Beyond Taktic® -Beekeeper-Funded Research
Beyond Taktic®
Beekeeper-funded Research
Randy Oliver
ScientificBeekeeping.com
First published in ABJ January 2017
The miticide Taktic has been the savior of the commercial bee industry since the early 2000s. But it may be time to move on. I’ve been experimenting with a promising potential replacement.
Our Situation
As I recently pointed out, there are signs that mites in areas of the U.S. are exhibiting some degree of resistance to Taktic’s active ingredient–amitraz. And since Taktic has been pulled from the U.S. market, some beekeepers are justifiably concerned that the EPA may stop looking the other way about them illegally using the product (Canada’s already hit one beekeeper with a hefty fine; no telling when some State enforcement branch will make an example of a U.S. beekeeper).
I’m freshly returned from the California State Beekeepers Assoc. conference, where Dr. Juliana Rangel presented the findings of her student Liz Walsh (who previously found negative effects on queens from residues of miticides in the comb). Liz recently found that field-realistic residues of amitraz in queen cell wax appeared to reduce the egg laying rate of queens reared in those cells. I’ve suspected something like this, since queen problems appear to have increased since the widespread adoption of amitraz as a miticide. Of further concern is that amitraz residues are increasingly being detected in U.S. honey. In any case, commercial beekeepers are (or I suspect will soon be) looking for alternatives to Taktic.
The ideal treatment
In this same issue of ABJ, I’m pushing our industry to get serious about shifting to mite-resistant stocks so that we can give up treatments altogether. But I know that my own operation would collapse if I were to attempt an abrupt transition, and have no doubt that most others would too. So although I don’t use amitraz in my own operation, I have a common interest with my professional brethren to find mite treatments that are cheap, don’t harm the bees, queen, or brood, and don’t get into the honey.
My sons and I have managed so far with Apiguard thymol gel, MAQS formic acid strips, and oxalic acid dribble (still not yet registered in California, thus unhappily making me a pesticide scofflaw too). I previously reported on last summer’s trials, in which we found Apiguard to be quite effective if applied in a 1½” rim. MAQS can also provide excellent mite reduction, although there are occasionally issues with it knocking out poor or aged queens (perhaps not a bad thing). We love oxalic acid, but neither the dribble nor vaporization is effective if the colony contains much brood.
As I reported last year, I had tried a new extended-release formulation of oxalic acid to overcome that problem [[1]]. In our initial small test, the results were so promising that I could hardly wait until this summer to work with it more seriously. The results are so encouraging that I wrote this lengthy step-by-step, picture-rich article to fully share what we learned.
Oxalic acid/glycerin strips
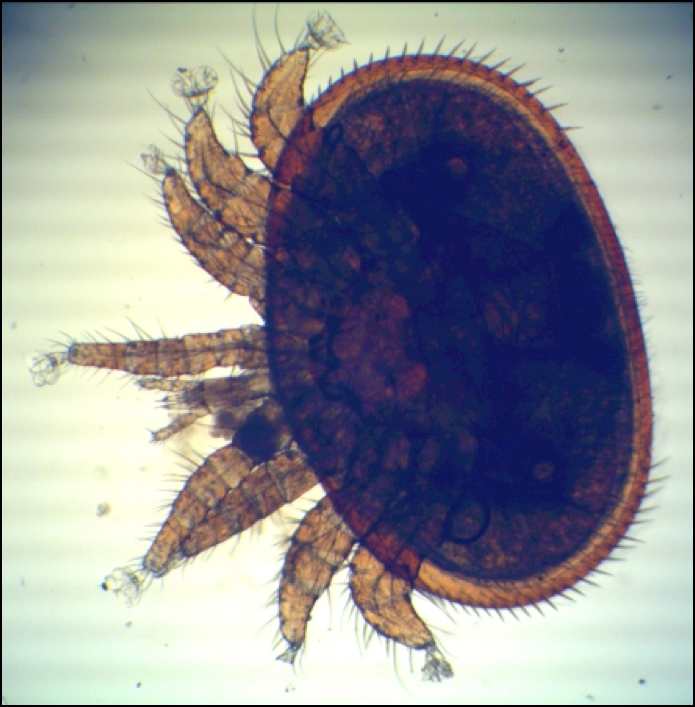
Oxalic acid dissolves to some extent in water, but has a notable fondness for glycerin. This makes food-grade glycerin an attractive carrier for oxalic acid in the hive, especially since its oily nature also confers upon it an affinity for bee (and mite) cuticle (Figs. 1 & 2).
