Sick Bees – Part 5: Multiple Infections
Sick Bees
Part 5
Multiple Infections
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in January 2011
Table of Contents
I ended the last installment of this series by asking the question, what happens when there are multiple parasites suppressing the bee immune system simultaneously? In this article I’d like to briefly discuss the complex interactions involved when bees are infected by more than one pathogen.
I have previously cited research by Diana Cox-Foster, Dennis vanEnglesdorp, Jay Evans, Jerry Bromenshenk, and other CCD researchers indicating that in collapsing colonies the bees were often being ravaged by infections of multiple pathogens (especially nosema and viruses). The question then is, whether it was the co-infection that initiated the collapse, or whether suppression of the bee immune response by environmental causes was allowing the explosive growth of opportunistic parasites to the point that the colony was simply overwhelmed?
Colonies of bees are invariably infected by several parasites at any time, but their normally robust colony- and individual-level immune systems generally either clear or suppress the infections (other than that of varroa) provided that environmental conditions are favorable. In the case of colony collapse, that normally effective immune function is clearly faltering. It appears to me then, that the key issue is to try to figure out just why the colony immune systems are failing.
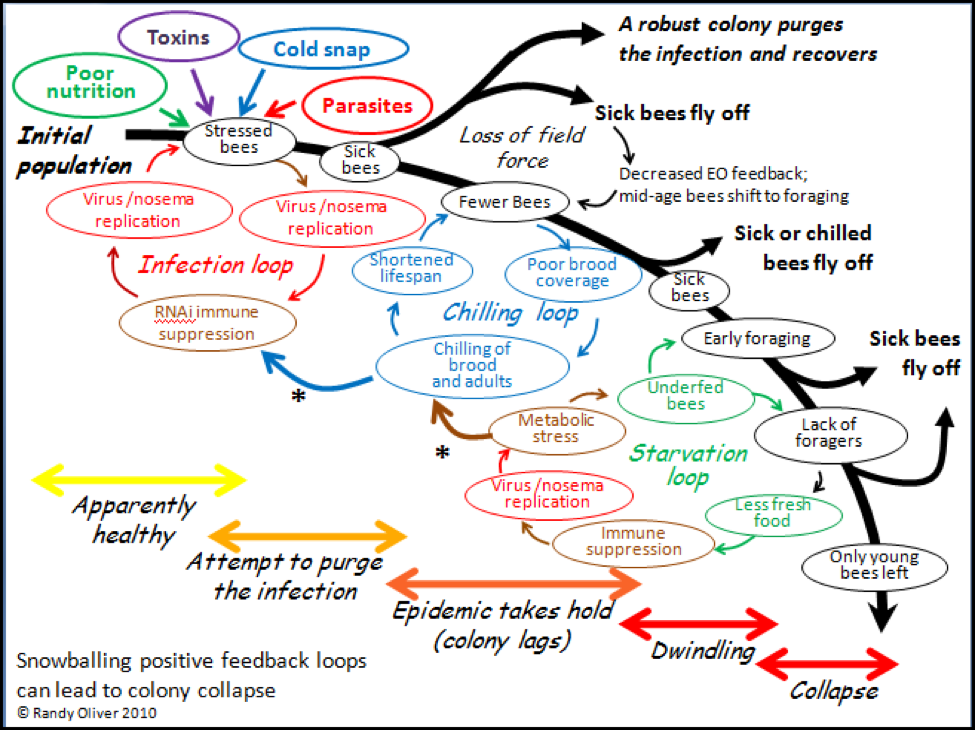
My proposed model (Fig. 5) suggests that it is due to positive feedback loops engendered by a combination of co-infection and environmental stresses (generally chilling and poor nutrition). It may not be so important as to which particular pathogens are involved, but rather that at some point the balance is tipped from the colony being able to purge the pathogens by altruistic self removal of infected workers, to a situation in which thermal and nutritional stress caused by infection eventually (or suddenly) leads to hive depopulation due to the exodus of sickened bees.
Since different CCD studies have found co-infections by different combinations of pathogens, my feeling is that colony collapse is a generic phenomenon, rather than something due to a specific culprit. It may be that we are looking at a “Which came first, the chicken or the egg?” phenomenon—the multiple pathogen infection or the immune suppression that led to it? To me, it appears to be a moot point; what is important is that once a co-infection starts, it may then become self perpetuating despite the return of favorable environmental conditions (as happened in my Beeologics trial last May, when colonies continued to collapse despite enjoying an excellent nectar and pollen flow, albeit punctuated by weekly chilling storms).
