First published in: American Bee Journal, October 2014
Effect of Amitraz on Buildup of Nucs
Randy Oliver
ScientificBeekeeping.com
First published in ABJ October 2014
Introduction and Objectives
I was surprised by the slow buildup and lack of drawing of foundation by the nucs in my 2013/14 Pollen Supp Trial. Although the colonies were started during a dearth, due to the near-continual feeding of syrup, I fully expected them to build more rapidly. They didn’t start to build to any extent until shortly after we removed the Apivar strips, but since I had not run a control group without Apivar, I had no way of knowing whether there was any connection.
As I mulled over the question, I came across additional information that furthered my curiosity as to whether amitraz might exhibit negative effects upon colonies (see Amitraz: Red Flags or Red Herrings? in this same issue).
So I decided to run a trial this summer to see whether the application of Apivar strips had a negative effect upon either colony buildup or the drawing of foundation.
Principal Investigator: Randy Oliver, assisted by Eric and Ian Oliver
Funding sources: I hope to be reimbursed for this trial by a grant from the North Dakota Department of Agriculture, solicited for me by the North Dakota Beekeepers Association.
Experimental Design:
- Set up 36 5-frame nucs with freshly-mated tested queens, of two different maternal lines, equalized for strength, in pairs by queen mother. All hives 10-frame deep singles.
- Reduce varroa levels to near nil by “natural” miticides known to leave few persistent residues.
- Randomly assign 1 hive in each pair to receive a single Apivar strip for the duration of the trial (Treatment); open the other hive in each pair but apply no strip (Controls).
- Add 5 frames of foundation and feed colonies for buildup, adding a second box of foundation to all when the first colony drew out its combs in the first box.
- Allow colonies to grow, and then grade them for gain in strength and amount of combs drawn.
Trial Log
June 4, 2014 Moved 20 recently-mated queenright nucs to the test yard, which already contained 20 similar nucs from a different queen mother (both Italian mongrels). All nucs oxalic’d at Day 19; all checked for good patterns of worker brood. The nucs were set in pairs, each pair consisting of two nucs headed by sister queens.
June 6 Equalized all into 5-frames in a single. Typically 4 frames with brood.
June 9 Equalized.
June 12 Equalized and treated all with ½ MAQS.
June 19 Checked one hive for mites—4 in ½ cup bee sample–too many.
June 20 Equalized. Treated all with 1 Hopguard II strip.
June 27 Colonies drawing and filling foundation well. Large hive-to-hive variation in the amount of chewed Hopguard paper at the front of hives. Took composite mite samples of ½ cup of bees from 6 pairs of hives. Only 2 mites total in >1800 bees.
June 30 Final equalization of colonies, checked for queenrightness (2 colonies superseding, removed them and 2 others from trial), removed excess frames of bees, leaving each colony with 5 frames of brood, covered solid with bees. Good nectar flow on.
Experimental note: These nucs were considerably stronger than those in the previous trial, since they started with 5 full frames of brood. The experiment also began earlier in the season (July 1 vs. Aug 8), and during a nectar flow rather than a dearth.
July 1 Time Point 0 Good nectar flow on. Overnight, nearly every colony had built several combs underneath the lids (Figs. 1, 2, and 3).
 Figure 1. All colonies were vigorously drawing comb on a natural nectar flow and ready to rock and roll. Remains of Hopguard II strip visible.
Figure 1. All colonies were vigorously drawing comb on a natural nectar flow and ready to rock and roll. Remains of Hopguard II strip visible.
 Figure 2. Colonies were in pairs, each pair headed by sister queens. We flipped a coin to decide which of each pair received an Apivar strip, and inserted one strip near the center of each treated hive. Here’s Ian adding the frames of wax-coated plastic foundation to the 5-frame nucs.
Figure 2. Colonies were in pairs, each pair headed by sister queens. We flipped a coin to decide which of each pair received an Apivar strip, and inserted one strip near the center of each treated hive. Here’s Ian adding the frames of wax-coated plastic foundation to the 5-frame nucs.
