Sick Bees – Part 17b: Nosema – The Smoldering Epidemic
Sick Bees 17: Nosema
The Smoldering Epidemic
Part B
First published in ABJ April 2012
Randy Oliver
ScientificBeekeeping.com
Why Would Nosema ceranae Not Cause Problems?
Understanding the Honey Bee Superorganism
You may have noticed that I’m doing a sort of “about face” in my assessment of the impact of Nosema ceranae upon colony health. I feel that I owe the reader an explanation. I just get this nagging feeling that there’s more to the invasion of this new parasite into the U.S. bee population than meets the eye. To truly understand the potential impact of nosema, we must look beyond its effects upon individual bees, and rather focus on its impact upon the superorganism that we call the honey bee colony.
The Scientific Method
Science is not about microscopes and laboratories (that’s technology); it is about the thought process that we use to make sense of our observations of the world around us. One can think in a scientific manner just as well while wearing dirty white coveralls as when wearing a clean white lab coat!
The true scientist holds no positions, frees himself of beliefs, and avoids any prejudices or biases. His only firm conviction is to remain completely open minded and objective in the never ending quest to understand why things are the way they are.
Science is based upon the free sharing of data (accurate observations) and its interpretation. Not every scientist will interpret the data in the same way. So long as good scientific method is followed, there is nothing wrong with floating highly controversial interpretations, and indeed good scientists relish having an established paradigm challenged.
As an aside, please realize that the editorial filtration process of scientific publication is hardly perfect. I’m personally sometimes dismayed by the poor quality of peer review of late, and have serious criticisms of both the methodology and interpretation in a number of recently published papers.
Warning: unless you are willing to research more deeply, I caution you to take any new scientific findings that get splashed across the headlines with a grain of salt, especially when “hot” topics, such as CCD, pesticides, the environment, or human health are involved!
(Back to the Scientific Method): any new interpretation as to why something is the way it is, or works the way it does, is subject to testing by proposing a “falsifiable” hypothesis. You can’t scientifically prove that anything is true (or that anything is “safe”); you can only “disprove” a hypothesis (such as that something does not cause measureable harm) by putting it to actual test in a well-designed experiment.
The best that you can do toward seeking truth is to find that the results of multiple experiments “support” your hypothesis. When a hypothesis has eventually been supported by enough robust data, then it is accepted as a scientific “theory”—a word that has much stronger meaning in science than it does in the common vernacular. A scientific theory becomes the paradigm by which the scientific community “understands” things—and is of course subject to revision should any new data come to light that falsify it.
When Dr. Mariano Higes found that Nosema ceranae was highly associated with the collapsing colonies that he observed in Spain, he proposed the hypothesis that the parasite was the cause. He further tested that hypothesis in various experiments by inoculating healthy colonies with spores, applying fumagillin or not, and then tracking the buildup of nosema and colony strength. He found that his results supported his hypothesis.
Other researchers, including myself, at first also found his hypothesis to be attractively plausible—it appeared to reflect the typical high mortality associated with the invasion of a naive host population by a novel parasite. However, when we sought to replicate Dr. Higes’ results in our own bees, we simply didn’t see a compelling cause and effect relationship, and as a result then questioned the validity of his hypothesis.
I myself fell into the skeptical camp; but I go out of my way to truly understand alternative viewpoints; to that end I have maintained a friendly ongoing conversation with Dr. Higes for the past five years—constantly challenging and questioning him. Such frank discussions are the best method to arrive at the actual truth of matters.
I want to be clear at this point that in this series I’m doing a lot of thinking aloud. I will try to be clear as to which conclusions (always subject to reevaluation) are based upon hard data and actual experimental testing; and which ideas or opinions are inferential—based upon suggestive data or observations. I also want to emphatically state that the evidence to date does not suggest to me that Nosema ceranae is directly responsible for either CCD or major colony losses; but it does appear to often be associated with them, and may be a contributor in some way. I’ll return to the subject of colony collapse in a later article.
Effect of the Invasion
So, how can we tell if the invasion by Nosema ceranae is having any substantial negative effect upon the health of our colonies? N. ceranae invaded East Coast apiaries as early as the mid 1980’s without anyone even noticing it, until it was discovered twenty years later by researchers investigating CCD. But then again, it was discovered in colonies suffering from CCD, which may be a telling point!
