A Test of The Drench Method for Nosema
February 23, 2011
A Test of the “Drench” Method of Nosema Treatment
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in Oct. 2008
In previous articles in this journal, I’ve summarized what we know about Nosema ceranae, its pathogenicity, detection, and treatment. I wondered whether N. ceranae would prove to be as virulent in North America as it appeared to be in Spain. There were also a number of unanswered questions as to the efficacy of treatments and the best ways to apply them. In this article I will report the results of a small field trial of “drench” treatments.
Introduction
Nosema ceranae is of great concern to the many beekeepers who have discovered that their bees are infected. It is indeed widespread, although I have sampled a number of operations in which it is virtually undetectable. Unfortunately, the standard treatment of fumagillin dissolved in heavy syrup has not always proved to be effective. In a trial that I ran last winter of several different ways of applying fumagillin, plus some alternative treatments (in prep), I was surprised to find that no treatment appeared to result in “clean” bees in the spring.
Since I now had a yard of nosema-infected bees going into a spring nectar flow, I was in a position faced by many beekeepers—how to treat your bees when it is impractical to feed two gallons of medicated heavy syrup. In my case, there was a nectar flow on, and the bees were not likely to take syrup consistently, or if they did, they would likely store it, rather than consume it. Some colonies were sick, and not likely to take syrup well. There are also other times when feeding gallons of medicated syrup is impractical, such as when the bees are storing honey, when the colonies are already plugged out with honey, or when feeding that much syrup is just too expensive. A concern of large commercial beekeepers is the potential degradation of fumagillin in their large tanks of syrup between the time that it is mixed, and when it is finally put into a feeder and fully taken by the bees—mixing fresh small batches that can be applied immediately alleviates this potential problem (this is not a problem if the syrup is fed within a day or two).
At these times, some beekeepers are using the “drench” method, in which the appropriate dose of fumagillin is dissolved in a cup or so of light syrup, and dribbled or sprinkled over the bees. The rationale is that the bees will lick up all the syrup off each other, and through the process of trophallaxis (food sharing), spread the active ingredient evenly through the colony, with minimal storage of the product.
An important, but frequently misunderstood, point is that drenching with a dose of strong medicated syrup does not necessarily mean that that you are increasing the dose of active ingredient (a.i.) to the colony—it simply means that you are giving the same dose in a lesser amount of syrup.
However, a single drench will only give a temporary dose. To control nosema, it appears that the treatment must be given continually over a period of at least one full brood cycle, in order to prevent reinfection of the new generation of bees. When treating with gallons of heavy syrup in the fall, it is easy to give a continuous dose to the bees as they eat their way through the stored medicated “honey.” In order to get the same effect with the drench method, it must be repeated more than once, and at frequent intervals. The question is: how frequently, and for how many doses? Higes (2006) found that treatment of colonies with 30mg of fumagillin a.i., repeated weekly four times was effective at controlling N. ceranae. However, note that it took until a week after the fourth treatment until no more spores were detected.
Fumagillin has clearly been demonstrated by others to be an effective therapeutic agent against infection by N. ceranae. However, when used as per label recommendations for control of N . apis (fed in gallons of syrup fall or spring), researchers (Williams 2008) and a number of beekeepers have reported that it is not always effective against N. ceranae. Williams also found that treatment, or lack thereof, the previous fall had little effect upon N. ceranae counts the next August (similar to previous experience with N. apis). Therefore, with the invasion of Nosema ceranae, beekeepers are now concerned about nosema spore counts during the summer.
Note that the efficacy of any particular treatment can be hard to determine, since spore counts can vary widely in individual colonies post treatment, and can drop spontaneously in untreated colonies during winter (Pernal 2008) or summer (Williams 2008).
I performed this experiment in order to answer two questions:
1. Would fumagillin, applied as a “drench” at the rate of 1 cup per 10-frame colony (30mg a.i. per colony), repeated weekly, stop an incipient N. ceranae infection in its tracks?
2. Would any other of several alternative treatments, applied similarly, be effective?
I assumed that if any treatments were really effective, then a small number of colonies would be enough to detect a difference in a “quick and dirty” field test.
Materials and Methods
Field Work
On May 8, 2008 we shook nosema-infested (previously confirmed to be N. ceranae by Dr. Robb Cramer) bees into a large bulk cage in order homogenize the infection level. The bees were shaken from two infected swarms that had been hived 2 weeks previously (and therefore consisted of aged bees) and from 6 additional infected colonies. The shook bee box was shaken to homogenize the bees, then allowed to set overnight.
Shaking bees from infected colonies into a bulk cage to homogenize the infection level.
