Extended-Release Oxalic Acid Progress Report #2
Extended-release Oxalic Acid Progress Report #2
Randy Oliver
ScientificBeekeeping.com
First published in ABJ October 2017
There has been a huge amount of interest in the extended-release application of oxalic acid for controlling varroa. I and my collaborators have been working hard to collect the data necessary get this treatment approved for use by U.S. beekeepers. We’ve still got lots to learn, but here’s an update.
Disclaimer and legality: The information in this article is solely to report on my beekeeper-funded progress (working in conjunction with ARS and others) towards getting this application method approved by EPA, and is not intended to promote illegal use in any way. I neither approve of, nor encourage, applications not sanctioned by local authorities [[1]]. This is not a “how to”—please do not write me asking how to apply it yourself until we have got the bugs worked out and gotten it registered.
Formal experiment #1
In order to get this application method of oxalic acid dissolved in glycerin (OA/gly) approved by EPA, we need to submit data to support its efficacy, lack of adverse effects upon the bee colony, and safety to the beekeeper/applicator and the honey consumer. To that end, myself, Jennifer Berry, and Drs. Jay Evans, Geoff Williams, and Meghan Galbraith are collecting data by performing controlled field trials in California (low humidity), Georgia (high humidity), Beltsville Maryland, and northerly Michigan.
Objectives:
The objectives of these experiments are to test various application methods over 42 days (two honey bee brood cycles; roughly 2.5 varroa reproductive cycles) for:
- Efficacy: To determine the efficacy of the treatment method at reducing the in-hive varroa infestation, compared to a solvent control (a towel with glycerin only), and a sham treatment (a towel only) [[2]].
- Adverse effects: To determine whether there are measureable or observable adverse effects upon the bee colony from treatment with oxalic/glycerin.
- Residues in honey: To determine whether treatment results in elevation of the naturally-occurring levels of oxalic acid in harvestable honey.
The above are abbreviated snips from the detailed protocols that we developed, based upon the minimal research that I had done up to actually initiating the trials (we’ve made last-minute changes all summer as we learn new things). For the California trial, I equalized 64 hives, entrances facing different directions, took mite washes from all, ranked them by starting mite count, and systematically assigned treatments so that each treatment was applied equally to the full range of mite infestation rates [[3]]. I set up the trial in my home yard, just at the beginning of what I expected would be a normal honey flow (Fig. 1).
Figure 1. A portion of the bear-fenced test yard at my home. All colonies were headed by queens in their second season in order to better detect any adverse effects, and equalized for weight as well as strength (~13 frames of bees), with each upper box containing 4 drawn combs, a drone frame, and 5 frames of foundation [[4]].
We decided to apply three half-towel strips, which (in the treatment group) held a total of ~18 g of oxalic acid in total, applied between the brood chambers (Fig. 2).
Update: We now prefer absorbent matrices other than shop towels, which have many disadvantages. Please refer to my more recent reports on extended-release oxalic acid.
Figure 2. Applying the lightly-saturated towels, with bee passageways between them. We used a formula that in previous testing the bees had slowly removed over the course of a month or so.
At three time points (start, mid, and end) we got up at dawn (before the clusters broke) and graded each colony for strength by counting the number of frame interspaces filled with bees (Fig. 3).
Figure 3. I graded the lower brood chamber; Eric and Ian (with flashlights) graded the upper, while our intern Rachel records the measurements).
Once graded for strength, we then accurately weighed each hive with a digital scale hung from an overhead hive loader (Fig. 4).
Figure 4. Bill is holding the boom steady while we weigh each hive to the tenth of a pound. The record keeper checks each hive number as we go—it’s critical not to make any errors during data collection.
I then inspected two frames of brood from each hive for adverse effects (and to check for queen rightness), and then shook a sample of bees for mite washing (Fig. 5).
Figure 5. As you can see, there were no noticeable adverse effects from the acidification of the hives.
But we weren’t done yet—we still needed to take samples of freshly-stored honey to be processed at Beltsville to determine their oxalic acid content (Fig. 6).
