Selective Breeding for Mite Resistance, Part 2; Mite Resistance and Genetic Expression
January 12, 2023
Contents
The Achilles’ heel of varroa. 2
Genotype vs. Individual- or colony-level phenotype. 2
An example of the differential expression of genes in the honey bee. 4
What Is our own bees’ mechanism(s) for Resistance?. 6
Testing for Uncapping behavior 9
Is there a Cost to the colony for resistance?. 10
Our Traditional Breeding Experiment 11
Selective Breeding for Mite Resistance, Part 2
Mite Resistance and Genetic Expression
First published in ABJ in July 2022
Randy Oliver
ScientificBeekeeping.com
A selective breeding program is all about genetics. But humans bred plants and animals for thousands of years before we knew anything about DNA or genetics. What breeders selected for were traits. So how are my own varroa-resistant colonies actually preventing varroa mites from building up in their hives? And why does the trait appear to exhibit low heritability?
I’ve already mentioned that I am selecting for varroa-resistant bees, as opposed to them being merely varroa-tolerant. Resistance to varroa takes place at the colony level. But that colony-level resistance depends upon the interactions of individual bees with individual mites.
It’s easy to visualize individual bees recognizing and attacking mites (so called “grooming” and/or “biting” behaviors), but this agile parasite has evolved to avoid harm. Varroa mites are able to survive for long periods in colonies of their original host (Apis cerana), despite the fact that cerana workers vigorously attack and groom mites not only off their own bodies, but also off their nestmates. So although grooming is certainly a useful behavior to select for, it’s unlikely that it alone would be enough to confer a colony with adequate resistance [[1], [2]].
The Achilles’ heel of varroa
All mites eventually die of old age. For mites to become a problem in the hive, they must reproduce more quickly than they die. Thus, as far as a honey bee colony is concerned, varroa’s Achilles’ heel is its ability to reproduce, especially in worker brood. Thwarting this may require a “team effort” by the bees. For example, a mite-infested larva or pupa might emit an olfactory signal, or even self-sacrifice (“social apoptosis”), a nurse bee would then need to recognize and respond to that signal and initiate uncapping of the cell, and then a different worker might either remove or cannibalize that infested pupa.
My point is that all of the above responsive behaviors are controlled by genetics, and may require the combined efforts of two or more workers (as well as the infested larva or pupa), each carrying different critical alleles (different “flavors” of any gene) that somehow code for the initiation of each specific behavior.
And then it gets even more complicated; it is not enough that a worker carry the right alleles (the worker’s genotype, or “library” of genetic instructions), those genes must then be read and expressed in the worker’s phenotype — its morphology, physiology, and behaviors. Molecular biologists now talk about the “transcriptome,” which reflects the genes that are being actively expressed from the DNA of the genome into different forms of RNA (the transcriptome) at any given time.
Practical application: The RNA in the transcriptome can code for the manufacture of proteins, or have regulatory functions. I suspect that resistance to varroa is gained mainly from shifts in the regulatory aspect of the transcriptome. Some genes being actively expressed in the transcriptome (at the moment) might be triggered by a nurse detecting a specific odor, which may then unleash “regulatory cascades” that result in the initiation of behaviors involved in mite resistance.
The coding and non-coding genes work together. For example, for a nurse bee to detect that a mite is reproducing under the capping of a cell may require an allele that specifically codes for the formation of an olfactory receptor protein which might bind to a specific odor emitted by immature mites (or a cuticular hydrocarbon from an infested pupa). Binding of that specific odor molecule at the tip of the nurse’s sensory papillae could then initiate a behavioral response cascade, resulting in that nurse bee initiating the chewing away of the capping.
Practical application: If you can’t smell it, you can’t respond to it! For a nurse to respond to a particular scent of a mite or an infested pupa, she must possess a specific olfactory receptor protein that binds to that scent molecule. The coding alleles for such critical receptor proteins may be rare in commercial bee stocks. But once detected, the alleles for the behavior of uncapping targeted pupa are already common in any breeding population, so it may take only the slightest genetic tweak to shift non-resistant bees toward resistance.
Genotype vs. Individual- or colony-level phenotype
Mechanisms for varroa resistance take place at both the individual worker (or larva) level, as well as at the colony-as-a-whole level. The phenotype is where the rubber of the genotype hits the road, and is where the end results of any particular assortment of alleles become evident.
