Sick Bees – Part 3: The Bee Immune System
Sick Bees—Part 3
The Bee Immune System
Randy Oliver
ScientificBeekeeping.com
First published in American Bee Journal October 2010
In my previous article, I proposed a model for the in-hive positive feedback loops that may lead to colony collapse. Before I can further explain the model, we must understand more precisely how the bee immune system functions, as it appears to be that the mechanisms leading to the disappearance of bees are rooted in an immune response gone awry.
The Honey Bee Immune System
The definitive sign of colony collapse is the often sudden “disappearance” of the worker force. This disappearance appears be mainly due to an aspect of the bee colony-level immune response that normally helps to purge an infection, but that when conditions favor certain positive feedback loops, can result in excessive or complete depopulation of the hive. To better understand what happens, we need to have a firm grasp of how the bee immune system functions.
The soundbite version that we all heard when the honey bee genome was sequenced—that bees had “fewer immune genes than other insects”—was rather misleading. The honey bee colony is a complex super-organism, with physical, chemical, and behavioral defenses at various levels—at that of the individual cell, the individual bee, the full colony, and at the level of the local population. Bees have a robust and effective immune system—they just do things a bit differently than the solitary flies to which they were compared.
Behavioral Boundary Barriers
A honey bee hive is a densely crowded, moist and warm environment with many closely related individuals. In other words, the perfect setting for parasites to exploit. Since honey bees have survived for millions of years, it’s evident that they have figured out ways to keep the ever present pathogens at bay.
The first line of defense is to avoid a fight in the first place. Bees do so by not allowing parasites to gain a foothold—they are relentless in their scrubbing and scouring of the inner surfaces of the nest cavity and in the grooming of their body surfaces (watch how amazingly quickly bees clean up any “accidents” in an observation hive!). They also sterilize all surfaces with antimicrobial secretions in their saliva (such as glucose oxidase, which produces the strong disinfectant hydrogen peroxide), and by “stealing” components of the plant immune system by gathering the highly antimicrobial resins found at leaf buds and wounds, and bringing them back to the hive—at which point they are called “propolis.” Bees use propolis to form an antibiotic envelope around the colony—they put a heavy layer around the entrance, coat the inner surfaces of the cavity, seal all crevices with it, and coat the face of the combs.
A returning forager with a load of antimicrobial plant resins on her leg. When the resins are scraped off and applied to the inner surfaces of the hive, they are then called “propolis.” The varnish of propolis creates a waterproof line of defense against parasites and pests. Photos by the author.
The coating of propolis has been demonstrated to inhibit AFB (Antúnez 2008), fungi, and wax moth; Marla Spivak has demonstrated that propolis from some regions is effective against varroa, and is investigating its effect on viruses. Of great interest is the recent finding (Simone 2009) that the presence of propolis appears to decrease the necessary investment in immune function of bees—thus, the bee colony, by self medicating itself with antimicrobial chemicals from plants, incurs less of a metabolic cost in fighting pathogens! Perhaps we have been shooting ourselves in the foot by breeding for bees that don’t use much propolis!
Update March 2018: Of interest is the observation that of the two species of honey bees that nest in cavities, only one–Apis mellifera–collects propolis. As I understand it A. cerana doesn’t.
In order to help prevent the spread of brood diseases, each larva is isolated into its own scrupulously-cleaned cell, which it later lines with a layer of silk cocoon, which helps to protect the pupa from contaminants in the cell wall.
The primary manner by which a pathogen can enter a colony is by hitching a ride back on or within a forager, which could pick it up from a flower contaminated by the last visiting insect, from water, or especially by robbing out a sick hive (is that stolen honey really worth it?). So the colony has “rules” to minimize the transmission of such incoming pathogens to the most critical and vulnerable parts of the hive—the queen and her broodnest.
Certain requisite forager behaviors set up “checkpoints” that serve to keep from introducing parasites or toxins into the broodnest. Foragers bring a flood of nectar back to the hive, which creates a major potential avenue for pathogen introduction. So let’s take a look at how bees process that nectar.
