Extended-Release Oxalic Acid Progress Report #3
Extended-Release Oxalic Acid Progress Report #3
Randy Oliver
ScientificBeekeeping.com
First published in ABJ January 2018
As the synthetic miticides predictably lose their effectiveness across the world, beekeepers are turning more and more toward oxalic acid to control varroa. Unfortunately, oxalic is not a very efficacious treatment when there is brood present.
To that end I am collaborating with USDA-ARS to petition EPA to add an extended-release application method for oxalic acid to the label.
Disclaimer and legality: The information in this article is solely to report on the progress of our beekeeper-funded research (working in conjunction with ARS and others) towards getting this application method approved by EPA. This report is not intended to promote illegal use in any way. This is not a “how to”—please do not write me asking how to apply it yourself until we have gotten the bugs worked out and it has been registered.
Field testing
We applied a number of single oxalic acid and glycerin (OA/gly) shop towels to strong hives shortly after their return from almonds (in early April, as an informal trial). We then checked back on them in late July, at which point any untreated colonies in my operation would typically exhibit alcohol wash counts in the 20-50 range (6-16 mites per 100 bees). This was confirmed by the fact that a number of other hives in the same yard, which had been started as April splits treated with an oxalic dribble during their induced brood break, also showed similar high counts.
By this time, the towels had been largely removed, and any remnants propolized over (Fig. 1).
Figure 1. One downside of the OA shop towels is that there are typically propolized remnants to scrape off the top bars. Fortunately, they are completely biodegradable, and can be safely discarded. I’d expect the amount of residues shown above to contain less oxalic acid than in a serving of spinach [[1]].
To our amazement, roughly half of the shop-towel-treated hives exhibited very low mite counts—zero to 5—suggesting that it takes the mites a long time to recover from a treatment (and that there is perhaps an extended residual effect). But this result was not consistent from hive to hive nor yard to yard—mite counts went up in some hives–so we still need to determine why the difference despite identical treatments.
The formal field trials
I’ve now completed two controlled field trials run during a dry California summer; the data is not yet in from the trials in humid Georgia. Please refer back to my previous reports for details [[2]]. For the second trial, I gave a second identical treatment to 23 of the colonies, which had previously received an initial treatment of 3 half sheets of OA/gly towels. Before applying this second round, I thoroughly scraped out any remainders of the first towels.
Practical application: the intent of this test was to see whether a repeat treatment resulted in increased efficacy, or adverse effects due to the prolonged exposure.
I split the remaining untreated control hives after the first trial into two groups—21 hives to receive 3 half OA/gly towels (for their first time), and 8 to serve as controls. By this time, I could see which of the control hives exhibited unusually high or low rates of mite growth, so I ranked them and used those in the midrange as controls for the second trial.
Scientific consideration: one must be careful when testing for the efficacy of varroa-control products, not to allow any control hives to collapse from mite infestation during the course of the trial, since drift of those mites will skew any results. So any high-mite colonies got placed into the treatment group. Note that this meant that in the second trial the treated hives were biased towards those in which mites had previously reproduced more rapidly (and vice versa for the controls), meaning that the results from the second trial would likely underestimate the true degree of efficacy of the treatment. Thus, any efficacy calculations would be conservative, which is fine for me.
I also ran 5 hives with three wide chipboard strips, saturated in OA/gly as per Maggi’s original formula (results not shown below). We ran the second trial for 40 days. Below are the results of both trials, all compiled into one chart spanning the entire 89 days (Fig. 2).