Resistance for varroa may involve the individual larva or pupa (by olfactory signals, social apoptosis, behavior, or gene expression [[3]]), or the behavioral actions of individual adults (grooming, uncapping, or hygienic behavior)[[4]], or take place at the colony level (temperature regulation, absconding, or brood management). All the above phenotypes result from the “expression” of the genotype. As summarized by a recent study that investigated SMR and VSH (Suppression of Mite Reproduction and Varroa-Sensitive Hygiene) [[5]]:
All of the above demonstrate that [mite non-reproduction] is a complex mechanism combining multi-factorial effects, such as adult bee behavior, brood and mite physiology, and bee and mite genetics.
Practical application: The above-cited study is open access, and is must reading for anyone engaged in a selective breeding program. The complexity of mite resistance may help to explain why it’s more difficult to breed for than some other traits.
Gene regulation
Left to themselves, honey bees have shown that they can fairly rapidly adapt to varroa. The process of selected breeding is simply human-directed evolution (forcing adaptation by the breeding population to our selective pressure). The fastest and most efficient way for a species to adapt to environmental changes (such as the gaining of a new parasite) is by gene regulation.
The above concept is explained by Boyle [[6]]:
For typical traits, even the most important loci in the genome have small effect sizes and that, together, … only explain a modest fraction of the predicted genetic variance … in contrast to Mendelian diseases — which are largely caused by protein-coding changes — complex traits are mainly driven by noncoding variants that presumably affect gene regulation. [Highlighting mine]
Albert [[7]] found that almost every gene is influenced by a complex set of regulatory regions all over the genome. Some hotspot regions (“expression quantitative trait loci”) were found to influence the expression of thousands of other genes. I suspect that the genetics of breeding for varroa resistance has mostly to do with selecting for the genetic regulation of alleles already existing in our breeding population (since we clearly see some existing colonies that are able to keep varroa in check) [[8]]. As stated eloquently by Alyson Ashe [[9]]:
The possibility of extreme phenotypes must lurk within the normal genome. These extreme phenotypes are only expressed when an environmental or genetic challenge is sufficient to reveal them. Therefore, the selection on the regulation of a gene network alone should be sufficient to produce the same extreme phenotype without change in the average genotype of the genes that directly contribute to a trait.
The Takeaway: The alleles of a few critical genes act as “switches” to influence or initiate regulatory cascades that control the expression of many other genes involved in physiology or behavior. Evolution may not depend upon novel mutations (adding new tools to the toolbox), but rather upon using the existing tools in the genome in new ways. In our selective breeding experiment we’re not hoping for “magic,” but simply selecting for colonies from our own stock that are using their existing toolboxes to effectively control varroa. In other words, simple “old school” selective breeding.
An example of the differential expression of genes in the honey bee
Allow me to offer a graphic example of how the genotype of a honey bee egg can be very differently expressed in the phenotype of the resulting adult. The one-celled zygote formed upon fertilization contains two sets of chromosomes — one from the queen, and one from a drone with which she mated. Let’s focus upon one matching pair of chromosomes in that zygote, which may have received from each parent a slightly different “spelling” (or not) of a single allele at one critical gene. That minuscule difference, coupled with the “environmental effect” of what the larva gets fed, can result in the formation of any of three strikingly different sexual phenotypes during the development of that egg into an adult.
Surprisingly, the default for any fertilized honey bee egg is to become a male (a viable diploid drone) [[10]]. Only if the matching pair of the Complementary Sex Determiner (csd) gene happens to carry two different alleles (out of over a hundred known), will the csd gene then produce messenger RNA to activate the Feminize gene (fem), which will then kick in to initiate a genetic “regulatory cascade” that results in the development of a female bee.
And once differentiated into a female, depending upon the “environmental influence” of how that now-female larva is fed, “she” can develop into either a worker or a queen (Figure x) [[11]]. Also surprising is that the young larval transcriptome is more influenced by diet than by sexual differentiation [[12]].
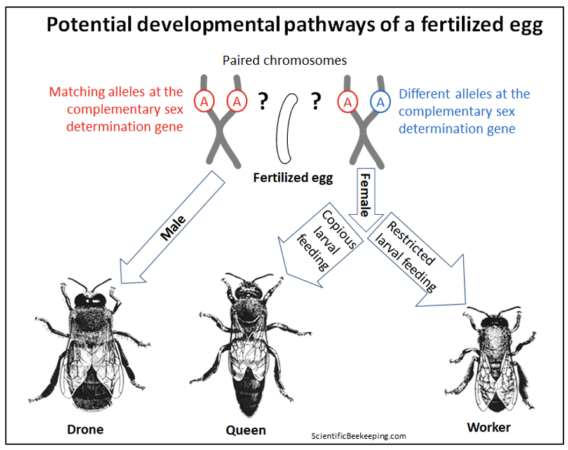
Fig. 1 A diagram of how a triggered genetic regulatory cascade, or the environmental effect of feeding, can differentiate the expression of the genes in a fertilized egg into developing into a drone, or either caste of female. Credits [[13]].