The next day, we set up 28 hives in a “U-shaped” row with entrances in alternating directions in or out. Each hive consisted of a single deep brood chamber over a screened bottom. Into each hive we placed a single brood frame, with approximately half the brood sealed, obtained from a nosema-infected colony, 4 new Dadant preassembled frames, and a caged queen fresh from a reputable queen producer (queens were from the same stock, but not necessarily sisters). We then sealed the hive entrances, and added 2 lbs of the shook bees to each hive. We took 8 samples of the shook bees to determine initial infection level. Each colony was given 1/6 gallon of 33% sucrose syrup in an inverted jar top feeder, and allowed to set for two days.
Dumping the 2 pounds of infected bees into a test hive. Each colony consisted of one frame of brood sandwiched between four frames of foundation, with a fresh queen.
On 5/11, we allowed the bees free flight, replaced the entrance blocks with entrance reducers (to reduce drift or robbing), exposed the candy of the queen cages, and fed ½ gallon of 1:1 sucrose syrup.
On 5/12, we checked colony strengths to make sure that all appeared equal, swapping one pair of colonies to equalize. Later in the day, colonies were numbered and assigned treatments in a randomized block design. Each block of 7 colonies received one each of 6 treatments, plus had a control colony.
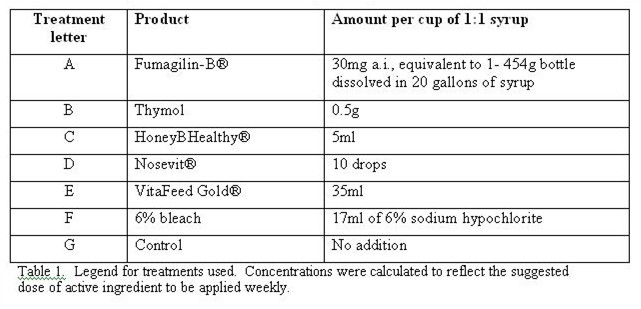
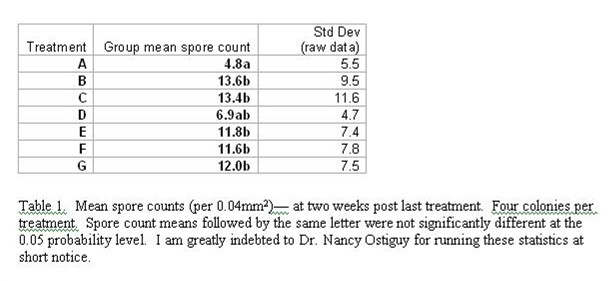
The treatments consisted of several products suggested to me by beekeepers and manufacturers as potential nosema treatments (Table 1).
Treatments tested—ready to be mixed fresh on the truck bed.
The product concentration was determined by manufacturer’s recommendation (label or personal communication) for amount of active ingredient to be applied to a 10-frame colony per week. This amount of a.i. was dissolved in syrup such that 1 cup of applied syrup would give the full dose to a 10-frame colony. In this trial, a full cup was not given to the small colonies, but the syrup was applied such that each frame of bees received a proportional dose.
The bleach concentration was suggested by Chilean beekeeper Juanse Barros, who has tested its application previously (pers. comm.). The thymol strength was 3 times as strong as that recommended by Yucel (2005), since the 0.44mM concentration that he used had not proven effective in other trials (pers obs; Dr. Jeff Pettis, pers comm).
The treatments were mixed fresh prior to each application, and applied first with a perforated-lid feeder jar, and subsequently with a modified plastic sports drink bottle with holes drilled in the lid. For the first (5/12) application, I held the frames of bees horizontally and drenched all the bees thoroughly. In subsequent applications (5/19, 5/26, 6/2) I left the combs in place and top drenched the bees, running the streams of syrup down each seam of bees from frame end to frame end. I gently spread frames when necessary to ensure complete coverage of the bees, in order to confirm queenrightness, and to check the general appearance of the brood. Note that all applications of treatments were “eyeballed” to approximate the correct dose per colony. Since the clusters varied greatly in density, especially on the foundation, I made sure that the outer layer of bees on each frame always was wetted. In checking the amount of syrup used, the doses were sometimes slightly over applied, rather than under applied, with the reasoning that excess would drop through the screened bottom and not be ingested.
Application of the first drench. I made sure that the bees were evenly wetted to ensure distribution of the treatment to the entire population.
