The Varroa Problem: Part 8 – Regulatory Cascades, Varroa Tolerance, and a Moon Shot
The Varroa Problem: Part 8
Regulatory Cascades, Varroa Tolerance, and a Moon Shot
Randy Oliver
ScientificBeekeeping.com
First published in ABJ June 2017
In writing this series, I skipped ahead over some details so that I could publish my suggestions for setting up a breeding program for mite resistance in time for this season’s queen rearing. I now return to pick up some of the pieces.
Do we need new genes for mite resistance?
In any selective breeding program, what you’ve got to work with is the innate amount of variability in heritable traits within the breeding population. And although we loosely speak of differences in “genes,” what we are really generally referring to are differences in the expression of genes. With regard to breeding for resistance to varroa, it is the expression of genes that result in phenotypic traits which we can observe and select for, such as morphology (the form and structure of the bees’ bodies), physiology (the biological functioning of the body, the immune system, pheromone production and olfactory sensing), and behavior (grooming, VSH, etc.).
It’s important to understand that there’s a big difference between genotype and phenotype. A bee’s inherited genes (the genotype) are like a library of instructions; the expression of those genes (the phenotype) is the choosing of which parts of the library to read, and then how those genes are used for the construction and function of the actual bee. This expression of the genetic material is done by genetic regulatory networks [[1]].
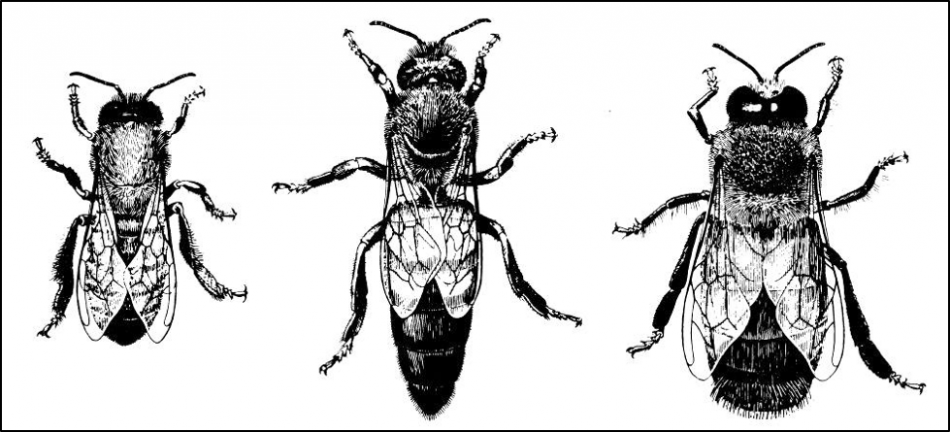
Think on this–any individual fertilized egg has the potential of developing into either a worker, a queen, or a diploid drone—there is absolutely no genetic difference (other than in the case of the females, which must exhibit slightly different alleles at only one single gene) [[2]]. By their looks and behavior, one might think that the three bees in Figure 1 below were different species entirely. Yet those differences in form and function were not due to any difference in their genetics whatsoever, but rather due to the initiation of different regulatory cascades of their identical genes.
Fig. 1. If one didn’t know better, it would appear that we were looking at three different species of bees, rather that the two castes of females and the male of Apis mellifera. This is an example of how identical genetics can be expressed in very different forms as a result of flipping switches in the regulatory networks. This detailed illustration is taken from Norbert Kauffeld [[3]].
And that’s only the half of it—the same identical genes also coded for the each developmental stage of the above bees—the larva, the pupa, and the adult again each look like entirely different organisms. And then once a worker emerges as an adult, her behavior and physiology can shift from being a nurse, a wax producer, a forager, a laying worker, or a long-lived diutinus bee. Have I made it clear that despite possessing identical genetics, one can get all sorts of forms and functions out of an egg’s genes simply by how the regulatory networks express those genes?
What biologists say is that the honey bee has great “phenotypic plasticity”—from any inherited set of genetic “instructions,” it can grow into different forms, or express different biological functions or behaviors.
Practical application: The point that I’m trying to make is that we may have all the “genes” necessary for mite resistance already built into the North American bee population—we may need only to select for heritable changes in the bees’ genetic or epigenetic regulatory systems. I will return to this concept near the end of this article.