First, foragers that become ill after tanking up on toxic nectar (or pesticides) simply don’t return to the hive. Those that do successfully return are still not allowed to directly deposit their nectar in the combs nor feed it to the brood—they must first pass it to a mid-aged receiver bee. Both the forager and the receiver use their proventriculus (meaning “in front of the stomach”) to remove pollen grains, dust, and infective spores from the nectar. This amazing structure consists of four fringed lips that “gulp” up and expel the nectar in the crop (or “honey sac”), filtering any particles out of it, kind of like a baleen whale filtering plankton from sea water. Some lines of bees are more efficient at filtering than others (Sturtevant 1953), which may thereby help to confer resistance to AFB and nosema upon their colony.
In addition to filtering out spores, bees secrete antimicrobial enzymes into the nectar. Not only that, but they also maintain cultures of two specific “friendly” lactic acid bacteria their crop (Forsgren 2010). These beneficial bacteria inhibit the growth of yeasts and disease-causing bacteria and fungi.
Pollen foragers, on the other hand, do directly deposit their loads at the periphery of the broodnest. Painstaking work by Martha Gilliam indicated that bees inoculate pollen loads with beneficial molds and bacteria that produce antibiotics that aid in the preservation of the beebread, and that are antagonistic to pathogenic organisms. The ARS Tucson Lab is currently following up on Gilliam’s groundbreaking research. To further isolate the brood from any pathogens in raw pollen, the beebread is then eaten and digested by nurse bees and converted into royal jelly (similar to the way in which a mammal converts raw food into milk), which contains antimicrobial fatty acids and peptides.
A forager covered by Scotch Broom pollen enters the broodnest to look for a cell in which to place her load. Pollen loads are a potential source of pathogens from other insects which may have previously visited the flower. Bees process the pollen first into beebread, then into antibiotic-rich jelly for feeding to nestmates. In this busy scene, two bees are engaging in trophallaxis at the lower right, to their left a pair are “antennating”—likely sharing pheromones. At the lower left, a worker has her head in a pollen cell; above her a varroa mite is hitching a ride on a bee’s thorax.
Hygienic Behavior
Should a larva sicken or die, it is removed by mid-aged undertaker bees (Arathi & Spivak 2001), which no longer engage in feeding of the brood. This disease resistance mechanism was first demonstrated by Park, and was termed “hygienic behavior” by Rothenbuhler (1956). Steve Taber (1982) promoted selectively breeding for it by using a “freeze killed” brood field assay. Marla Spivak has since picked up the ball, and has encouraged a number of commercial queen producers to start selecting for this desirable trait.
There are apparently at least seven genes involved in hygienic behavior (Lapidge 2002); the critical component appears to be the ability to detect the odor of sick brood, so that it can be removed before it becomes infective (Wilson-Rich & Spivak 2009). Because of this time constraint, the authors suggest that bees be selected for what they term “rapid-hygienic” behavior. Note that such removal of dying brood can result in an irregular pattern of uneven-aged brood (“shotgun pattern” or “spotty brood”), and may be an indication of an otherwise invisible virus infection.
Evans (2006) puts the effectiveness of colony cleanliness into perspective:
A testament to this hygiene is the fact that, even when facing severe colony-level infections by bacterial pathogens such as [AFB] (for which < 10 spores are normally fatal to young larvae)…, the vast majority of larvae show no signs of exposure.
Other Behaviors
Several bee pathogens are sensitive to temperature, and the individual bee or the colony may create a “fever” to kill nosema (Martín-Hernández 2009), chalkbrood (Starks 2000), or even mites (Currie 2008). As indicated in my model for colony collapse, chilling and the lack of thermoregulation may play a major role in the multiplication of pathogens and the bee immune response to them.
Finally, bees may even sacrifice themselves or abandon their hives in order to try to get the upper hand against parasites. The natural host of varroa, Apis cerana, simply absconds should the mite population build up (Anon 1990), thereby leaving many mites behind in the brood. Both the Savannah bee (A.m. scutellata) and the Africanized honey bee are also noted for their swarming and absconding behavior. European honeybees rarely abscond, but in my experience it appears that colonies heavily parasitized by varroa or N. ceranae may swarm more readily. This may be a behavior to try to get away from parasite buildup–swarms seem to build up exceptionally well on new, fresh, pathogen-free combs.