 Figure 3. Start of trial, treatment randomly assigned to each pair. I would have preferred a less-crowded test yard, but this one was near home.
Figure 3. Start of trial, treatment randomly assigned to each pair. I would have preferred a less-crowded test yard, but this one was near home.
July 7 Spot checked. Some colonies drawing foundation, nectar flow tapering off. Fed each colony ½ gal of 1:1 sucrose syrup.
July 21 All colonies appeared strong and building. The strongest had all 10 frames drawn and were heavy; the weakest had not yet drawn all 10 frames, nor covered them. The main flow is tapering off. Fed ½ gal 1:1 sucrose syrup. Added deep super of foundation to each colony.
Experimental note: the strong nectar flow after the start of the trial was unexpected, based upon the previous history of this yard.
July 24 Fed ½ gal 1:1 sucrose syrup.
Aug 8 Time Point 1 Main flow appears to be over, but still mixed pollens coming in. Since there were large differences in strength, we decided to perform the end point assessment. Graded all colonies (by Randy) in morning (by 9:15), blinded as to treatment. Graded for seams of bees and number of well/drawn combs of foundation (out of possible 15).
Results
The final grading took place at Day 38. The Control colonies averaged 8.8 (SEM 0.3) seams of bees; the Apivar-treated colonies averaged 8.5 (0.3). There was no significant difference between the two (Mann-Whitney p = 0.61), as reflected in the histogram of colony strengths in Fig. 4.
 Figure 4. There was no apparent difference in buildup due to treatment with Apivar.
Figure 4. There was no apparent difference in buildup due to treatment with Apivar.
There was no difference between groups in the number of combs of foundation drawn– 5.9 (SEM 0.6) in the Controls; 5.7 (0.5) in the Apivar-treated group.
Discussion
The application of one Apivar strip to a strong 5-frame nuc on a decent nectar flow did not appear to inhibit either colony buildup or the drawing of foundation. I am toying with continuing this trial, periodically replacing the Apivar strips, through next spring, to see whether there are any other observable effects upon the colonies.
Acknowledgements
Thanks to Bonnie and Brent Woodworth and the North Dakota Beekeepers Association for their support of my research.
First published in: American Bee Journal, December 2013
BEEKEEPER-FUNDED RESEARCH
Does Oxalic Acid Treatment of Nucs Affect Honey Production?
Randy Oliver
ScientificBeekeeping.com
Originally published in ABJ Dec 2013
Last year I ran two trials to see whether I could take advantage of the brief window of opportunity which occurs 19 days after starting nucs with queen cells, during which the mites are forced out of the brood. I detailed the theory, timing, and procedure in a previous article [1]. The result was that treating nucs with an oxalic dribble was highly effective against varroa, and appeared to lead to increased colony strength. However, in one replicate, it appeared that the oxalic treatment may have reduced subsequent honey production. So I ran another trial this spring to confirm whether that was indeed the case.
Introduction
Varroa management is much easier if you begin the season with mites reduced to the lowest possible level. The problem is that colonies in the springtime are full of brood, which provides the mite with a place to hide from treatments. By inducing a period of broodlessness, the beekeeper can force all the mites out onto the adult bees, where they are exposed to the action of the mite treatment of your choice.
A very brief period of such broodlessness occurs when one makes up nucs using queen cells (as opposed to with a caged queen). With careful timing, one can obtain a high efficacy mite kill by applying a safe, inexpensive, and quick treatment of oxalic acid dribbled in sugar syrup. Although oxalic acid is considered to be a “natural” and “organic” treatment, a concentration high enough to kill mites is somewhat stressful to bees. The point of treatment, though, is that the short-term stress from the treatment should be more than compensated for by the long-term benefits to colony health and productivity by the knockback of the mite infestation rate.
This experiment was designed to test whether such an oxalic dribble of nucs would negatively affect the honey production of treated colonies in the ensuing months.
Materials and Methods/Trial Log
April 14, 2013 Made up a batch of 4-frame nucs, Italian stock, set in their own yard in the Sierra Foothills.
April 15 Installed 10-day queen cells from single mother.