The effects of infection by the new nosema seem, in general, pretty similar to those of its cousin, although it appears to cause somewhat more gut damage, and may be a bit more resistant to fumagillin. The most notable aspect that is different about N. ceranae is that it apparently “has better mechanisms to evade host immunity to allow for faster growth and reproductive capacity than N. apis” (Chen 2009). Antúnez (2009) found that it up- and down regulates bee immune response genes differently than its cousin. Plus it is able to thrive over a wider range of temperature (Martín-Hernández 2009), so it exerts its negative influence over a more prolonged period each year. I suspect that it also has better mechanisms for transmission from bee to bee. All the above differences make it a more virulent pathogen –in the sense that it reproduces more efficiently, rather than necessarily causing increased individual bee or colony mortality.
Then Again, Why Would Nosema ceranae Not Cause Problems?
With the majority of U.S. bee samples currently being infected by nosema (presumably Nosema ceranae), it seems to me that perhaps the question that we should be asking ourselves is, “Why wouldn’t we expect this level of infection to be causing problems?”
There is a vast body of “classical” research on the fundamental negative effects of Nosema apis infection upon colony health and productivity. Nosema is an age-old nemesis of beekeepers. Why would we not expect similar effects due to the new nosema, which is even more successful at infecting bees?
Understanding Nosema
Nosema is adapted to turn a bee into a spore-producing factory; there is no benefit to the parasite in killing the bee. And therein lays the problem, because it makes nosema so insidious and unnoticeable. But a widespread increase in the prevalence of such an insidious infection could still exert major effects upon colony buildup, production, and survival.
Understanding the Honey Bee Superorganism
In order to understand the effect of nosema upon the colony, one must stop thinking of the honey bee as merely an insect. Rather, we must think of it at the level of the superorganism, similar to an intelligent, warm-blooded, fast-growing ten-pound animal. But not just any animal; specifically one whose rapid growth makes it ravenous for energy and protein—exactly the precious commodities that nosema steals from the colony.
So I did some research on the effects of gut parasites upon other animals. In humans, microsporidian infection of the gut results in malabsorption of nutrients (Kotler 1999). It is no surprise that a common result of gut parasitism is reduced growth rate and poor energy metabolism, due to less efficient digestion and utilization of food rations (McRae 1993). This made me think that I should compare the normal growth rate of the honey bee colony to that of other livestock. So I looked for a similar-sized, exceptionally fast-growing organism. I arrived at the modern day broiler chicken.
When I ran a farm store some thirty years ago, a broiler took 19 weeks to grow from egg to slaughter. Today, with better rations and breeds selected for rapid growth, it only takes about six weeks to grow the same chicken! As with the bee, this incredibly rapid growth rate requires a high energy, high protein diet, which must be optimally digested and utilized.
Next I downloaded data for the growth rate of a broiler, and transformed it into graphical form (Fig. 1).
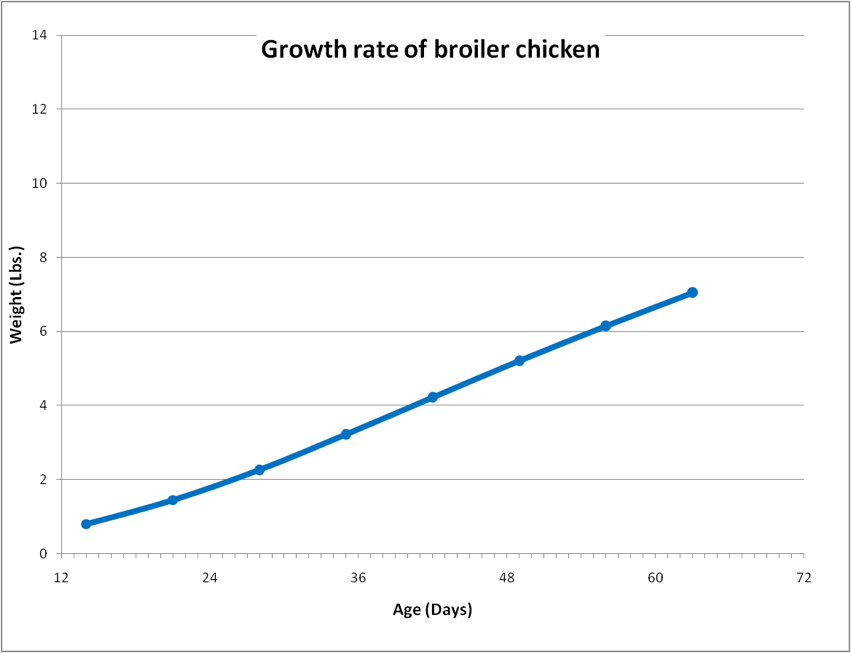 Figure 1. The growth rate of a modern broiler chicken. The chicken grows from 2 lbs to 7 lbs in 36 days. For perspective, the growth slope for a normal 12-month-old human child would appear dead level on the scale of this chart. Data from Jacob 2011, CDC 2012.
