Sick Bees – Part 1
Sick Bees: Part 1
Randy Oliver
ScientificBeekeeping.com
First published in American Bee Journal August 2010

Most any long-time beekeeper has noticed that bees are simply not as healthy as they used to be, and that we have been plagued with a spate of unusual colony collapses in recent years. As luck would have it, I’ve been an intimate witness to the experimentally-induced collapse of colonies in a field trial this winter and spring. My observations have helped me to better understand how such collapses play out.
Sick Bees
We generally assume that our bees are healthy, as long as they are building normally and producing honey. The reality is that there is a constant battle going on in the hive between the bees and the parasites that seek to exploit them (note that to a biologist, the word “parasite” includes all the pathogenic mites, fungi, bacteria, and viruses). I’d like to quote excerpts from an address given in 1977 by the late, great bee pathologist, Dr. Leslie Bailey:
“Bees flourish in spite of the beekeeper, and this is because they are not domesticated….Indeed, they must have their freedom to survive [no one has yet figured out how to keep bees alive without access to natural forage].
Anyone who relies on the old accounts of bee diseases, and even on the most up-to-date books on beekeeping, will conclude that when bees are not obviously sick they must be free of pathogens; conversely, when a visibly sick colony is found to be infected with a pathogen this will be blamed for causing the disease…When I began work on bee diseases, this was the general idea, and I fully expected to cause sickness easily for my experiments. However, my bees usually continued to look well. It was not that the agents for these alleged diseases were not in my bees; I could find them quite easily, but usually they didn’t produce any striking symptoms. The fact soon became clear that most, probably all of the wide variety of bee pathogens can occur in colonies that nevertheless can continue to appear healthy….
A good beekeeper understands how certain activities in beekeeping can aggravate and spread infections of all kinds. These practices include disturbing colonies unduly, hindering their normal development, keeping too many close together, keeping them in districts of poor nectar-flows, …dispersing brood in them, distributing contaminated combs to other colonies, and feeding unsuitable sugar preparations” (Fig. 1).
Figure 1. Colonies being “stimulated” in California prior to almond bloom. (Practical tip:) The feeding of syrup will induce these bees to break cluster and shift from the long-lived “winter bee” state to “forager” status. It will also encourage them to wear themselves out by engaging in fruitless foraging in an unfriendly environment. Such stimulation prior to natural pollen flows is very stressful to the colony. Due to drift and robbing in the crowded yard, pathogens can quickly spread and flourish. Photos by the author.
**********************************************
Update March 2013: My report of Dr. Leslie Bailey’s demise was greatly exaggerated! I received the following note from Dr. Peter Tomkins at the Rothamsted research station:
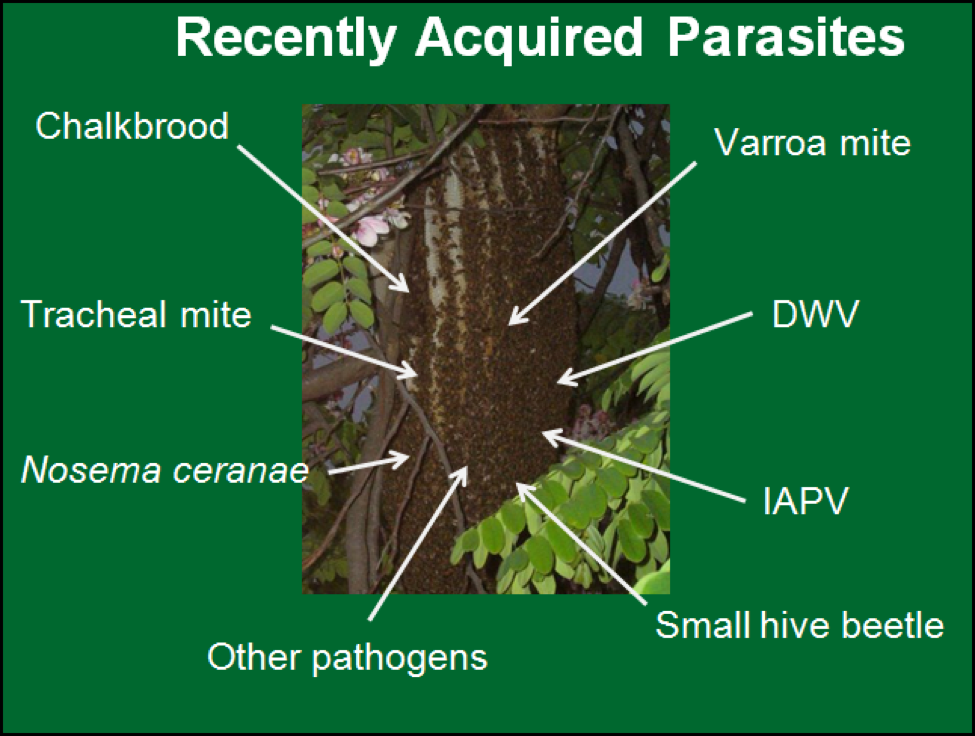
And that was back in the “good old days” of beekeeping, before I had ever heard of tracheal mite, chalkbrood, varroa, or bee viruses! Since I first started keeping bees, all the new pathogens in Figure 2 have arrived one by one. Such an onslaught of parasites is a tremendous evolutionary challenge to any species, since each new pathogen changes the equilibrium of existing host/parasite relationships, and the dynamics and efficacy of the bee immune response.