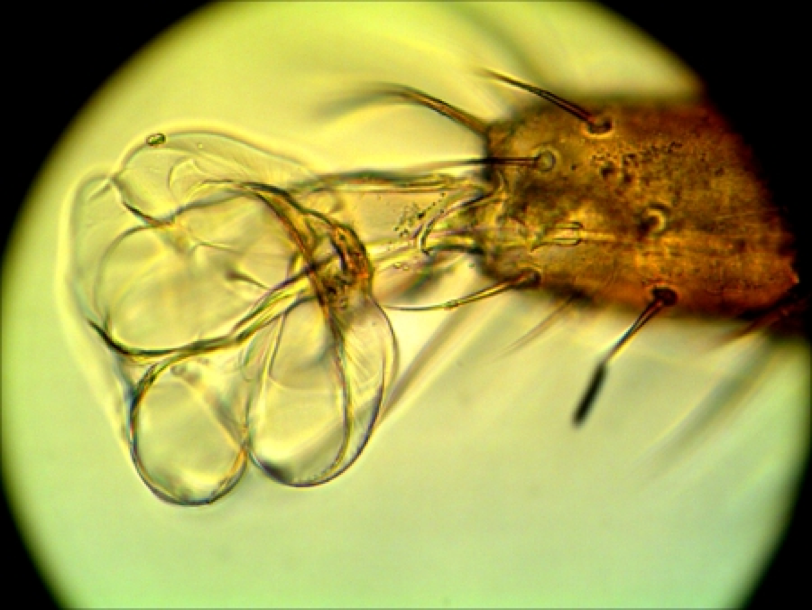
Figure 1. These wonderful photomicrographs by German beekeeper Bernhard Heuvel show the sticky tarsal pads which the mite uses to get a grip on the bee. Photos courtesy of Bernhard Heuvel.
Figure 2. It has yet to be confirmed, but perhaps, as it moves over a bee carrying oxalic residues on its exoskeleton, the mite absorbs the acid through the thin cuticle of these pads. (Findings by Dr. Dennis van Engelsdorp suggest that acids may also affect the mites’ sensory papillae.)
Since my first trial of OA/gly strips last fall (using Fernando Estaban’s formula), my good friend Juanse Barros in Chile tested them extensively, and was also impressed.
Practical application: by dissolving oxalic acid into glycerin, and then saturating a cardboard strip with the solution, one can obtain an extended release application method for the acid into the hive, thus continually killing mites over more than one reproductive cycle, thereby overcoming the limitation of the dribble or vaporization application methods.
By this time, an Argentine group started selling a formulation, Aluen CAP. The product was extensively tested by Matías Maggi [[2]], with astounding results—excellent mite control, no adverse effects on the bees, and no residues in the honey (plus it’s considered as an “organic” treatment). Almost too good to be true! So when it came time for late summer mite control, I was ready to test it more extensively.
Trial #1
We set up a yard of 28 hives of moderate strength, containing 2nd-yr queens, in which we had intentionally allowed mites to build to high levels (I wanted mite-stressed, old-queen hives to best detect any adverse effects from the treatments).
Scientific note: one gets much better efficacy data if treatments are tested on colonies that have high mite counts at the beginning of the trial. However, if the trial is to run for an extended period, the issue arises that the untreated controls may collapse from mite overload before the trial is over. But this creates another potential issue when one later applies the Henderson-Tilton formula to calculate treatment efficacy. This is because of mite drift from the high-mite test hives into the low-mite controls. The movement of mites carried by drifting bees is akin to the process of diffusion—invariably moving from hives with high mite density to those with lower mite density. This can result in increasing the apparent rate of mite increase in the Control hives relative to that of the Treated hives, thereby artificially skewing the final calculation of efficacy upward. Thus, in calculating efficacy, one should be sure to limit the apparent rate of mite growth in the Controls against the biologically-limited maximum daily intrinsic rate of increase [[3]].
