Old Bees/ Cold bees/ No bees? Part 2
Old Bees/ Cold Bees/ No Bees? Part 2
© Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in July 2008
One day during his tenure as a professor, Albert Einstein was visited by a student. “The questions on this year’s exam are the same as last year’s!” the young man exclaimed.
“Yes,” Einstein answered, “but this year all the answers are different.”
Beekeepers today may feel that they are in a similar situation. Colony management that was previously successful may no long work! Here at Next Year We’re Going to Make the Big Bucks Apiaries, unexpected losses of colonies have been eating up our profit margin. We’ve been making increase like crazy each spring, only see some fail to build, or crash during fall or winter.
However, being the optimist that I am, I think that I’m beginning to get a handle on the situation!
Last month, I was describing how a forager has limited longevity at best. Even that brief lifespan can be shortened further by disease or poor nutrition.
Protein levels
Protein intake of newly-emerged bees has recently been correlated with their subsequent behavior (Nelson, et al 2007). The authors found that bees with low vitellogenin levels begin foraging earlier in life, and tend to forage more for nectar than pollen. This is an insidious effect of lack of pollen in a colony—that the bees will begin to forage, and thus begin aging, sooner in life. (Research on this topic is ongoing, and sometimes contradictory—see Matilla and Otis 2006).
The “salary” that the hard-working foragers get from the colony is a daily allotment of protein-rich jelly. Schmickl & Crailsheim (2004) point out that although the older bees acting as foragers collect the pollen, most of it is eaten by the nurse bees. The nurse bees then process it into protein-rich jelly, which is then fed back to returning foragers to fulfill their protein requirements. Since the forager immune system and antioxidant system is dependent upon vitellogenin, their lifespan as seniors is partially limited by how well the younger bees feed them.
Predictably, the nurse bees prioritize first the hungry older larvae (since the colony has invested considerable resources in them). Foragers will feel the pinch during a protein shortage. Indeed, this appears to be part of a feedback loop that stimulates foragers to collect more pollen.
So imagine the situation during a drought—foragers work extra hard to locate pollen, yet there is not enough to spare to keep their own body protein levels up. Ditto when inclement weather during broodrearing keeps the foragers inside. The colony eats up all available pollen in a few days. The nurse bees cut back on the jelly fed to brood, may cannibalize eggs and younger larvae, and are ultimately forced to steal protein from their own bodies to continue feeding the oldest brood to maturity. No jelly will be left for the foragers. The bottom line is that when pollen resources get scarce, the foragers may suffer from lack of protein. This could again shorten their lifespan.
From a practical standpoint, it is important to remember that a low protein level in a colony will not only initiate premature aging of the bees, but will depress the immune system of the colony overall, thus making the colony as a whole more susceptible to any diseases.
As an aside, we beekeepers must be careful when feeding our bees protein supplements or sugar syrup. Poor quality ingredients–such as aged soy flour, or “off spec” HFCS can be quite toxic to bees. In addition, foragers can be poisoned by mycotoxins (fungal toxins) in fermented nectar or sugar syrup, leading to large losses (Manning 2008).
General infections
Sick bees are short-lived bees. Bees that had to fight infection (bacterial or viral) or parasitism (varroa) when they were in the larval or pupal stage may never reach their full potential as adults. Adult bees then have their own set of problems. A forager carrying a varroa mite, or infected with nosema has less chance of returning from each foray than an unparasitized bee. Or perhaps they don’t return on purpose— “This behavior can be interpreted as suicidal pathogen removal, serving as a disease defense mechanism which reduces the colony’s load of parasites or pathogens” (Kralj, et al 2006). Nature has likely selected for a behavior in which dying foragers remove themselves from the hive, rather than forcing undertaker bees to carry them out.
When a bee is “challenged” by a pathogen or parasite, it activates its immune system. This process is metabolically expensive (Moret & Schmid-Hempel 2000). A bee (or colony) fighting a disease requires more food, and is not as productive. The extra metabolic effort required for cold-weather flight may prove to be more than it can summon. For an older bee with a low vitellogenin titer, similar to an elderly malnourished human, a normally minor disease may be fatal.