My point is that any genome has the potential to develop into substantially different morphological or behavioral phenotypes, depending upon which genetic “switches” are flipped. A honey bee embryo exhibiting virtually identical genetics (with only the slightest difference of a single allele at one regulatory gene) has the potential to develop into either a drone, a queen, or a worker. Further regulation of the transcriptome can then shift that worker from acting as a jelly-producing in-hive nurse, to a wax-producing “mid-age” bee, a guard at the entrance, or eventually into a specialized free-flying pollen, nectar, water, or propolis forager.
Practical application: The above is an example of how differential expression of a fixed set of genes can result in vastly different phenotypes (morphologically, physiologically, or behaviorally). Breeding for mite resistance may not be so much about finding novel genes, but rather about selecting for bloodlines that differentially express existing regulatory genes in response to certain cues (such as the odor of varroa mating pheromone, or mite-stressed pupae). Slight differences in how even a single patriline of workers in a colony flip their genetic “regulatory switches” may allow the colony as a whole to exhibit resistance to varroa.
An addition following a Letter to the Editor:
I was in no way trying to suggest that diploid drones were “normal.” Drones are of course normally haploid, coming from unfertilized eggs, and diploid drone larvae are normally quickly consumed by the nurse bees. Since, as you say yourself that “It is fairly common knowledge that the common male drone bee is from a HAPLOID zygote,” I mistakenly assumed that my readers would know that, and it didn’t occur to either me or the Editor that further explanation was necessary.
The point of my example was that any fertilized egg has the “choice” of either of two (as termed by Herrmann1) “sex options” — taking either of two different developmental pathways resulting in the development of either male or female organs and body morphologies. Which pathway it takes is determined solely by whether it carries the same, or a different, pair of alleles at a single gene.
Thank you for bringing this possible confusion to my attention; I will add this correction to the article when I post it to my website.
Regarding your claim that I failed to attribute credit, I make a point of always acknowledging the source of images in my illustrations, as I did for this one in the legend. One cannot cite USDA as an author or illustrator, one must cite the human being who actually created the work, which I did.
1Herrmann, M, et al (2005) Characters that differ between diploid and haploid honey bee (Apis mellifera) drones. Genet. Mol. Res. 4(4): 624-641.
What Is our own bees’ mechanism(s) for Resistance?
I don’t limit the “genetic creativity” of my bees by telling them how to do their job; I just fire all the ones that don’t get the job done. After a few years of strong selective pressure, I observe quite a bit of ”bald brood” (Figure 2).

Fig. 2 “Bald brood,” likely indicating uncapping/recapping behavior, involves some workers (perhaps only a patriline of full sisters fathered by a single drone) chewing the cappings off apparently-healthy sealed pupae, but not necessarily removing those pupae (removal would be termed “Varroa Sensitive Hygiene” (VSH) [[14]]). The pupae may then be capped back over, perhaps by a different patriline of workers (recapping is a common trait).
Uncapping/recapping behavior was described in 1998 in Brazilian Africanized honey bees by Corrêa-Marques and de Jong [[15]], elaborated on by Harris, Danka, and Villa in 2010 [[16]], recently identified as a common trait for resistance in bee populations around the world by Stephen Martin in 2020 [[17]], and elaborated upon by Melissa Oddie in 2021 [[18]].
Since I keep an eye on the first brood patterns of our new queens (in order to look for signs of excessive inbreeding resulting in diploid drones), I note that the first patterns are generally quite solid (Figure 3).

Fig. 3 I monitor the brood patterns of our new queens each year to make sure that we’re not inbreeding excessively, which would result in spotty brood patterns due to diploid drones.
Compare the above to a typical pattern of one of our mite-resistant colonies later in the season (Figure 4).

Fig. 4 Although strong and healthy, with a mite wash count of zero, note the typical presence of open cells in this resistant colony later in the season. I suspect that the open cells have to do with some aspect of mite resistance, such as VSH, perhaps following uncapping behavior.
Practical application: I rarely observed bald brood before varroa, but now see it regularly in our own resistant colonies. But it’s not always easy to observe, because if a colony holds its infestation low, there won’t be many infested cells at any time. But what I do see is that there appears to be some threshold of mite infestation at which uncapping and removal of infested pupae really kicks in, especially in my resistant colonies.