Figure 6. Tara using a disposable spoon and pipette to take a honey sample.
Initial results
I’ve only analyzed the data to date for the California trial, but unfortunately can’t publish it here, since it is going to be included in a peer-reviewed scientific publication. Suffice to say that there were absolutely no observable effects from treatment on colony strength or weight gain (which was minimal, due to a heat wave knocking out our honey flow). Nor did I observe any adverse effects upon colony health or the brood pattern.
Efficacy at Reducing the Mite Level
The oxalic treatment reduced the rate of mite buildup by the midpoint grading, and by the end point (at 48 days) resulted in 94% efficacy at reducing the median mite count [[5]]. The mite count dropped nicely in 23 out of 24 hives, but surprisingly, skyrocketed in a single treated hive [[6]].
Informal testing in California
We had also informally treated several yards of of hives early in the season [[7]], giving each hive a single full OA shop towel, and are currently checking mite counts—in some yards, the single treatment held mite counts to surprisingly low levels (a number with zeroes and ones), but in other yards the treatment only reduced the expected climb–but the reduction was generally substantial. Of note is that in our August inspections of those OA-treated hives (all with 2nd year queens), they were some of the best-looking hives in our operation.
Practical application: as did Maggi [[8]], we’ve clearly demonstrated proof of concept—that a single extended-release oxalic acid treatment can bring mite counts down to close to zero, without any apparent adverse side effects to the colony. But applied as above, the results may be inconsistent—the method still needs fine-tuning for improvement.
An Important Finding
Of greatest interest to me were the hive-to-hive differences in the amounts of the towels removed by the bees. The bees removed the dry (sham) towels in about a day, and the glycerin-alone towels within a few days. But there was wide variation in how they dealt with the OA/gly towels (Fig. 7). By 48 days, some had removed 100%, and in others 0% (mean 46%, median 38% removal, by eyeballed estimates).
Figure 7. The colonies, although all roughly the same strength and condition, reacted very differently to the OA/gly towels—some chewing away every bit, others barely touching them. At 48 days, the remnants of the towels in the hives appeared “drier”—indicating that even if not removed, the glycerin and OA had been tracked or rubbed off on the bees.
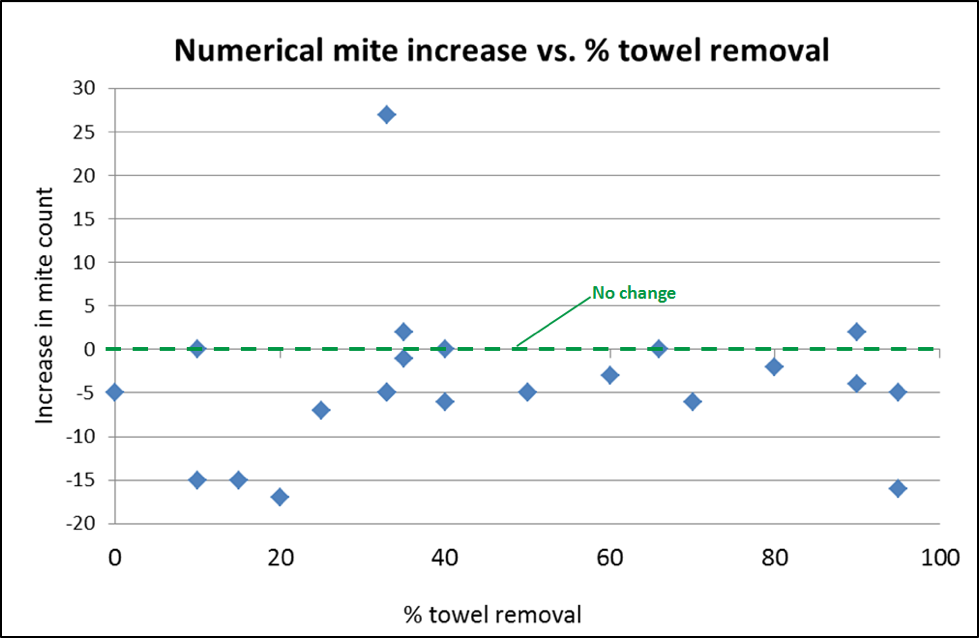
Aha, I thought—let me plot out the percentage of towels removed vs. the reduction in mite counts [[9]]. This might tell us whether my assumption that the distribution of OA throughout the hive was dependent upon the chewing and dragging out of the cellulose matrix was correct (Fig. 8).