The Problem of Co-infection
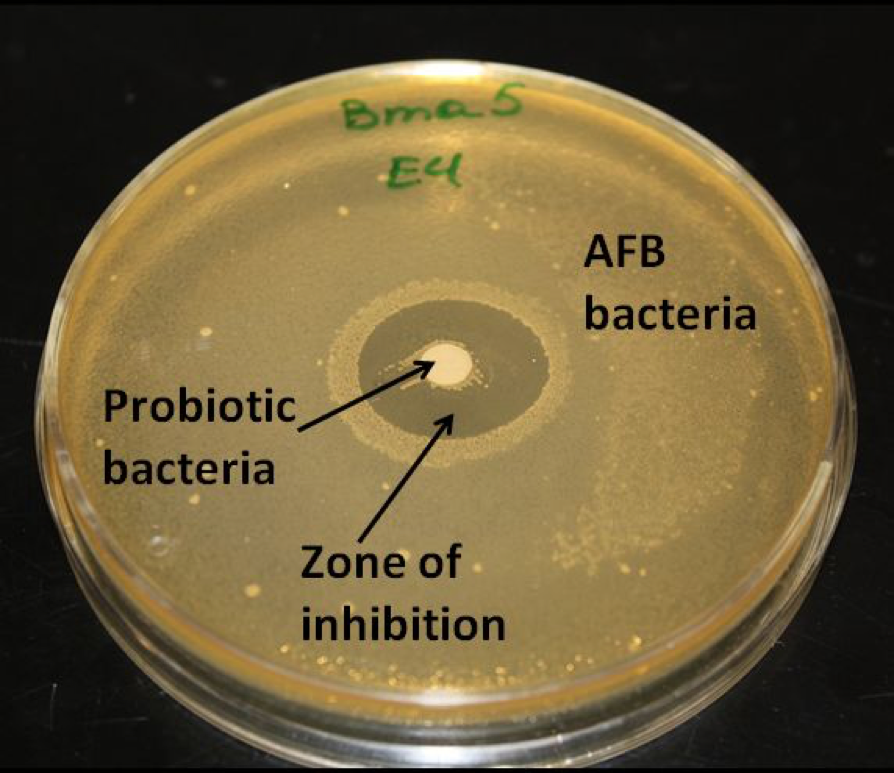
Every single honey bee is co-infected by more than one species of bacteria, generally more than one species of virus, and often nosema, fungi, “amoeba”, and trypanosomes as well. But they normally don’t get noticeably sick from such infections! In the first place, some of the bacteria are highly beneficial endosymbionts, without which bees would be vulnerable to yeasts, AFB, EFB, and Chalkbrood (Fig. 1)(Reynaldi 2004; Nayudu 2009; Forsgren 2010).
Figure 1. The beneficial bee gut bacteria suppress the growth of pathogenic bacteria and yeasts. In this petri dish, Dr. Eva Forsgren inoculated the agar with AFB bacteria, and placed a disc of beneficial bacteria in the center. The growth of AFB bacteria was inhibited by the presence of the beneficial bacteria. Photo courtesy Dr. Eva Forsgren.
There is a pitched and dynamic battle going on every second in the bee gut between parasites that would exploit the bee for their own advantage, and endosymbionts that protect the bee for their own, or mutual, benefit. The bee’s immune system is faced with the difficult task of recognizing (and cooperating with) the beneficials, while still fighting the bad guys; and the bad guys don’t play fair! The immune system is actually forced to act more akin to a bouncer at a club, rather than as an indiscriminant defense force which attempts to kill all foreigners (Travis 2009).
