The “Nosema Twins” – Part 1
© Randy Oliver
It appears that a new bully has moved into town, and we didn’t even realize it! Maybe now we can explain some of the nasty things that have been going on.
As of a few months ago, I knew diddly squat about nosema. Sure, I bought Fumidil-B some twenty years ago when I was shipping queens, but never even used up the bottle! In California bees, nosema is usually not a problem. That is, until some beekeepers started noticing changes in their bees a few years ago. Was something new afoot?
The European honey bee has a longtime association with the familiar midgut parasite Nosema apis. N. apis is a relatively benign parasite in warmer climes where bees can fly freely for cleansing flights during winter, and generally only becomes a problem where bees are restricted from such flights by long, cold winters.
Nosema spores spread by being ingested by foragers at water sources contaminated by bee droppings. Such older bees are short lived anyway, so the occasional infection is no big deal. Infected bees also tend to altruistically prevent spreading the infection by flying away and not returning to the hive. Kralj (2006) refers to this behavior as “suicidal pathogen removal”.
Nosema becomes a problem, however, when newly-emerged bees ingest spores. When this happens, the infection messes up their ability to digest pollen, and thus keeps them from ever developing their hypopharyngeal glands. So how does a “house bee” get infected? This happens when extended cold or rainy weather prevents cleansing flights. But when a bee’s got dysentery from nosema she just can’t hold it. The house bees then say “OhMyGod, somebody’s pooped in the hive!” The insidious thing is that cleanup is a job delegated to newly emerged bees, who then ingest the spores in the process, and the infection thus moves from older bees to the very youngest. When this happens, nosema can go epidemic in the hive, with dire results. Infected queens can be superceded, and unless the newly-reared virgin replacement can fly, the colony will go queenless.
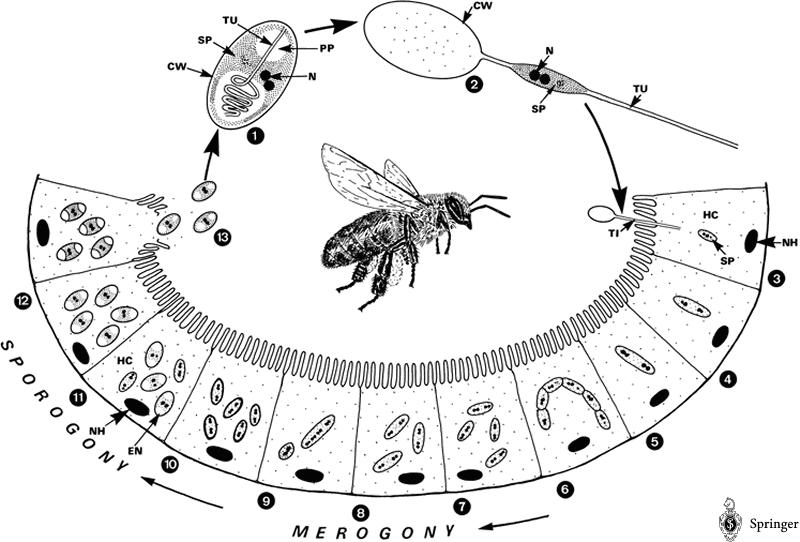
Life cycle of Nosema apis. The spore injects its contents into a gut epithelial cell, multiplies, and eventually causes the cell to burst and release the new spores back into the gut. Nosema can also reproduce “vegetatively” cell to cell. © Springer Life Sciences
Even more importantly, the life spans of infected young bees can be reduced by up to 78%, plus they are unable to feed brood! At this point the death rate of the bees exceeds the birth rate, and the colony collapses. “In a typical case of a colony being depleted because of a Nosema [apis] infection, the queen can be observed surrounded by a few bees, confusedly attending to brood that is already sealed” (Anon 2004). (Does this sound familiar to anyone? Note that the remaining newly-emerged bees may not test positive for nosema yet—allowing the culprit to go undetected).
