The Primer Pheromones and Managing the Labor Pool – Part 1
The Primer Pheromones and Managing the Labor Pool: Part 1
Randy Oliver
ScientificBeekeeping.com
First published in American Bee Journal April 2010
I’ve spent my adult life trying to understand the minds of women and bees; I find female bees to be far more predictable. In many aspects of bee behavior, there are relatively straightforward stimulus/response effects. In this article I hope to summarize the current state of knowledge on how the colony allocates its work force, and what initiates the various forms of colony reproduction. The better we understand what drives colony behaviors, the better beekeepers we can be! With that point in mind, I’ve started inserting “Practical Applications” into my articles, to show how the understanding of bee biology is the basis for practical management decisions—some standard maxims, some perhaps more innovative or alternative.
The Thinking Colony
We humans tend to anthropomorphize animal behavior, which is often just plain silly. The most caring, gentle, nurturing beekeeper can pamper a hive to no end, yet will still have her friendship rewarded with a volley of stings to the face should she move a bit too quickly. Bees simply do not “think” like humans, and the romantic notion that a colony “deliberates, feels, or plans” is merely metaphorical.
Lest I sound callous, a bee colony sure acts as though it “thinks,” but one must realize that any actual action is performed by individual bees responding to simple stimuli—any “thought” would only apply to the behavior of a single bee. The amazing thing is that the overall result is a democratic and wondrous organization of colony function.
Let me give you an example. Every beekeeper is familiar with how a frame from the center of the broodnest looks—an oval of brood, with an arc of pollen above it, then open nectar or honey, then capped honey at the top and sides (Fig. 1). Consistent, predictable, and clearly designed for efficiency. But think about it—it’s pitch black inside the hive, and no single bee has much of an idea what is going on even as close as an inch away. So how the heck do they create such a consistent pattern without a plan or foreman?
Figure 1. A frame of brood generally has a consistent pattern—an arc of pollen above the brood, open nectar above that, and capped honey at the top and sides. Note how, in response to smoke, some bees have run to gorge on open nectar.
A groundbreaking study by Dr. Scott Camazine (1990, 2001) details how such apparently coordinated efforts can be produced simply by each individual bee following simple “context-dependent behaviors.” The term that is used for such a process is “self organization of biological systems.”
Dr. Brian Johnson (2009a) recently created a model for the formation of the typical broodframe pattern that expands upon Camazine’s work. Johnson’s model incorporates four context-dependent behaviors, with bees moving randomly until they find what they are seeking. Greatly simplified, the behaviors are:
1. The queen lays an egg if she finds an empty cell that is within four cells of another brood cell. She starts near the center of the frame, and quickens her movement if she finds herself walking over cells of nectar.
2. Pollen foragers seek cells near open brood in which to store pollen, otherwise they deposit it randomly in an empty cell.
3. Nectar receivers walk upward until they find an empty or partially full cell in which to unload.
4. Nurses search randomly for food, starting in the brood nest (note that the model does not incorporate a sense of smell).
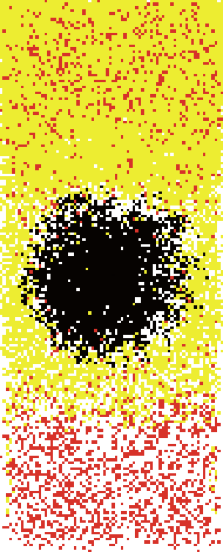
Figure 2 is a series of plots from a computer simulation produced by Johnson’s model. Note how closely it approximates the “real thing.” His paper is freely downloadable, and worth the read, as are two other recent papers (Johnson 2009b, 2010).