Figure 8. So much for my assumption—there was no correlation between the increase or decrease of mite counts and the percentage of the towels removed by the bees! It’s easy to see the one outlier hive in which the mite counts went up substantially—in all the other treated hives, the counts remained the same or decreased (relative to the horizontal zero line indicating no change).
Practical application: I had gone through conniptions in order to figure out a rate of towel saturation that would encourage the bees to chew and remove the towels. And now I see that we got just as good mite reduction when the bees simply walked over the horizontally-laid towels. This indicates that the efficacy previously demonstrated by Maggi and myself from the cardboard strips, was apparently not due to their being laboriously hung between the frames, but might be attained just as well by simply laying the strips across the top bars of the lower brood chamber (far less labor, and far easier to remove after treatment). This also means that we’re not limited to the small amount of oxalic acid that could be dissolved into the limited amount of glycerin used to partially saturate the towels.
This is an exciting discovery—we now know that an OA/gly-saturated cellulose matrix (not necessarily a towel) laid flat across the top bars of the lower brood chamber appears to do the trick—we can avoid laboriously inserting (and later removing) 8 strips between the frames. So now it’s back to the drawing board to work out the details.
A second trial
We’d already planned to run follow-up late-season trials (to compare to the early-season results), and I was already 5 days into mine—this time repeating the application of OA/gly towels to one test group, to determine the effects of long-term application (compared to a single treatment), and an untreated control group.
It occurred to me that perhaps I had gone off on a wrong tangent–assuming that Maggi’s strips required insertion between the frames for efficacy. What if we simply laid them across the top bars [[10]]? And what if instead of 8 narrow strips, we simply applied 3 wider strips? So I quickly modified our protocols, and we are all now in the process of testing strips of heavy “chipboard” solid cardboard, cut to 4” x 10.5” (in order to leave plenty of bee passageways between the brood chambers—see Fig. 9).
Figure 9. OA/gly-saturated chipboard strips 20 days after application. We saturated the strips in the same formula of OA/gly used by Maggi in Argentina. There was some chewing in some of the hives–but not in all–similar to what I’d previously observed with hung strips.
The use of fully-saturated chipboard allows for a greater amount of OA to be applied to each hive (over that in the lightly-saturated shop towels that we’d been testing–~50 g compared to 18 g, but less than the 80 g when using the 8 Maggi strips). The three wide strips have a total surface area of only 75% of that of the 8 Maggi strips—thus a lower overall exposure to the colony to the OA/gly solution.
These strips also contain a lower concentration of OA relative to glycerin than that which we’ve been using in the shop towels—my thought being that extra glycerin might be of benefit in the distribution of OA throughout the hive (Table 1).
Table 1. Comparison of OA concentrations in various tested formulations. The Argentina “kitchen” formulation is commonly used by beekeepers in that country to soak their own strips [[11]]. In our “dry towel” formulation, crystals of OA cover the surface of the towels at low humidity—more so than with the 28% OA strips..
| “Dry” towel | Maggi | Our chipboard | Argentina “kitchen” | |
| Gly: OA | 13 mL: 12 g | 20 mL: 10 g | 100 mL: 50 g | 1000 g: 600 g |
| g glycerin (@1.26 g/mL | 16.38 | 25.2 | 126 | 1000 |
| g OA | 12 | 10 | 50 | 600 |
| g total | 28.38 | 35.2 | 176 | 1600 |
| % OA w:w | 42% | 28% | 28% | 38% |
Acidified Hives
OK, you have every reason to ask, won’t this extended application of OA/gly acidify the hives to the detriment of the bees? After all, repeated oxalic dribbles have been shown to cause adverse effects [[12]], although OA applied by vaporization appears to be easier on the bees [[13]]. To my surprise, until just yesterday, I hadn’t noticed any adverse effects from my experiments with extended-release OA/glycerin (Fig. 10).