We’ve been discussing bee nutrition. Here’s an important point from Dr. Ingemar Fries: “Under conditions where old infected bees must rejuvenate the production capacity of their hypopharyngeal glands – nosema disease may be devastating.” This means that if you want a colony to brood up after a dearth, or after winter, and the population consists of older bees infected with nosema, they aren’t going to be able to do it, no matter how well they are fed!
The frustrating thing about nosema is that “the majority of Nosema-infected colonies will appear normal, with no obvious signs of disease even when the disease is sufficient to cause significant losses in honey production and pollination efficiency” (Hornitzky 2005). In general, Nosema apis has historically been a serious problem only in areas with cold winters, and then was most noticeable in early spring. However, in the last several years, we’ve started seeing unusual nosema problems during summer…
Nosema ceranae—the “new” kid on the block
Nosema ceranae grabbed headlines in the U.S. in April of this year (Raven 2007) when Drs. Joe DeRisi and Don Ganem of U.C. San Francisco were given samples of CCD bees by the Army virologists working with Dr. Jerry Bromenshenk. DeRisi, who had helped solve the SARS disease mystery, analyzed the bees, and was surprised to find that they contained considerable DNA from N. ceranae—a parasite previously unreported in North America.
At that point, the USARS CCD team announced they had already been preparing a paper to formally announce that ceranae had been in the U.S. since at least 1995 (based upon analysis of stored samples of bees) (Chen 2007). This new bug had only been first named in 1996 by Dr. Ingemar Fries, but wasn’t detected in Apis mellifera until 2004 in Vietnam, then in Taiwan, and by 2006 was reported to be widespread in Europe, Asia, Israel, the Caribbean, and North and South America (Paxton 2007, Klee, et al. 2007). Amazingly, in a few short years N. ceranae appears to have supplanted N. apis throughout much of North America and the world! In many areas, it is now difficult to find the previously common N. apis!
So ceranae snuck in under our noses, spread widely, and now researchers worldwide are scrambling feverishly to find out answers about this new, and apparently different acting, species. Once again, just as with varroa, our poor bees are being forced to evolve yet another new host/parasite relationship (not to mention Israeli Acute Paralysis Virus). How did ceranae get here? Cox-Foster (2007) found it in imported Chinese royal jelly used by queen producers, but Williams’s (2007) genetic sequencing indicates that the likely origin was from Europe.
Is N. ceranae going to be (or is it already) a problem? I’ve spoken or corresponded with scientists and beekeepers worldwide, and the answer is: we don’t know enough, and not all agree. There are currently several camps, based upon their favorite suspects for colony collapses. One camp (led by Spanish researchers) believes that ceranae is more virulent than apis, and is trashing bees throughout Europe. Other researchers are more conservative, and suggest that ceranae may be relatively benign unless unfavorable weather conditions prevail, especially when they cause nutritional stress. Others suggest that ceranae might be an opportunistic pathogen that thrives once the bees’ immune systems are impaired by pesticide, viral, or other unknown stresses.
The $64,000 question is: “Is N. ceranae the cause of CCD?” The very short answer is that the Cox-Foster team found it in 100% of their CCD samples, yet have additional evidence to suggest that it may only be a player, not the underlying cause. I do not want to enter into the CCD debate here (and I greatly respect the work of the CCD scientists), but there is considerable evidence from the rest of the world that N. ceranae is likely responsible for a substantial proportion of the colony collapses in those countries in which it is found. Indeed, Martin-Hernandez (2007) states: “The relative risk of bee depopulation observed in colonies with either both species or only N. ceranae (almost six times greater than those with negative PCR results), indicates a significant causative association between the presence of N. ceranae and the development of hive depopulation.” How’s that for conservative “science-speak”?
The authors have a stunning graph that shows how nosema infections in Spain have changed over the course of seven years. Up through 1999, infections peaked in December and March, and were quite low the rest of the year. However, the lows filled in progressively each year, until by 2005 nosema infection level was nearly constant through the entire year! The bees no longer get a break.