The trial began with good flight weather, and a light nectar flow, but soon turned hot, and then too cold for flight. On 5/23, we fed each colony ½ gal of 1:1 syrup to avoid starvation. This poor weather may have been fortuitous, since it stressed the colonies with cold conditions and poor nutrition, both of which may promote nosema virulence. Colonies were additionally fed ½ gal of syrup on 6/3 and 6/14. By 6/16 there was a good nectar and pollen flow on.
Subsequent treatments were applied with a modified sports drink bottle. An air vent hole under my index finger was used to control the flow. It was not difficult to evenly drench these small colonies, so that all bees received likely were exposed to the active ingredient.
Samples of adult bees were taken into 70% isopropanol in clean bottles near mid day from the entrance with a vacuum (Oliver 2008) on 5/27 and 6/16, plus a sample was taken from the brood frames on 6/16. After sampling on 6/16 (2 weeks after the last treatment), the colonies were given an additional 5 frames of foundation, and the entrance reducers removed.
On 7/23, upon noticing that there was substantial difference in colony weights and strengths, we weighed each colony, and took adult bee samples from the 6 lightest, and 5 heaviest colonies.
Lab Analysis
The number of bees in each sample was counted, the drained bees placed into a ½ pint mason jar, and water was added at the rate of 1ml per bee. We screwed a kitchen blender blade base onto the jar, and blended with two 5-second pulses at the “chop” setting, allowing the blades to come to rest between pulses.
All equipment was rinsed thoroughly with tap water between samples (samples were tested to ensure that there was no spore cross contamination). I counted two samples of spores from each bee sample at 400x, using a Hausser Bright-Line hemacytometer; infection level was calculated as per Cantwell (1970).
Results
On 5/27 (the day after a treatment) we noted significant adult bee mortality in front of the colonies treated with VitaFeed Gold. Those colonies also had minimal forager flight that day, compared to other colonies.
On 6/2 we noted that the colonies receiving bleach and the controls appeared to have the most brood.
By 6/16 there was substantial difference in colony strength increase, and number of frames drawn and filled. It appeared that the stronger colonies tended to have more solid brood patterns. Three colonies went queenless after the 6-week count, and another by 8 weeks.
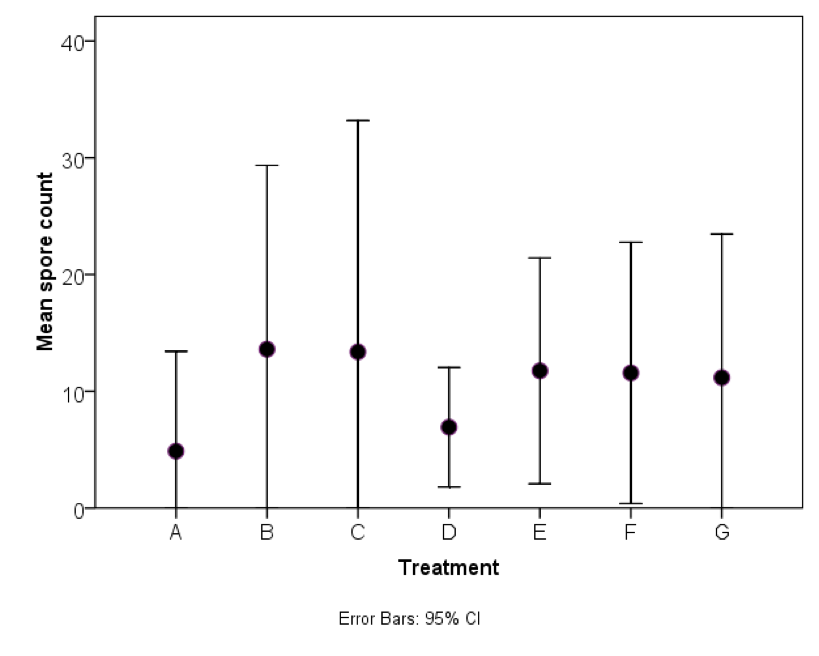
Initial infection level of the shook bees was 0.15 million spores per bee (std dev 0.09). Levels two weeks post the last treatment varied from zero to 7.3 million spores per bee (Figures 1and 2, and Table 1). Please note that all results are given in unconverted spore counts per 16 hemacytometer squares—to convert to millions of spores per bee, divide by 4. Colonies receiving fumagillin had significantly lower spore counts than the controls or other treatments except Nosevit (P < 0.05). There was no statistical difference between the fumagillin and Nosevit treatments.
Figure 1. Infection level two weeks after four weekly treatments. A = Fumagilin-B, B = thymol, C = HoneyBHealthy, D = Nosevit, E = VitaFeed Gold, F= bleach, G = Control. The arrow at the far left indicates initial infection level (all results are unconverted raw data counts of number of spores per 16 hemacytometer squares (total 0.04mm2)—divide by 4 to obtain million spores per bee). Note that no treatment eliminated Nosema ceranae, but that spore counts were somewhat reduced in treatments A and D.
