorganisms or digested pollen grains (Figs. 5-7).
Figure 5. Pollen grains in aged beebread under light microscopy. Note the relative absence of yeast or bacteria, and the unbreached (not misshapen) exines and intact colored contents of the pollen grains.
Figure 6. Pollen grains from beebread that I diluted with a weak sugar solution and allowed to ferment for 10 days. Note the abundant yeast and bacterial cells in the background (unfortunately there was not enough depth of field to bring both the pollen and microbes simultaneously into focus). Despite being exposed to over a week of vigorous aerobic fermentation, these pollen grains remain intact and undigested. Compare these intact grains to those below.
Figure 7. A sample of digested pollen from the hindgut of a nurse bee. Note how most of the pollen shells are empty (no longer yellow inside) and distorted, indicating digestion of the contents.
Conclusion: the bee midgut can quickly empty pollen grains and digest the contents. But such digestion does not occur when bacteria and yeast ferment pollen into beebread.
Dr. Anderson’s Conclusions
Our combined results do not support the hypothesis that hive-stored pollen of honey bees involves nutrient conversion or predigestion by microbes prior to consumption.
- The bacterial communities found in hive-stored pollen did not differ from those of newly collected pollen, but both sample types varied significantly by season. This result indicates the lack of an emergent ‘core’ bacterial community co-evolved to predigest pollen.
- Relative to other plant material involving microbial digestion or extensive fermentation, hive-stored pollen contains very few microbes.
- The absolute number of bacteria in hive-stored pollen decreases with storage time, indicating that it is not a suitable medium for microbial growth.
- The preferential consumption of freshly collected pollen indicates that bees have not evolved to rely on microbes or other time-related factors for pollen predigestion.
- The microbe to pollen grain ratio is many orders of magnitude removed from that required to alter hive-stored pollen.
- Regardless of sampled season or the taxonomic character of microbial communities, microscopic examination revealed no intermediate stage of pollen digestion in hive-stored pollen.
Based on these collective findings, we suggest that stored pollen is a preservative environment governed largely by nonmicrobial additions of nectar, honey and bee glandular secretions.
Summary
Flowers and pollinators have coevolved over millions of years, and along with them microbes always at the ready to consume them, or perhaps establish symbiotic relationships. Since microbes are invisible to the naked eye, it is difficult for us to grasp their degree of prevalence in the environment, or to understand their contribution to bee nutrition and health.
There may be a million bacteria (along with yeasts and other fungi) per milliliter in nectar when bees first collect it—fresh nectar is often already starting to ferment [18]. Pollen contains somewhat fewer microbes, but is still very biologically active when first gathered. The greatest concentration of microbes in the hive is in the residual undigested material in the bees’ hindguts—that of a single bee may contain hundreds of millions of bacteria [19].
Kirk points out that the fluctuation of pollen availability has likely “selected for the quick turnover of the most readily available pollen nutrients into a ‘nutritional reservoir’ of living tissue (i.e. larva and worker fat bodies). In such a state, nutritional reserves are better protected from microbial digestion, more quickly shared among hive members and easier to digest than hive-stored pollen.”
The above observation reminds us that hungry nurse bees readily consume larvae that they had previously been lavishing with jelly when it was abundant. The larvae thus function as a living protein reserve that through cannibalism can be quickly converted back into jelly or used to replenish fat bodies.
The honey bee, similar to humans, stores surplus food (that can’t be immediately consumed) for later consumption (mainly to survive the winter). Like us, bees have figured out similar ways to prevent that food from being decomposed by microbes. Both nectar and pollen are prepared for prolonged storage by using a combination of high sugar concentration for osmotic preservative effect (similar to making jam), acidification via bacterial lactic acid production (pickling), and the addition of salivary antimicrobial compounds such as glucose oxidase (food preservatives).
Two key bacteria in this preservation process are the acid-resistant and osmotolerant [20] Lactobacillus kunkeei and Parasaccharibacter apium, both of which appear to be instrumental in hive hygiene, food storage, and larval health.
The microbial community in the hive is dynamic and evolving, not only in the gut of every bee during its short life, but also hour by hour during the fermentation of beebread. The microbiomes of the hive rapidly “adapt to changing diets and conditions not only by shifting community membership but also by changing gene content via horizontal gene transfer.” Bacteria, by continually swapping genes, can adapt and evolve at a rate inconceivable to humans.