Figure 2. Bee parasites introduced into the U.S. since the 1960’s. It is amazing that the honey bee is resilient enough to deal with at least eight new pathogens in so short a time! We older beekeepers reminisce about the “good old days” when all we worried about was AFB (not yet antibiotic resistant).
Likely as a result of bees trying to come to terms with the new parasites, nowadays I’m seeing bee diseases that I’ve never seen before (or at least haven’t seen since bees suffered from Parasitic Mite Syndrome shortly after varroa arrived) (Figures 3 and 4). Plus, I suffered the unfortunate experience a few years ago of watching my colonies go through a period of dwindling and die offs that fit the symptoms of what was later named “colony collapse disorder” (CCD).
Figure 3. Sick brood suffering from what is termed “EFB-like” symptoms. Note the “corn yellow” larvae, the slumping white propupae, the sunken capping, and the partially removed sick larvae. Colonies in my operation with these symptoms flounder.
Figure 4. Sudden death of adult bees. Common in my yards in both California and Nevada, on various crops and at different times of the year. Generally there will suddenly be a pound or two of dead bees in front of one or a few hives in a yard. Then the “disease” disappears just as quickly, and the colony recovers. I typically do not find nosema spores in the dead bees. In the one instance where I was able to put samples of dying bees onto dry ice, there were no detectable pesticide residues, and viral testing was inconclusive.
This is not just the grousing of an aging beekeeper—I feel that the fact that our bees are having to come to terms with a slew of new parasites has a great deal to do with the case of the…
“Disappearing” Bees
I started writing this article some time ago, thinking that I would first introduce the reader to the aspects of honey bee biology that lead to colony collapses. Unfortunately, I got caught up in my obsessive thoroughness (as I am want to do), and in simply trying to introduce you to the critical function of ethyl oleate in colony collapse, I wound up writing the previous four-part series on the primer pheromones! This led me to realize that if I kept it up at that rate, it would be a year before I finally got to the actual mechanism of collapse. So I’m jumping ahead now, and going to describe collapses, and then follow with more detailed analyses of the components involved.
Beekeepers are used to colonies perishing from starvation, queenlessness, AFB, nosema, or varroa. In these cases, each sort of deadout exhibits a signature of distinct symptoms. What has caught our attention about CCD is that it seems “different.” The first reports were of a sudden “disappearance” of the adult workforce, leaving behind plenty of stores and large areas of brood (vanEnglesdorp 2006). A later description (Debnam 2009) describes the slow (often two-year) progression of the symptoms. In the experimental yard mentioned above, I was able to observe both the rapid and slow collapse of colonies.
There is nothing new about the phenomenon of sudden colony collapses (Underwood & vanEnglesdorp 2007). The historical descriptions of the symptoms of such collapse events were often strikingly similar to those that have occurred in the past few years. Note that they occurred prior to either parasitic mite or Nosema ceranae arriving in this country, and before the invention of cell phones or neonicotinoid insecticides!
Therefore, I have a hard time swallowing that CCD is necessarily caused by an entirely novel factor, but am guessing that the recent collapses are likely a variation on an old theme. In fact, the speculation about the cause of CCD reminds me of the blind men with the elephant—many have allowed their research specialty, personal biases, or favorite pet peeves to focus on a single culprit.