Efficacy
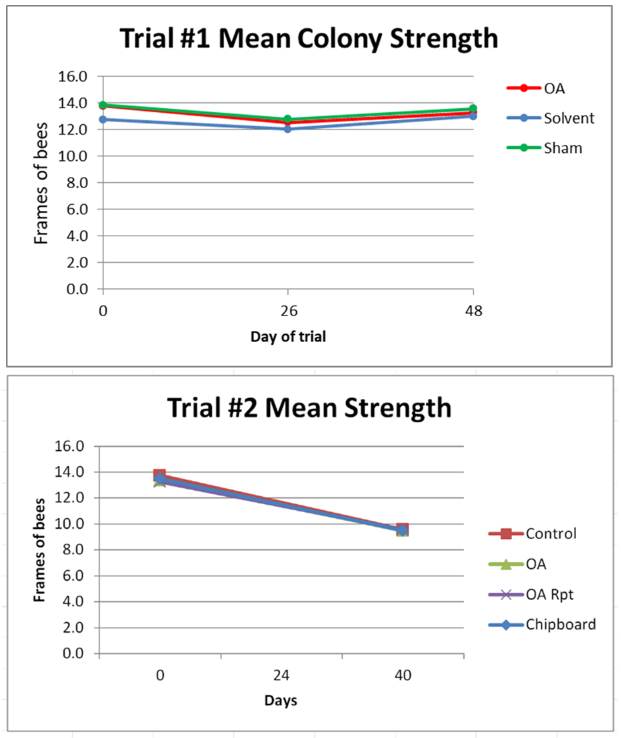
Figure 2. The rates of mite growth in the control groups was as expected. On the other hand, the mite infestation rates were greatly reduced in the treatment groups. Surprisingly, the calculated efficacies of mite reduction [[3]] for Trial #1, Trial #2, and Overall were all identical at 94%. This puts this application method on par with the best mite treatments on the market, but at a far lower cost, and without the concern of leaving persistent residues in the combs [[4]]. Median values [[5]], with SEMs indicated.
I was impressed by the degree of mite control across the board, but there were a few outliers. Five (of 21) hives in the second treated group still had mite counts between 9 and 14 at 40 days, but based upon an observation that I’ll detail later, I have reason to suspect that they would have gone down with time.
Practical application: with this particular application method (3 half towels containing 18 g OA & 19.5 mL gly in total), a single treatment resulted in good efficacy; a repeated treatment in even more consistent efficacy.
| TAKE HOME MESSAGE: OA/gly does not result in quick mite knockdown—it appears that it may best be applied as an early-season treatment, and at the very latest, prior to the end of July.
|
ADVERSE EFFECTS
I was especially curious as to whether the colonies receiving two consecutive OA/gly treatments in Trial #2 would cause any adverse effects. Apparently not (Figs. 3 & 4).
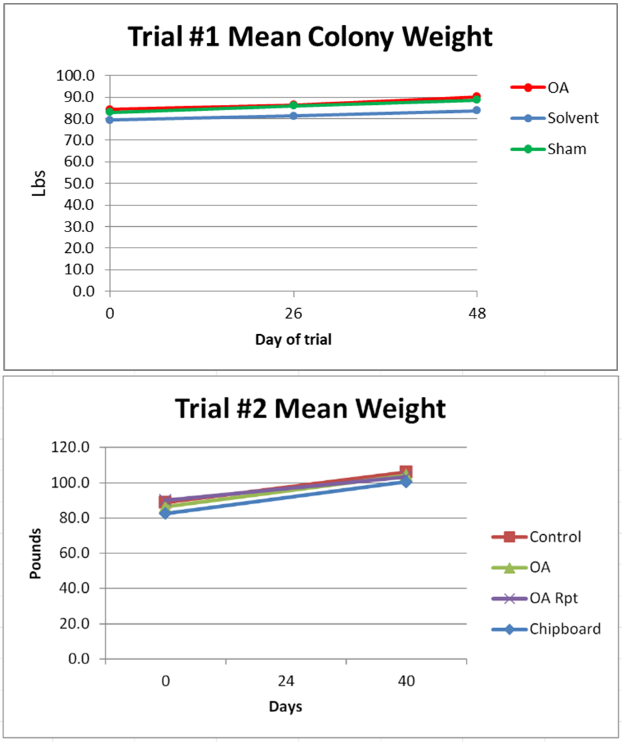
Figure 3. Due to the heat wave and drought, similar to the rest of our hives in the area, the colonies did not grow in either trial. There was no apparent effect in either trial from the treatments upon colony strength, despite the early brood kill from the chipboard strips and the extended exposure in the OA Repeat group.
As far are further monitoring of colony strengths, we ended the trials at the end of August so that we could begin our critical September stimulative feeding. I’m continuing to track a number of the OA Repeat colonies (with their 2nd-year queens) over the winter—they still look good as of mid-November.
Figure 4. Nor was there any apparent effect upon weight gain—due to the hot and dry conditions, there was scant weight gain in most hives.