Figure 2. Mean spore counts (per 0.04mm2)— for treatments. Due to high variability, and small number of colonies in each treatment (n = 4), note that all treatments fell within the ranges of the others. Courtesy Dr. Colin Henderson.
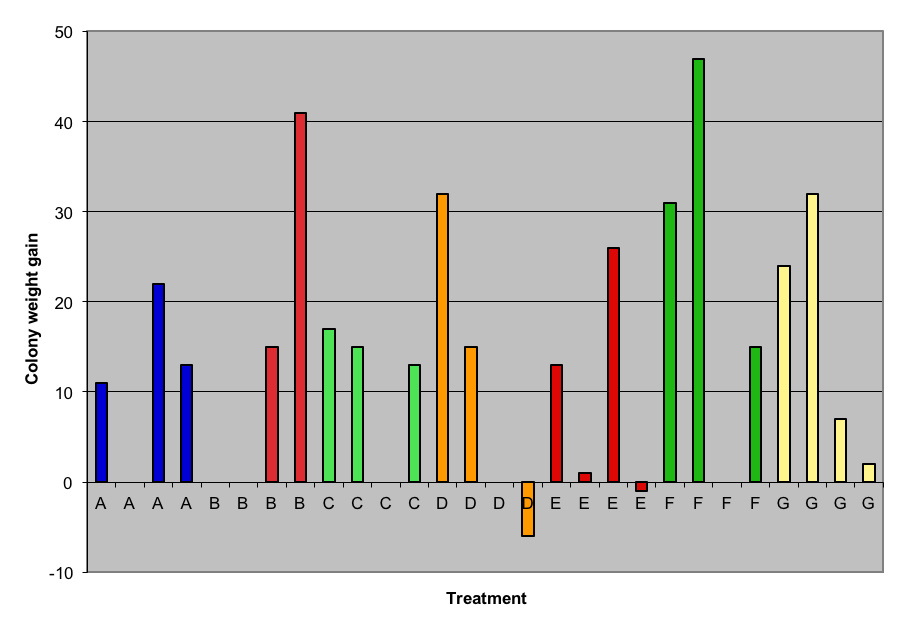
Although I had not initially planned on taking colony weights, they varied so much at 73 days, that we weighed each hive. The initial tare weights were not taken; however they would have all been expected to be about 68 lbs, since all colonies started with the same equipment, 2 lbs of bees, and a single comb of brood and honey. The final weight gain of surviving queenright colonies is shown in Figure 3 (weight gain strongly reflected colony strength). No clear pattern emerged.
Figure 3. Colony weight gain (which reflected colony strength) vs. treatment, at 73 days. Again, note the large differences in the yellow control group. Some colonies are missing due to queenlessness or weakness. No clear-cut benefit is apparent from any treatment. (See Table 1 or Fig. 1 for treatment legend).
The last measurement that I took was to see if there was a difference in nosema infection level between the heaviest and weakest colonies at 73 days (Figure 4). Curiously, the stronger colonies had higher infection levels.
Figure 4. Colony weight gain vs. spore count (per 0.04mm2)—. I was curious as to whether the nosema infection affected the weight gain of colonies (weight gain largely reflected colony strength). This graph plotted the spore counts at 73 days of the 5 heaviest and lightest colonies. Surprisingly, the stronger colonies had higher spore counts (in the range of 2 million spores per bee).
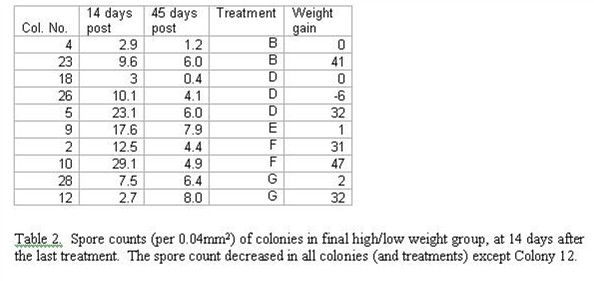
I compared the spore counts of the eleven colonies measured on Day 45 post last treatment with their counts on Day 14 post. All had gone down (despite lack of further treatments), except in colony no. 12 (Table 2).