Figure 1. The growth rate of a modern broiler chicken. The chicken grows from 2 lbs to 7 lbs in 36 days. For perspective, the growth slope for a normal 12-month-old human child would appear dead level on the scale of this chart. Data from Jacob 2011, CDC 2012.
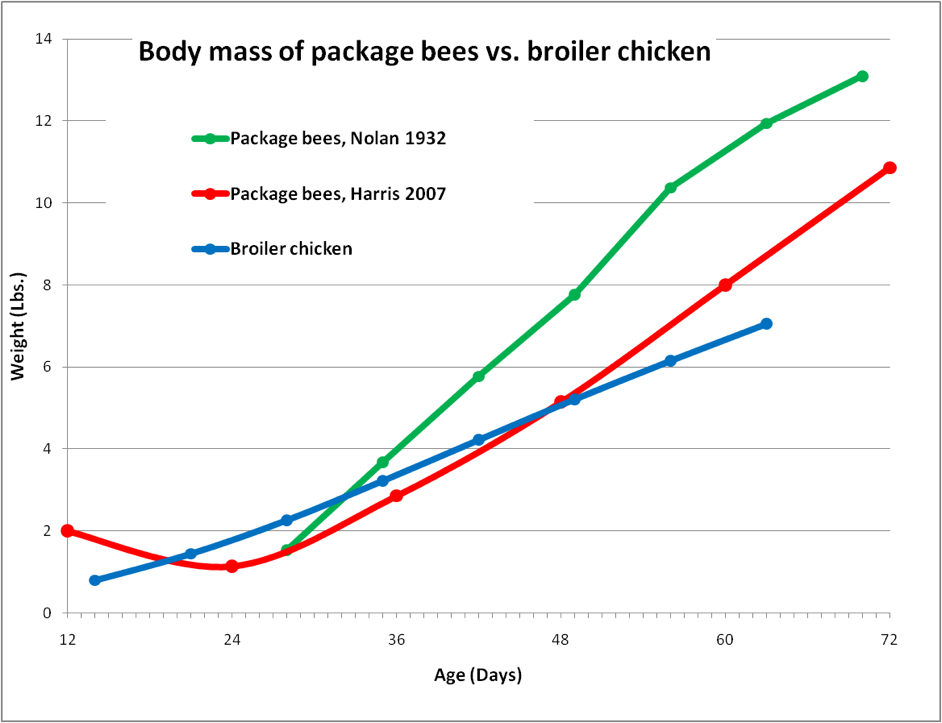
Since the chicken’s weight crosses the 2-lb line early in this graph, we can handily compare its growth rate to that of a small colony of bees—a freshly-hived 2-lb package. In the following chart, I took data from two studies that measured package bee growth over time, and overlaid them on top of the chicken growth plot.
Figure 2. Comparison between the weight gain of package bees vs. that of a broiler chicken. The red curve shows how a package loses population until the first brood emerges. After that point, packages grow considerably faster than even the fastest-growing chicken! If our human child were to grow as rapidly as a bee colony, its weight would increase from 24 lbs to 250 lbs in the two months following its first birthday! Data for 3-lb packages calculated from Nolan (1932) and Harris (2008),
Clearly, a bee colony grows at an amazing rate. But that ain’t the half of it! The chicken has the immense advantages of being penned in a warm room and provided with optimally-formulated chow, and maintains a compact body size, insulated by feathers. On the other hand, the industrious bee colony has to forage over a dozen square miles, spending a tremendous amount of energy in the process, as well as wasting a vast amount of body heat to the environment.