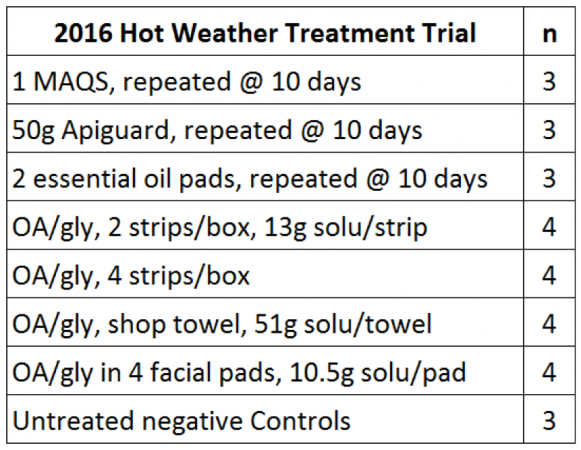
We ran positive controls of Apiguard and MAQS, as well as untreated negative Control hives (Table 1, Figs. 3-7). Since this was intended to be only a preliminary and exploratory “quick and dirty” testing of various methods of application of the OA/gly formulation, we used low numbers of hives in each group. What I was looking for were substantial and consistent differences rather than trying to tease out slight statistically significant effects.
Table 1. The treatments. This was a preliminary “quick and dirty” trial, so we used low n’s. We used a slightly different OA/gly ratio than did Maggi [[4]].
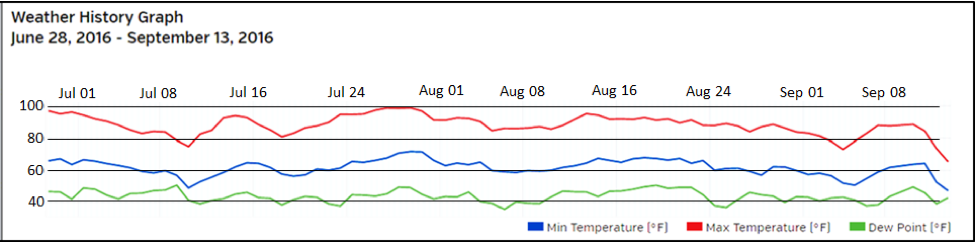
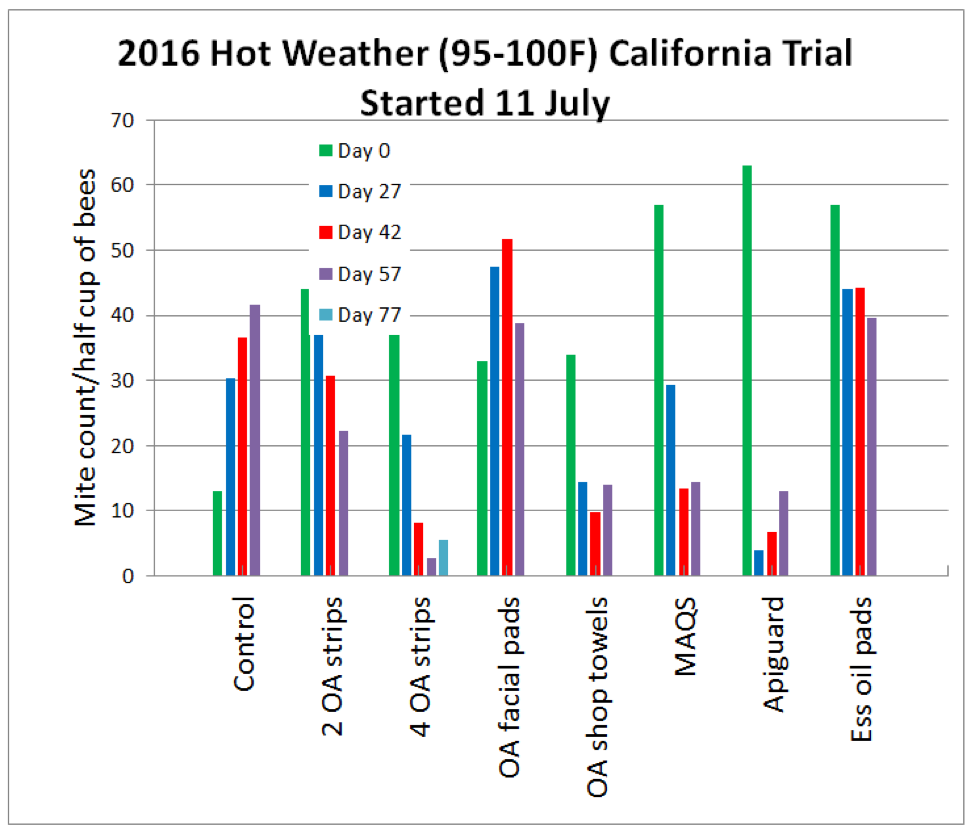
We waited for a window of hot weather, since that is often a limiting factor for us (Fig. 3).
Figure 3. The trial ran from June 28 through September 13, with daytime temperatures often in the high 90s.
Figure 4. All hives were double deeps. Here we’re placing two strips per box, over brood frames. For an absorbent cardboard, I used strips cut from egg carton lids.

Figure 5. Here we’re placing 4 strips per box (8 per hive), distributed for maximum bee exposure.
Figure 6. I was curious whether the bees would tug the OA/gly-soaked cotton fibers from facial pads and thus drag the solution over the bodies of other bees. (Result: they didn’t touch the pads).
Figure 7. The beekeepers’ favorite, the blue shop towel. I wondered whether bees would expose themselves to the OA/gly in the process of removing the towel. (One result: it did not help to cut slits in the towel—that allowed the bees to remove it too quickly).
Results of the first trial
As expected, the mites increased at a normal rate in the Controls, and Apiguard reduced mite levels quickly, after which they resumed a normal climb. The oxalic strips took longer to drop the mite counts, taking two months for full effect (Figs. 8 & 9).
Figure 8. The alcohol counts for the groups at each time point. I took Day 77 counts only for the 4-strip group—note how the mite count had begun to climb, suggesting that the effect of the strips starts to wear out after two months. See the next figure for normalized results for easier comparison of treatment effect.