Resistance vs. tolerance
Another important detail that I’d like to cover is the difference between two often-confused terms—resistance and tolerance. These two terms have well-defined biological meanings [[4]]:
Tolerance: the ability of a host to limit the damage caused by a given parasite burden.
Resistance: the ability of a host to limit parasite burden (read that, the buildup of the mite level).
We don’t need to breed for varroa-tolerant bees—our bees were already quite tolerant when varroa first invaded. Back then, we could allow varroa to build up until the colony was literally crawling with mites, and notice no sign of harm so long as we hit the hive with a single treatment of Apistan each fall [[5]].
All that changed as the viruses evolved to take advantage of varroa as a vector. Dr. Stephen Martin was the first to deduce that it was an in-hive epidemic of virus, rather than the mite, that was the real problem, although he had a helluva time convincing the research community that this was true [[6]].
After a few years of evolution, in most every country (South Africa being an exception) we watched Deformed Wing Virus (DWV) evolve into a more virulent form [[7]], and colonies would collapse at mite levels that were previously well-tolerated [[8]].
Practical application: By definition, varroa-tolerant bees do not suppress mite buildup. So what we’re really talking about when we speak of tolerance is the ability to not be harmed by mite-vectored viruses.
Examples of tolerance
I observe signs of tolerance in the feral Africanized bees, in some colonies of Primorsky Russians, and in the occasional hive of my own Italian stock. Such tolerant colonies sometimes have quite high mite levels, but their brood patterns remain solid and healthy, and there is no sign of deformed wings or illness in the adult workers. Technically, although these bees might be called varroa tolerant, in fact such tolerance is likely due to their being virus-resistant (see [[9]]).
Alternatively, the bee might collaborate with a benign virus in order to confer resistance to a more virulent strain. Strains of virus compete against each other, and a recent study [[10]] suggests that by harboring a benign strain of DWV, that benign strain can outcompete the virulent strain (I’ve recently coordinated sampling from across the U.S. to determine whether this is happening here). Thus, a line of bees might be able to inherit tolerance to DWV by inheriting the protective benign strain transovarially from their queen.
Practical application: of course, breeding for tolerance of varroa-vectored virus(es) would be a good thing, but due to varroa’s exponential growth, the mite would eventually cause even tolerant colonies to collapse.
And here is where strict interpretation of definitions gets sticky. Nature (the environment) may select for some combination of both tolerance and resistance. As Sorci [[11]] points out:
The task of the immune system is not necessarily to clear the infection. In many cases, it might be more rewarding to coexist with the parasite instead of declaring the war.
A case in point is the host/parasite relationship between varroa and its natural host, Apis cerana. The bee apparently found it to be most adaptive to not waste any more energy in resistance than necessary to hold varroa to a tolerable level.
Keep in mind that Sorci also notes that:
An alternative view suggests that tolerance reduces the cost of virulence traits for highly exploitative parasite strains (infected hosts tolerate the infection and the parasite achieves its transmission). Therefore, tolerant hosts might actually select for more virulent parasites.
Practical application: although “varroa tolerance” has a nice ring to it, we must keep in mind that we want bees that tolerate varroa only at a very low level in the hive.
An inherent problem with managed bees
Dr. Tom Seeley, in two recent papers, has pointed out that there are substantial differences between wild and managed bee colonies [[12]]. Studying feral colonies, as well as unmanaged colonies maintained in a single Langstroth deep, Seeley and his team found that those colonies somehow manage to restrict varroa buildup—thus by definition, a form of resistance. Such suppression of varroa buildup appears to be due to those colonies’ restriction of broodrearing and frequent swarming [[13]]. Note that both the Africanized ferals and Primorsky Russian bees also swarm frequently, plus shut down broodrearing during pollen dearths, resulting in reduced mite buildup.
Figure 2. A varroa restaurant. Those beautiful brood patterns that we so love can also be considered as all-you-can eat buffets for the varroa mite. This is why our strongest colonies often have the worst problems with mites come fall.
Practical application: As I’ve pointed out before, we beekeepers are part of the problem (Fig. 2). When we manage our hives for productivity (by encouraging early spring buildup, large broodnests, and by restricting swarming), we are also producing more varroa food (sealed brood) over a longer period of time.