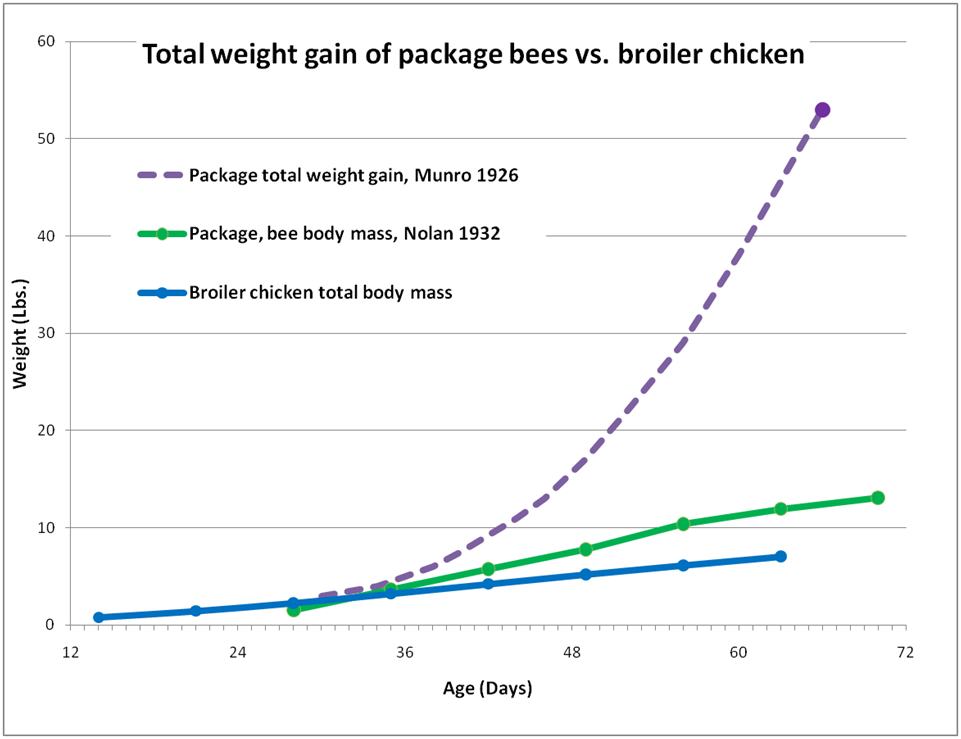
But I’m not done yet! If the chicken manages to store any excess energy or protein, it puts it on as fat or muscle—which then adds to its body weight. The analogous storage “tissues” of the bee colony would be the honey and beebread accumulated in the combs, but the above graph doesn’t reflect this fact. So let’s adjust the graph to take into account the bees’ stores (Fig. 3).
Figure 3. Total weight gain (including honey and beebread) of a 3-lb package installed two weeks before the main flow, compared to the gain in body mass alone of bees or a broiler chicken. When we measure total colony weight gain, the bees leave the broiler in the dust! The end points of the dotted line are actual data Munro (1926); I estimated the intermediate curve based upon measurements by Nolan.
By the end of the above chart, the broiler was essentially done growing. On the other hand, the colony was approaching its maximum population, but it was hardly done “growing.” In the next two months, it gained yet another 218 pounds! It is not unheard of for a colony with a bee body mass of 12 lbs to gather, process, and stockpile its body weight in surplus honey each day!
So what’s my point? It’s that we beekeepers expect our bees to perform a feat of rapid growth beyond the capability of perhaps any other animal! But the bee superorganism can only pull this prodigious feat off by being extremely efficient at digesting and utilizing protein and sugar (energy). That is why it is completely dependent upon two of the richest foods in nature—pollen and nectar. A bee colony would starve to death on the sorts of diets that most organisms are adapted to.
A strong, foraging colony during spring or early summer must consume and efficiently process, every single week, a minimum of 2-3 pounds of high-protein pollen plus several (about 10-15) pounds of sugar. By comparison, a rapidly-growing chicken eats only about 2-3 lbs. of total dry ration; a similar sized growing cat eats only about a pound of dry chow a week.
The point: The goal of a colony is to convert nectar and pollen into bees and honey. The main deleterious effect of nosema may not be bee mortality, but rather the fact that infection suppresses efficient food conversion.
Nosema and Energy Metabolism
The point of the above graphs is that we beekeepers are keeping an exceptional animal! In order for a colony to perform to our expectations, it can’t afford to be handicapped by a parasite that messes with its digestion or saps its energy. Yet nosema does exactly that!
Dr. Dhruba Naug and Chris Mayack (2009) have been pioneers in this avenue of research. Allow me to quote some excerpts from their paper:
“Parasites are dependent on their hosts for energy to reproduce and can exert a significant nutritional stress on them. Energetic demand placed on the host is especially high in cases where the parasite-host complex is less co-evolved” [perhaps as in the case of N. ceranae and Apis mellifera?].
“Some pathogens such as microsporidians are particularly severe on their hosts in terms of exerting an energetic stress because they lack mitochondria and therefore have little metabolic ability themselves” [nosema is unable to digest sugar by itself, and steals energy directly from the bees’ metabolic pathway].
“These results demonstrate that energetic stress is the probable cause of the shortened life span observed in infected bees.”
The authors also noted that bees exhibiting an infection of only a quarter of a million spores per bee were hungrier than uninfected bees. They exhibited greater responsiveness to sugar and consumed about half again as much sugar per day.