Figure 10. Two typical brood combs from the upper box of the shown hive after 75 days of continuous exposure from two back-to-back OA/gly treatments. The OA/gly-treated colonies are generally the best-looking colonies in my operation, as far as strength, brood survival, and brood patterns.
It appears that bee colonies tolerate continually-acidified hives quite well, so long as the OA isn’t applied in sugar syrup [[14]]. But oxalic is a relatively strong acid, so there must be a limit. Since EPA likes to see data that tests the effects of a stratified range of doses in order to determine the NOAEL and LD50 levels, we had planned to eventually perform such tests. But I may have inadvertently stumbled onto that dose…
A surprising result
When I did midpoint mite counts for the second trial yesterday, in which I had a group of hives treated with the chipboard strips described above, for the first time I noticed what appeared to be adverse effects upon the colonies from OA/gly. The clusters in those hives tended to move to the lower box, and there appeared to be substantial larval mortality and shot brood patterns (Fig. 11).
Figure 11. At 20 days after application, all 5 of the hives in which I applied the chipboard strips exhibited elevated mortality of the young larvae, resulting in the shot brood patterns above. Surprisingly, despite the apparent adverse effects to the brood, mite counts by this time were not reduced to any extent.
The chipboard stock has no odor or unusual taste that I can detect, so I strongly suspect that this effect is due to the treatment. I’m totally surprised, since I did not observe such an effect when I applied an even greater amount of OA/gly in 8 hung strips in my tests last summer (nor did Maggi). Perhaps the adverse effect was due to the fact that I swapped out the recently-applied towels for fresh chipboard strips after 5 days. I’ll experiment to figure out what happened.
Anyway, it will be necessary for us to determine the dose and concentration at which oxalic acid in glycerin causes adverse effects to the colony, and the above observations are a step in that direction.
Question: Since the bees are blocked from contact with a substantial proportion the undersides of horizontal strips due to their resting on the top bars, it would seem that the bees would have been exposed to far more OA with the hung strip applications, yet the horizontal strips appear to be releasing a stronger dose. We clearly need to investigate this more deeply!
Mode of distribution and action
Here’s the problem—we really don’t yet understand the mode of action of OA against varroa, nor how it gets mechanically transferred from the strips to the mites. Initial research by Milani [[15]] found that the addition of glycerin to oxalic acid was synergistic, acting as a humectant to absorb water from the air (which would thus allow the OA to dissociate and free its biologically-active acidic hydronium ions). In trials in petri dishes, he found that the addition of glycerol roughly tripled the lethality of OA to mites allowed to crawl over the treated surface. The higher the humidity, the better that glycerin absorbed water from the air (this is why we need to test this treatment in both low- and high-humidity environments) [[16]]. Of note, Milani also observed that “mites laying on their back on the treated surface often showed lesser signs of injury”—strongly suggesting that the OA was better absorbed by the mites’ moist tarsal pads.
A recent study by Papežíková [[17]] showed that varroa can be killed by feeding on bees that had consumed OA (as well as by walking over finely crystallized OA from vaporization). Nanetti [[18]], using radioactive tracer, demonstrated that OA clearly gets into the bees’ bodies when dribbled in sugar syrup, and that they mostly clear it from their systems within days. So there is the possibility that bees ingest some of the OA/gly, and thus expose feeding mites to it.
Or perhaps after walking over the OA/gly/water-saturated surface, the bees inadvertently transfer the solution to their bodies as they groom themselves, thus exposing any phoretic mites to the acid. Or maybe it only affects mites that walk over the body of bees (or comb surfaces) contaminated with the solution. Clearly, I’m unclear as to the exact path by which the OA/gly in these strip applications gets to the mites, why it kills them, and exactly how important is the ratio of glycerin to OA.