On the other hand, as someone who makes his living from supplying large colonies to the almond orchards, by selling excess bees, and by producing honey, I would go broke if I kept only small, swarmy colonies. Thus, I definitely want to breed for resistance to varroa buildup, since so long as mite levels are low, there’s really no need for tolerance.
That then introduces another concept—intolerance. Intolerance of varroa (and viruses) at the individual bee level can result in resistance at the colony level.
intolerance as form of resistance
The flip side of tolerance would be “intolerance” (I’m using the term “intolerance” here in a way that has not yet been vetted by the research community). Intolerance can actually result in a very strong form of resistance. For example, let’s say that there was a line of bees exhibiting the trait of being completely intolerant of allowing a mite to hitchhike on their bodies—to the extent that such a bee would immediately rush to the hive entrance and commit “altruist self removal” (fly off and not return). Such an individual-level trait would, at the colony level, make it impossible for varroa to ever establish a population in the hive. End of The Varroa Problem.
An evolutionary point: as recently pointed out to me by Dr. David De Jong, the intolerance of drone pupae to damage by varroa (drones damaged by varroa during pupal development stand little chance of successfully mating), exerts very strong selective pressure for unmanaged bee populations to evolve resistance to varroa.
Returning to my hypothetical example of varroa-intolerant workers, most species do not have the option of exhibiting such extreme intolerance of parasites, since self sacrifice prior to reproduction would be an evolutionary dead end. Not so for social insects.
In the superorganism that we know as the honey bee “colony,” any single bee is somewhat analogous to a single cell of a multicellular organism. From an evolutionary perspective, the survival of that bee’s genetics (carried by its sister queens and brother drones) is more important than its own survival as an individual. Thus, any member can and will sacrifice itself for the good of the colony. We’re all well aware that a worker will not hesitate to sacrifice itself in defense of the colony by stinging, drones do it in the act of mating, and even a failing queen can give her workers a signal to supersede her. Even more regularly, any sick worker will commit altruistic self removal at the drop of a hat— should it start to feel sick, it simply flies out of the hive, never to return [[14]].
Anyway, the most effective mite-resistance trait yet identified, extreme varroa-sensitive hygiene (VSH), turns out to be based upon the intolerance of pupae to being bitten by varroa, as recently elucidated in a fascinating study. The discovery process of the details of the mechanisms involved in VSH makes for an interesting story.
VSH: A story of discovery
(For those interested in deeper information, refer to my end notes throughout the following discussion).
We’ve long known that varroa’s original host, Apis cerana (Fig. 3.), exhibits strong resistance to the mite—as evidence by its ability to prevent the mite from building up to damaging levels in the hive. The Asian bee utilizes a variety of behaviors to suppress mite buildup, such as fervent grooming behavior, frequent swarming, and absconding [[15]]. However, its main mechanism of resistance appears to be due to the fact that varroa rarely attempts to reproduce in its worker brood, thus limiting its reproduction to cerana’s limited amount of drone brood [[16]].
Figure 3. Queen and workers of the Asian bee, Apis cerana, the original host of varroa. Unlike Apis mellifera, the Asian bee coevolved with varroa, and exhibits strong resistance to the mite. Photograph by Azman [[17]].
Practical application: the Achille’s heel of varroa is its need for abundant brood cells in which it can successfully reproduce. The question then is why the mite does not normally attempt to reproduce in Apis cerana worker brood? There must be some evolutionary reason. If we could figure that reason out, we might be able to help our bees to conquer varroa.
It’s been clearly demonstrated that a foundress mite can differentiate between worker and drone prepupae by their pheromonal and cuticular odors [[18]], but why do they avoid cerana worker cells, and even if they enter one, tend not to ovulate? Evolutionarily, there must have been an adaptive reason for the mites not to do so. Could it be that because ceranae was able to make it futile for varroa to attempt to reproduce in worker brood, that it would then be nonadaptive for the mite to expose itself to the danger of doing so? A potential mechanism to achieve this futility might be the observed high degree of hygienic behavior (Fig. 4) [[19]] exhibited by cerana toward infested worker brood.
Figure 4. Bees exhibiting some degree of varroa-sensitive hygiene (VSH), as evidenced by the white pupae being chewed out (indicating that the pupae were being removed while still alive). However, as evidenced by the presence of at least five infested cells in this photo (as well as the mite on the bee’s thorax), this colony’s VSH behavior appears to have been too little, too late. Also note the small inspection hole chewed through the cell cap directly above the mite-carrying bee.