Figure 9. I normalized the results (so that they all start at 100%) and added standard error bars. Of the oxalic acid treatments, the winner was the 4-strip group, although the shop towels didn’t do too badly.
I inspected the colonies regularly throughout the course of the trial. At these high temperatures, both the Apiguard and mixed essential oil treatments were hard on the bees (colonies tended to move their broodnests away from both treatments) [[5]], although the Apiguard hives quickly recovered once the bees removed the last of the gel after 20 days. The efficacy of the mixed essential oil pads was unimpressive. It was (not unexpectedly) too hot for MAQS, even applied as single strips, which set the colonies back, although treatment did reduce the mite level.
I was pretty stoked about the 4 OA/gly strip treatment–not only did it control the mites (with time), but the colonies thrived during treatment (Figs. 10 & 11).
Figure 10. A brood frame with an OA/gly strip at right, two weeks after insertion. The colonies consistently reared brood right up to the strips. To my surprise, the bees and brood appeared to thrive in the acid-rich environment for the duration of the summer, and were some of my best looking hives come fall. .
Figure 11. There was little chewing of the strips, and all remained intact for the entire summer (still tasting of acid). This created the problem of needing to remove the strips one by one.
Although our results confirmed those of Maggi, with the OA/gly strips fully living up to expectations, this method of application came with some major problems:
Three problems
Problem #1—the labor involved: When I excitedly showed the results to my son Eric, he rained on my parade with some simple arithmetic: 4 strips per box, 8 strips per hive, 1500 hives to treat = 12,000 strips to make, insert one at a time, then pry out one at a time for disposal (wearing nitrile gloves at every step). This treatment wasn’t going to fly, not at our labor costs.