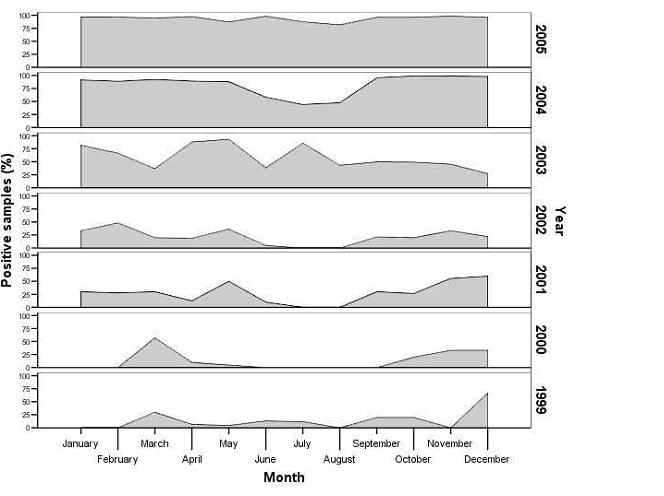
Percent Nosema-positive samples, by month, in Spain for the years 1999-2005. Based upon samples received in the Bee Pathology Laboratory (after Martin-Hernandez, et al. 2007).
“Good old” N. apis requires the bees to spread it by defecation during winter, and does not do well in hot weather (Manning 2007), so it is usually a rather benign parasite, when bees are kept in their normal climatic range. The level of infected bees peaks in early spring, then drops close to zero by June, and stays low until next winter. The yearly drop in infection generally allows the colonies to recover. However, N. ceranae is a very different animal! It appears to peak in summer. As best I can tell, beekeepers in every country where ceranae has been identified have been reporting that they now have nosema problems during summer.
I can’t help but to share with you with this scary tidbit: Delaguila (2006), in developing methods to culture N. ceranae in vitro, found that “as mammal cells were infected at 37°C [human body temperature], this opens up the possibility of [it] being a source of human microsporidiosis.” Yet another reason to keep our fingers out of our mouths when in the beeyard!
Symptoms and consequences of N. ceranae infection
One European researcher feels that we have been so distracted by varroa, that we have simply overlooked the poor buildup, queen failures, poor honey crops, and colony collapse due to N. ceranae. Other scapegoats have been fingered. French beekeepers, who have been experiencing major colony losses in recent years, blamed the neonicitonoid pesticides, and got them banned in their country. However, at the last meeting of the “Bee Losses Group” held at Wageningen (Holland) last March, the French research group stated that the pesticide hypothesis had finally been discarded because none of the research could establish a relationship between colony deaths and the crops treated with imidacloprid or fipronil. N. ceranae has now been found to be well established in France (Chauzat 2007), and is currently a major suspect, as it is in Spain. The similarity between the French die offs and the effects of N. ceranae are striking.
Side note: I’m not in any way trying to denigrate the effects of pesticides on our bees, and fully support further research on the subject—especially with regard to the neonicitinoids.
So are we having the same problems in the U.S. as they are in Europe? One consideration is that the haplotype of N. ceranae found in Minnesota and Canada is different than that found in Spain, Germany, or China, and may differ in virulence (Williams 2007). The American bee research establishment appears to have been caught as much by surprise by ceranae as everyone else, and is just now gearing up to try to find answers to our questions.
Researchers have found ceranae spore counts to explode in a matter of days. Higes (2007) saw 100% mortality of inoculated bees within 8 days in the lab! Yet, on the other hand, ceranae has been in the U.S. since at least 1995, and there are still plenty of colonies of bees still alive! Clearly, ceranae is not invincible, as far as our bees are concerned. It’s been noted that various races of bees vary in their natural resistance to N. apis—perhaps the same will hold true for ceranae. In its original host, Apis cerana, N. ceranae is often isolated to islands of single infected epithelial cells. In Apis mellifera, however, ceranae runs amok from cell to cell and quickly spreads. Perhaps some of our bees have developed similar resistance.