Perhaps the most important question of interest is, what cues mite-resistant bees to uncap and remove infested worker pupae? A great deal of meticulous research (sometimes with conflicting findings) has gone into answering this question. Aumeier [[20]] found that it wasn’t the movement of mites that triggered VSH, that Africanized bees often removed the mites without damaging the brood, and that the trigger appeared to be olfactory. They concluded that:
In the case of mite-infested brood in A. mellifera colonies, however, the scent or signs of life of the parasite appear to have low importance as recognition cues.
Shortly afterward, the Le Conte lab [[21]] found that there were differences in the volatile compounds within infested cells compared to uninfested cells, some of which bees could detect, and some of which resistant bees appeared to exhibit greater antennal response to. But this did not appear to be the odor of the mite itself, and his group later discovered that a mite quickly absorbs the odor of its host [[22]]. This “olfactory camouflage” helps prevent the bees from detecting a mite hidden on an adult body (preventing grooming response), or beneath the cell cap.
So the question remained whether the trigger for VSH was the actual detection of varroa (or varroa reproduction [[23]]), or a signal from the infested pupa, as suggested by Nazzi [[24]]:
[Some] olfactory cues coming from the infested larva may be involved in the recognition of infested cells by bees.
Then Schöning [[25]] demonstrated that the putative “varroa-sensitive” hygienic behavior was actually triggered by olfactory signals put off by “damaged” brood, including that damaged by an uncontrolled infection by DWV:
Our results suggest that bees show selective, damage-dependent hygienic behaviour, which may be an economic way for colonies to cope with mite infestation.
Recently, Mondet performed a brilliant study, from which she was able to elaborate on the olfactory mechanism involved in VSH [[26]]. She found that virus-infected and developmentally-delayed prepupae and pupa are sacrificed due to their emitting of altered brood ester pheromone (BEP)—more so when infected by Kashmir Bee Virus (KBV) than with DWV. Since she, despite being a scientist, is able to compose sentences comprehensible to the lay reader, I’ll allow her to speak for herself in some snips that I’ve lifted from her paper:
Here we show that varroa-infested brood produce uniquely identifiable cues that could be used by VSH-performing bees to identify with high specificity which brood cells to sacrifice. This selective elimination of mite-infested brood is a disease resistance strategy analogous to programmed cell death [remember this term], where young bees likely to be highly dysfunctional as adults are sacrificed for the greater good of the colony.
The results also imply that infested brood has a much better chance of escaping VSH detection with DWV infection than with KBV infection, thereby providing a mechanistic explanation for both the gradual disappearance of ABPV complex (i.e. KBV) and the long-term persistence of DWV in newly varroa-infested colonies.
A significant additional factor is that VSH behaviour appears also to be dependent on the sensory acuity of the detecting bees, which may be compromised by the same viruses facilitating the detection of varroa-infested brood. Thus, VSH behaviour itself may also break down through varroa-transmitted virus epidemics, placing limits on its ability to control mite infestation [refer to Fig. 4 above].
Practical application: by quick removal of any prepupae or pupa that emits an olfactory signal of being sick or developmentally delayed, a colony could prevent the reproduction of any mite that transmitted a virulent virus or otherwise “damaged” its pupal host. Due to the evolutionary penalty of non reproduction in such colonies of virus–intolerant pupae, varroa would never want to vector any virulent virus that sickened its pupa, since this would bring the mite’s reproductive success to zero (refer back to my second quote from Sorci).
Thus, those studying VSH figured out that it appears to mainly be an olfactory signal from a sick or damaged pupa that triggers VSH behavior, rather the bees detecting that a mite was in the cell, as opposed to “normal” hygienic behavior (“HYG,” as determined by the freeze- or prick-killed brood assay) in which the nurses detect the odor of decomposing brood [[27]].
Practical application: VSH is a composite behavior that involves a suite of traits. The most important is for a distressed prepupae or pupa to emit a “sacrifice me for the good of the colony” signal to the nurses. Then there must be a proportion of bees of nursing age that regularly chew holes in the cappings of occupied pupal cells to “sniff” inside (and another behavior to reseal them). Those hypersensitive bees must then possess the antennal receptors to detect the pupal olfactory signals, and for those odors to then cue the behavior of a hygienic removal response. The beauty of VSH is that not every bee in the hive needs to exhibit every trait—only a proportion of the population needs to exhibit each individual trait involving in serving as “hygiene police.”