Colonies may also “blame the queen” should they get sick, and attempt to supersede her—leaving behind a new queen with a potentially more successful mix of genes. The downside to this behavior is that the remaining colony in the hive is dependent upon a newly-emerging queen successfully mating and taking over egglaying duties. Unfortunately, in such a sick colony, there is a substantial chance that the new queen may emerge already infected (say with DWV or Black Queen Cell Virus, which is strongly associated with nosema infection). Or, the supersedure queen may simply not be able to find enough healthy drones for proper mating. In either case, the end result would be a queenless colony. The increased viral loads since the arrival of varroa (along with the associated miticide residues) may help to explain the greater degree of queenlessness that we observe in our hives compared to twenty years ago.
Colonies in temperate climates normally undergo a massive loss of older cohorts of bees in both early spring and fall. Note that these are the same periods of time in which nosema builds up. It may be that the colony uses these “cleansings” of old, infected bees to flush parasite epidemics prior to summer buildup or overwintering. I will return later to the individual behavior of sick bees removing themselves from the hive.
Mechanical and Physiological Barriers
The bees’ first line of defense is simply to avoid, exclude, remove, or kill the bad guys before they can actually infect a bee. The bee is well protected from most pathogens by its strong, waterproof cuticle. As long as this biologically-active armor remains unbroken, viruses and bacteria are generally held at bay (I will revisit the significance of the breaching of this armor by the varroa mite). Even the trachea (breathing tubes) are lined with (a water permeable) cuticle. Should the cuticle be injured, the exposed hemolymph (the fluid filling the insect body cavity functions as both “blood” and intracellular lymph, but does not carry oxygen) quickly clots in a manner similar to that of mammals. Immune cells (hemocytes) engulf any foreign invaders at the wound site; then a chemical cascade initiating with the enzyme phenoloxidase melanizes the clot into an inert and impermeable barrier. Note that rough handling of bees breaks off their body “hairs” and exposes the haemolymph temporarily—this brief breach may allow viruses to enter the bee’s body.
The weakest chink in the bees’ armor is the gut, the place where the inside of the bee meets the myriad potential pathogens from the outside world. The gut must juggle being a barrier to invading parasites of all sorts, yet still afford permeability for the digestion of nutrients. The foregut (that portion from the mouth to the stomach) is lined with a cuticle that can slough off should pathogenic bacteria (as opposed to beneficial bacteria) attach to it. I’ve already described the action of the proventriculus, which would then filter such sloughed lining into the midgut.
Insects protect the midgut with a neat trick—they line it with a protective chitinous inner sleeve called the peritrophic membrane, which helps to protect the delicate midgut epithelium from both spiky pollen grains as well as potential invaders (nosema spores, when they germinate, must harpoon their polar bodies through the peritrophic membrane and spear an epithelial cell in order to infect the bee). The gut lumen is chemically hostile to pathogens—it is mildly acidic, and the epithelial cells produce digestive enzymes and defensive peptides and binding proteins. Nevertheless, the gut still appears to be the main avenue through which AFB, EFB, chalkbrood, and viruses infect the bee.
Individual Systemic Immune Response
The insect “innate” immune system is basically similar to that of humans (Kavanagh 2007) although insects do not exhibit as complex an “adaptive” or “acquired” immunity” (such as our formation of antibodies specific to new pathogens). Since some of the terminology may not be familiar to the reader, I will diagram it below.