Retesting the argentine chipboard formulation
OK, how about those chipboard strips? To my complete surprise, despite causing the only adverse effect that I’ve observed (to the brood), they utterly failed at reducing mite counts. I have no explanation for this result, since I previously got good results when similar strips were hung over the frames (note that the chipboard strips were saturated with a different formulation than were the shop towels). So we made up a fresh batch of strips, using chipboard from another source, again using the 2 parts glycerin: 1 part oxalic acid ratio from Maggi (Fig. 5). We applied these to hives in other yards.
Figure 5. I questioned whether the adverse effect or poor efficacy from the chipboard strips was due to a problem with the paper [[6]]. So we made a fresh batch, using office-grade chipboard. We applied from 1 to 3 strips per hive to a number of hives, proportional to their mite level.
It was now late summer, so I placed pollen sub right between and over the strips to encourage the nurse bees to walk over the strips (Fig. 6).
Figure 6. You can see the remnants of recently-consumed pollen sub on these strips. Again, to my complete surprise, even three strips had little effect on mite counts. This makes me strongly suspect that the oxalic-to-glycerin ratio is important.
Questions Remaining
The optimal formulation
After such dismal results from flat-laid high-glycerin strips, as well as the observation that the degree of chewing of the towels didn’t make a difference, I strongly suspect that there are two critical factors involved:
- The ratio of oxalic acid to glycerin, and
- The degree of glycerin saturation of the cellulose substrate.
To that end, I’m now in the middle of incubator trials to test the above (Table 1).
The above matrix consists of formulas for a half shop towel (which absorb roughly 8 mL of liquid), and holds the oxalic content fixed. It does not cover all the possible ratios to test—for example, I’d also like to test 4:4:0, but it’s a start. In my preliminary trials (Fig. 7), I found that a towel lightly saturated with glycerin alone, caused the bees to lose traction on the side of the cup, so I need to figure out how much towel to insert into a cup cage (or provide better footing).
Figure 7. A preliminary “quick and dirty” trial. The cage at the left contains a towel with glycerin alone—after a few hours I added a dry leaf for the bees to climb upon to reach their syrup. The middle cup contains a dry towel covered with oxalic acid crystals (Formula A), which didn’t appear to harm the bees in the three days that I ran this trial. The third cup is a control with filter paper on the bottom.
The question of resistance
One colony in the repeated treatment group maintained high mite counts through the end of Trial #2. This concerned me greatly, since I wondered whether I’d found a bloodline of mites that were resistant to oxalic acid. So I continued to monitor that hive after the completion of the trial—its mite count eventually dropped to only 2 by early October. Whew! I have no explanation as to why the delay.
I’m continually asked about whether varroa has the potential to evolve resistance to the essential oils or organic acids. This is actually a pretty easy question to answer—all one need do is to Google “pests of thyme” (or rhubarb) in order to see that this is likely biologically possible. That said, varroa may have difficulty evolving resistance to oxalic acid, since the main mechanism of exposure appears to be through its sticky tarsal pads. Were the mite to develop less sticky or more acid-resistant pads, it might not be able to cling so tightly to the bee, thus allowing bees to more easily groom off their unwanted hitchhikers.
Modeling the effect of OA/gly application upon varroa
Based upon my mite model, I developed a second simplified model to plot out the day-by-day effect of oxalic acid dribble or vapor treatments (coming next month). It occurred to me that I could also use the same model to back-calculate the effect of an extended-release oxalic acid treatment [[7]]. I first tried entering a continual day-after-day kill of 20% of the phoretic mites—the simulation predicted that after a month, the slow additional attrition would result in 88% efficacy, and by the end of two months, 98% efficacy at reducing the mite population.
But it’s doubtful that the oxalic would actually maintain the same kill rate over time. It’s more likely that it would take some time for the bees walking over and under the towels to inadvertently transfer it to their bodies via grooming, thus “acidifying” the hive and exposing the mites to the acid (few mites would be expected to walk over a towel on their own accord). I’d also expect the amount of acid in the hive to peak at some point, and then decline as the acid dissipated, or the towels were removed or propolized over.