Problem #2—disposal: We’d need to deal with 12,000 strips of hazardous waste. The spent strips still contain enough acid that you can’t be casual with them–you don’t want to touch them with your bare hands or hive tool, nor toss them into the back of the truck, since they’d corrode the bed.
Problem #3—pest resistance management: I don’t want to apply this (or any) treatment continuously, since I’d then be selecting for oxalic-resistant mites. I want an application method that the bees will remove by themselves after 30 days.
More experimentation
By this time I was networking with a few other beekeepers in testing the strips. Laying the strips across the top bars (similar to the facial pad application above), resulted in poor mite kill, since the bees simply avoided the strips or pads. Bees apparently don’t like glycerin-saturated cellulose—in another test that I ran, they even steered clear of cotton pads soaked in plain glycerin. I needed to go back to the drawing board.
If you refer back to Fig. 9, you can see that application via blue shop towel was pretty efficacious. So I starting experimenting with various towels, fabrics, expanded packing cardboard, and other substrates, as well as with different concentrations of oxalic acid in the glycerin (since one can easily make a much more concentrated solution than that used in Argentina)(Figs. 12 & 13).
Figure 12. I tested various types of substrates, and various concentrations of oxalic acid. What soon became apparent was that it was critical to arrive at a delivery method in which the bees would chew and remove the substrate, thus getting the acid solution onto their bodies.
Figure 13. The trick appeared to be to reduce the amount of glycerin saturation of the strips. Here you can see that when I reduced the amount of saturation of the towel to only 25%, that the bees would chew and remove it. That’s a piece of slotted, expanded Kraft paper at the top, tested with the hope that the bees would crawl through the holes and get the solution on their bodies (they didn’t).
One would think that it would be easy to simply dilute the OA/gly solution with water to create low-glycerin towels. I’ll save you the trouble—the addition of water causes the OA to come out of solution. There is some interesting chemistry involved [[6]].
The most promising application method was to mix a very concentrated solution of OA in glycerin, allow shop towels to soak it up, and then to press the solution out of the towels until only a fraction remains (this also has the effect of dissolving much of the blue pigment out of the towels) (Figs. 14 & 15).
Figure 14. This photo, by my collaborator Aaron Bergman, shows the drier texture of a pressed towel (compare to the towel in Fig. 7).
Figure 15. In this ironically humorous photo, one of my collaborators is using a container of Taktic as a weight in order to press the OA/gly solution out of a stack of towels—perhaps akin to forcing someone to dig their own grave?
The Last trial—August through September
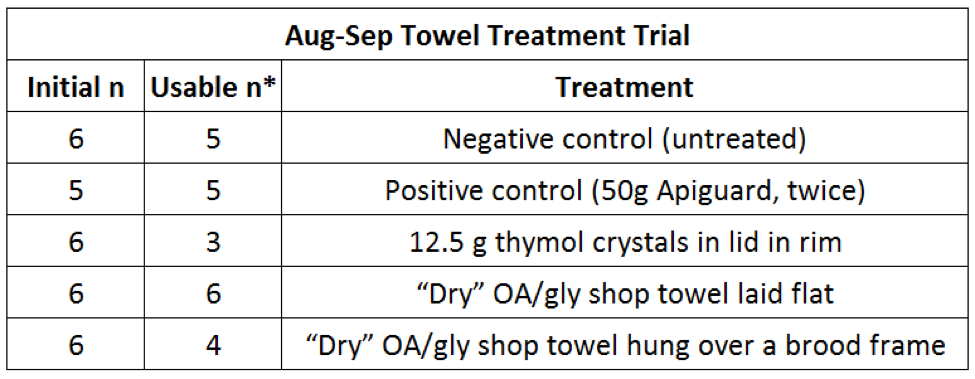
As September approached, the pool of 200 untreated high-mite hives that I’d set aside for trials began to dwindle, so I brought the remaining 40 into a single yard for a final experiment. In this quick and dirty trial I wanted to see how well my “dry” (squeezed out) shop towel formulation would work. At this point perhaps I should point out the total amount of OA applied per hive by various application methods (Tables 2 & 3).