I have noticed that bald brood appears to occur in patches, which is explained by an illuminative paper by Grindrod and Martin [[19]]:
These findings are important as they suggest first that all colonies have the ability to detect and thus potentially to remove mite infested brood, and secondly that whether a cell is checked for Varroa is influenced by the infestation status of its surrounding cells.
Grindrod’s findings suggest that the olfactory cue from mite-infested cells may trigger, at least in certain workers, upregulation of their inspection and uncapping behaviors, similar to how other environmental cues alter bee behavior.
Practical application: It’s not clear whether uncapping behavior or even the removal of infested pupae results in the death of any adult mites in the cells, but it likely decreases their overall reproductive success (varroa’s Achilles’ heel).
Testing for Uncapping behavior
During visual inspection, such as in the above photos, one may see uncapped pupae and/or scattered open cells, but you can’t tell the degree to which cells have been uncapped and then recapped without the removal of the pupae. Finding that out requires removing the cappings over sealed brood, and inspecting for signs of being recapped. When recapped in a dark comb, the underside of the capping of a recapped cell will look darker, due to the light-colored silk cocoon having been chewed away.
This process is tedious to do with forceps. In discussion with other researchers, I’ve tried using molten beeswax and gauze or sticky tape to remove a large number of cappings at once. But neither worked well, for various reasons. Luckily, I got a tip from Dr. Ralph Büchler to try using depiliatory strips [[20]], which work as well to remove cappings as they do for bikini waxing (Figure 5).
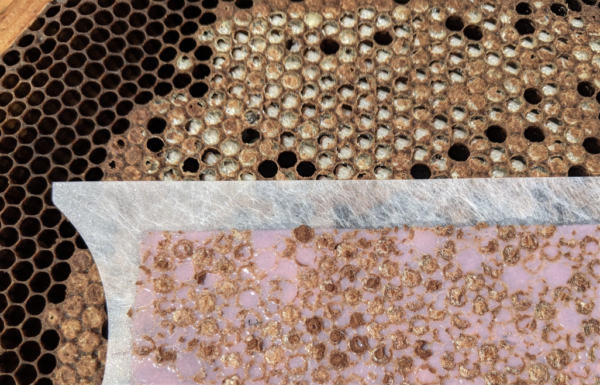
Fig. 5 This assay must be performed on capped pupae (capped larvae may not have yet spun their cocoons). Look for a patch of pupae at pink-eyed stage, so that the nurses have had plenty of time to detect any cue for uncapping. It’s easy to pick out the dark recaps, compared to the intact undersides still showing light-colored silk cocoons.
Practical application: I’ve only just begun to experiment with this assay, and may write more about it as I learn more. It could be a quick assessment method for mite resistance. Let me be clear that this is only one mechanism for resistance that happens to stand out in our colonies — I have no idea how many different mechanisms that they are actually using (or whether all bloodlines are using the same mechanisms).
Of course I’m curious as to how our resistant colonies keep varroa under control, but as concluded by Eynard [[21]]:
[Mite Non-Reproduction] measurement remains one of the few measurements for varroa resistance in honey bee populations, which can be achieved in the field on a relatively large scale. Although time consuming and tedious to implement, it also gives a lot of different information which can help us to better understand the control mechanisms that bees use to counteract the varroa mite. [Boldface mine]
In our selective breeding program, we do observe a great deal of uncapping behavior and some VSH. But because a resistant colony contains very few mites, it would indeed be tedious to confirm. I’ll leave it up to someone else to figure out how our zero-count colonies manage to control the mite.
Is there a Cost to the colony for resistance?
One advantage of uncapping/recapping behavior for mite resistance is that there is not as much cost to the colony as from brood sacrifice, such as with “social apoptosis” or Varroa Sensitive Hygiene [[22]]. I don’t see any negative tradeoff in productivity or gentleness in our most-resistant colonies — they’re often the most productive hives in a yard. This could be due to their not having to deal with having their all-important fat bodies getting destroyed by the mites, as well as benefitting from not having mites injecting their weakened bodies with viruses.
Our Traditional Breeding Experiment
It’s clear that mite resistance is attainable, but it may take a while to “fix” the trait in our breeding population [[23]]. We’re gonna stick with Harbo and Harris’s suggestion to simply identify those colonies that by some means are able to prevent varroa from building up over the course of the season [[24]]:
We define mite resistance as the ability of a colony of honey bees to impede the growth of a population of V. jacobsoni. With this definition, a highly resistant colony of bees would cause a mite population to decline and then to either disappear or be maintained at a very low level. This is the breeding objective.