Parasites
As I mentioned earlier, colony collapse events have a recurrent history—which suggests that weather and/or the subtle effects of well-established pathogens are involved. However, in the last 25 years three new serious players have entered the picture: tracheal mites in 1984, varroa mites in 1988, and Nosema ceranae (apparently) sometime in the past decade. These parasites did not enter the scene quietly, but rather have each wreaked havoc. In the aftermath, they have added continual new stresses to our beleaguered bees, by weakening them, suppressing their immune systems, creating points of entry for pathogens, and adding entirely new vectors for viruses. Therefore, the current widespread collapses could be caused by the action of historical environmental stresses and pathogens, exacerbated by the additional parasite stresses.
The internal parasite, nosema, has long been called the “invisible killer” —since by shortening forager lifespan, it can devastate a colony without visible symptoms. Dr. Mariano Higes has detailed the pathology of N. ceranae that can lead to colony collapse. Nosema alone can be bad for a colony, but in concert with poor nutrition and/or viruses, it can be devastating. Indeed, some bee viruses are only found in conjunction with nosema infection.
Nosema can have an additional effect upon colony population. Some older research (Fisher 1964) suggested that nosema infection increases the level of juvenile hormone (JH) in the insect. In the case of honey bees, JH is antagonistic to vitellogenin, and higher levels of JH would cause premature aging.
I’ve corresponded with JH expert Dr. Zachary Huang, and vitellogenin expert Dr. Gro Amdam about this. Dr. Huang has unpublished data showing that nosema does not produce JH directly, but that infected bees can indeed have elevated JH titers, which cause bees to begin foraging earlier. (In some colonies, bees do not respond to the infection by showing earlier foraging, but he did not examine these colonies to see if the infected bees also had higher JH titers). The actual mechanism has not been determined, but likely involves vitellogenin, just as in healthy bees.
The colony-to-colony difference in response to nosema infection is notable, since it may explain why nosema is harder on some colonies than others. Anything that induces bees in a colony to begin foraging at an earlier age, will accelerate the aging of the workers, and restrain population growth.
Parasitic mites have also clearly demonstrated their impact upon bee longevity. Both varroa and tracheal mites can cause colony depopulation. The tracheal mite is especially rough on wintering bees, and the varroa mite on fall and winter bees. However, it is possible that neither mite kills the colony directly, but rather initiates a viral epidemic that polishes the bees off.
From a practical standpoint, it is moot whether the parasite actually kills a colony, or rather just sets it up for a fatal blow from one or more viruses. Control the parasite, and the bees can generally keep viral infections to a low level on their own.
Viruses
Bees are ubiquitously afflicted by continuously morphing RNA viruses, much as beekeepers are afflicted by the continuously morphing cold and flu RNA viruses. However, bee viruses tend to lie latent in the bees—only occasionally causing observable illness. In this manner they act more analogously to herpes viruses—virtually all humans carry them, but they only flare up when we are stressed, infected by another disease, or immune compromised.
Bee viruses were a relatively unimportant issue to beekeepers until the arrival of varroa. Then we quickly discovered that the combination of varroa and nearly any virus can be lethal. Dr. Norman Carreck (2008) writes:
“Infestation by Varroa and subsequent infection by ABPV and KBV can lead to many of the symptoms associated with CCD, namely the spectacular and rapid loss of strong colonies, leaving empty hives with just the queen and a few workers remaining.”
So the obvious question is, can a normally latent virus, or one of its mutants, flare up periodically to cause an epidemic (or more properly, epizootic) in the bee population, perhaps due to an earlier nutritional stress event, and especially with the assistance of nosema? Such a virus could be newly identified, such as Israeli Acute Paralysis Virus (IAPV—still a suspect in my book), or an old timer like Sacbrood Virus (SBV).
Sacbrood is a good example of how the effect of a virus can be overlooked. We generally think of sacbrood as an uncommon virus of bee larvae in spring and summer, generally associated with poor weather or nutritional stress. In actuality, it has always been an extremely common virus, and can be found in adult bees on all continents at high frequency throughout the year (Tentcheva 2004, Köglberger 2005, Berenyi 2006, Nielson 2008). It can exist as an inapparent infection in pupae, which then emerge as infected adults (Dall 1985).
Not surprisingly, sacbrood is commonly found in collapsing colonies (Kulincevic 1984, Cox-Foster 2007, Bromenshenk 2008). However, one would expect such a common virus to be found in any survey—that doesn’t necessarily mean that it is creating the problem.