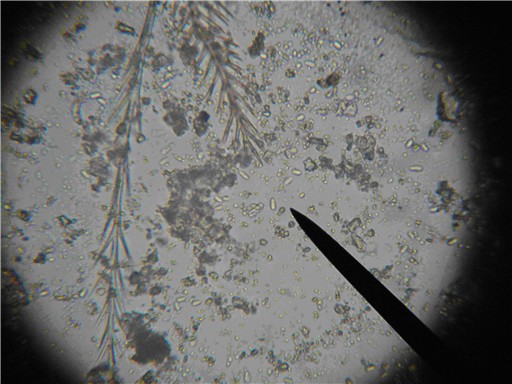
Nosema spores, species unknown, with bee setae (brushy hairs) for scale. Photomicrograph of a wet mount of a macerated bee abdomen at 400x. The spores are the oblong capsules that appear to glow in the center (three are off the tip of the pointer). This bee is sick. Photo by the author.
Unfortunately, there is yet another reason that ceranae could affect our bees more than apis did. Otteni (2004) found the gut epithelium to be an effective barrier against a virus. Remember earlier when I described that bees digest food by shedding the epithelial cells of their guts? This normal shedding mitigates the effect of N. apis infection, since infected cells may well have been eventually shed anyway. Gliñski & Jarosz (2001, a good read) also describe how the resulting scar tissue is more resistant to further infection (but may eventually give way to bacteria). They also make clear that a damaged gut is the prime route of entry for viruses. Problem is, Higes (2007) found that N. ceranae destroys not only the epithelial cells, but also the basal cells of the gut. To me, it brings up the question, “does a nonfatal ceranae infection make the gut barrier permeable to other infections, including viruses?”
Let’s play detective. Carreck (1997) states that “three viruses almost invariably multiply only in those individual bees which are also infected with Nosema apis” (Black Queen Cell Virus is especially noted for this association). Inactive viruses in a bee can be initiated to begin reproducing if triggered by another pathogen entering the bee’s hemolymph (Yang & Cox-Foster 2005). Could ceranae be doing just that, or opening the door for gut bacteria and/or fungi to enter the body cavity? Bauer (1998) found that “viral infectivity [in gypsy moths] increased 10-fold when larvae were preinfected with Nosema sp.” Oh, how complex this all gets, especially with our old “friend” varroa shutting down the bees’ immune systems and vectoring viruses.
Martin-Hernandez (2007) states that “pathological data from Spanish apiaries affected by N. ceranae do not show a fast acting, short duration syndrome. On the contrary, only nonspecific symptoms, such as a gradual depopulation, higher autumn/winter colony death or low honey production are associated with the presence of this parasite. None of the dysentery or crawling bee behaviour usually related with Nosema apis infection has been reported. A similar syndrome has recently been reported from France and called ‘dry nosemosis’.”
So if there is no dysentery, how does ceranae spread from bee to bee? Higes (2007) found that infected foragers contaminate the pollen they collect with spores, apparently when they moisten the pellets with nectar from their crops. This contaminated pollen is then delivered to the brood nest, where it is consumed by young bees. This important discovery could explain why ceranae can be so prevalent during summer. The oldest, most spore-infested bees transmit spores to the food that the youngest bees are about to eat. As if this weren’t enough, stored beebread pollen has been found to have virus particles in the various layers (Cox-Foster 2005), so the young bees could simultaneously ingest both N. ceranae spores plus virions. The young bees might be doomed from their first meal!

Are these bees eating pollen that is contaminated with Nosema ceranae and virions?
Are you putting the pieces together? Note the positive feedback—nutritionally-stressed bees can’t fight nosema, then fill full of spores, and contaminate the bee bread of the colony. The young bees that eat that bee bread are immediately infected and are soon unable to digest their food, and thus are even more nutritionally stressed. Then these poor short-lived bees are unable to provide jelly to the queen, foragers, and especially the brood. The poorly-fed brood then emerges already nutritionally stressed, and may not even live long enough to become productive foragers. Without foragers, the colony is toast!