    |
| Day 1 | Day 4 | Day 7 | Day 14 |
| Figure 2. A simulation of the formation of the typical brood nest pattern during a honeyflow, with rain on days 9-11. White = empty cells, yellow = honey, red = pollen, and black = brood. Note how in this simple “self-organizing” model, pollen is stored initially mainly below the brood, but is then consumed during the rainy period, and by Day 14 is beginning to form the typical band above the brood. My appreciation to Dr. Brian Johnson for running these plots for this article. For details, see Johnson (2009a). |
Practical application (beginners): Note how the behavior of nectar receivers is to walk upward until they find drawn comb in which to unload. However, they do not “expect” there to be empty comb above the honey band at the top of the brood frames. Therefore, if you place a super of foundation above a queen excluder, the bees may ignore it, yet fill every cell of the brood chamber below the excluder with nectar before they will work up through the excluder. It helps to either pull the excluder temporarily, or to “bait” the super with a drawn comb (or better yet, frames of brood), or to reverse the brood chambers to make sure that there is not a band of honey directly below the excluder.
Practical application (comb honey producers): Similarly to the tip above, crowding the broodnest into a single, so that the brood displaces the honey band, will encourage the bees to fill comb honey supers directly above the excluder.
Practical application (pollinators): Dr. Frank Eischen (in prep) found that “the crescent-shaped space above the broodnest that is used for pollen storage is a critical factor in driving pollen collection” in almond orchards. Colonies that plug out have little reason to collect pollen.
What scientists often do when they want to understand some aspect of bee behavior is to take a reductionist approach, and try to determine the “proximate cause” (the trigger that leads directly to an outcome) for that behavior. The point of models, such as Johnson’s above, is to test our understanding of the “cause and effect,” “stimulus/response,” or “activator/inhibitor” processes in a system, by seeing if the model replicates what we observe in nature.
In this article, I am going to take a similar approach, and look at the mechanisms involved in the management of the major functions necessary for colony success: broodrearing, foraging, supersedure, swarming, and wintering. Surprisingly, it appears that the proximate causes and regulation of all these behaviors are largely controlled by pollen income, the in-hive transfer of jelly, and three primer pheromones!
Colony Communication & Pheromones
Definitions: Any substance, produced by an organism, that provokes a response in another organism is generically called a semiochemical; if the response is in an individual of the same species, it is called a pheromone; if it benefits another species (such as a parasite) it is called a kairomone (I mention this one because you will be hearing much more about kairomones when we get to varroa behavior).
The communication of information is the fabric of bee society, largely taking place within the dark confines of the hive. It is important to realize that the vast majority of every bee’s life is spent in complete, or near complete darkness. Since sight is useless under such conditions, bees must communicate by vibration and sound (waggle and tremble dances, buzz run, piping, etc.), touch (stop signal, shaking signal), smell, and taste. I’m going to leave the discussion of their senses of vibration and touch for another time, and focus on taste and smell.
The economic news of the hive is largely communicated by the sharing of food, and especially by the bees’ exquisite sense of smell. Any beekeeper is familiar with the rich aromas of the hive–beeswax, propolis, floral scents of the nectar and pollen, and of the bees themselves. However, we are virtually “deaf and blind” to the major “language” of the colony—the pheromones. Of the numerous (over 50) pheromonal components produced by bees, we humans generally only perceive two at best—the “banana-rose” odor of alarm pheromone, and the “lemon geranium” smell of the orientation pheromone.
Even a cursory review of the honeybee’s chemical communication repertoire reveals a rich vocabulary. Work by Drs. Yves LeConte and Tanya Pankiw have demonstrated how the major pheromones are composed of several different components (generally aromatic esters) which can vary in proportion, depending upon what the bee “wants to say.” Think of the individual components as words that can be used to phrase sentences, complete with nuance and emphasis. For example, the proportion of each component of brood pheromone differs between that of male or female larvae; young or older larvae; hungry larvae, satiated larvae; or those ready to be capped over (Table 1).
Table 1. Components of the brood pheromone from two different-aged larvae. Note how greatly the proportions of some components differ. All the compounds are esters—formed by combining an alcohol (in these cases ethyl or methyl alcohols) and an organic acid. These particular esters are all formed from long-chain fatty acids typically found in vegetable oils (linoleic, oleic, and palmitic acids)–thus they are not very volatile. From United States Patent US6595828.