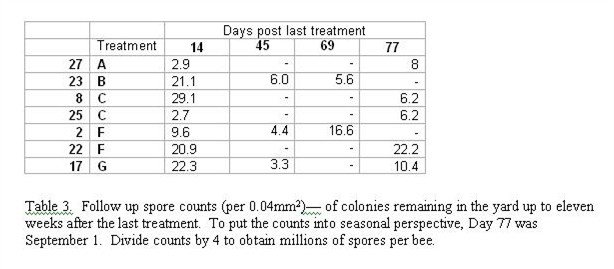
Finally, after seeing the above progression of the infection, I sampled the few remaining colonies in the yard on Day 69 or 77 (Table 3). Unfortunately, most of the strongest colonies (other than 2 and 23) had been moved into production. There was no obviously discernable pattern of progression of infection.
Discussion
This experiment was designed to test the effectiveness of repeated drench treatments to control Nosema ceranae in a California apiary. A potential problem in nosema trials is the effect of spore contamination of the combs when established colonies are used. I attempted to minimize this and other variables by using homogenized shook bees, new equipment, and similar fresh queens. The only starting variable out of my control in this trial was any spore contamination of the single brood frame added to each colony. However, I made the point of taking healthy-looking brood frames from colonies with relatively similar levels of infection. I felt that the addition of this frame was appropriate, in order to allow fresh brood to emerge during the treatment period, to see if treatment would prevent infection of these bees. At the 6-week sample, few of the original shook bees would be expected to be still surviving, and thus the sampled bees should reflect bees that had emerged during the treatment period.
Despite all these cautions, I was surprised by the extreme differences within all groups. Note the variability of the control group in both spore counts and weight gain. These results indicate that there is an inherent variability of colonies, even when started with near identical bees and equipment, which can easily overshadow the effect of treatment.
Also note that of 28 queens from a reputable queen producer, four failed in the second month. I do not know if this was due to nosema, or to other queen issues.
I used a simplified method of grinding the bees in the blender in order both to save time, and to obtain a more uniform release of spores. I have observed that hand grinding of samples of more than a few bees allowed too much room for error in releasing spores from all the bee guts. The only drawback that I found to this method was the excessive foam in some samples—but with practice, a relatively foam-free sample could be taken with a plastic 10µl loop for transfer to the counting chamber. A detailed description of the method can be found at www.scientificbeekeeping.com.
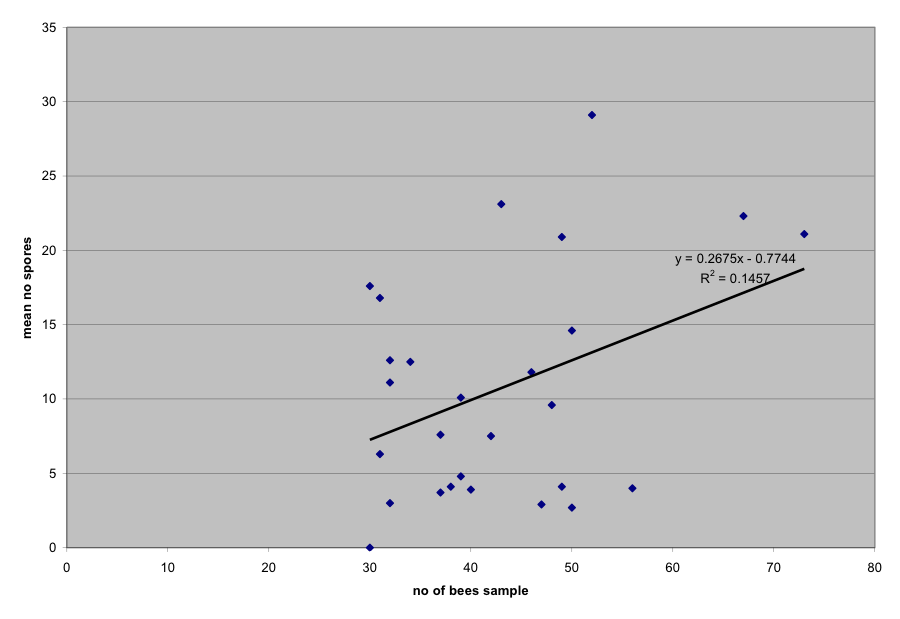
When I was faced with the decision as to whether I should process all the bees in each sample (most accurate), or to only pick an equal number out of each sample (more comparable), I chose to use the whole sample. In a previous article, I explained the critical nature of sample size. I have also found (pers obs) that if I take sub samples of 25 bees from a large sample of infected bees, that many sub samples will have low spore counts, but the occasional sample may be 10 times higher. Apparently, a single highly-infected bee will skew an entire sample. This effect apparently happened in this trial (Fig. 5).