So let’s play Sherlock Holmes, and briefly sift through the evidence for or against the various suspects that have made the news. What I’d like to do is to apply Koch’s Postulates (the accepted scientific method for identifying the causal agent of a disease).
Koch’s Postulates (simplified)
1. Is the suspect factor always associated with the disease?
2. Will the suspect factor always create the same symptoms?
Let me make a quick list of suspects that do not meet both of the above postulates, since they either do not always show up in analyses of collapsed colonies, and/or do not always create CCD-like collapses when applied to colonies: cell phones or electromagnetic radiation, varroa or tracheal mites, genetically modified crops, or any particular pesticides.
That is not to say that at least some of the above could not be contributing factors. And this may be a key point—the current level of colony losses (not just CCD-like collapses) indeed appears to be higher than the “norm.” So it would be wise to see what novel contributing factors might be increasing the degree of losses.
What’s Changed?
I’ve been running bees in roughly the same manner for thirty years: I pollinate almonds in February, then to prunes, then home to make splits which build up on the foothill honeyflow, then to Nevada for irrigated alfalfa for the late summer, fatten them up on Rabbitbrush in fall, and then winter them back in the foothills. Worked like a charm, year after year.
The introduction of tracheal mite hit me hard, but I quickly recovered with resistant stock. Varroa brought me to my knees; recovery was much harder, but I was still eventually able to greatly expand my operation.
However, things were no longer the same. Queens would fail, and colonies would go queenless. Winter losses were higher. Beekeeping was just tougher. Dave Mendes notes that “bees are simply more ‘fragile’ than they used to be.”
Then in 2004 and 2005 my bees weren’t right. At first they just didn’t build up normally, and often exhibited the odd diseases in the photos above. Then they suffered serious fall and winter collapses (a fellow beekeeper in the same area lost thousands of colonies, but oddly, not so another buddy with yards alongside ours!). We erroneously blamed it on the mosquito spraying due to the West Nile Virus scare—further investigation did not support that link. And when I asked the growers, they hadn’t changed seed type nor pesticide use (most didn’t use any) for years. I couldn’t keep my numbers up, and was puzzled and distraught.
Then Dave Hackenberg made the news when he suffered unusual colony collapses in 2006. The point that he made was not that he suffered losses, but rather that the degree of the losses and way in which colonies collapsed, was unlike anything he had ever seen before. Something had changed!
What bugs commercial beekeepers is when folk blame long-time practices for this new problem: we’ve been trucking bees, using plastic foundation, feeding syrup, and had varroa mites for some time, and our bees generally thrived (as long as we kept mite levels down). So I’d discount any purported cause that hadn’t changed shortly prior to the appearance of CCD.
Some changes that did occur in that time frame were:
1. Additional colony stress due to the resurgence of the varroa mite and buildup of miticides. When mite levels increase, colonies die. At the time when colony collapses were increasing, some miticides had begun to fail, which then led to higher mite levels, the use of stronger miticide doses, and the use of multiple miticides. This resulted in increasingly high levels of comb contamination from those beekeeper-applied miticides (BAM’s)—the most common pesticides now found in hives (Mullin 2010; imidacloprid, by the way, was detected in only 1% of U.S. samples). Indeed several blue-ribbon studies from throughout the world have linked colony deaths with high levels of (often illegally applied) BAM’s. (However, this wasn’t the case with me, since I had eschewed synthetic miticides since the year 2000).
2. Large-scale unfavorable weather events preceded many reported collapses—mainly droughts in the Midwest, South, Texas, and California, very wet summer weather in the Midwest and East last year, and unusual frosts in the southerly states. Poor weather during expected bloom periods causes major nutritional stress for the bees. Perhaps more importantly, drought and wet summers create two serious problems related to Deformed Wing Virus (DWV): colonies don’t build large enough to outbreed varroa, and beekeepers may be tempted to put off mite treatment in order to get that last super of honey. If mites are not controlled by the end of August, the colony may enter winter with eventually fatal DWV levels (Martin 2010). (I don’t feel that abnormal weather was a factor in my losses).
Figure 5. These stacks of empty pallets indicate the degree of losses suffered by one beekeeper this winter in California—the deadout boxes were shipped home separately to save space. He attributes the losses largely to “natural events”—poor weather that left the colonies in a stressed condition. He has subsequently restocked the equipment. The ability of beekeepers to recover from such misfortune, although at staggering financial expense and lost income, tends to mask the serious costs of maintaining a bee operation these days. Photo by Dr. Jerry Bromenshenk.