But get this: in my own operation, I have historically rarely seen sacbrood. Yet the past two years, I’ve seen it commonly. Notably, I’m seeing it with regularity in colonies that are collapsing, or recovering from collapse. These observations certainly make me a bit suspicious!
SBV is only noticed when it kills last-instar larvae (which die stretched out on their backs, with their heads upturned—see Goodwin (2003) to download photos). It is normally spread as workers remove infected larvae, and get exposed to the highly-infected ecdysial fluid under the skin. Only young workers are easily infected by ingesting the virus―but these are the very workers that typically clean cells. Once infected, the nurse bees or foragers can spread the virus through their saliva, jelly, and stored pollen. Shen, et al (2005), additionally found that SBV can be transmitted by the queen to her eggs, and likely by the varroa mite. I will go into more detail about bee viruses later, but first let me quote from the legendary bee pathologist, Dr. Lesley Bailey (1972):
“sacbrood virus accumulates in the brains of infected bees…without causing symptoms [emphasis mine]. However, infected individuals fly earlier in life than healthy bees and infected foragers fail to collect pollen….The few infected bees that gather pollen contaminate their loads with much sacbrood virus. Infection…much shortens the…lives of workers that have eaten pollen.”
Bailey found that the median number of days for foragers to failure to return to the colony went from 14 days for healthy bees, down to 5 days for SBV-infected foragers! This fact could have major implications on colony population (recall Figure 3), and there are no symptoms for infected adults other than that they just disappear. Now I am not suggesting that Sacbrood Virus is the cause of CCD, but merely pointing out how easy it is to overlook the potential contribution of an inapparent infection by a virus.
Yet another tidbit from the paper was especially striking to me: “Infected workers…are unable to maintain the usual metabolic rates of bees at temperatures below 35ºC [brood nest temperature], or to resist chilling.” Remember how colonies often dwindle during cold spells? This brings us to the subject of…
Hot-Blooded Ladies
The honey bee is a tropical insect that has adapted to temperate climates, much as humans have done—by living in heated shelters. Harvard zoologist Bernd Heinrich describes bee thermal strategies in two fascinating books: The Thermal Warriors and Bumblebee Economics. Unlike most insects, honey bees normally maintain body temperatures above ambient temperature, both individually, and as a colony. They do so in a clever way—they can “uncouple” their wings from the massive flight muscles in the thorax, and shiver―much as we shiver to warm up. However, bees have refined their shivering to such an extent, that they do it without visible shaking.
And shiver they do. They shiver to keep the brood nest at 94ºF. Individual bees shiver to maintain a flight muscle temperature of at least 85ºF, below which they are unable to fly. A bee readying itself to take off shivers to warm its flight motor up to about 100ºF, and typically maintains it at about 95ºF. Bees at the outside of a cluster hold their temperature to a minimum of 41ºF, since below that temperature they are too cold to initiate shivering, and will die.
Although the bee’s thorax is covered with an insulating pile, it will still chill quickly in cool air if it is not constantly generating heat. Due to the laws of thermodynamics, for every 20ºF that the ambient temperature drops, the bee needs to work about twice as hard to stay warm. As I mentioned before, foraging in cool weather is very wearing on the bees!
When bees are returning from foraging during a cool day, one can see them occasionally stop to “rest.” They are hardly resting! Rather, they have lost too much heat from their flight motor (thoracic muscles) due to the 15 mph “wind” passing over their bodies as they fly. They need to stop to warm back up. Indeed, an apparently resting bee may be working its flight muscles harder than it would while flying!
A bee stores about 15 minutes worth of fuel in its flight muscles, and about another 15 minutes worth in its blood (Southwick 1992). Once these sources are depleted, it is dependent upon whatever nectar it has in the honey sac—and can fly much faster if the sac contains high-sugar nectar. Should a forager run out of sugar fuel while it is flying or shivering, it will die in the field.