May 2 Checked nucs for queenrightness; good take. Equalized the queenright nucs to 5 frames of bees using frames from the queenless nucs. Total count 37 colonies. There was some comb whitening from the spring honey flow and the colonies appeared healthy. I flipped a coin to randomly assign the hives to be treated (n = 19) and applied approx. 5 mL of 3.2% w:v oxalic syrup [2] applied by a garden sprayer set to a slow stream (field calibrated with a graduated cylinder).
May 10 Worked all colonies into singles, rechecking for queenrightness and brood health; added drawn brood combs and recorded starting weight of each hive. Most colonies were roughly equal in strength—a strong 5 frames of bees with 4 frames of brood. Colonies had been set back slightly by poor weather last week, but were showing a fair nectar shake this day. Good weather in forecast; the main honey flow about to begin.
May 11 In order to minimize the effect of any introduced nosema or EFB on the drawn comb, and to stimulate the colonies, I fed all ½ gal 1:1 sucrose syrup containing 95mg fumagillin and 100mg OTC per hive.
May 20 Blackberries (our main source of nectar) were coming into bloom, but disappointingly, there was only minimal nectar shake. Most colonies 6-10 frames strength. Weighed hives again (Fig. 1) and added a weighed deep second deep of foundation to all.

Figure 1. My sons Eric and Ian weighing a hive on a digital scale (placed under the empty package cage).
May 30 Unfortunately, it is clear that our expected main flow is not happening, apparently due to the winter drought. Fed ½ gal 1:1 syrup to stimulate the bees to forage.
June 10 Fed ½ gal 1:1 syrup
June 14 Blackberry bloom mostly over, minor nectar shake. Weighed all hives for the final time. Checked the two anomalous light-weight hives and censored both; one had gone queenless, and one had requeened itself.
Results
There was, as we typically observe, a wide colony-to-colony variation in weight gain (Fig. 2).
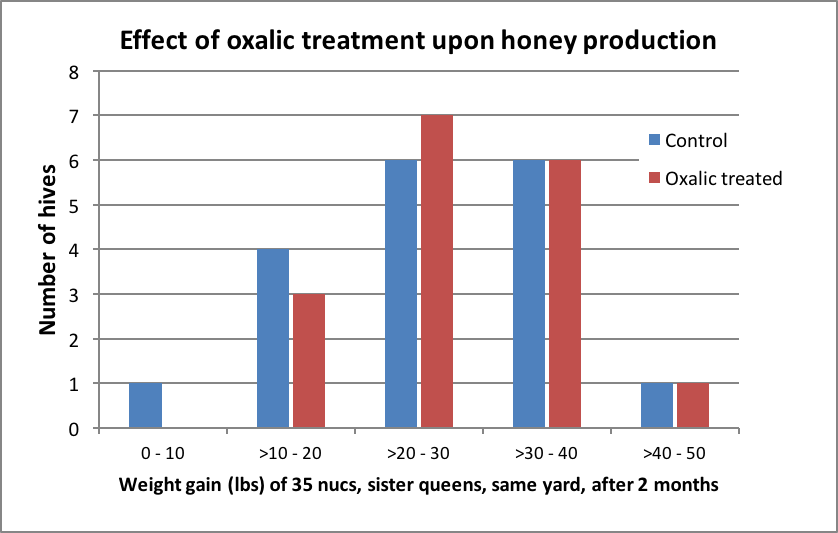
Figure 2. The amount of weight gain per hive (treated and controls combined) followed a normal distribution. Mean weight gain was 26 lbs (median 27 lbs). The highest producing colony was in the oxalic group. The three half gallons of fed syrup contained a total of 7.5 lbs of sugar per hive, which would be expected to have contributed to that amount of the weight gain. This was a very poor honey crop, but likely adequate to determine whether oxalic treatment substantially affected honey production.
At the first weighing (Day 25), the treated colonies, on average, put on slightly less weight, but the difference was not statistically significant (Fig. 3). At the second weighing (Day 59) the treated hives weighed slightly more on average, but again the difference was not statistically significant.