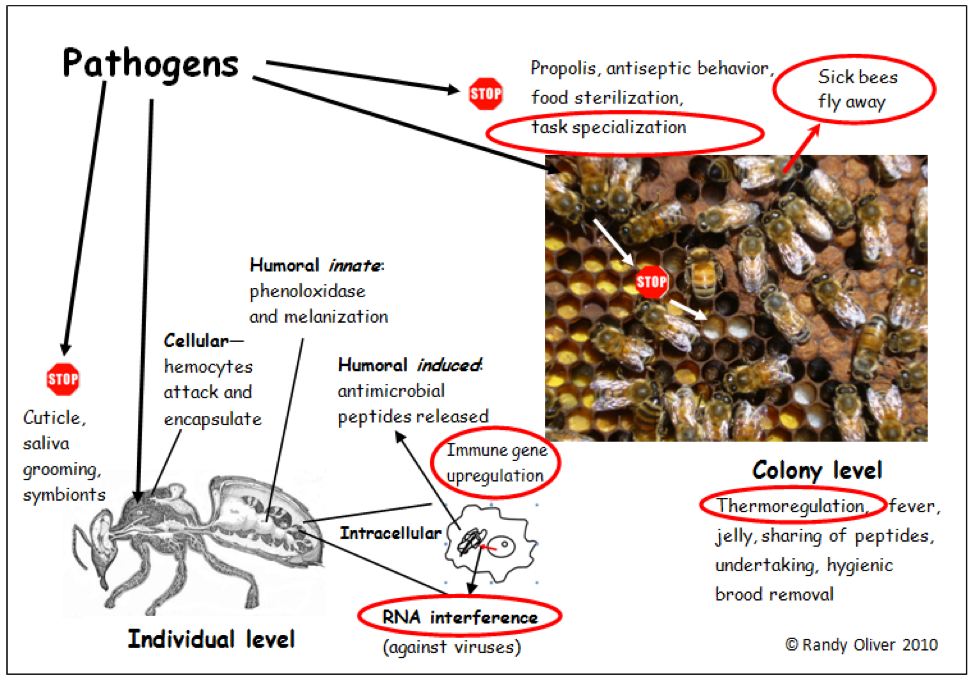
A simplified schematic diagram of the honey bee immune system. The various components work together against the wide variety of pathogens that bees encounter. The “stop” signs indicate barriers to pathogen introduction. The functions circled in red are involved in my model of colony collapse. Bee cross section from Snodgrass 1910.
The first order of individual immune response is to recognize “self” from “foreign.” The bee immune system recognizes invaders by keying in on specific proteins present on each type of pathogen (i.e., the surface proteins of bacteria are different than those of say, fungi). Bees have “constitutive” or “innate” defenses that are always present and at the ready, such as roaming hemocyte cells and enzymes in the hemolymph. The hemocytes recognize invaders and signal the enzyme phenoloxidase to start a cascade of chemical response, generally ending in either the engulfing or encapsulating of the invader, killing it with a barrage of powerful chemicals (e.g., quinines, phenols, and reactive oxygen species). The hemocytes involved may them apoptose (kill themselves) and be coated with dark melanin polymers (you can see these dark areas in white dead pupae being removed by bees).
This innate defense system works fast—Haine (2008) found that the mealworm beetle can nearly clear its system of an injection of 4 million bacteria in about 30 minutes! During this quick response (pay attention here, as we are getting to a potentially important aspect of colony collapse), the hemocytes release chemicals that penetrate the cell nuclei, and cause them to upregulate certain immune response genes, which then transcribe RNA messengers to exit the nucleus, and then move to the ribosomes to translate the genetic instructions into antimicrobial peptides. This is called the “induced” response, and takes at least 1-3 hours to get going (after 99.5% of the bacteria have already been killed), and 12-48 hours to reach peak levels. The induced response can last for weeks, and it appears that these peptides can be passed to nestmates to confer them resistance prior to being infected (something that solitary insects can’t do).
Note that the antimicrobial peptides are produced largely in the fat bodies—so there would be less of this sort of response in forager bees, which don’t maintain their fat bodies. This makes sense, since foragers aren’t expected to live for long. However, keep in mind that the bees in protein-hungry colonies are unable to develop their fat bodies fully—this one point where nutrition ties in to immunity.
Surprisingly, Jay Evans found that these genes are not upregulated in bees from CCD colonies, even though the bees are full of pathogens! There are a few potential explanations for this finding that come to mind:
- The bee hemocytes are not recognizing the pathogens as foreign (suppression of recognition systems, perhaps by viruses?).
- The colonies could be protein-starved.
- Something is suppressing the transcription of the genes, or their translation to peptides. Note that viruses can do this very thing, which I feel may be a big clue!
Haine and her collaborators suggest something very interesting:
Our experiments showed that those bacteria that survived exposure to the insect’s constitutive immune response were subsequently more resistant to it. These results imply that induced antimicrobial compounds function primarily to protect the insect against the bacteria that persist within their body, rather than to clear microbial infections.
Two arguments favor our idea that long-lasting antimicrobial activity has evolved as part of a two-stage process, preventing resistance evolution in bacteria and/or managing persistent infections. First, bacteria readily evolve resistance against individual antimicrobial peptides in isolation, and recent work [Pham 2007] suggests that phagocytic haemocytes are responsible for the immune reaction against secondary infections in insects.