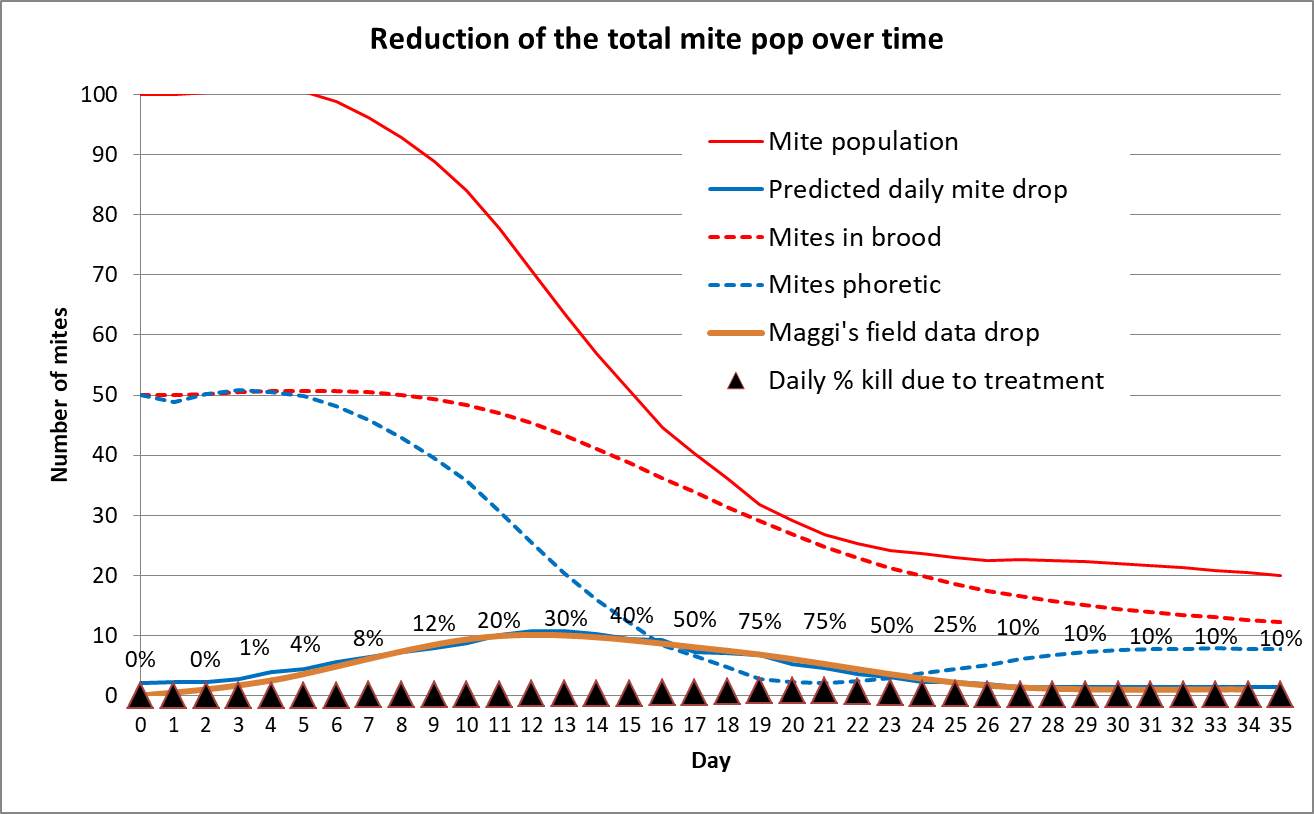
What I needed to model this was field data of daily mite drops after applying an OA/gly treatment. Not having taken daily mite drop counts myself, I worked up Maggi’s [[8]] data for the weekly counts from his OA/gly treated hives. Mite fall after applying the treatment typically peaked in his monitored hives during the second and third week, and then declined to low levels. So I plotted an averaged curve of his data into my simple model, and then hand adjusted the daily phoretic mite kill rates until they closely matched the field data (Fig. 8).
Figure 8. I plotted Maggi’s data on the brown line, and then back calculated the daily mite kill rate by hand matching the somewhat hidden solid blue curve. This modelling suggests that it typically takes about a week for the oxalic acid to spread onto the bees, after which it starts killing mites at a fairly high rate for a couple of weeks. By three weeks, the overall mite population is greatly reduced, and then continues to decline for some number of weeks thereafter, since the potentially reproducing mites in the brood are not being replenished.