A landmark study
This finally brings my story to the landmark paper published last year. It finally answered why varroa strains adapted to Apis cerana avoid reproducing in worker cells—it’s because the worker pupae are completely intolerant of being fed upon by a mite—whether they get infected by a virus or not.
Practical application: as pointed out by Mondet, viruses cleverly fight back with subterfuge. So simple emission of virus-infection-induced pheromones may not be enough.
This brings us back to the concept of programmed cellular death (apoptosis) mentioned above. This naturally occurs in any multicellular organism during the process of development or tissue rejuvenation. It is also used as an immune defense mechanism. Let’s say that a virus or nosema manages to invade a bee’s intestinal cell. That cell, in order to prevent the pathogen from multiplying, immediately sacrifices itself [[28]]. Thus, by such apoptotic cellular self sacrifice, the bee may avoid becoming seriously infected by the pathogen.
In the bee hive, in which any individual is expendable, the altruistic suicide of any individual for the good of the colony could be considered as a form of social apoptosis [[29]], a little-used term recently resurrected by Dr. Paul Page and collaborators [[30]]. They discovered that it is the social apoptosis exhibited by Apis cerana pupae that makes its varroa-sensitive hygiene so effective—so efficient that it’s not in the mite’s interest to even attempt to reproduce in worker brood. Page found that A. cerana worker pupae are completely intolerant of being fed upon by varroa. If a mite makes the mistake of feeding on a worker (rather than a drone) pupa, the pupa emits a “sacrifice me” signal, and then simply rolls over and dies, thus preventing the mite from reproducing in that cell. In contrast, A. mellifera pupae tolerate being fed upon by a mite, and generally go on to emerge as adults (so it is clearly to the benefit of varroa to parasitize mellifera worker brood).
Even more remarkable is that when Page experimented by piercing the skin of cerana and mellifera pupae with a sterile glass pipette drawn to the size of a mite’s mouthparts, that even though most cerana pupae, and nearly all mellifera pupa exhibited the ability to heal themselves, the cerana pupae nevertheless apparently emitted a “remove me” signal to the nurses. This sort of self sacrifice thus results in cerana’s well-known efficiency at VSH in their worker brood—they don’t need to detect the mite, their brood tells them if they need to be sacrificed.
Update March 2018: The study by Page mentioned above was only the first of what I hope will be a series by a collaboration of researchers whose names I’ve included in full for their open-access paper below [[31]]. I applaud these researchers for finally clarifying critical aspects of the methods by which Apis cerana has successfully achieved a workable host/parasite relationship with varroa. I feel that they have now presented perhaps the most important and applicable findings in over a decade towards our goal of coming to terms with the mite. The team, through well-designed experiments, has found that the Korean haplotype of Varroa destructor that now plagues beekeepers across the world can indeed successfully reproduce in the worker brood of Apis cerana, and that they main method by which cerana is able to control the mite is by the “susceptibility” or self-sacrifice of parasitized brood (I prefer the term “intolerance”). In the eusocial superorganism that we know as the honey bee colony, such self-sacrifice of the first individuals to be attacked by a parasite can allow the colony as a whole to avoid a damaging infestation. The team has now also described a relatively simple assay for us to select for “varroa proof” bees.
Practical application: by being completely intolerant of varroa parasitism (by not only emitting a “sacrifice me” signal, but also by then rolling over and dying), A. cerana worker pupae limit varroa reproduction to the drone brood only, where the colony can then further limit the mite’s success. The implications of this discovery are immense. If our bees exhibited a similar trait, varroa would also be forced to limit its reproduction to the drone brood. This would be a huge step toward mite-resistant bees (and counteract the evolutionary pressure for varroa to shift its preference to worker brood in Apis mellifera) [[32]].
Practical application: In order for altruistic apoptosis to be effective, not every single pupa needs to exhibit the trait. Similar to the “herd immunity” reached by vaccination of roughly 90% of a population, one can work out the math for what proportion of pupae would need to exhibit self sacrifice in order to bring overall varroa reproductive success to zero. By my modeling, cutting the overall reproductive success of the mite by 70% (of current “normal” values) in worker brood alone would lead to a net decline of the mite population.