3. More expansive monoculture and herbicide use, which results in incomplete bee nutrition, due to the elimination of the weeds that previously provided good forage to honey bees. There is also a shift in the types of pesticides applied–many environmentally more benign, but at the cost of being systemic in the pollen and nectar. Also of particular concern is the effect of the new classes of fungicides upon colony health—note that boscalid (Pristine, first registered for use in 2004) causes major problems to beekeepers in almonds, and is also widely sprayed during bloom on apples, sunflower, canola, and a host of other crops. (Again, other than in almonds, I had always avoided crops with any pesticides).
4. The shift of beekeeping to larger-scale operations (at least in the U.S.), and especially the industry shift to almond pollination midwinter. The homogenization of bee pathogens from throughout the country occurs each year in the California almond orchards. The changes in management necessary to fill contracts for strong colonies in February, especially following a poor season, may be challenging, and not all beekeepers manage to stay on top of colony health and nutrition. (Being a California beekeeper, I’d always managed my bees for almond pollination).
5. The introduction of Nosema ceranae. What can I say? Being infected by nosema all through the year just can’t be good for bees, and Dr. Mariano Higes’ work strongly suggests that it was associated in the unusual losses of colonies in Mediterranean Europe about that time. However, archived samples from Dave Hackenberg’s operation in 1985 tested positive N. ceranae, but Dave didn’t notice CCD problems until 2006! But apparently N. ceranae did not become widespread in the U.S. until about 2004 (Evans 2010)—which certainly puts it on the short list of suspects. (When I tested my colonies for N. ceranae in 2006, only some had it, but not at high levels—it was too late by that time to test the deadouts from the previous years).
5. New or more virulent strains of bee viruses. Varroa changed everything about virus presence, transmission, and virulence in bees. Note that widespread colony losses have only been reported from countries is which varroa is a problem (Neumann 2010). Colonies without mites may be virus free (Highfield 2009), but up to 100% of colonies with varroa may be infected by one or more viruses, even if there are no apparent symptoms (Tentcheva 2004). The scary thing about viruses is that they can suppress certain aspects of the immune system, and interact with each other, and with nosema!
So could new virus strains, such as Israeli Acute Paralysis Virus (IAPV) be the problem? And where would they come from? Some have pointed the finger at Australian imports, but there are two flaws that I see in that logic—collapses started prior to the first imports in 2005, and Canadian beekeepers imported plenty of Aussie bees, but did not suffer concurrent massive losses. However, I don’t want to let imports off scot-free. Dr. Elke Genersch (2010) points out that “Repeating previously observed scenarios, the dramatic increase in emerging virus diseases in the honey bee may still be worsened by the continuing development of international exchanges and the potential dissemination of still undiscovered viruses or other agents that may favor their active multiplication.”
New virus strains may have come from elsewhere, but can also arise spontaneously through their rapid evolution (like human flu viruses), or by passing through an alternate host (such as bumblebees or yellowjackets or perhaps varroa). In fact, the bee viruses actually exist as “virus clouds” of slightly differing, constantly changing strains, generally becoming more virulent each generation until they reach an equilibrium (which changes again each time the bees gain a new parasite)( Agudelo-Romero 2008).
I’ve been noticing such changes in my own bees. I saw more sacbrood (some of it odd looking) in those two years than I had ever seen before, more unusual larval deaths, and also more symptoms of Chronic Bee Paralysis Virus (hairless black trembling bees). There were sudden losses of adult bees as illustrated in Figure 4, suggesting rapid-acting viruses. But most notable was that the epidemiology of deformed wing virus (DWV) appeared to have changed—it no longer required high mite levels to show evidence of symptoms.
The case for fingering viruses as a prime suspect involved in colony collapse is strong. It’s been well established that it is one or more viruses that finally take down a varroa-infested colony (in which the adult bees also “disappear” suddenly—but, in the case of DWV, leave clear symptoms in the brood).
There are a number of other pieces of evidence that implicate viruses:
- Most emerging infectious diseases on Earth are caused by viruses.
- The disorder appears to be transmissible from one colony to the next (vanEngelsdorp 2009), and can spread from yard to yard (Debnam 2009). Only a disease caused by an infectious, transmissible pathogen would be expected to behave in such a manner. By process of elimination, the causal parasite would likely be a virus, since parasites other than viruses would be fairly readily detected (OK, could be a viroid or even a prion if you want to get picky).