Table 2. In the dribble and sublimation applications, only a small amount of oxalic acid is applied in a “flash-type” short-term treatment. The Aluen CAP strips used by Maggi are an extended-release treatment, apparently (based upon mite drop) releasing OA for three weeks to a month (with some amount of unreleased OA residue remaining in the strips). The pressed “dry” shop towel, which is removed by the bees over the course of about a month, contains less OA, and some is contained in the towel trash carried out of the hive.
In another trial (we ran a number of controlled trials of mite treatments this summer), I applied OA dribble, using glycerin instead of sugar as the humectant (as a number of beekeepers in Italy are currently doing). Even after three weekly dribbles, the degree of mite reduction was unimpressive (ditto for other applications of thymol dissolved in glycerin, on facial pads, as well as other application methods of Apiguard).
Table 3. We tested two application methods of the “dry” towels—either laid across the top bars of the lower hive body, or hung over a central brood frame in the lower box. I also tested simply placing thymol crystals in a 70 mm jar lid, covered by 1/8” hardware cloth, in order to see whether simple thymol evaporation would be as effective as an Apiguard treatment. *Some of the Control and Thymol Crystal hives died from mites; 2 of the Hung towels dropped and were censored.
The hung towels were a pain to apply—requiring pulling out a frame in order to make space. But the bees did not avoid them, and continued to rear brood “beneath” them (Fig. 16).
Figure 16. Bees rearing brood “beneath” a hung shop towel. This method certainly forced rubbing of the bees against the towel, and resulted in good exposure to OA. Unfortunately, the bees chewed the top edges of some, causing them to drop to the hive floors, where they then lay untouched.
Figure 17. Bees readily chewed the “dry” towels, and in the process apparently exposed the mites to OA. I included this photo since beekeepers love to see pictures of dead mites. I didn’t take sticky board counts, but Maggi’s data indicate that the Aluen strips cause serious mite drop for three to four weeks.
Figure 18. As I hoped, the bees removed the “dry” towels, dragging pieces of them through the brood nest, which likely helped to distribute the OA/gly solution to other bees’ bodies. This photo is of a hive given a “wet” towel, with slits cut into it at time of application, which resulted in excessively quick removal.
Figure 19. Typical beautiful brood pattern during treatment with a “dry” towel. Photo courtesy Aaron Bergman.
Figure 20. The bees work at the “dry” towels at a nice rate, thus exposing themselves to OA.
Figure 21. As I hoped, colonies removed most of the flat-laid towels in about a month, with some degree of propolisation. I’ve got ideas to reduce that problem. In any case, the objective of removal in about a month was largely met—any remaining residues could safely be scraped from the top bars and discarded on the ground (although you’d want to rinse your hive tool afterward). Such “pesticide waste” is about as dangerous as is lemon slices, and readily biodegrades.
Figure 22. During hot weather (and thus high temperatures in the rim space), the thymol crystals quickly melted, but despite the temperature, evaporated only to minimal extent (I’ve confirmed this in other tests). Bees build a propolis wall around thymol vapors, but I could easily smell thymol every time that I opened the hive cover (there was always plenty of clear vertical space above the screen). It appears that the efficacy of Apiguard is due to the bees coming in physical contact with the granules as they remove them from the hive, rather than from vaporization of the thymol.