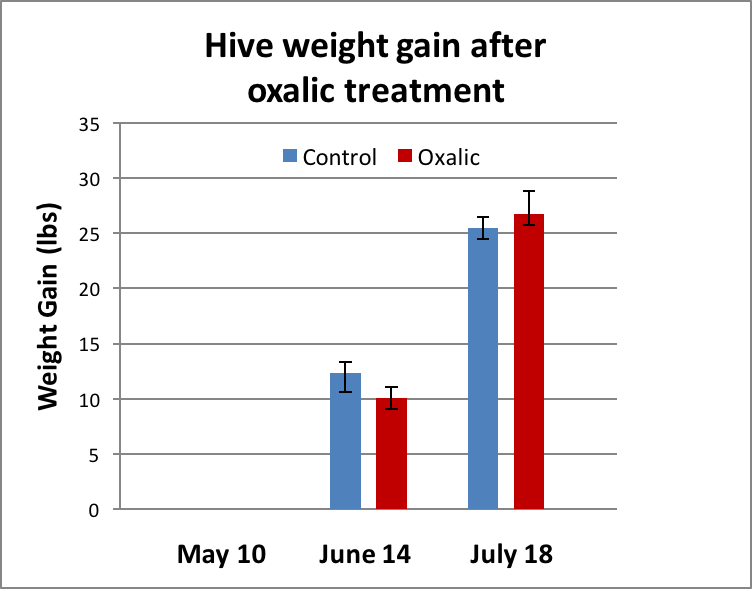
Figure 3. There was no significant difference in weight gain between groups at either time point.
Discussion
The typical great degree of colony variation, even in hives started with sister queens and kept in the same yard, often makes it difficult to tease out any minor effect of treatment. However, in particular trial there did not appear to be any substantial negative effect due to oxalic acid treatment.
The efficacy of treatment, as shown in the original trials, could be improved by blasting the parent hives with formic acid prior to making up the nucs. Any loss of the old queens would not be important, since the colonies were going to be nuked up anyway. I suspect that such a one-two combination of “natural” treatments would result in very low mite levels in the nucs.
Other treatments, such as Hopguard strips or powdered sugar dusting, might also be possibilities for working into this sort of mite management. All told, I find that this simple mite management method is safe and inexpensive, does not leave persistent residues in the combs, and does not appear to negatively affect honey production. In my own operation, we have now treated over 2000 nucs in this manner, and are pleased with the results.
Acknowledgements
Thanks to local beekeepers Fosten Wilson and Rick Blaski for their help in the field. Funding for this experiment came from beekeeper donations to ScientificBeekeeping.com, with special thanks to the Beekeepers Association of Southern California and to the beekeepers contracting with Scientific Ag Company. I am happy to share the raw data from any beekeeper-funded trials.
References
1 Simple Early Treatment of Nucs Against Varroa, ABJ April 2012
2 https://scientificbeekeeping.com/oxalic-acid-treatment-table/
 Figure 1. All colonies were vigorously drawing comb on a natural nectar flow and ready to rock and roll. Remains of Hopguard II strip visible.
Figure 1. All colonies were vigorously drawing comb on a natural nectar flow and ready to rock and roll. Remains of Hopguard II strip visible. Figure 2. Colonies were in pairs, each pair headed by sister queens. We flipped a coin to decide which of each pair received an Apivar strip, and inserted one strip near the center of each treated hive. Here’s Ian adding the frames of wax-coated plastic foundation to the 5-frame nucs.
Figure 2. Colonies were in pairs, each pair headed by sister queens. We flipped a coin to decide which of each pair received an Apivar strip, and inserted one strip near the center of each treated hive. Here’s Ian adding the frames of wax-coated plastic foundation to the 5-frame nucs. Figure 3. Start of trial, treatment randomly assigned to each pair. I would have preferred a less-crowded test yard, but this one was near home.
Figure 3. Start of trial, treatment randomly assigned to each pair. I would have preferred a less-crowded test yard, but this one was near home. Figure 4. There was no apparent difference in buildup due to treatment with Apivar.
Figure 4. There was no apparent difference in buildup due to treatment with Apivar.