Pheromones differ greatly in their degree of volatility—some, such as alarm pheromone, evaporate quickly, waft through the air, and then dissipate. Others are more “oily” and need to be physically transferred from bee to bee. In general, the more volatile act as “releaser” pheromones that elicit short-term behavioral responses (such as homing toward orientation pheromone). The less volatile function as “primer” pheromones, which cause longer-term physiological changes, such as ovary suppression in workers, change in brain function, or vitellogenin production in nurse bees. In some cases (such as with brood or alarm pheromones) a single pheromone can elicit both behavioral and physiological effects (Alaux 2007, LeConte 2001).
To an individual bee (which has a sense of smell that’s better than a bloodhound’s), the sum of all the aromas of the hive create a unique and ever changing olfactory assessment of the colony milieu. A bee doesn’t need to read the paper to get the news and an economic report—that information is continually shared in the gossip of ritualistic trophallaxis.
Trophallaxis
It’s easy for us to understand how volatile pheromones can waft through the hive carried in the circulating air, but the means of transport of the nonvolatiles is truly amazing. We’ve all seen bees sharing food on the comb via the face-to-face ritual of trophallaxis. In one classic study (Nixon & Ribbands 1952), researchers found that a colony given a few teaspoons of radioactively labeled syrup spread it evenly among 98% of the sampled nestmates within 48 hours. The efficient circulation of pheromones in the bee superorganism via trophallaxis is akin to the circulation of hormones in the blood of the human circulatory system.
Practical application: when one gives medications to a colony, they are generally quickly spread to all members. The amount of syrup, sugar dust, or other vehicle is generally not as critical as that of getting the right dose of active ingredient into the hive in one form or another.
Most everyone assumes that trophallaxis is all about the exchange of food. Surprisingly, researchers have found that in the vast majority of trophallactic interactions, no food is exchanged whatsoever! Most of the time, the bees are only passing pheromones, largely via the tapping of their antennae. The hundreds of thousands of trophallactic interactions in a hive each day function as a vast and intricate interactive communication network (Fig. 3).
Practical application: each time you smoke a colony, the communication network is disrupted, since smoke affects the bee sense of smell (Visscher 1995). There is little research as to the effects of various medications, essential oils, or pesticides on colony communication.
Figure 3. Bees engaged in trophallaxis. Note the touching of antennae, which serves to transfer pheromones from bee to bee. The group at the bottom appear to be sharing food; the pair at the upper left are sharing only information. Photo courtesy Dr. Zachary Huang.
Let’s return to the workings of the bee economy. Johnson’s model for the arrangement of brood and pollen was relatively simple. Pheromonal communication is far more complex. In an excellent review of the subject, Slessor, Winston, and LeConte (2005) state:
“Recent studies have demonstrated a remarkable and unexpected complexity in social insect pheromone communication, particularly for honeybees…. The intricate interactions characteristic of social insects demand a complex language, based on specialized chemical signals that provide a syntax that is deeper in complexity and richer in nuance than previously imagined.”
The complexity and nuance mentioned clearly apply to two of the multicomponent primer pheromones—brood pheromone and queen pheromone. Please realize that we’ve only scratched the surface in our understanding of this pheromonal communication interplay. Allow me to quote from Sarah Kocher’s (2009) doctoral dissertation:
“The results of these studies demonstrate that the chemical communication system between honey bee queens and workers acts a dialog, rather than a simple, static signal-response system, and that variation in pheromone production and response both play a critical role in modulating queen-worker interactions within the hive.”
Please humor me as I give you an analogy. Let’s say that you ask a woman, “How are you feeling?” A simple sentence, composed of simple words. But imagine the differences in meaning depending upon which word you accent. If you accentuate the first word, you sound like a doctor. Stress the second, and you are a friend asking for an update. The third, you are comparing her feelings to yours. Emphasize the last to express concern for her emotions (believe me, I’ve learned to watch inflection carefully!).