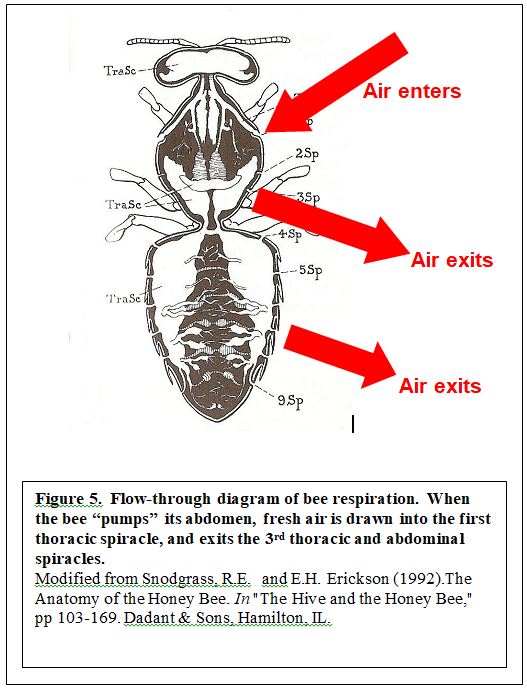
So how can you tell if a bee is really resting, or whether it is working hard to warm itself up? Simple: look at its abdomen. From the drawing in Figure 5, you can see that much of the bee’s abdomen is taken up with air sacs. These sacs function as pumps to move fresh air efficiently through the bee (more efficiently than our own lungs). Insects obtain oxygen, and dump carbon dioxide by using branched tubes called trachea that open to the outside air at holes called spiracles on the sides of their bodies—three pairs on the thorax, and six on the abdomen.
In order to ventilate, the bee “pumps” its abdomen like an accordion, and opens and closes its spiracles so that it sucks air into the tracheal sacs in the thorax, and expels it from the abdominal spiracles (Stoffolano nd). The largest intake spiracle is the first thoracic, and it is screened by “hairs” to prevent the entry of dust and parasites (although the tracheal mite can enter this spiracle in newly-emerged bees of susceptible stocks).
The illustration above may mainly apply to a bee that has high respiratory needs, such as when flying or producing heat. Otherwise, the the breathing may occur mainly in the thorax (Bailey 1954), with air entering through the first thoracic spiracle, and exiting through the third thoracic spiracle (rather than out the abdominal spiracles).
So if an apparently “resting” bee is pumping its abdomen, you know that it is in actuality working as hard as it can to warm up—pumping oxygen to its flight muscles, and carbon dioxide out. This one-way flow-through system of ventilation is extremely efficient. Indeed, when not flying or shivering, the bee stops the pumping action in order to minimize its tissue exposure to harmful oxygen.
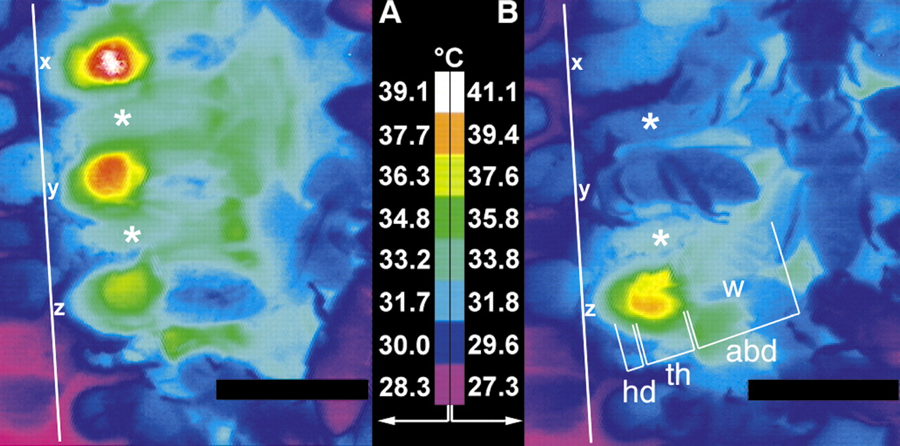
The bee has a clever countercurrent heat exchange system at its waist (the petiole) which prevents thoracic heat from being lost to the abdomen. The abdomen remains unheated. However, the bee instead uses its haemolymph (blood) to pump heat to the head! By placing its head or thorax against a cell wall or capping, the bee can transfer considerable heat to the brood (Figure 6).
Figure 6. At left is a top-view thermograph of three bees inside empty cells adjacent to brood. The upper bee is generating the most heat. Note how the heat transfers to the head. At right is a side view of two bees in cells. The upper is resting, the lower generating heat. The asterisks mark the walls of adjacent pupae. The white line is the comb midrib. From Marco Kleinhenz, Brigitte Bujok, Stefan Fuchs and Jürgen Tautz (2003) Hot bees in empty broodnest cells: heating from within © 2003 The Company of Biologists Ltd, by permission.