Figure 5. Spore count vs. sample size. Note that the more bees taken in a sample, the higher the spore count tends to be (P < .05). This is apparently due to a sporadic highly-infected bee being included in the sample.
I suggest that other researchers pay attention to this phenomenon, since it means that previous research with the typical 10-bee sample may lead to erroneous conclusions unless many samples are taken. There is likely an optimum number of bees to include in a sample. That number is apparently greater than 30, and discovery statistics suggest that it is likely in the range of 60 bees per sample. Unfortunately, collecting 60 returning forager bees from a weak colony can be nearly impossible, even when the entrance is blocked off in the middle of a good foraging day (pers obs).
I must confess, that based upon others’ successes with fumagillin against N. ceranae (Higes 2006, Williams 2008, Pernal (in press)) that I expected to have found better control of nosema by this product. Although one fumagillin-treated colony had zero spore counts, I will also confess that that I wouldn’t swear that that count wasn’t in error, since it was the first slide that I processed that day, and in my memory, seemed unusually free of debris.
Due to the small number of colonies tested, and the extreme variability of spore counts, I hesitate to even compare treatment results. However, the mean counts of the fumagillin and Nosevit treatments were lower, although Nosevit was not significantly lower (and fumagillin was only found to be significantly lower depending upon the analysis used). Therefore, this small trial did not result in a robust confirmation of efficacy for any treatment. Update: Let me reword the previous sentence! What this trial demonstrated was that none of the tested treatments, applied at label or suggested rate of active ingredient claimed to be effective when fed in syrup, were effective when applied as a drench with the same amount of active ingredient. These results do not reflect upon the efficacy of any of the products when given to the colony in sugar syrup feed.
The results for the compound Nosevit, however, pique my interest, since it is widely used in Central Europe, and is a natural extract of an oak bark (Joe Carson, pers comm). Its mode of action is not understood, but oak bark has long been used as a source of tannins. Tannins are bitter plant polyphenols that either bind and precipitate or shrink proteins. Tannins are used in medicine to form a protective leathery layer over mouth wounds, and for their anti-inflammatory effect in irritated bowel disorders (Wikipedia 2008). It will be of great interest to discover whether they have effect upon the bee gut or nosema germination.
Another observation of note are the results of the HoneyBHealthy treatment. HBH is an emulsified mixture of spearmint and lemongrass oils, and is widely used by beekeepers in the hope that it will control nosema infections. The concentration of product used in this trial was recommended by the manufacturer, Bob Noell. In two HBH-treated colonies spore counts were quite low. However, in the other two, they were among the highest!
The lack of nosema eradication by any treatment is surprising, given that the colonies were treated four times a week apart, and under near ideal conditions for coverage of the bees with the medication. It suggests to me that we need to look more carefully at improving the efficacy of drench treatment. It also suggests that those beekeepers who are splashing on a single drench treatment, and expecting it to do more than give them exercise, are wasting their time.
As I mentioned in a previous article, fumagillin has previously been proven effective in various forms of application (including dusting with powdered sugar), so it would at least have been expected to perform better in this trial. Perhaps the hungry bees did not store enough product between drenchings to realize a continuous treatment to their guts. Or perhaps the dosage of active ingredient simply wasn’t enough. The concentration that I used gave each colony the equivalent of 30mg a.i. per 10-frame colony per dose, as per Higes’ successful trial. The Medivet label says to mix 4.5g of product into a gallon of syrup, which would result in 94.5mg of active ingredient. If a strong 10-frame colony were to consume that gallon over the course of a week, they would receive the full 94.5 mg per week—better than three times the strength that I applied, yet still within the range of 30-100mg per dose suggested by previous researchers (Wyborth 1987).
While I am loathe to report the “off label” use of medications, in this case the trial and error experimentation by beekeepers may be ahead of the controlled trials by researchers. The problem is that there is no medication labeled for Nosema ceranae—Fumagilin-B is labeled for N. apis only. Control of N. ceranae by using treatments designed for N. apis has sometimes been disappointing. In any case, some commercial beekeepers are using a more concentrated drench—a large bottle of Fumagilin dissolved in 5-7 gallons of syrup, and repeated three times at 7-10 days. Unfortunately, those I’ve spoken with couldn’t confirm whether the treatment actually reduced spore counts relative to control colonies, although the beekeepers felt better because they had done something (perhaps a sympathetic placebo effect).
The colony weight gains (Fig 3, which were measured as an afterthought) were not instructive, either. No product appeared to really help or hurt colony performance in this small trial.