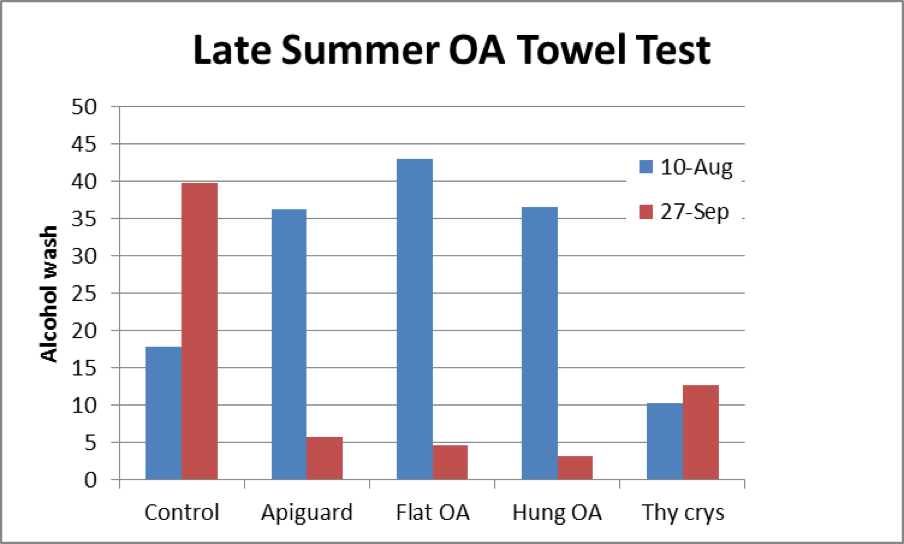
Figure 23. Results of the trial. Mite counts in the Controls went up as expected. The OA/gly towels performed as well as Apiguard. The thymol crystals were of little benefit. I hesitate to offer Henderson-Tilton calculations of efficacy (due to the small n’s), but for the flat towels it was 95%.
Practical application: beekeepers have been looking for an inexpensive, fairly rapid acting, easy to apply miticide that doesn’t leave residues in the combs or the honey, and that doesn’t slow colony growth or harm the queen. Oxalic acid dissolved in glycerin, applied on a removable substrate, appears to fit the bill. Applied on a shop towel, it causes immediate mite kill, and then continues to kill mites over a period of a month—spanning approximately two varroa reproductive cycles.
Conclusion
I’m sharing the results of my preliminary experimentation so that others can use what I’ve learned, and further experiment at improving this delivery method. I expect that next season many of us will be using oxalic acid instead of less desirable miticides.
There remains the problem, however, that this method of application is not currently legal in the U.S. I’m currently working to see whether we can get this method added to the existing label, which allows only for dribble, vaporization, and spraying (Fig. 24).
Figure 24. Oxalic acid is not yet registered as a varroacide in all states. If it’s not yet registered in your state, start making phone calls.
It is especially important to beekeepers to pay attention the last part of the label—RESISTANCE MANAGEMENT (enlarged in the box below). Beekeepers have proven to be quite adept at breeding varroa resistant to any miticide (in the case of coumaphos, it took us only three years). Since we really don’t know the exact mode(s) of action of oxalic acid upon varroa, there’s no telling how readily the mite will be able to evolve resistance. The scary thing is that there’s not much margin of safety between the dose of oxalic that kills mites, and that which kills bees—so if mites develop even marginal resistance, we’d lose oxalic as a viable treatment.
All indications are that the OA/gly strip or towel application method, despite containing fairly large amounts of acid, are not harmful to the hive. Indeed, as best I can tell, the bees thrive in the slightly acidified environment. But if beekeepers leave oxalic strips in their hives 365 days a year, or even rely upon it as their only treatment, without rotation of other modes of action, they will be applying strong selective pressure for oxalic-resistant mites, thereby screwing it up for the rest of us.
Practical application: OA/gly towels appear to be a dream come true for beekeepers (Fig.25). Please don’t screw up a good thing by overusing them! Rotate treatments—using biotechnical methods, formic acid, thymol, and perhaps Apivar strips to delay the development of oxalic-resistant mites.
Figure 25. I strongly suspect that the trio above is going to become a treatment of choice for many beekeepers, both recreational and commercial. Let’s hope that we can get the method quickly approved by the EPA!
The Formula
It is only legal to use oxalic acid that has the EPA label on the package, and it is a violation of Federal law to use the product in a manner inconsistent with its labeling. I am not in any way suggesting that beekeepers mix or apply this formulation unless it is registered for such use in your State.