Practical application: One might ask, “Can we import the “intolerance genes” from Apis cerana into Apis mellifera?” Please think back to my discussion of genetic regulation at the beginning of this article. Our North American bee population already possess all the traits necessary for the social apoptosis and VSH exhibited by the pupae of Apis cerana—all that we need to do is to select for those that upregulate those traits.
Some of Apis cerana’s other tricks
OK, so perhaps we’ve now figured out the last piece of the puzzle as to how Apis cerana evolutionarily forced varroa to eschew worker brood (which is apparently why varroa was not originally a problem when it was first introduced into Apis mellifera colonies in Asia).
So the question then is what steps cerana then takes to limit varroa’s reproduction in its drone brood (other than by simply only rearing drone brood on occasion). It may have to do with the fact that A. ceranae leaves an interesting ventilation hole in the center of the cap over a drone cell (Fig. 5), thus allowing the nurses to easily smell what’s happening inside [[33]].
Figure 5. The pores in the cappings of Apis cerana drone cells. These pores allow both required ventilation through the extra-thick cap, as well as a means for the workers to get a whiff of what’s going on inside. Photo by Dr. Nikolaus Koeniger, by permission.
Should a drone pupa become sickened, that drone can then emit a pheromonal signal to the nurse bees to close the pore, resulting in the entombing both the sick pupa as well as any mites in that cell (thus putting an end to that specific mite bloodline). This puts strong evolutionary pressure upon the mites to not vector any viruses that might sicken the drone brood, nor to harm those drones in any other manner [[34]].
Practical application: a willingness by the drone pupae to perform self sacrifice again results in very strong selective pressure for varroa to be a benign parasite.
The “Moon Shot”
The groundbreaking finding by Paul Page and team (mentioned above) of what appears to be the main varroa-resistance mechanism employed by Apis cerana—“taking one for the team” to prevent oneself from being used as food for baby mites—opens my eyes to a “moonshot opportunity.” It occurs to me that we perhaps needn’t resign ourselves to being stuck with varroa at all! Natural selection may result in bees that resist varroa to the point that the damage caused by the mite is tolerable—since it might not be worth it to the bees to fight for total eradication. But that doesn’t mean that it wouldn’t be worth it for a breeder to do so.
What if we were able to select for a strain of bees in which 90% of both workers and drones exhibited self sacrifice if wounded by a mite during their pupal stage? This would absolutely arrest mite reproduction, and varroa would be completely purged from that bee population within a season—and even a stray mite could never be successful at reinvasion.
Practical application: I can see no biological reason that we couldn’t breed a bee with supersensitive pupae—pupae whose motto was, “We’re not gonna take it!” All that we’d need to screen for would be pupae that committed social apoptosis should a mite bite them.
Since such wounding of a pupae while protected by its cocoon would only occur in the case of invasion by a foundress mite, I can’t see any reason for there to be a downside for the pupae to have such a hair-trigger response to wounding (it doesn’t seem to hurt Apis cerana). So here’s my suggestion:
The Moon Shot Challenge: we could offer a $100,000 prize for the first team to breed a line of bees that exhibits complete intolerance of varroa due to pupal self sacrifice. The trait could be relatively easily selected for by the simple wounding technique used by the researchers. My pie-in-the-sky dream would be that we might be able to then cross those bees to other lines, and completely purge varroa from our bee population—forever [[35]] (hey, it doesn’t hurt to dream…).
Acknowledgements
Thanks to my research collaborator Peter Borst. And to all the dedicated bee researchers who have devoted their careers to helping to save our honey bee from the plight of varroa.
Notes and citations
[1] I’m not going to go any deeper into this fascinating subject. For an introduction, refer to https://en.wikipedia.org/wiki/Gene_regulatory_network
[2] Hold it, you say—diploid drones are inviable. Not exactly true; see
Woyke, J (1969) A method of rearing diploid drones in a honeybee colony. J. Apic. Res. 8(2) : 65-74.