Even with the variation of meaning demonstrated above, that’s only the half of it! You must put any of those inflections into context. Is she having a bad day? Did you have an argument yesterday? Is she exhausted, excited, ill, angry, lonely, emotional, hormonal, or feeling loving at the moment? (Or any of the gazillion other unfathomable “primers” that affect how a woman takes anything that a man says).
This is apparently how it is with bee pheromonal communication. Any exchange of information via pheromones can exhibit inflection, and is highly context dependent—the message can be tweaked by either the sender or the recipient. Although we use the term “brood pheromone” generically, a hungry young larva makes a very different statement than does an older larva ready to pupate; and the responses to the same pheromonal composition will be quite different from a nurse bee or a forager (foragers ignore larvae).
Perhaps because of that complexity, it appears that we can explain much of the communication involved in organization of the bee economy based solely upon three primer pheromones of paramount importance: brood pheromone, queen pheromone, and ethyl oleate produced by foragers.
I’m out of space now; next month I’ll diagram just how the system works…
Acknowledgements
I could not begin to compile the information necessary for my writing without the steady assistance and suggestions by my friend and collaborator Peter Loring Borst, to whom I am again indebted. I would also be greatly remiss not to tip my hat to that great bee researcher, Dr. Tom Seeley, for his immense influence in the field of bee behavior (both Scott Camazine and Brian Johnson were PhD students of his!).
References
Alaux, C. and G.E. Robinson 2007. Alarm pheromone induces immediate-early gene expression and slow behavioral response in honey bees. J. Chem. Ecol. 33: 1346-1350.
Camazine, S., J. Sneyd, M. J. Jenkins, and J. D. Murray (1990) A mathematical model of self-organized pattern formation on the combs of honeybee colonies. J. Theor. Biol. 147: 553–571.
Camazine, S., Deneubourg, J., Franks, N. R., Sneyd, J., Theraulaz, G. & Bonabeau, E. (2001) Self-organization in biological systems. Princeton,NJ: Princeton University Press.
Johnson, BR (2009a) Pattern formation on the combs of honeybees: increasing fitness by coupling self-organization with templates. Proceedings of the Royal Society B: Biological Sciences 276:255–261. Free download at http://rspb.royalsocietypublishing.org/content/276/1655/255.full.pdf+html
Johnson, BR (2009b) A self-organizing model for task allocation via frequent task quitting and random walks in the honeybee. The American Naturalist 174(4): 537–547.
Kocher (nee Ayroles) (2009) Molecular and physiological mechanisms underlying chemical communication in the honey bee, Apis mellifera. http://www.lib.ncsu.edu/theses/available/etd-08112009-235911/
LeConte Y, Mohammedi A, Robinson GE (2001) Primer effects of a brood pheromone on honeybee behavioural development. Proc R Soc Lond B 268:1–6.
Nixon, HL and CR Ribbands (1952) Food transmission within the honeybee community. Proceedings of the Royal Society B 140: 43-50.
Visscher, P.K., R.S. Vetter and G.E. Robinson. 1995. Alarm pheromone perception in honey bees is decreased by smoke (Hymenoptera: Apidae). J. Insect Behav. 8: 11-18.
Suggested Review Papers
I recommend the following for those who wish to delve deeper.
Johnson, BR (2010) Division of labor in honeybees: form, function, and proximate mechanisms (Review). Behav Ecol Sociobiol 64:305–316. Free download. (Broken Link!) http://berkeley.academia.edu/documents/0065/9441/Johnson_2010a.pdf
Moritz, RFA (1994) Nourishment and sociality in honeybees. Pp 345-390 in Hunt, JH and CA Nalepa, eds. Nourishment and Evolution in Insect Societies. Westview Press, Boulder, CO.
Pankiw, T (2004) Cued in: honey bee pheromones as information flow and collective decision-making. Apidologie 35: 217–226
Slessor KN, ML Winston, Y LeConte (2005) Pheromone communication in the honeybee (Apis mellifera L.). J Chem Ecol. 2005;31:2731–2745.