At this point, I suggest that if you haven’t yet read Bernd Heinrich’s (2004) seminal book “Bumblebee Economics” that you stop right now and order it! Heinrich clearly explains how bee survival is all about energy balance and efficiency. This readable and fascinating book will give you a far greater appreciation and understanding of the economics of the hive, and of ecology in general.
Take home message: As Bernd Heinrich explains, for a colony to be successful, every worker bee must, over the course of its adult life, not only repay the colony’s investment of protein and energy used in rearing that bee to adulthood, but must then additionally forage for enough resources to support itself and to provide for additional broodrearing. Prior to its death, it will ideally produce a surplus of energy stores in the form of honey, which the colony can later use to survive dearth or winter. Any bee that, due to being parasitized by nosema, is unable to fulfill the above responsibilities would then be a net liability, rather than an asset, to the colony as a whole.
Heinrich focused upon bumblebees, but other authors soon followed suit with studies on honey bees. One excellent model of honey bee economics was published by Jon Harrison and Jennifer Fewell (2002). They worked up calculations for net forager caloric gain to the colony after subtracting the costs of colony metabolism and the energy necessary for foraging flight (Table 1).
| Energetics of ‘typical foraging’ for Apis mellifera at an air temperature of 86 F | |
| Nectar load | 30ml |
| Nectar energetic content | 9 J/ml (50% sugar) |
| Energetic reward per trip | 270 J |
| Flight metabolic rate | 2.5 J/bee min |
| Trip duration | 30 min |
| Cost per trip | 75 J |
| Net gain per trip | 195 J; 6.5 J/min |
| Trips per day | 12 |
| Reward per day | 3240 J |
| Cost per day during flight | 900 J |
| In-hive metabolic rate | 0.16 J/bee min |
| Daily in-hive metabolism of forager | 173 J/day |
| Metabolic cost per forager day | 1073 J/day |
| Net gain rate per forager day | 2167 J/day |
| Hive bees fed per forager | 9.4 |
| % of bees which forage | 10 |
| % of total colony energy spent foraging | 30 |
Table 1. The economy of bee foraging energy gains vs. costs. Table modified after Harrison and Fewell (2002) by permission. By their calculations, in warm weather, the net energetic gain per forager per day is about 2167 Joules, which translates (by my calculations) to 1/50th of a teaspoon of stored honey per day, or a bit less than 4 lbs of honey for a colony with 10,000 foragers.
Harrison and Fewell’s excellent model was a great starting point for me to try to gauge the effect of nosema infection upon colony weight gain. I know how my readers just love when I take the burden off their TV-addled brains and grind through the math for them, so I entered all of the data from the table above into my own spreadsheet. Luckily, my son volunteered to drive, so I was able to figure out most of the equations while we were hauling a load of hives down to almonds.
When I created a spreadsheet for a colony of 40,000 bees (about 23 frames of bees), assuming that a quarter of them were foragers (Winston 1987; a larger proportion than estimated by Harrison), the resulting daily weight gain or loss figures didn’t necessarily match those that I typically observe in the field (about a pound a day weight loss when confined by rain, and about 5 lbs per day net gain during a decent honey flow). So I adjusted the assumptions using other researchers’ measurements (Southwick 2001; Woods 2005) until the model better reflected field reality.
I got some interesting results. In warm weather, when there is only enough of a nectar flow such that the colony is just holding its own (neither gaining nor losing weight), one forager is essentially gathering enough nectar to feed itself, plus about three house bees, which kinda makes sense if a quarter of the bees are foragers! (A larger proportion may shift to foraging during an intense flow (Oliver 2010)).
However, given the exact same colony, with the same nectar income, but on a cool day, the colony will lose over a pound of weight a day, due mainly to the increased metabolic cost of foraging at lower air temperature.
Pay attention: This is likely a significant point to keep in mind, as nosema infection appears to mainly be a problem in cool weather. The energy economy of a bee colony is much more tenuous when bees must forage in cold air.
OK, now let’s go back to warm weather, again with enough of a light nectar flow that the colony is just able to hold its weight. Then add a nosema infection to the equation, such that half the field force is infected; and guesstimate that the cost of infection results in a 50% increase to the metabolic demand of the infected foragers (Mayack 2009, Martín-Hernández 2011). Without changing anything about the foraging trips or bloom, and without any bee mortality, the cost of the infection would result in about a half pound weight loss for the colony a day!
The infection above would be completely invisible to the beekeeper—the bee and brood population would be exactly the same, the number of foragers and the nectar income would be exactly the same, but the added metabolic cost of the nosema infection to only half the foragers (1/8th of the colony population) would cause that colony to lose significant weight rather than holding its own.