However, there are also surprising, and seemingly contrary, observations from Spain. Infected colonies may actually increase the number of frames of brood, even when the climatic conditions are not the optimum for broodrearing! Also, ceranae infection does not disappear during good foraging periods, when plenty of pollen and nectar is being collected. In Spain, nutritional stress (at least at colony level) does not appear to be a prerequisite for N. ceranae problems—however, I wonder whether the disease itself creates enough nutritional stress to start a feedback loop. (The astute reader may have noted the similarity of the above observations to CCD symptoms).
On the subject of CCD, we’ve had recurrent bouts of colony collapses for as far back as anyone can remember. One would do well to read Andy Nachbauer’s (1996) column, written about beekeeping prior to varroa, N. ceranae, imidacloprid, or IAPV, in which he describes “Stress Accelerated Decline” (seems that there’s very little new under the sun, as far as beekeeping is concerned). Even if CCD proves to be due to something novel, beekeepers will still have to deal with recurrent weather-modulated nutritional stress events. When bees are stressed, nosema and viruses thrive, and colonies collapse. We can’t do much about the weather or viruses, but we can sure help our bees with nutrition, and by keeping an eye on nosema, whether it be apis or ceranae.
I have great faith in the bee researchers to eventually figure out how we can deal with Nosema ceranae. Currently, there are many unanswered questions (heck, we don’t even know some of the questions to ask!) about this critter, its interactions with nutrition, varroa, and other pathogens, or how it fits into the CCD picture. I’m in no way trying to second guess the CCD researchers, but one can’t help but notice how the symptoms of ceranae fit closely to many of the problems that beekeepers have reported, and appear to coincide with its invasion of the continent. Independent of CCD, though, yet another added burden has clearly been placed upon our poor bees, and beekeepers need to be aware of it. However, I’m always the optimist, and have no doubt that we and our bees will pull through once again.
Upcoming: In subsequent articles I will describe management techniques and treatments for nosema, including alternatives to fumigillin. Then I will cover sampling methods and considerations, with a photo essay on simplified techniques for taking spore counts yourself.
References and Resources
A wonderful drawing of Nosema life cycle:(Broken Link!) http://parasitology.informatik.uni-wuerzburg.de/login/b/me14229.png.php
For great photomicrographs of nosema, see the ppt presentation by Dr. Ingemar Fries:
(Broken Link!)
http://www.dipucordoba.es/medioambiente/pdf/XJornadasApiPonencia01.pdf
A great review on N. apis from a California perspective: Mussen, Eric (2002) Diagnosing and Treating Nosema Disease. (Broken Link! http://entomology.ucdavis.edu/faculty/Mussen/beebriefs/Nosema_Disease.pdf
An excellent review on N. ceranae: Flottum, K. (2007) Know about Nosema ceranae. Bee Culture 135(5): 12-14.
A good Australian summary on N. apis (Broken Link!) http://www.rirdc.gov.au/reports/HBE/05-055.pdf
Anon (2004) Nosemosis of Bees in Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 5th Ed. World Organisation for Animal Health (Broken Link!) http://www.oie.int/eng/Normes/mmanual/A_00123.htm
Bauer, Leah S.; Miller, Deborah L.; Maddox, Joseph V.; McManus, Michael L. (1998) Interaction between a Nosema sp. (Microspora: Nosematidae) and Nuclear Polyhedrosis Virus Infecting the Gypsy Moth, Lymantria dispar (Lepidoptera: Lymantriidae). Journal of Invertebrate Pathology. Vol. 74 no.1.:p. 147-153.
Carreck, N. (1997) The IACR-Rothamsted Varroa Project. http://www.ibiblio.org/pub/academic/agriculture/entomology/beekeeping/general/biology/varroa_virus.txt
Chauzat M-P, Mariano Higes, Raquel Martín-Hernández, Aranzazu Meana, Nicolas Cougoule and Jean-Paul Faucon (2007) Presence of Nosema ceranae in French honey bee colonies. Journal of Apicultural Research 46(2): 127–128
Chen, Yanping , (Broken Link!) Jay D Evans , I Bart Smith , Jeffery S Pettis (2007) Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J Invertebr Pathol. 2007 Aug 6; : 17880997
Cox-Foster (2005) Colony Collapse Disorder—Determining the causes. http://www.ucs.iastate.edu/mnet/_repository/2005/plantbee/pdf/prese ntations/coxfoster.pdf
Cox-Foster, and others too numerous to mention (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318(5848): 283-287.