The ability to transfer heat to the head allows honey bees to perform another neat trick—they can fly at temperatures that would kill most insects (up to 113ºF). They do so by using their hot head as a radiator, and if necessary, exuding a droplet of nectar from their mouth to cool by evaporation! I am struck by what an amazing insect the bee is—it can maintain a constant body temperature similar to ours, cool itself when necessary, transfer heat to its offspring, and regulate the amount of oxygen that its tissues are exposed to!
OK, I’ve digressed, so let me return again to the question, What factor(s) could prevent the return of a bee that was initially healthy enough to fly away from the hive? Obviously, a pesticide kill, but those instances are generally pretty clear, and there are often piles of twitching bees in front of the hive. Instead, perhaps we should focus on the ability to fly. Sudden depopulation of a colony with no dead bees present, means that the bees must have flown away, and not flown back.
The non-returning bees were healthy enough rev up their flight motor and fly out, so were unlikely to have suddenly succumbed to mortality. More plausibly, they were simply unable to get their wing muscles back up to takeoff temperature once they cooled after leaving the warm colony. A bee can raise its thoracic temperature roughly 30ºF above the ambient temperature while it is flying. That means that if it is flying in 55º weather, that once it leaves the warmth of the hive, it will barely be able to keep its wing muscles up to their minimum operating temperature (85ºF)—hence bees don’t fly much at temperatures below 55 degrees. And if they do, they often don’t return.
So it appears that we should be looking for factors that affect ability of a bee to warm up its thoracic flight muscles. The most likely are age, poor nutrition, and/or disease—especially any disease that affects the flight musculature, the nerves that control it, or its energy conversion. The preliminary CCD report (van Englesdorp, et al 2007) describes some apparent pathologies of the flight muscles, including “white nodules” and “crystalline arrays.” And remember Bailey’s findings detailed earlier, that bees infected with sacbrood virus chilled more quickly, and were unable to maintain normal metabolic rates once cooled below broodnest temperature. I’m eager to hear of any research as to how any particular pathogen might accelerate the “aging” of the flight muscles.
Whatever the specific mechanism, the most likely reason that bees fly out, but don’t return, is simply that once out of the hive, they couldn’t generate flight muscle temperatures necessary for the return trip.
This brings up the additional question as to whether some factor is causing such flight-impaired bees to leave the colony at unfavorable temperatures. An intriguing hypothesis is based upon the observations that prior to some recent collapses, the colonies appeared “restless,” bees may move away from the brood area toward the entrance(s), or that the combs may become repellent to bees. A pathogen or condition that changes the behavior of the bees to exit the hive at an early age, to initiate forays at inappropriate temperatures, or to abandon their hive and drift into others could certainly bring about a depopulation.
The Bottom Line
The dwindling of healthy-appearing colonies appears to be largely a function of the combined effects of the age of onset of foraging (or exit from the hive), and the number of days that the foragers then survive. Should the average age for forager “failure to return” drop to only a few days, the colony population dynamics go seriously into the red, and we observe that the colony “dwindles” as younger and younger bees are forced to shift from nursing responsibilities to foraging.
Several factors can promote early foraging, or accelerate the aging of foragers―especially poor nutrition or nosema, which also increase their vulnerability to disease and stress. In some collapses, something appears to cause a restlessness of the bees, or a repellency of the combs.
Some diseases, especially nosema, mites, and viruses, decrease the survivability of foragers without any apparent symptoms. The effect of any of these factors is greatest when foragers leave the warmth of the hive in cool weather, lose body heat, and are later unable to warm up for the return flight. Hence, spring and fall dwindling are often observed at the onset of cool weather events.
This is unfortunate for beekeepers preparing for almond pollination. A cold snap in fall or close to beginning of bloom can turn of profitable-looking yard of bees into a bunch of dinks seemingly overnight! We can only hope that further understanding of the causes leading up to such collapses, can help us to avert them in the future.
I have spoken with a number of beekeepers who were successful at taking strong colonies to almonds this season. There doesn’t appear to be any single formula or magical potion for success, but rather, common sense husbandry may be the best approach:
· Be diligent with varroa! Don’t let levels ever get high. Any number of methods will work to control the mite. But definitely get mite levels way down mid August at the latest. This will help keep viruses in check.
· Monitor nosema infestation, and treat in a timely manner if appropriate. Especially check colonies that fail to build normally.