It gets even worse in cool weather. Everything else remaining the same except for the greater heat loss from the foragers to the cool air (I’m ignoring any additional heat loss by the cluster), the colony would now lose over a pound a day—more than it would if the foragers were simply kept in by poor weather! The model suggests that the impact of nosema infection upon energy dynamics will be most substantial during cool weather or in times of nectar dearth when bees are engaged in fruitless foraging (like sitting in the orchards just prior to almond bloom).
Keep in mind that in recent years, surveyed U.S. beekeepers most often ranked colony starvation as the major cause of winter losses (vanEngelsdorp 2012). I’m thinking, if nosema infection results in less honey being stored over the season, and less efficient metabolism of that honey in cool weather, then perhaps nosema could be an indirect factor in these starvation losses.
You may be wondering what the model predicts for the impact of nosema infection during the main honey flow. Things change quite a bit when colonies are large, the weather is warm, the days are long, and foraging trips are richly rewarded. In a strong nectar flow in warm weather, the model predicts that a 50% infection rate of the foragers would not suppress honey production to any great degree.
But that’s only half the story—because by that time, nosema may have already done its damage during the colony buildup period prior to the main flow.
Nosema and Protein Metabolism
So let’s look at the main limiting factor for colony buildup. Colony buildup, given enough available honey, is limited by the protein income from pollen, and then the ability of young bees to efficiently convert that pollen into jelly.
It may be that the main problem with nosema infection is its impact on the protein dynamics of the hive. Not only do the foragers have a more difficult time energetically in foraging for pollen, and a reduced flight range, but the colony may “starve” for protein despite its being brought in, if infected nurse bees can’t efficiently convert it to jelly. There is a fairly rapid turnover of protein within bee body tissues (Crailsheim 1986), so any hampering of protein processing could really throw a stick into the gears of the hive economy.
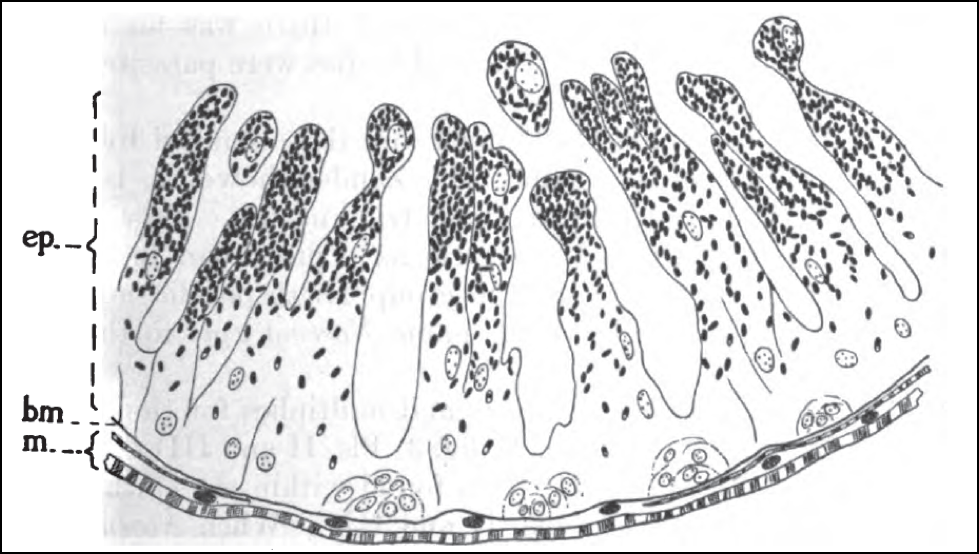
Nosema infection of the gut cells has an insidious effect. Not only does it reduce the ability of the gut to digest pollen and then absorb its nutrients, but it diverts protein that would normally go to jelly production into the replacement of damaged gut cells (Fig. 4). As a result, the hypopharyngeal glands tend to “dry up” in infected bees, and they can no longer feed the queen nor the brood.
Figure 4. Cross section of the midgut wall of a bee infected by nosema. The parasite infects the epithelial cells (ep) which form the intestinal villi (the finger-shaped projections through which nutrients are absorbed). The epithelial cells naturally break off from the tips of the villi and are replenished by fresh cells regenerated at the basal membrane (bm), which N. ceranae may also infect. Infected mature epithelial cells fill with nosema spores (dark ovals), which are released when those cells are shed into the gut lumen. There is a greater overturn of the epithelial layer in infected bees, as the bee tries to generate cells faster than nosema can infect them. This increased replacement rate requires the diversion of protein that would normally go into other tissues or jelly production. Drawing by G.F. White (1919) Nosema-Disease. USDA Bulletin No. 780.