Delaguila C , F. Izquierdo, P.G. Palencia, R. Martin , M. Higes, S. Fenoy (2006) First Steps Towards The In vitro Cultivation of Nosema ceranae. Proceedings of the Second European Conference of Apidology EurBee
Fries I, R Martín,A Meana, P García-Palencia,M Higes (2006) Natural infections of Nosema ceranae in European honey bees. Journal of Apicultural Research 45(3): 230–233 (2006)
Flottum, K (2007) Know about Nosema ceranae. Bee Culture 135(5): 12-13.
Gliñski, Z & J. Jarosz (2001) Infection and immunity in the honey bee Apis mellifera. Apiacta 36(1), 12 – 24.
Higes M, Garcia-Palencia P, Martin-Hernandez R and Meana A. (2007) Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J Invertebr Pathol. 94(3):211-7
Higes, Mariano , Raquel Martín-Hernández , Encarna Garrido-Bailón , Pilar García-Palencia , Aránzazu Meana (2007) Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J Invertebr Pathol. 2007 Jun 20; : 17651750
Higes M, Martín R, Meana A., J (2006) Nosema ceranae, a new microsporidian parasite in honeybees in Europe, Invertebr Pathol. 92(2):93-5.
Hornitzky (2005) Nosema Disease in Honeybees. (Broken Link!) http://www.rirdc.gov.au/reports/HBE/05-055.pdf
Klee, J., et al. (2007) Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol.. doi:10.1016/
Kralj J, S. Fuchs, J. Tautz (2006) Disease removal by altered flight behavior of forager honey bees (Apis mellifera) infested with Nosema apis. Proceedings of the Second European Conference of Apidology EurBee
Manning R,, Kate Lancaster, April Rutkay and Linda Eaton (2007) Survey of feral honey bee (Apis mellifera) colonies for Nosema apis in Western Australia. Australian Journal of Experimental Agriculture (47): 883–886. www.publish.csiro.au/journals/ajea
Martín-Hernández, Raquel, Aránzazu Meana, Lourdes Prieto, Amparo Martínez Salvador, Encarna Garrido-Bailón, and Mariano Higes (2007) The outcome of the colonization of Apis mellifera by Nosema ceranae. Applied and Environmental Microbiology http://aem.asm.org/cgi/content/abstract/AEM.00270-07v1
Nachbauer, Andy (1996) SAD & BAD Bees (Broken Link!) http://www.beesource.com/pov/andy/sad_bad.htm
Otteni, M & W. Ritter (2004) Effects of the acute paralysis virus on honey bees (Apis mellifera l.) infested by Nosema apis Z. Apiacta 39: 91-97.
Paxton, RJ (2007) Nosema ceranae – a new threat to Apis mellifera honey bees. Bees for Development Journal 81.
Raven, W. (2007) Bee deviled: Why are our honeybees vanishing? UCSF Sleuths Identify Suspects in Mystery of Vanishing Honeybees. UCFS Today (Broken Link!) http://pub.ucsf.edu/today/cache/feature/200704251.html
Rice, RN (2001) Nosema in honeybees, Genetic variation and control. Pub 01/46 Rural Industries Research and Development Corp, Australia
(Broken Link!) http://www.rirdc.gov.au/reports/HBE/01-046.pdf Nosema Disease in Honeybees
Sanford, MT (2007) A new nosema. Bee Culture 135(2): 18-20.
Williams, G.R. et al., (2007) First detection of Nosema ceranae, a microsporidian parasite of European honey bees (Apis mellifera), in Canada and central USA., J. Invertebr. Pathol. (2007), doi:10.1016/j.jip.2007.08.005
Yang X, Cox-Foster DL (2005) Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification. Proc Natl Acad Sci U S A 2005, 102:7470-7475.