This finding suggests two thoughts relevant to bee health—(1) that the lack of antimicrobial gene upregulation in CCD hives would make the bees more vulnerable to secondary pathogens (I’ll get back to this later), and (2) any bee ever bitten by a mite will need to expend energy for the rest of its life to maintain the upregulated peptides.
As bees “age,” they shift their immune response tactics from hemocytic (cellular) response more toward phenoloxidase-based immunity (Schmid 2008). The implications of this finding are not completely clear (e.g., why do bees allocate the job of cell cleaning to newly-emerged bees?) This again ties in to the best use of resources—an aged bee has little reason to ramp up a long-term metabolically-expensive immune response. However, this finding may have implications in collapsing colonies, in which younger and younger bees must take the place of foragers. Wilson-Rich (2009) make an interesting observation:
Because nurse bees are not as immunologically competent as foraging bees, they are not as well equipped to combat the increased pathogen exposure that older foragers encounter. In the absence of foragers, younger nurse bees prematurely transition to precocious foragers…This behavioral shift is likely to negatively impact colony fitness, regardless of the pathogen pressure….In this light, foraging bees may play a similar role as vaccinated individuals in a population by providing a type of herd immunity [via trophallaxis?], and in their absence the disease resistance capacity of the group is likely compromised.
This “herd immunity” concept is interesting, as Traniello (2002) found that termites (which live in a social structure similar to that of bees) appear to be able to “prime” other nestmates’ immune response to pathogens. This avenue of immunity is still speculative for bees, but I’m guessing that they still have a lot of tricks up their sleeves that we have yet to discover! O.K., I know–more than you wanted to know about the bee immune system. But the mystery of colony collapse starts to fade the more one understands bee immunity. I’m going to break here, but we’ll get into some real meat in the next article.
Update 28 January 2013
A study by Bull (2012), in which newly-emerged or forager bees were experimentally infected by a pathogenic fungus, found that young bees were more susceptible to infection than the older bees. Of interest is that the older bees did not appear to upregulate their immune response to the degree that the young bees did, suggesting that they are innately more resistant to allowing an infection to get started.
Bull JC, Ryabov EV, Prince G, Mead A, Zhang C, et al. (2012) A Strong Immune Response in Young Adult Honeybees Masks Their Increased Susceptibility to Infection Compared to Older Bees. PLoS Pathog 8(12): e1003083. doi:10.1371/journal.ppat.1003083
References
I am deeply indebted to the authors of the following reviews, which I recommend for further reading:
Evans, JD, & M Spivak (2009) Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. doi:10.1016/j.jip.2009.06.019 Free download
Evans, JD, et al (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Molecular Biology 15(5): 645–656.
Siva-Jothy, MT, Y Moret,and J Rolff (2005) Insect Immunity: An Evolutionary Ecology Perspective. ADVANCES IN INSECT PHYSIOLOGY VOL. 32: 1-47. Free download, google the title
Wilson-Rich, N, M Spivak, NH Fefferman, and PT Starks (2009) Genetic, Individual, and Group Facilitation of Disease Resistance in Insect Societies. Annu. Rev. Entomol. 54:405–23. Free http://www.extension.umn.edu/honeybees/components/pdfs/annurev.ento.53.103106.093301.pdf
Schmidt-Hemple, P (1998) Parasites of Social Insects. Princeton Univ. Press
Citations
Anon (1990) Beekeeping with oriental honeybees (Apis cerana). FAO Agricultural Services Bulletin 68/4
Antúnez, K., Harriet, J., Gende, L., Maggi, M., Eguaras, M., Zunino, P., 2008. Efficacy of natural propolis extract in the control of American foulbrood. Veterinary Microbiology 131, 324–331.
Arathi, HS, and M Spivak (2001) Influence of colony genotypic composition on the performance of hygienic behaviour in the honeybee, Apis mellifera L. Animal Behaviour 62: 57-66.
Berthoud, H, A Imdorf, M Haueter and J-D Charrière (2006) Colony mortality and bee viruses. Proceedings of the Second European Conference of Apidology EurBee Prague (Czech Republic) 10-16 September 2006
Chen, YP and R Siede (2007) Honey Bee Viruses. Advances in Virus Research 70: 33-80.