So let’s look at the protein cost of nosema to colony buildup. In Figure 2, those pre-varroa colonies built up damn quick! They multiplied their populations fivefold in two brood cycles! That works out to a daily intrinsic rate of increase (r) of 1.04 (1.04% compounded daily for 42 days equals 5x increase).
So let’s factor in the potential drain to colony protein dynamics due to nosema. Unfortunately, I’m going to have to guesstimate here, since I haven’t found any studies in which the jelly production suppression due to nosema infection was clearly quantified. So I’m going to assume for modeling purposes that any badly-infected bee removes one “bee share” of contribution toward brood production.
So, let’s say that a quarter of the bees in the hive were infected; which would then depress the intrinsic rate of increase by a quarter, from 4% to 3% (r of 1.04 to 1.03). At that rate, a 2-lb package, instead of growing into a 24-frame honey-producing monster by the end of 10 weeks, would cover only 17 frames—you’d only get 2/3rds of normal colony growth! And that’s not even taking into effect the increased nosema-induced mortality of the package bees prior to the first brood emerging.
And yet again, the colony would appear to be perfectly healthy, with no brood mortality nor dead bees evident–it would just seem a bit lethargic in buildup. This is why nosema is called the “invisible disease.” And about half of all U.S. colonies now test positive for Nosema ceranae to some degree! It sure makes me wonder if we haven’t been paying enough attention to this new parasite.
Fundamental concept: honey bee colonies are by necessity voracious consumers of high-protein, high-energy food. Anything that affects the digestion and utilization of that food will negatively affect colony buildup and survival. Nosema siphons off a share of that protein and energy.
Practical application: Porrini (2011) and other researchers have found that infected bees can live nearly as long as uninfected workers provided that they receive plenty of protein. But at the same time, nosema spore counts get higher in protein-fed bees. I’d personally worry more about protein deficiency than spore counts! Making sure that your bees get plenty of nutritious pollen or supplement can greatly help to mitigate the deleterious effects of nosema infection.
Nosema and Colony Population Dynamics
You may have noticed that in this article I’m ignoring any increased worker mortality due to nosema. As I explained before, it is not in the interest of the parasite to bring about the death of its host. Rather, the effect of nosema is to turn a colony from a honey-producing factory into a spore-producing factory.
Should there be good weather and plenty of pollen and nectar, and if the colony has a vigorous queen, it can typically purge itself of all but a residual level of nosema infection. However, that colony may not be the sort of robust, productive colony that we are accustomed to. Not only is the colony handicapped by energy and protein competition with the parasite, but infected young bees tend to shift to foraging behavior earlier in life. Since the clock for bee “aging” doesn’t start to tick until a bee begins foraging, such a shift to earlier foraging means that the colony population buildup rate (the slope in the earlier graphs) would be further suppressed due to decreased worker mean lifespan. Again, this would not be due to direct bee mortality due to infection, but from reduced overall lifespan as a result of premature foraging.
“Fragile” Bees
And how about those “fragile” bees that we keep hearing about, that no longer recover from pesticides or viral infections the way that they used to? Could nosema be involved? I’ve seen the spore count reports from a number of commercial operations. In light of what I’m learning about the effects of nosema upon colony buildup, it may not be surprising that their colonies don’t rebound as well as they used to! It may simply no longer be possible to run bees to back to back in pesticide-laden pollination contracts without helping the bees in some way to get ahead of nosema.
Bottom line: These insidious effects of Nosema ceranae may well be related to why today’s successful commercial beekeeper is forced to requeen two or three times annually, to feed more syrup, and to feed much more supplemental protein. Let me state emphatically that I’m not sure about this, but the pieces sure seem to fit together!
We still have much to learn about the effects of Nosema ceranae infection upon colony energy, protein, and immune dynamics. I commend U.S. researchers Chris Mayack, Dhruba Naug, Ann Gibbs, Michael Goblirsch, Zachary Huang, Marla Spivak, and Frank Eischen for their work on this avenue of research (my apologies to those I’ve left out). I especially wish to thank Mariano Higes’ team for putting together an overall picture of the possible impacts of infection, well-reviewed by Raquel Martín-Hernández (2011).
Perspective
I apologize to my readers that this article is lacking in direct practical applications, other than that I suggest that perhaps we should start paying a bit more attention to Nosema ceranae. I realize that the tone of this article may lead some to freak out about this parasite, but I wish to emphatically state that that is not what I had in mind!