As best I can tell, the default for any bee egg (fertilized or unfertilized) is to develop into a male. Only if that egg is fertilized by a sperm carrying an allele at the complimentary sex determination (csd) gene that differs from that of the egg itself will the egg develop into a female. It is the presence of two different alleles at the csd locus that triggers another gene—feminizer (fem)—to initiate the regulatory cascade for the larva to develop into a female. Without two different alleles at the csd locus, a fertilized egg will turn into a diploid drone, the larva of which is normally quickly consumed by a nurse.
[3] Kauffeld, NM (1980) Seasonal cycle of activities in honey bee colonies. In Beekeeping in the United States, Agricultural Handbook 335, USDA.
[4] Schneider, DS & JS Ayres (2008) Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases Nature Reviews/Immunology 8: 889-895.
Råberg, L, et al (2009) Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364: 37–49.
[5] During a visit to New Zealand in 2011, I was reminded of those days when I asked commercial beekeepers when they treated for mites. Their answer was invariably, “When we see mites crawling all over the bees.” It seemed like it had been ages since U.S. beekeepers had that luxury.
[6] Pers. comm., although he finally got it accepted for publication in his groundbreaking paper: Martin, SJ (2001) The role of Varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. Journal of Applied Ecology 53: 105–112. (This paper is what really kicked off my decision to start hitting the books and better educate myself about varroa). Open access. His modeling was further elaborated in:
Sumpter DJ & SJ Martin (2004) The dynamics of virus epidemics in Varroa-infested honey bee colonies. J Anim Ecol 73(1):51–63.
[7] Martin, SJ, et al (2012) Global honey bee viral landscape altered by a parasitic mite. Science 336(6086): 1304-1306.
[8] It’s still an open question as to why Apis mellifera scutellata colonies can tolerate high varroa counts. It appears to have something to do with lack of virulent DWV and other viruses (viruses are seldom detected in South African bees):
Mortensen, AN, et al (2016) Differences in Varroa destructor infestation rates of two indigenous subspecies of Apis mellifera in the Republic of South Africa. Exp Appl Acarol 68:509–515.
[9] Maori, E, et al (2007) Reciprocal sequence exchange between non-retro viruses and hosts leading to the appearance of new host phenotypes. Virology 362: 342 – 349. Short version: by incorporating a bit of viral genetic material into their own DNA, the bees can confer upon themselves a degree of resistance to that specific virus.
[10] Mordecai, GJ, et al (2015) Superinfection exclusion and the long-term survival of honey bees in Varroa-infested colonies. The ISME Journal 1 – 10. Open access
[11] Sorci, G (2013) Immunity, resistance and tolerance in bird–parasite interactions. Parasite Immunology 35(11): 350–361.
[12] Seeley, T (2017) Darwinian beekeeping: An evolutionary approach to apiculture. ABJ 157(3): 277–282.
Loftus JC, et al (2016) How Honey Bee Colonies Survive in the Wild: Testing the Importance of Small Nests and Frequent Swarming. PLoS ONE 11(3): e0150362. doi:10.1371/ journal.pone.0150362
[13] I’ve gone over the Seeley data with him and his team. In their experiment with commercial bee stock, the mite appeared to build up at a normal rate in the small hives, but is then got set back from time to time. Those setbacks did not occur in the larger hives.
[14] Rueppell, O, et al (2010) Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evol. Biol. 23: 1538–1546.
[15] Rath W (1999) Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie 30(2-3): 97-110.
[16] Reviewed in Boecking, O & M Spivak (1999) Behavioural defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30: 141–158.
[17] https://commons.wikimedia.org/wiki/File:Apis_cerana_queen_2010-04-30-_027.jpg
[18] Le Conte, Y, et al (1989) Attraction of the parasitic mite Varroa to the drone larvae of honey bees by simple aliphatic esters. Science 245: 638-639.
The late Dr. Peter Teal of ARS identified the pheromone that attracted varroa specifically to drone brood (I was fortunate to see his data and discuss this with him prior to his untimely death); unfortunately, his research was never published in a scientific journal. See: Luring varroa mites to their doom. https://agresearchmag.ars.usda.gov/AR/archive/2009/Jul/mites0709.pdf
[19] One of the pioneers in breeding for hygienic behavior and VSH was Dr. Marla Spivak: Spivak, M (1996) Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 27: 245-260.
[20] Aumeier, P & P Rosenkranz (2001) Scent or movement of Varroa destructor mites does not elicit hygienic behaviour by Africanized and Carniolan honey bees. Apidologie 32(3) :253-263.