Exactly! Funded researchers can perform the tedious work. I just define the job description for our bees, and breed only from those that do the job, as evidenced by, however they do it, of maintaining mite counts of zero!
References
[1] Vandame, R, et al (2002). Parasitism in the social bee Apis mellifera: quantifying costs and benefits of behavioral resistance to Varroa destructor mites. Apidologie 33(5): 433-445.
[2] Borba R, et al. (2022) Phenomic analysis of the honey bee pathogen-web and its dynamics on colony productivity, health and social immunity behaviors. PLoS ONE 17(1): e0263273. “… our data failed to show any significant relationship between damaged mites and the mite infestation indices measured.”
[3] Conlon, B, et al (2019). A gene for resistance to the Varroa mite (Acari) in honey bee (Apis mellifera ) pupae. Molecular Ecology. 28. 10.1111/mec.15080.
[4] Scannapieco, A, et al (2016). Individual precocity, temporal persistence, and task-specialization of hygienic bees from selected colonies of Apis mellifera. Journal of Apicultural Science 60(1): 63–74.
[5] Eynard, S, et al (2020) Descriptive analysis of the Varroa non-reproduction trait in honey bee colonies and association with other traits related to Varroa resistance. Insects 11(8): 492.
[6] Boyle, E, et al (2017) An expanded view of complex traits: from polygenic to omnigenic. Perspective 169 (7): 1177-1186.
[7] Albert, FW, et al (2018) Genetics of trans-regulatory variation in gene expression. Elife 7:e35471. doi: 10.7554/eLife.35471.
[8] Albert, F & L Kruglyak (2015) The role of regulatory variation in complex traits and disease. Nature Reviews/Genetics 16:197.
[9] Ashe, A, et al (2021) How does epigenetics influence the course of evolution? Phil. Trans. R. Soc. B3762020011120200111.
[10] Gempe, T, et al (2009) Sex determination in honeybees: Two separate mechanisms induce and maintain the female pathway. PLoS Biol 7(10): e1000222. doi:10.1371/journal.pbio.1000222. “in the absence of the female-specifying signal, the male variant is produced that is the default regulatory state.”
[11] The developing worker larva is also affected by the presence of queen pheromone. Woyciechowski, M, et al (2017) Honeybee worker larvae perceive queen pheromones in their food. Apidologie 48: 144–149.
[12] He, X-J, et al (2019) A comparison of honeybee (Apis mellifera) queen, worker and drone larvae by RNA-Seq. Insect Science 26: 499–509. “For young larvae (2-day-old) environmental factors such as larval diet have a greater effect on gene expression profiles than ploidy or sex determination.”
[13] I arbitrarily placed the csd gene on a fanciful chromosome. Bee drawings from: Kauffeld, M (1980) Seasonal cycle of activities in honey bee colonies. In, Beekeeping in the United States, Agriculture Handbook 335: 30-32.
[14] Harris J, R Danka, J Villa (2021) Honey bees (Hymenoptera: Apidae) with the trait of Varroa sensitive hygiene remove brood with all reproductive stages of Varroa mites (Mesostigmata: Varroidae). Ann Entomol Soc Am. 2010; 103(2):146–52.
[15] Corrêa-Marques, M, D de Jong (1998) Uncapping of worker bee brood, a component of the hygienic behavior of Africanized honey bees against the mite Varroa jacobsoni Oudemans. Apidologie 29 (3): 283-289.
[16] J Harris, R Danka, J Villa (2010) Honey bees (Hymenoptera: Apidae) with the trait of varroa sensitive hygiene remove brood with all reproductive stages of varroa mites (Mesostigmata: Varroidae). Annals of the Entomological Society of America 103(2): 146–152.
[17] Martin, S, et al (2020) Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 51: 369–381.
[18] Oddie, M, et al. (2021) Reproductive success of the parasitic mite (Varroa destructor) is lower in honeybee colonies that target infested cells with recapping. Sci Rep 11: 9133.
[19] Grindrod, I & S Martin (2021). Spatial distribution of recapping behaviour indicates clustering around Varroa infested cells. Journal of Apicultural Research 60(5); 707-716.
[20] I used Veet brand “Ready-to-use wax strip kit” (a gel adhesive used without heating).
[21] Eynard, S, et al (2020) op cit.
[22] Vandame, R, et al (2002) op cit.
[23] By us continually removing from the breeding population any queens who apparently didn’t carry alleles that conferred resistance to her colony.
[24] Harbo, JR & JW Harris (1999) Selecting honey bees for resistance to Varroa jacobsoni. Apidologie 30: 183-196.