For each towel (1 towel per hive):
Wearing nitrile gloves and eye protection, measure 25 mL of food-grade glycerin, and heat it to the temperature of hot coffee in the microwave (easiest) or on the stove. Weigh out 25 g of oxalic acid dihydrate (wood bleach), and stir it into the hot glycerin until it is fully dissolved (you can reheat, but don’t bring it to a boil [[7]]). This will produce enough solution to saturate 1 shop towel (multiply these figures by the number of towels that you wish to prepare). Soak a stack of towels in the warm solution until they are all fully saturated [[8]]. Then place them in a tray with a catch drain, and squeeze or press them until you’ve recovered half the solution (it will be surprisingly blue, and can be reused). The final “dry” towel will hold about 25 g of solution, and weigh about 31 g.
Handle the towels with nitrile gloves (as the OA/gly solution sticks readily to your skin, and can easily be transferred to everything and anything you touch!). Luckily, it washes off easily with warm water. I am not suggesting that you actually do this, but it is easy to check for residues on your fingers by seeing if they taste like lemon juice. Oxalic acid can be easily neutralized by baking soda dissolved in water.
Note: it is possible to dissolve a greater amount of OA into the glycerin (I’ll experiment next season). I suspect that this application method can still be improved on.
Practical caution: I’ve only tested this application method on colonies during the dry California summer. Fernando Esteban tells me that during damp winter conditions, the oxalic acid may crystallize on the surface of strips and cause problems to the bees (such as wing damage). I have not yet had the chance to test myself.
Treatments are only buying us time
I must temper my enthusiasm about OA/gly by reminding you that this is still only a stopgap flyswatter as far in the long term picture of varroa management. We need to start demanding of our queen producers that we want, and are willing to pay for, truly mite-resistant stock. In my concurrent series I will lay out how our industry can realistically do that. We only need guidance from scientists—we can do the work ourselves. Stay tuned.
Aknowledgements
This research was funded by donations to ScientificBeekeeping.com. If you find such research to be of value, feel free to donate at the website. Thank you to those of you who have contributed, as it is very costly to perform such research, not only in labor, but in resultant loss of those colonies that I intentionally allow to reach high mite levels for research purposes (we blew off nearly 200 hives for research purposes this season alone). I could not manage all the work without the skilled assistance of my sons Eric and Ian. Thanks to collaborating beekeepers Fernando Esteban, Juanse Barros, Aaron Bergman, Richard Hyde, Kenny Reed, and Charles Linder. And to organic chemists Mark Burlingame, Richard Cryberg, and Edmond Stark.
Notes and Citations
[1] Suggested to me by Fernando Esteban of Argentina.
[2] Maggi, M, et al (2015) A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 47(4): 596–605.
[3] During periods in which colonies are full of brood and the bee population is expanding, the daily intrinsic rate of increase (without drift) is limited to around 0.021 (for use in the formula Pt = P0 *ert), and will be roughly reflected in alcohol wash counts. I will elaborate upon this in an upcoming installment.
[4] Maggi used strips each containing 10g of OA and 20g of glycerin. We used a common “kitchen formulation” from Argentina of a 6:10 ratio. Thus, Maggi applied 40g of OA per single-box hive. With the 4-strip/box treatment, we applied a total of 39g of OA, essentially the same amount as Maggi. The 2-strip/box treatment was roughly half that dose.
[5] Although humans enjoy the aromas of some essential oils, we should keep in mind that plants generally produce these oils in order to repel insects or other herbivores. Bees clearly do not like some essential oils, and my impression was that the colonies were stressed by their presence.
[6] I’ve researched the literature, and consulted at length with a few organic chemists. The oxalic/glycerin/water/temperature/time chemistry is complex. There may be reversible esterification occurring to some extent, and reversible solubility issues.
[7] At boiling, the water molecules unbind from the oxalic dihydrate, and esterification and other reactions begin.
[8] Two InterDesign Refrigerator and Freezer Storage Organizer Tray for Kitchen, 12″ x 2″ x 14.5,” work very well for soaking and draining—they stack, and fit a shop towel nicely.