The comparison of spore counts in the strongest and weakest colonies (Fig. 4) might come as a surprise, since the literature clearly indicates that nosema infection should hamper a colony’s ability to build up. So why would the stronger colonies have higher spore counts? The first explanation that comes to my mind is that some colonies are “bee collectors.” This phenomenon has been documented by Dr. Jerry Bromenshenk (pers comm) by using bee counters at the entrances to colonies. Some colonies in a yard (perhaps due to queen pheromone or colony odor) consistently attract more bees than they lose. Most colonies lose more bees than they attract. Location in the apiary is not a factor.
If that were the case in this trial, the collector colonies may have continually “stolen” foragers from the other colonies. Since foragers typically have the highest spore counts of bees (due to their age), that might tend to ramp up the spore count of the samples from those colonies. Certainly, other explanations might be equally viable. I attempted to mark foragers with fluorescent spray paint to test the drifting hypothesis, but apparently need to refine my painting technique, as the bees groomed it off fairly quickly.
Since nosema did not appear to be main factor in colony buildup in this experiment, what was going on? Although the queens were all fresh and from the same line, perhaps it was simply due to queen variability. We also noted that the stronger colonies appeared to have less spotty brood patterns. Although there were no overt symptoms of brood disease, I currently have samples in to two labs for further testing for viruses or other pathogens.
The take home message to beekeepers is that while the “drench” method of application of nosema medication holds promise, it does not appear to have a consistent enough effect at the doses of active ingredients given in this experiment to arrest the development of nosema infection, even in small colonies on mostly clean combs. By all means don’t expect one or two drench treatments at these concentrations to do much! Note that nosema levels increased in virtually every colony, and that few beekeepers could afford to drench weekly for four consecutive weeks. Note also that some colonies in the control group did not increase nosema levels to a great extent, despite lack of any treatment.
The other take home is that fumagillin, and possibly Nosevit, appear to be effective against N. ceranae to some extent when applied as drenches. Further research should be undertaken to determine the appropriate doses for each (and especially to confirm the efficacy of Nosevit). Since this trial, recommendations from Europe for Nosevit are now 15-20 drops per colony in 12 oz of syrup—1½ to double the amount that I used (Carson 2008, pers comm). Nosevit is available in this country from Joe Carson (see References), and has the advantages of being relatively inexpensive. It is widely accepted in Europe in countries where synthetic antibiotics are frowned upon. I have not seen data on either its toxicity or possible residue levels in honey.
Since the effect of fumagillin was so weak in this trial, I would not use these results to discount any possible effect of the other treatments. I suggest that further research be done on these products at stronger dosages applied by drench. HoneyBHealthy is being widely used due to anecdotal reports of efficacy, and Vitafeed Gold has data to support it. Recent research by Maistrello (2008) found thymol to be effective against N. ceranae when fed in candy. However, by my calculations, the amount of active ingredient per bee in their caged bee trial was not much more than I applied in this trial.
Another question is whether the spore count would have dropped if given more time than two weeks after treatment. That question is partially answered by the measurements taken of the eleven colonies at 45, 69, and 77 days post last treatment (Tables 2 and 3). At 45 days post, counts had gone down in all colonies except one, which was in the untreated control group. However, another control colony was also measured, and its count had gone down. No individual treatment stood out in the limited 45-day count, so there is little evidence of efficacy of any treatment at 45 days post last treatment. By eleven weeks post treatment, spore counts varied greatly—some going up, some down. However, note that if the raw spore counts are converted to millions of spores per bee (by dividing by 4), no colony measured had counts much over 5 million.
Counts may have gone down due to other causes, such as the return of good weather with a concurrent nectar/pollen flow. Williams, et al (2008) noted that N. ceranae spore counts dropped during the summer in untreated colonies. Note that spore counts in this trial went up during poor foraging weather regardless of treatment, then generally went down during the following good nectar/pollen flow, despite lack of treatment.
Another question is whether the infection was raging within the colony, and not evidenced by the older foragers sampled. Since I also took samples from within all colonies at day 45, by brushing bees from the outside frame, I checked a number of those samples from colonies that had high spore counts at the entrance. All checked within-colony samples showed virtually no spores (I did not count all inside samples, since I got negatives on the first several). This observation is in accordance with previous observations (pers obs; Martin-Hernandez 2006) that “inside” samples can easily miss nosema infections, since the bees are too young.
At the American Bee Research Conference, Drs. Higes and Meana detailed how N. ceranae moves into the population of house bees in the latter stages of the disease. Since I detected virtually no spores in the bees on the outside comb, the indication is that the disease had not progressed far in these colonies, and was only affecting older foragers. However, Higes found that the spore count jumps only in last two months before the collapse in fall. He found in Spain that in cold season colony collapses, >50% of workers are infected, and average spore counts are greater than 10 million spores per bee. In warm season collapses, however, less than 50% of the workers are infected, and spore counts are lower than 10 million.