- The sterilization or “resting” of combs from failed hives decreased the incidence of collapse following reintroduction of fresh bees. Bee viruses tend to become noninfective when dried (Bailey 1967; and from personal experience with DWV deadouts).
- Analysis of the bee immune response in CCD colonies indicated that the main infection was likely viral (Johnson 2009). The same study found that DWV and a number of similar viruses were more abundant in CCD bees, and that the bees exhibited “all the signs of death by massive virus replication.” On the other hand, Bromenshenk (2010) found that DWV was negatively correlated with collapses in the operations that he sampled (another virus appeared to be involved)!
- Titers of some viruses are strongly correlated with colony losses in Europe and the U.S. (Highfield 2009, Berthoud 2010, Evans 2010) (but different viruses appear to be associated with losses in different areas, and at different times of the year). vanEngelsdorp (2009) found that “colonies co-infected with 4 or more viruses were 3.7 times more frequent in CCD colonies than in control colonies.”
It sure appears to me that viruses look pretty guilty, and the results of our field trial strongly support this hypothesis. But viruses generally don’t take down a colony on their own, or there wouldn’t be any colonies left alive today! Colonies appear to normally be able to “purge” a within-hive virus epidemic (Bailey 1983), unless it passes a certain threshold of numbers of bees infected (Sumpter 2004). Or, perhaps we should more clearly define what a viral “epidemic” is. Sumpter states: “An epidemic can have a range of severities, an epidemic may mean the virus persists at a low level with only a small proportion of bees being infected,…or it may mean the virus has spread through the whole colony.”
So the question to me is, what factors or circumstances will kick the low-level viral epidemics that our colonies normally experience, into the sort of raging epidemic that results in rapid colony collapse?
Some clues come from beekeeper observations. In a recent self-reported survey, U.S. beekeepers ranked the following factors as contributing to colony losses (top to bottom): starvation, queens, weather, mites, weak in fall, Nosema, management, CCD, and pesticides (vanEngelsdorp 2010). Please note that colonies fail for many reasons, and that most deadouts are not the result of CCD. This can make the investigation of the cause(s) of CCD difficult, especially when the Government is offering financial compensation to beekeepers who claim that their losses were caused by CCD! The above survey results indicate that most beekeepers will honestly report the true causes of their losses, to the best of their knowledge. And of the causes, four factors clearly stand out, both historically and associated with recent collapses.
The “Four Horsemen of Bee Apocalypse”
Poor nutrition, due to drought, extended rain, lack of bloom, or crop monoculture. Also includes starvation from lack of nectar or honey. I’ve written at length about bee nutrition–any fool can keep bees alive during a good nectar and pollen flow! It’s generally when bees become nutritionally stressed that they get sick.
Cold snaps in spring or fall, when the colony is not in winter cluster. When I look at the historical accounts of collapse events, a cold snap often jumps out. Honey bees are tropical animals, and the European honey bee has adapted to life in temperate climates by living in insulated cavities, and by forming a heated winter cluster consisting of long-lived, stress-resistant “winter bees.” A cold snap when a colony is not prepared for it can chill the brood and stress the workers (especially by depressing their suppression of viruses, nosema, and chalkbrood).
Toxic chemical stress—this can be from natural plant toxins (Fig. 6), heavy metals, environmental pollutants (such as PCB’s), any sort of pesticide, or beekeeper-applied miticides. Bees have evolved the ability to detoxify many chemicals (including pesticides), but that ability is depressed when they are nutritionally stressed or cold.
Figure 6. Honey bees have needed to deal with environmental toxins long before humans started using pesticides. This forager may be unwittingly poisoning its colony by working California Buckeye. Generally, my bees take the nectar, but do not collect the toxic pollen (the literature is unclear as to whether the nectar is also toxic). In years when the Buckeye products are not diluted by other nectars and pollens, this natural toxin can devastate colonies.
Parasite stress, especially infection with multiple parasites (remember, this includes viruses). There are several pieces of evidence linking parasites to CCD, notably that excessive numbers and variety of parasites often exist in the sick colonies (Cox-Foster 2007).
The first three “Horsemen” (nutrition, chill, and toxins) don’t normally directly take down a colony (except in the case of severe starvation or pesticide poisoning)–it generally takes a combination of more than one factor to do the job, as long as the bee stock is robust and genetically diverse.