· Don’t baby colonies that aren’t thriving, or have spotty brood. Kill or requeen them! (Some successful beekeepers requeen more than once a year!) Get sick colonies off to a hospital yard.
· Maintain good colony nutrition with regard to pollen, especially in late summer and fall. Move to better pasture, or feed your bees if necessary.
· It may be wise to maintain genetic diversity in your operation, since colonies vary in their resistance to different pathogens. Naturally resistant stocks go a long way toward success.
References
Allsopp, M (2008) Tracheal Mites. http://www.arc.agric.za/home.asp?PID=1&ToolID=63&ItemID=3081
Amdam, G. V., K. Norberg, A. Hagen, and S. W. Omholt (2003) Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. U.S.A. 100: 1799Ð1802.
Bailey, L & EFW Fernando (1972) Effects of sacbrood virus on adult honey-bees. Ann. Appl. Biol. 72:27-35.
Carreck, N (2008) CCD – A View From Across The Pond. Bee Culture Jan 2008
Bailey, L (1954) The respiratory currents in the tracheal system of the adult honey-bee. J. Exp. Biol. 31: 589-93.
Berényi, O, T Bakonyi, I Derakhshifar, H Köglberger, N Nowotny (2006) Occurrence
of six honeybee virus in diseased Austrian apiaries. Appl Environ Microbiol 72, 2414-2420.
Bromenshenk, J (2008) pers comm.
Burmester, T. 2005. A welcome shortage of breath. Nature. 433: 471-472
Corona, M, KA. Hughes, DB Weaverd and GE Robinson (2005) Gene expression patterns associated with queen honey bee longevity. Mechanisms of Ageing and Development 126(11): 1230-1238
Cox-Foster, D, et al. (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318(5848): 283-287.
Cox-Foster, D, et al (2007) Supporting Online Material for A Metagenomic Survey of Microbes in Honey Bee Colony Collapse Disorder. www.sciencemag.org/cgi/content/full/1146498/DC1
Dall, D. J. (1985). Inapparent infection of honey bee pupae by Kashmir and sacbrood bee viruses in Australia. Ann Appl Biol 106, 461–468.
Fisher, FM Jr and RC Sanborn (1964) Nosema as a source of juvenile hormone in parasitized insects. Biol Bull 126: 235-252.
Fluri, P, M. Lüscher, H. Wille and L. Gerig (1982) Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees Journal of Insect Physiology Volume 28, Issue 1, Pages 61-68
Fridovich, I. 1977. Oxygen is toxic! Bioscience 27: 462-466.
Goodwin, R.M., Taylor, M.A. (2003) Diagnosis of Common Honey Bee Brood Diseases and Parasitic Mite Syndrome. http://www.hortresearch.co.nz/files/science/biosecurity/227525-Bee-Pamphletpths-small.pdf
Heinrich, B (1996) The Thermal Warriors. Harvard University Press
Heinrich, B (2004) Bumblebee Economics. Harvard University Press
Hetz, S.K. and T.J. Bradley. 2005. Insects breathe discontinuously to avoid oxygen toxicity. Nature 433: 516-519.
Higginson, AD and FS Gilbert (2004) Paying for nectar with wingbeats: a new model of honeybee foraging Proc Biol Sci. 271(1557):2595-603.
Kleinhenz, M, B Bujok, S Fuchs and J Tautz (2003) Hot bees in empty broodnest cells: heating from within. The Journal of Experimental Biology 206, 4217-4231.
Köglberger, H, I Derakhshifar, J Kolodziejek, H Homola, and N Nowotny (2006) Prevalence of six honeybee viruses in beehives collected at different Austrian locations during different seasons, and correlation with non-viral disease. Proceedings of the Second European Conference of Apidology EurBee
Kralj J, S. Fuchs, J. Tautz (2006) Disease removal by altered flight behavior of forager honey bees (Apis mellifera) infested with Nosema apis. Proceedings of the Second European Conference of Apidology EurBee
Kronenberg, F & HC Heller (1982) Colonial thermoregulation in honey bees (Apis mellifera) -. J Comp Physiol 148:65-76
Kulincevic, JM, WC Rothenbuhler, TE Rinderer (1984) Disappearing disease: III. A comparison of seven different stocks of the honey bee (Apis mellifera). Research bulletin 1160, Ohio State University
Manning, R (2008) The Effect of High and Low Fat Pollens on Honeybee Longevity. RIRDC Publication No 08/031.