The above simulation suggests that the treatment works by slow attrition. I’m skeptical, however, of the indicated track of the phoretic mite population (blue dotted line), which suggests that an alcohol wash at three weeks would be close to zero. I need to confirm by taking alcohol wash counts every few days.
Scientific request: We still have a lot to learn about this treatment, and it would be of great value to confirm the validity of the simulation above. I haven’t had the time to do daily mite drop counts or alcohol washes on OA/gly treated hives. If anyone has done so, please send me the data.
TAKE HOME MESSAGE: OA/gly is an extended application method that takes several weeks for full effect.
Wrap up
To date, we’ve only gotten results from field testing in a dry climate, and only for three month’s exposure. So far:
- We’ve demonstrated proof of concept, as did Maggi with his strips, that OA/gly applied on a cellulose substrate can clearly control varroa, although it takes several weeks to do so.
- It appears that the Argentine formula does not control varroa when applied in wide horizontal strips.
- We don’t yet know the effect of ambient humidity upon efficacy or adverse effects.
- We still don’t know if there are any “legacy” adverse effects over the winter in a hive that was treated during the summer.
- We don’t know the ideal ratio of OA to glycerin, nor the ideal degree of saturation of the substrate. I hope to answer that question by this time next year.
Overall, I’m very encouraged by the results, and can see how this hive-friendly treatment could be an important component of our mite management strategies once approved (I will still rotate with thymol and formic acid, and continue to select for mite-resistant stock). Once my collaborators work up their data, we’ll see whether we’re ready to submit to EPA, or whether we’ll need to run another round of field trials.
My research is funded solely by donations from beekeepers—feel free to contribute to this exciting project. You can donate at ScientificBeekeeping.com.
A note to those wishing to help: If you are a student, researcher, or beekeeper who can run incubator trials, or obtain an experimental use permit to run field trials (and have a number of hives at your disposal), let me know, and I can suggest some experiments to run. I am in no way promoting applications not sanctioned by your State Lead Agency [[9]].
Acknowledgements
Thank you to Jay Evans and USDA ARS, Jennifer Berry, Geoff Williams, and to those who have donated to support this research.
Notes and citations
[1] A serving of spinach can contain up to 1 g of OA. The full towels contained 8 g. There was well less than a tenth of the towel remaining, and even those residues would have already lost some amount of their OA to the bee traffic (otherwise the treatment would have no effect upon the mites).
[2] https://scientificbeekeeping.com/extended-release-oxalic-acid-progress-report/
https://scientificbeekeeping.com/extended-release-oxalic-acid-progress-report-2/
[3] Using the Henderson-Tilton formula.
[4] Unlike the persistent comb residues from lipophilic synthetic miticides or thymol, analyses of combs and honey samples after oxalic treatment by sugar dribble or vaporization show minimal residues of OA, which is a natural component of honey. Maggi also found few residues in honey following OA/gly treatment. Schneider found only minor residues in the wax and wood following dribble applications of OA in glycerin.
Schneider, S (2015) Subletale Wirkungen von Oxalsäure in Kombination mit Zuckerwasser oder Glycerin auf Apis mellifera: Untersuchung der Toxizität, der Pharmakodynamik, des Verhaltens und der Lebensdauer, sowie der Rückstände auf Bienen und Beutenmaterial. Doctoral Dissertation, Freien Universität Berlin. Refer to Abb. 114
[5] There were a few outlier counts, so the median values better represented the results. I show the standard errors of the means.
[6] I also ran cage trial tests of the original chipboard without any OA or glycerin and observed no adverse effect upon adult bees.
[7] Funny how the mind works (or doesn’t). After working most of a day creating and using the new model to create vaporization treatment graphs, the thought of using it to back calculate the kill rates for OA/gly came to me at 4:00 in the morning two days later.
[8] Maggi, M, et al (2015) A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 47(4): 596–605.
[9] If you wish to experiment yourself, please check with your State Lead Agency for pesticide regulation to see whether you need to obtain an experimental use permit. Please do not bother your EPA district office—the EPA Registration department would prefer to handle the process without needless confusion.