In any case, with this method of application of OA, rather than the effect being due to a sudden and short-term kill of the mites that are phoretic at the time of application (as with dribble or vaporization), the extended-release OA/gly application works by causing increased mite attrition over 3-4 weeks.
Practical application: it’s pretty clear that we’re going to need to test various concentrations of OA to glycerin, as well as the optimal dose of OA per hive, and the optimal surface area of the cellulose strips.
Development of mite resistance to oxalic acid
I’m acutely aware of a warning pointed out by Milani back in 2001 [[19]]:
Among substances of natural origin used against V. destructor, the risk of resistance is higher for oxalic acid. High efficacy in the absence of capped brood, ease of use of the application by trickling [not to mention vaporization or OA/gly strips], extremely low cost, independence from the temperature and shortage of alternatives might make treatments with this acid widely used in the future and thus increase selection pressure for resistance.
Maggi [[20]] showed evidence that varroa does not appear to be able to rapidly develop resistance to oxalic acid. This may be because of the strong physiochemical effects of acids upon the mite–rather than it targeting a specific protein or neuroreceptor. For example, even the most antibiotic-resistant bacteria still succumb to the deadly chemical sodium tallowate—commonly known as “soap.”
That said, I’m finding the mite counts in one of my test hives to be of concern. Hive #45 was the only OA-treated hive in which the mite count rose in Trial #1. At 48 days, I reapplied fresh shop towels, yet its high mite count remained the same after 25 more days. Is it possible that there is a clonal matriline of mites in that hive that are resistant to oxalic acid? I sure hope not (and will keep checking).
Update: without further treatment, the mite count of this colony had dropped to only 2 the last time I checked. Apparently the mites were not resistant afterall.
Practical application: I share the concern of Milani that since OA is so cheap and easy to use for varroa control, that beekeepers will overuse it, and thus quickly breed for resistant mites. On the other hand, plants have been fighting herbivores for hundreds of millions of years, and have become master chemists at producing compounds toxic to arthropods. There may be too great a fitness tradeoff for varroa to develop resistance to strong physiochemically-active natural plant toxins such as thymol or oxalic acid (e.g., hardening their shells or tarsal pads would make the mites less nimble on the bees, and more susceptible to grooming). It’s impossible to predict evolution—so no telling whether oxalic acid and acidified hives will be our salvation, or merely another short-term solution until we switch to mite-resistant bees.
A game changer?
I strongly suspect that extended-release oxalic acid in glycerin is going to be a game changer in varroa management. My sons, after checking mite counts in some yards today, are already asking me whether we should start incorporating it into our mite management strategy when it becomes legal. Cross your fingers that Dr. Jay Evans of USDA ARS, I, and our collaborators can figure out an optimal application method quickly, and present supportive data to EPA for it to be listed as an approved application method.
A note to those wishing to help: if you are a student, researcher, or beekeeper who can obtain an experimental use permit and have a number of hives at your disposal, let me know, and I can suggest some experiments to run.
Acknowledgements
Thank you to Dr. Jay Evans and USDA ARS, and to those who have donated to support this research. And I couldn’t have done it without the help of my sons Eric and Ian, my technicians Tara McKinnon and Rachel Woodward, and assistance from Bill Mills, Cory Dodgson, and Sean Cullers.
A thank you and goodby to editor Joe Graham
And I’d like to give heartfelt thanks to Editor Joe Graham, who has so helpfully allowed me to grace the pages of the American Bee Journal this past decade. I admire Joe for his sober assessment of the problems facing our industry these past tumultuous years, and for providing a reliable source of information to the beekeeping community in the form of this magazine, his ABJ Extras, and his revised editions of The Hive and the Honey Bee. Joe, we all wish you a well-earned and enjoyable retirement!
Notes and citations
[1] With each experiment, we are learning more about how best to apply this treatment—it is not a done thing, so please do not ask me for recipes. If you wish to experiment yourself, please check with your State Lead Agency for pesticide regulation to see whether you need to obtain an experimental use permit. Please do not bother your EPA district office—the EPA Registration department would prefer to handle the process without needless confusion.