Matilla, RM and GW Otis (2006) The effects of pollen availability during larval development on the behaviour and physiology of spring-reared honey bee workers. Apidologie 37:533–546
Schmickl, K & K Crailsheim (2004) Inner nest homeostasis in a changing environment with special emphasis on honey bee brood nursing and pollen supply. Apidologie 35: 249–263
Seehuus, SC, et al. (2006) Reproductive protein protects functionally sterile honey bee workers from oxidative stress. PNAS 103(4): 962-967.
Shen, M L Cui, N Ostiguy and D Cox-Foster (2005) Intricate transmission routes and interactions between picorna-like viruses (Kashmir bee virus and sacbrood virus) with the honeybee host and the parasitic varroa mite. Journal of General Virology 86: 2281–2289
Nelson, CM, KE Ihle, MK Fondrk, RE Page Jr., GV Amdam (2007) The Gene vitellogenin Has Multiple Coordinating Effects on Social Organization. Public Library of Science http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0050062
Neukirch, A (1982) Dependence of the life span of the honeybee (Apis mellifica) upon flight performance and energy consumption. Journal of Comparative Physiology 146(1): 35-40.
Nielsen, SL, M Nicolaisen, P Kryger (2008) Incidence of acute bee paralysis virus, black queen cell virus, chronic bee paralysis virus, deformed wing virus, Kashmir bee virus and sacbrood virus in honey bees (Apis mellifera) in Denmark. Apidologie 39 DOI: 10.1051/apido:2008007
Remolina SC, Hafez DM, Robinson GE, Hughes KA (2007) Senescence in the worker honey bee Apis mellifera. J Insect Physiol. 53(10):1027-33.
Seehuus S, Norberg K, Krekling T, Fondrk K, Amdam GV. (2007) Immunogold localization of vitellogenin in the ovaries, hypopharyngeal glands and head fat bodies of honeybee workers, Apis mellifera. Journal of Insect Science 7:52, available online: insectscience.org/7.52
Seeley, T (1995) The Wisdom of the Hive. Harvard University Press.
Speakman, JR (2005) Body size, energy metabolism and lifespan. Journal of Experimental Biology 208, 1717-1730
Stills, S. (1966) For what it’s worth. Recorded by Buffalo Springfield
Stoffolano, J (nd) Respiratory or ventilatory system http://www.faculty.ucr.edu/~insects/pages/teachingresources/stoffolano/14.pdf
Southwick, E (1992) The physiology and social physiology of the honey bee. In The Hive and the Honey Bee. Dadant and Sons
Tentcheva, D, L Gauthier, N Zappulla, B Dainat, F Cousserans, M E Colin, and M Bergoin (2004) Prevalence and Seasonal Variations of Six Bee Viruses in Apis mellifera L. and Varroa destructor Mite Populations in France. Applied and Environmental Microbiology, December 2004, p. 7185-7191, Vol. 70, No. 12
Tribe, MA and DE Ashhurst (1972) Biochemical and structural variations in the flight muscle mitochondria of aging blowflies, Calliphora erythrocephala. J. Cell Sci. 10: 443-469
Van Engelsdorp, D, D Cox Foster, M Frazier , N Ostiguy, J Hayes (2006) “Fall-Dwindle Disease”: Investigations into the causes of sudden and alarming colony losses experienced by beekeepers in the fall of 2006.
http://www.ento.psu.edu/MAAREC/pressReleases/FallDwindleUpdate0107.pdf
van Nerum, K, and H Buelens (1997) Hypoxia-Controlled Winter Metabolism in Honeybees (Apis mellifera). Comparative Biochemistry and Physiology 117(4):445-455
Whitfield, CW, Y Ben-Shahar, C Brillet, I Leoncini, D Crauser, Y LeConte, S Rodriguez-Zas, and GE Robinson (2006) Genomic dissection of behavioral maturation in the honey bee. PNAS 103 (44): 16068–16075.
Wilson, WT and DM Menapace (1979) Disappearing disease of honey bees: a survey of the United States, Part 1. ABJ 119:118-119.
Yannick ,M and P Schmid-Hempel (2000) Survival for Immunity: The Price of Immune System Activation for Bumblebee Workers Science 10 290: 1166-1168