It is likely that the drench method is the most cost effective method of treating nosema in some situations. The results of this trial indicate that drenching at either the label recommendations, or even at the current research recommendations of 30mg a.i. per dose are inadequate to arrest Nosema ceranae reproduction in late spring. Any beekeeper drenching at this rate would likely be wasting his time and money. I did not test drenching at the rates of 75 or 120 mg a.i. per dose (one 454g bottle of Fumagilin-B in 8 or 5 gallons, respectively, of syrup), which many commercial beekeepers feel is effective. Clearly, further research is called for to determine the optimum dosage, frequency, and number of treatments.
We are grasping for answers in dealing with this new parasite, and I’ve spoken with other researchers. New questions arise if we start using regular summer treatments. Dr. Robert Cramer notes that nosema does not exist in a vacuum–it is associated with a suite of beneficial and/or pathogenic bacteria, fungi, and viruses. Any treatment may affect any or all of them. Dr. Marla Spivak wonders whether regular summertime nosema treatments will contaminate honey, or cause nosema resistance to the medications. We don’t know the long term effects of any kind of treatment (although fumagillin has a good track record to date). Finally, Geoffrey Williams questions whether treatment with fumagillin may give a competitive edge to N. ceranae over N. apis. At this time, there are still more questions than clear answers as to Nosema ceranae virulence, threshold treatment levels, effective treatment doses, and optimal application methods.
Acknowledgments
I would like to earnestly express my gratitude to those who are collaborating with me on this research: Drs. Nancy Ostiguy (PSU); Jerry Bromenshenk, Colin Henderson, and Robert Cramer (MSU): Jay Evans and Blaise LeBlanc (USDA), Michelle Flenniken (UCD), Richard Rogers (Wildwood Labs), and of course, my mentor Eric Mussen (UCD). I also appreciate input from Dr. Marla Spivak, and from those commercial beekeepers who share their field experiences with me. Any faults in this paper are mine alone, and do not reflect upon my collaborators. Funding for this project is currently being arranged.
References
This, and all my other articles, can be accessed conveniently at my website www.scientificbeekeeping.com.
Cantwell GE (1970) Standard methods for counting nosema spores. American Bee Journal 110(6), 222–223.
Carson, Joe AlaskaHeavenlyHoney@hotmail.comThis e-mail address is being protected from spam bots, you need JavaScript enabled to view it ,(907) 727-8200.
Eischen, F (2008) American Bee Research Conference
Higes M, Martín-Hernández R, Garrido-Bailón E, Meana A (2006) An approach to Nosema ceranae control with fumagillin in field conditions. Proceedings of the Second European Conference of Apidology EurBee Prague (Czech Republic) 10-16 September 2006
Maistrello, L, M Lodesani, C Costa, F Leonardi, G Marani, M Caldon, F Mutinelli and A Granato (2008) Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera) Apidologie 39 (4) 436-445.
Martín-Hernández Raquel, Higes Mariano, Garrido M. Encarnación, Meana Aranzazu (2006) Influence of sampling in the detection of Nosema ceranae spores. . Proceedings of the Second European Conference of Apidology EurBee Prague (Czech Republic) 10-16 September 2006
Oliver, R (2008) The Suck-a-Bee. ABJ 148(8): 719-721.
Pajuelo, AG, C Torres and FJ Orantes Bermejo (2008) Colony losses: a double blind trial on the influence of supplementary protein nutrition and preventative treatment with fumagillin against Nosema ceranae. Journal of Apicultural Research 47(1): 84-86.
Williams, G.R. et al.,(2008) Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)?., J. Invertebr. Pathol. (2008), doi:10.1016/j.jip.2008.04.005
Topolska, G, and A Hartwig (2005) Diagnosis of Nosema apis infection by investigations of two kinds of samples: dead bees and live bees. J. Apic. Science 49(2):75-78.
Wikipedia (2008) Tannins. http://en.wikipedia.org/wiki/Tannins
Wyborth, MH, & DM McCutcheon (1987) A comparison of dry and wet fumagillin treatments for spring nosema disease suppression of overwintered colonies. ABJ 127: 207-209.
Yucel, B & M Dogaroglu (2005) The impact of Nosema apis Z. infestation of honey bee (Apis mellifera L.) colonies after using different treatment methods and their effects on the population levels of workers and honey production on consecutive years. Pakistan Journal of Biological Sciences 8(8): 1142-1145.