Currie, R (2008) Summary of Take Home Messages- CHC – AGM January 2008, Canadian Honey Council.
Evans, J.D., Armstrong, T.-N., 2006. Antagonistic interactions between honey bee bacterial symbionts and implications for disease. BMC Ecology 6.
Evans, JD and JS Pettis (2005) Colony-level impacts of immune responsiveness in honey bees, Apis mellifera. Evolution 59(10):2270-2274.
Genersch E, Ashiralieva A, Fries I (2005) Strain- and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing American foulbrood disease in honeybees. Appl Environ Microbiol. 71(11):7551-5.
Gilliam, M (1997) Identification and roles of non-pathogenic microflora associated with honey bees. FEMS Microbiology Letters 155: 1-10.
Haine, ER, Y Moret, MT Siva-Jothy, J Rolff (2008) Antimicrobial defense and persistent infection in insects. Science 322: 1257-1259.
Kavanagh, K and EP Reeves (2007) Insect and Mammalian Innate Immune Responses Are Much Alike. Microbe 2(12): 596-599.
Kralj, J, and S Fuchs (2009) Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 41(1): 21-28.
(Broken Link!) Lapidge, KL BP Oldroyd, M Spivak (2002) Seven suggestive quantitative trait loci influence hygienic behavior of honey bees. Naturwissenschaften 89(12):565-8.
Martin, SJ, BV Ball,and NL Carreck (2010) Prevalence and persistence of deformed wing virus (DWV) in untreated or acaricide-treated Varroa destructor infested honey bee (Apis mellifera) colonies. Journal of Apicultural Research 49(1): 72-79.
Martín-Hernández, R, et al. (2009) Effect of Temperature on the Biotic Potential of Honeybee Microsporidia. Appl Environ Microbiol. 75(8): 2554–2557.
Nachbaur, A (1996) SAD & BAD Bees. http://www.beesource.com/point-of-view/andy-nachbaur/sad-bad-bees/
Naug, D, and S Camazine (2002) The role of colony organization on pathogen transmission in social insects. Journal of Theoretical Biology 215: 427–439.
Park, O., Pellet, F., Paddock, F., 1937. Disease resistance and American foulbrood. Journal of Economic Entomology 30, 504–512.
Pham LN, MS Dionne, M Shirasu-Hiza, DS Schneider (2007) A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007 Mar;3(3):e26.
Pruijssers, A and MR Strand (2006) PTP-H2 and PTP-H3 from Microplitis demolitor Bracovirus Localize to Focal Adhesions and are Anti-Phagocytic in Insect Immune Cells. J. Virol. doi:10.1128/JVI.02189-06
Richard, F-J, A Aubert, and CM Grozinger (2008) Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biology 6:50.
Rothenbuhler, W.C., Thompson, V.C., 1956. Resistance to American foulbrood in honey bees: I. Differential survival of larvae of different genetic lines. Journal of Economic Entomology 49, 470–475.
Schmid MR, Brockmann A, Pirk CW, Stanley DW, Tautz J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J Insect Physiol. 2008;54:439–444.
Simone, M., J Evans, M Spivak (2009) Resin collection and social immunity in honey bees. Evolution 63-11: 3016–3022.
Snodgrass, RE (1910) The Anatomy of the honey bee. US Government Printing Office.
Starks, PT, CA Blackie, TD Seeley, (2000) Fever in honeybee colonies. Naturwissenschaften 87, 229–231.
Sturtevant AP & IL Revell (1953) Reduction of Bacillus larvae spores in liquid food of honey bees by action of the honey stopper, and its relation to the development of American foulbrood, J. Econ. Entomol. 46, 855–860.
Taber S, (1982) Breeding for disease resistance. American Bee Journal 122(3):177-179.
Traniello J, Rosengaus R, Savoie K. 2002. The development of immunity in a social insect: evidence for the group facilitation of disease. Proc. Natl. Acad. Sci. USA 99(10):6838–42.
Wilson-Rich, N., ST Dres, PT Starks (2008). The ontogeny of immunity: development of innate immune strength in the honey bee (Apis mellifera). Journal of Insect Physiology 54: 1392–1399.