That leaves parasites to apply the coup de grâce. So which parasites can cause symptoms similar to CCD? There are four that stand out:
1. Viruses—covered above, perhaps a new strain.
2. Tracheal mite, but it simply isn’t found to any extent in CCD colonies.
3. Nosema apis—the “old” nosema. This long-time scourge of the honey bee may cause colony collapse: “In a typical case of a colony being depleted because of a Nosema infection [normally in winter or spring], the queen can be observed surrounded by a few bees, confusedly attending to brood that is already sealed” (Anon 2004). However, there is generally distinct dysentery, and dwindling of the colony, and it normally doesn’t kill strong, healthy colonies with adequate nutrition.
4. Nosema ceranae—A great body of research by Dr. Mariano Higes (2010) supports the hypothesis that N. ceranae causes colony collapses. However, the progression of disease as he describes it is not universally observed by either other researchers or beekeepers (nor in my own test yards), so there are likely other factors at play (let me be clear that I greatly respect the work of the Higes team, and that I correspond regularly with Dr. Higes and find him to be most helpful in trying to resolve this quandary). A number of researchers have suggested that poor bee nutrition and/or weather play a major role in the pathogenicity of N. ceranae (Pajuelo 2008).
So could one or both of the nosema “cousins” be involved in CCD? Cox-Foster (2007) found both species of nosema in all CCD colonies, with N. apis at surprisingly high levels. vanEngelsdorp (2009) found that “Co-infection with both Nosema species was 2.6 times greater in CCD colonies when compared to control colonies.”
Then at the American Beekeeping Federation conference early this year, Dr. Jerry Bromenshenk stated that his extensive data from CCD sampling supported a dual-pathogen (virus + nosema) hypothesis, which was later echoed by a presentation this May by Dr. Jay Evans (2010).
One clue may be that nosema and viruses often go hand in hand—some viruses are only found in association with nosema, perhaps because a nosema infection breaches the integrity of the normal effectiveness of the gut wall as a virus barrier. However, clinching the virus/nosema link is difficult—the detection of viruses depends largely upon experienced technique and having the proper primers at hand, so it is easy for some viruses to go undetected. There is also another critical point to keep in mind—that both bee viruses and N. ceranae are constantly changing by recombination of their genes (de Miranda 2009; Sagastume 2010), so the virulence of the parasites could vary from month to month, area to area, and colony to colony!
So, can researchers induce CCD by inoculation with viruses (and fulfill Koch’s second postulate)? Dr. Diana Cox-Foster (2008) inoculated nucs in a greenhouse, and found that “rapidly increased death of bees was observed within a week after feeding the colony sugar-water containing the IAPV….Although these symptoms were consistent with reported symptoms of CCD, effects on the brood were not like that observed in CCD colonies. In CCD colonies, brood appears to remain healthy and strong, with the primary death being observed in adult bees.” I will return to this observation later, as I do not feel that it precludes IAPV from causing CCD-like symptoms, however, in later surveys in other areas, IAPV was not found in the majority of collapsing colonies, so is unlikely that IAPV alone is the major cause of CCD.
In my own trials with caged bees in an incubator, inoculation with a purified cocktail of mixed viruses, originally extracted from collapsing colonies, caused rapid and near complete mortality of the bees in some cages at about nine days. Dr. Wayne Hunter confirmed that this is normal. In my field trial, feeding the same cocktail in syrup also initiated collapse of colonies within ten days, some failing rapidly, some suffering an agonizingly slow death over the course of months! This is a very important point—that oral ingestion of viruses by adult bees can cause rapid illness and death. I will expound upon this point later in this series.
OK, so we’ve got some likely suspects. In the next installment, I will explain two critical aspects of the honey bee immune response that can lead to colony collapse, detail how the “Four Horsemen” fit in, and present a model of the process.
Acknowledgements
I could not begin to make sense of all this without personal communications with a number of researchers in the field. I wish to thank Nitzan Paldi of Beeologics, Jay Evans, Jerry Bromenshenk, Robert Cramer, Dennis Anderson, Mariano Higes, Rob Currie, Steve Pernal, Medhat Nasr, Dennis vanEngelsdorp, Jeff Pettis and Eric Mussen (apologies to the many others who have taken time to answer my questions). I’m also greatly appreciative for the financial donations from Ray Olivarez, Joe Traynor, Paul Limbach, and numerous small contributors, without whose support I simply couldn’t devote the time involved in my research.