[21] Martin, C, et al (2002) Potential mechanism for detection by Apis mellifera of the parasitic mite Varroa destructor inside sealed brood cells. Physiological Entomology 27: 175-188. Open access.
[22] Le Conte Y, et al (2015) Varroa destructor changes its cuticular hydrocarbons to mimic new hosts. Biol. Lett. 11: 20150233. Open access.
[23] It initially appeared that the bees detected when the foundress began to reproduce in the cell, but this doesn’t appear to be the case, although it’s possible that bees might be able to detect the odor of a pupal wound, mite feces, or mite mating pheromones.
Harbo, JR & JW Harris (2005) Suppressed mite reproduction explained by the behaviour of adult bees. Journal of Apicultural Research 44(1): 21–23.
[24] Nazzi, F, et al (2004) A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 35: 65–70. Nazzi was hardly the first to suggest this—it’s also mentioned by Rath and others.
[25] Schöning, C, et al (2012) Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. The Journal of Experimental Biology 215: 264-271.
[26] Mondet, F, et al (2016) Specific cues associated with honey bee social defence against Varroa destructor infested brood. Nature Scientific Reports 6:25444 DOI: 10.1038/srep25444. Open access.
[27] The discovery process of the differences between “normal” hygienic behavior (HYG), SMR, and VSH has been fascinating to follow.
Ibrahim, A & M Spivak (2006) The relationship between hygienic behavior and suppression of mite reproduction as honey bee (Apis mellifera) mechanisms of resistance to Varroa destructor. Apidologie 37: 31–40.
After the above paper, USDA dropped the term “SMR” and started using VSH. And although selecting for HYG in a mite-resistance breeding program does not appear to be as effective as selecting for VSH, it does appear to offer some benefit:
Al Toufailia, HM, et al (2014) Towards integrated control of varroa: effect of variation in hygienic behaviour among honey bee colonies on mite population increase and deformed wing virus incidence. Journal of Apicultural Research 53(5): 555-562.
As far as I can tell, the jury’s still out on the trait of SMR (suppression of mite reproduction)–which is also biologically plausible.
[28] The pathogens, not unexpectedly, have evolved to secrete proteins to suppress such apoptosis. This may be why infection by nosema (which suppresses apoptosis of the intestinal cells) may open the door for bees to become infected by Black Queen Cell Virus.
[29] “Social apoptosis” appears to be a relatively new term, with few hits in a Google search. It appears to have first been used in scientific literature (and in regard to honey bees) by:
Rueppell, O, et al (2004) From Genes to Societies. Sci. Aging Knowl. Environ. 2004(5): 4.
[30] Page, P, et al (2016) Social apoptosis in honey bee superorganisms. Nature Scientific Reports 6:27210 Open access at: http://www.nature.com/articles/srep27210 I feel that this may be one of the most important discoveries in our fight against varroa.
[31] Zheguang Lin, Yao Qin, Paul Page, Shuai Wang, Li Li, Zhengsheng Wen, Fuliang Hu, Peter Neumann, Huoqing Zheng, and Vincent Dietemann (2018) Reproduction of parasitic mites Varroa destructor in original and new honeybee hosts. Ecology and Evolution DOI: 10.1002/ece3.3802
[32] We may be seeing varroa evolve towards a preference for worker brood in Apis mellifera. I’m simply not seeing many mites in my drone brood trap frames. And a recent study from South Africa suggests that the mites there are shifting their preference away from drone brood to worker brood:
Strauss, U, et al (2016) Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology 143: 374–387.
[33] Hänel, H & F Ruttner (1985) The origin of the pore in the drone cell capping of Apis cerana Fabr. Apidologie16(2): 157-164.
[34] It appears to actually be a bit more complicated, with variation between races of Apis cerana. See:
Boecking, O (1999) Sealing up and non-removal of diseased and Varroa jacobsoni infested drone brood cells is part of the hygienic behaviour in Apis cerana. Journal ofApicultural Research, 38(3-4):159-168,
[35] I’m fully aware that the evolutionary response by varroa would be to evolve chemicals to suppress the apoptosis response. And that once varroa pressure was off, that natural selection would back off on maintaining extreme pupal sensitivity. But I don’t see that either of these facts are reasons not to pursue this line of breeding.