[2] EPA needs eliminate the possibility that any observed effects were due only to the glycerin or the towel material itself.
[3] This helps to minimize the effect of differences in colony-to-colony mite buildup rates.
[4] I did this to minimize any restrictions upon colony buildup or honey production due to any colony “plugging out” during the course of the trial.
[5] Due to the single outlier, the mean mite count was skewed, so I used the Henderson-Tilton formula to calculate the efficacy of treatment based upon the medians.
[6] Even more surprising is that I retreated that hive in the second trial, and its mite count held steady at 75 days. I have no idea what’s happening in that one hive (I’ve inspected it closely a few times), but I’m sure hoping that it’s not an indication that some varroa matrilines exhibit resistance to oxalic acid! We’ll see what happens over the next few weeks.
[7] Having received a permit from California Department of Pesticide Regulation.
[8] Maggi, M, et al (2015) A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 47(4): 596–605.
[9] Since there were counts of zero, I couldn’t calculate percentage reductions, so used absolute values.
[10] Another California beekeeper reported to me that he’d had good luck laying the strips horizontally.
[11] Fernando Esteban, pers. comm
[12] Hatjina F & L Haristos (2005) Indirect effects of oxalic acid administered by trickling method on honey bee brood. Journal of Apiculture Research. 44(4): 172-174.
Schneider, S, et al (2012) Sub lethal effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae): changes in behaviour and longevity. Apidologie 43: 218.
[13] Al Toufalia H, et al (2016) Towards integrated control of varroa: 2) comparing application methods and doses of oxalic acid on the mortality of phoretic Varroa destructor mites and their honey bee hosts. Journal of Apicultural Research. 54(2): 109-121.
[14] I’m in no way a promoter of the mystical and esoteric practices of biodynamic beekeeping, but when researching oxalic acid and bees, I found that the founder of the movement, Rudolf Steiner, in his Lecture IX about bees, spoke at length about how “In Nature formic acid is continually being prepared from oxalic acid.“ He waxed eloquent about the importance of oxalic and formic acid: “Thus we can say that for the earth also, formic acid is the basis for earth-soul and earth-spirit.” He even mentions the importance of oxalic acid and glycerin.
To quote from Steiner: “Oxalic acid is essential for all that has life. Wherever there is life, there is oxalic acid, an etheric body. The etheric body brings it about that the oxalic acid is renewed. But the oxalic acid never becomes a formic acid that can be used by the human or animal organism unless it is first transformed by an astral body from oxalic into formic acid.”
http://www.biobees.com/library/biodynamics/SteinerBeeLectures.pdf
[15] Milani, N (2001) Activity of oxalic and citric acids on the mite Varroa destructor in laboratory assays. Apidologie 32: 127–138.
[16] From Milani: “The data on the relative humidity in equilibrium with saturated solutions of these substances explain the mechanism of this synergism: oxalic acid does not absorb water vapour from the atmosphere at R.H. below 86% but when mixed with sucrose it does at R.H. > 69%. Thus at 75% R.H. the deposit of oxalic acid and sucrose is constituted by highly concentrated droplets of a liquid, but not at 42% R.H.; the addition of glycerol has the same effect also at 42% R.H., since glycerol is always hygroscopic. Deposits constituted by droplets of liquid were more active than dry deposits. Effect of glycerol was higher at 42% than at 75%; the solution is more diluted at the higher R.H.”
[17] Papežíková, I, et al (2017): Effect of oxalic acid on the mite Varroa destructor and its host the honey bee Apis mellifera. Journal of Apicultural Research DOI: 10.1080/00218839.2017.1327937
[18] Nanetti, A (2003) Pharmacodynamics of oxalic acid in the honey bee colony. http://www.apimondia.com/apiacta/slovenia/en/nanetti.pdf
[19] Milani, N (2001) op cit.
[20] Maggi, MD, et al (2017) The susceptibility of Varroa destructor against oxalic acid: a study case. Bulletin of Insectology 70 (1): (preprint).