In the extended flower-pollinator community, pollinators vector microbes (including pathogens) with a remarkably high degree of efficiency. Graystock [21] found surprisingly efficient transmission of pathogens from bee to bee during flower visitations—the same would be expected to apply to hive or gut bacteria. This is due to the fact that the toilet hygiene of flying insects is rudimentary at best, resulting in plenty of fecal bacteria being inadvertently transferred to flower surfaces during pollinator visits (after all, just think about the degree of pollen transfer from flower to flower). And such transmission can be interspecific—any bee, fly, butterfly, beetle, or wasp can carry floral or pathogenic microbes from one flower to another. The total amount of microbial interchange within the entire flower/pollinator environment is mind boggling to consider, and makes for a highly dynamic system of microbial and pathogen interactions that we are only beginning to understand.
Next
The biological and chemical processes that take place during the fermentation of beebread, yeasts and fungi, probiotics, and the Anderson labs’ current research projects.
References and Notes
[1] Published as Anderson, KE, MJ CarrolL, T Sheehan, BM Mott, P Maes and V Corby-Harris (2014) Hive-stored pollen of honey bees: many lines of evidence are consistent with pollen preservation, not nutrient conversion. Molecular Ecology 23: 5904–5917.
[2] Mainly from the above citation, but also from other published and as yet unpublished research. I also took the liberty to edit or reorder the quotations from his papers for readability.
[3] Vásquez A, Olofsson TC (2011) The honey crop – the holy grail when antibiotics fail. Microbiology Today, 38, 226–229.
[4] Fridman S, et al (2012) Bacterial communities in floral nectar. Environ. Microbiol. Rep 4:97–104.
[5] See my previous articles in this series.
[6] Disayathanoowat, T, et al (2012) T-RFLP analysis of bacterial communities in the midguts of Apis mellifera and Apis cerana honey bees in Thailand. Journal of Apicultural Research 51(4): 312-319.
[7] Don’t be grossed out—the same thing happens with humans and many other animals.
[8] Blum JE, et al (2013) Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. mBio 4(6):e00860-13. doi: 10.1128/mBio.00860-13
[9] Salubrious–healthful, beneficial, wholesome. Man, I gotta add this descriptive word to my vocabulary!
[10] I couldn’t wait.
[11] Again, I’ve yet to see any hard data that any probiotic currently on the market is of benefit to bees. But I wouldn’t be the least surprised if some were developed in the near future. But don’t just go feeding any untested probiotic—a recent study found that some can seriously harm bees:
Ptaszyńska, AA, et al (2015) Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Parasitol Res DOI 10.1007/s00436-015-4761-z Open access.
[12] Kirk shared the raw data so that I could chart it out in a manner more understandable to the layman. See the original paper for his graph, which contains more information.
[13] Or perhaps a preference for fresh, or slightly fermented, pollen. The overall expected consumption rate for each age class of beebread predicted to be consumed at random based on its abundance on the frames was 9.3%. Compare that to the measured consumption rates shown. Kirk has shown me additional (as yet unpublished) data gathered this spring that shows that the relative consumption rate continues to drop for beebread aged 6, 7, or more days.
Note: despite the observation that bees clearly prefer fresher beebread to older, due to the overwhelming presence of aged beebread in the combs of the colonies he observed, the majority of total beebread consumed (other than the unmeasured consumption of freshly-gathered pollen) was over 96 hrs old, suggesting perhaps that bees make an effort to “rotate the stores.”
[14] Roulston, THI and JH Cane (2000) Pollen nutritional content and digestibility for animals. Plant Systematics 222: 187–209.
[15] By my math.
[16] Klungness L. M., Peng Y.-S. (1983) A scanning electron microscopic study of pollen loads collected and stored by honeybees. J. Apicul. Res. 22: 264-271.
[17] Peng YS, ME Nasr, et al (1985) The digestion of dandelion pollen by adult worker honeybees. Physiol. Entomol. 10: 75-82.
[18] Castillo, C, et al (2012) Seasonal variation in the titers and biosynthesis of the primer pheromone ethyl oleate in honey bees. J Insect Physiol 58(8):1112-21.
[19] Estimate, Kirk Anderson, pers comm.
[20] Osmotolerance is the ability to live in high-sugar environment that would dessicate other microbes.
[21] Graystock P, et al (2015) Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. B 282: 20151371. http://dx.doi.org/10.1098/rspb.2015.1371
Tags: beebread, digestion, DNA sequencing, Dr. Anderson, fermentation, hive-stored pollen, microbe biomass