References
Anon (2004) Nosemosis of Bees in Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 5th Ed. World Organisation for Animal Health http://www.oie.int/eng/Normes/mmanual/A_00123.htm
Bailey, L (1977) Good beekeeping and bee health. A lecture given to the Central Association of Bee-keepers on 1st October 1977.
Bailey, L, BV Ball and J. N. Perry (1983) Association of viruses with two protozoal pathogens of the honey bee. Ann. appl. Biol.103:13-20.
Bailey, L. (1967) The incidence of virus diseases in the honey bee. Ann. appl. Biol. 60: 43-48.
Berthoud, H, A Imdorf, M Haueter, S Radloff and P Neumann (2010) Virus infections and winter losses of honey bee colonies (Apis mellifera). Journal of Apicultural Research 49(1): 60-65
Bromenshenk, J (2010) Breakthroughs on CCD research. Presentation to the American Beekeeping Federation, Orlando.
Cox-Foster, DL (2008) Colony collapse disorder—determination of role of pathogens in unique-colony losses of honey bees and funding of workshop on CCD http://www.reeis.usda.gov/web/crisprojectpages/212157.html
Cox-Foster, DL, et al. (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318(5848): 283-287.
de Miranda, JR, E Genersch (2009) Deformed wing virus. Journal of Invertebrate Pathology 103: 548-561.
Debnam, S, D Westervelt, J Bromenshenk, R Oliver (2009) Colony Collapse Disorder Symptoms. Bee Culture Feb 2009 https://www.beeculture.com/storycms/index.cfm?cat=Story&recordID=629
Evans, JD (2010) Interview on ASMnewsroom. http://www.ustream.tv/recorded/7203825
Genersch, E and M Aubert (2010) Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet. Res. (2010) 41:54
Higes, M, R Martín-Hernández and A Meana (2010) Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 41: 375–392.
Johnson, RM, et al (2009) Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera). PNAS 106(35): 14790-14795.
Martin, SJ, BV Ball and NL Carreck (2010) Prevalence and persistence of deformed wing virus (DWV) in untreated or acaricide-treated Varroa destructor infested honey bee (Apis mellifera) colonies. Journal of Apicultural Research 49(1): 72-79
Mullin CA, M Frazier, JL Frazier, S Ashcraft , R Simonds, et al. (2010) High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE 5(3): e9754. doi:10.1371/journal.pone.0009754
Neumann P., NL Carreck. (2010) Honey bee colony losses, J. Apic. Res. 49, 1–6.
Pajuelo, AG, C Torres and FJ Orantes Bermejo (2008) Colony losses: a double blind trial on the influence of supplementary protein nutrition and preventative treatment with fumagillin against Nosema ceranae. Journal of Apicultural Research 47(1): 84-86.
Sagastume, S, C del Águila, R Martín-Hernández, M 7 Higes, and N Henriques-Gil (2010) Polymorphism and recombination for rDNA in the putatively asexual 3 microsporidian Nosema ceranae, a pathogen of honey bees. Environmental Microbiology and Environmental Microbiology Reports, in press.
Sumpter, DJT and SJ Martin (2004) The dynamics of virus epidemics in Varroa-infested honey bee colonies. Journal of Animal Ecology 73: 51–63.
Tentcheva, D., et al (2004) Prevalence and Seasonal Variations of Six Bee Viruses in Apis mellifera L. and Varroa destructor Mite Populations in France. Appl. Envir. Microbiol. 70: 7185 – 7191.
Underwood, R., D. vanEngelsdorp 2007 Colony Collapse Disorder: Have we seen this Before? Bee Culture 135(7) 13-18.
vanEngelsdorp, D, et al (2006) Fall Dwindle Disease: A preliminary report. maarec.psu.edu/pressReleases/FallDwindleUpdate0107.pdf
vanEngelsdorp D, JD Evans, C Saegerman, C Mullin, E Haubruge, et al. (2009) Colony Collapse Disorder: A Descriptive Study. PLoS ONE 4(8): e6481. doi:10.1371/journal.pone.0006481
vanEngelsdorp D., J Hayes, RM Underwood, JS Petti (2010) A survey of honey bee colony losses in the United States, fall 2008 to spring 2009, J. Apic. Res. 49, 7–14.