Lest I overplay the consequences of the presence of N. ceranae, remember that I’ve successfully run my California operation for many years without using any medications against nosema, so I’m certainly not recommending that you blindly start dumping medications into your hives. This winter is the first time that I’ve used any treatments—and I only treated colonies that clearly had problems.
Not all scientific studies have found benefit to treatment. Traver (2011) reports that in Virginia “we observed very little impact of Nosema infections on either colony growth or productivity, suggesting that even though we found higher levels of infection, treatment is not necessary.”
I’ve personally watched colonies with mean forager spore counts in the 5M range build up explosively and put on good honey crops, provided that forage conditions were good. But keep in mind that when I measured those counts, I wasn’t yet determining nosema prevalence (the proportion of foragers infected), so I really don’t know just how badly those colonies were actually infected.
I know of large commercial operators whose colonies had sky-high spore counts in spring, yet went on to be extremely productive without treatment.
On the other hand, in the majority my strongest colonies this December, zero out of 10 sampled bees were infected by nosema, and in no case were more than 1 out of 10 infected. But nosema prevalence was typically higher in my weakest colonies.
Somehow my strongest colonies appear to be holding their own against nosema just fine without treatment. I plan to test again at the end of almond pollination to see how they are faring at that time, as last year spore counts were quite high in the bee net samples from my returning truckloads.
Please be clear that I don’t want to sound alarmist about N. ceranae, yet I feel a responsibility to my readers to keep them abreast with recent research. In this article I’ve been thinking aloud, and have yet to reach firm conclusions. However, the evidence continues to mount that N. ceranae may be more of a problem than it originally appeared. I will go into more detail in subsequent articles.
References
Antúnez, K., et al (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol 11(9):2284-90.
CDC (2012) http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5909a1.htm
Chen YP, et al. (2009) Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera. J Euk Microbiol 56: 142–147.
Crailsheim K (1986) Dependence of protein metabolism on age and season in the honeybee (Apis mellifica carnica Pollm). J Insect Physiol 32: 629-634.
Harris, JL (2008) Development of honey bee colonies initiated from package bees on the northern Great Plains of North America. Journal of Apicultural Research and Bee World 47(2): 141–150.
Harrison, JF and JH Fewell (2002) Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comparative Biochemistry and Physiology Part A 133: 323–333.
Heinrich, B (1979, 2004) Bumblebee Economics. Harvard University Press
Jacob, J, et al (2011) How much will my chickens eat? University of Kentucky Cooperative Extension (Broken Link!) http://www2.ca.uky.edu/afspoultry-files/pubs/How_much_will_my_chicken_eat.pdf
Kottler, DP and JM Orstein (1999) Clinical syndromes associated with microsporidosis. In Wittner and Weiss, eds. The Microsporidia and Microsporidiosis. ASM Press.
MacRae, JC (1993) Metabolic consequences of intestinal parasitism. Proceedings of the Nutrition Society 52: 121-130.
Martín-Hernández, R., et al (2009) Effect of temperature on the biotic potential of honeybee microsporidia. Applied and Environmental Microbiology 75(8): 2554–2557.
Martín-Hernández, R, et al (2011) Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol Res.109(3):605-12.
Mayack, C and D Naug (2009) Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invert. Path. 100(3): 185-188.
Munro, JA (1926) N. Dak. Beekeeper’s Assoc. News Letter 4(1); cited in Nolan (1932).
Nolan, WJ (1932) The development of package-bee colonies. USDA Technical Bulletin No. 309. Available online from Google Books.
Oliver, R (2010) https://scientificbeekeeping.com/the-primer-pheromones-and-managing-the-labor-pool-part-3/
Porrini, MP (2011) Nosema ceranae development in Apis mellifera: influence of diet and infective inoculum. Journal of Apicultural Research 50(1): 35-41.
Southwick, EE and D Pimentel (1981) Energy efficiency of honey production by bees. BioScience 31(10): 730-732.
Traver, BE, MR Williams, RD Fell (2012) Comparison of within hive sampling and seasonal activity of Nosema ceranae in honey bee colonies, Journal of Invertebrate Pathology 109(2): 187–193.
vanEngelsdorp, D, et al (2012) A national survey of managed honey bee 2010-11 winter colony losses in the USA: results from the Bee Informed Partnership. J. Apic.Res. 51(1):115 – 124.
White, GF (1919) Nosema-Disease. USDA Bulletin No. 780.
Winston, ML (1987) The Biology of the Honey Bee. Harvard Univ. Press
Woods, W.A., B Heinrich, RD Stevenson (2005) Honeybee flight metabolic rate: does it depend upon air temperature? Journal of Experimental Biology 208, 1161–1173.