At this point I should define some terms (Table 1):
| Table 1. Definitions of Parasite Relationships | |
| Parasite | Any organism that lives on or in a host, and obtains nourishment from the host is called a “parasite.” |
| Parasitism | A relationship wherein the parasite benefits at the expense of the host. Most bee viruses and nosema fit this bill. |
| Pathogen | A parasite that causes disease in the host. Many normally “benign” bee parasites have the potential of acting as pathogens, especially if the bees are stressed. |
| Endosymbiont | An organism that lives inside another (endo = within; sym = together; biosis = living). Generally used for those exhibiting mutualism. |
| Mutualism | A relationship in which both the host and parasite benefit (such as the Lactobacillus bacteria which suppress yeasts and foulbrood; or gut bacteria that produce critical nutrients for the host). |
| Commensalism | A relationship wherein the parasite neither benefits nor harms the host (e.g., many gut bacteria). |
| Semelparous parasite | A parasite that is an “obligate killer.” I.e., the parasite needs to kill the host to complete its life cycle and to transmit to another host. AFB, chalkbrood, and (often) varroa and Sacbrood act as semelparous parasites. Semelparous parasites are generally easy to diagnose by the presence of dead brood or colony collapse. |
| Virulence | In this discussion, “virulence” is the capacity to cause disease. An “avirulent” parasite doesn’t normally cause disease. |
A very important point in the above table is that most bee parasites are not semelparous at the colony level, and do not benefit from actually killing a colony (AFB and varroa are notable exceptions). Several common bee parasites are semelparous at the individual bee level—e.g., chalkbrood must kill the pupa in order to sporulate. I can’t think of any example, however, of any bee parasite that benefits by actually killing adult bees. Neither nosema nor bee viruses are necessarily semelparous—they do not benefit by killing either an individual bee nor the colony. In fact, it is generally to their advantage for the bee and colony to remain healthy so that they can continue to produce and transmit spores and virions!
There is a fine line between a parasite acting as a pathogen, or being considered as a beneficial symbiont (reviewed by Rigaud 2010). The symbionts run something akin to a “protection racket,” in which they demand a payment to protect the host from other parasites. The payment is generally in the form of some nutrient provided by the host (in the case of bees, sugar is likely the preferred payment, but it could be in the form of a protein or some limited nutrient). To the bee, a payment of a tiny bit of sugar and protein in return for protection from AFB, EFB, and Chalkbrood is a pretty good deal! A promising avenue of research would be to investigate just how the beneficial gut bacteria derive the necessary protein in their diet—do they extract it from the vitellogenin-rich jelly fed to all other bees by the nurse bees? And if so, what happens to those bacteria when the flow of jelly is diminished in a nutritionally-stressed colony?
The endosymbiotic bacteria of bees likely form “biofilms” in the gut and other surfaces which assist the bees in avoiding infection by more virulent bacteria, fungi, and likely viruses. Recent studies have found that the bacteria in such films actually communicate with the host and each other, and can cooperate by coordinating the release of proteins (Kleerebezum 1997, West 2007). Although bacteria have not been implicated in CCD, they are fairly well studied, and exhibit good examples of the kinds of unseen skirmishes that are going on within a bee’s gut.
The Battle of the Brood Pathogens
The complexity of the battles raging within the bee gut becomes mind-boggling! Take, for instance, the wrestling match between the beneficial endosymbiotic bacteria, the virulent bacteria that cause AFB and EFB, and the fungus that causes chalkbrood. Friendly bacteria such as Bacillus subtilis, Lactobacillus and Bifidobacterium inhibit the growth of the pathogenic organisms by secreting antibacterial and antifungal substances (Reynaldi 2004; Forsgren 2010).
Should the causative agent of EFB (Melissococcus pluton) manage to infect a larva, it appears to open the door for co-infection by at least five species of opportunistic bacteria commonly associated with the disease (Alippi 1999). However, EFB and AFB are never found in the same larva—AFB bacteria secrete a substance that suppresses the growth of EFB and a whole slew of other bacteria (Holst 1946, Shimanuki 1992).
And it’s even more complex than that! The causative agent of AFB is a particular subspecies of Paenibacillus larvae; there are many different strains of that subspecies, which then vary at least tenfold in their virulence (Genersch 2005). To add to the confusion, the strains of Paenibacillus differ in their susceptibility to different phage (bacteria-killing) viruses, and duke it out by infecting one another!
The Chalkbrood fungus muscles in by producing linoleic acid, which has been shown to inhibit both AFB and EFB bacteria (Feldlaufer 1993). So the hierarchy of bee brood diseases appears to be: chalkbrood fungus weighing in as the toughest, followed by AFB, with EFB getting the bronze. Not that any beekeeper is rooting for Chalkbrood, but sometimes an enemy of your enemy might be an ally!
One aspect of the beneficial gut endosymbionts that needs much more study is the effect of antibiotics on the tenuous balance between the good vs. the bad bacteria. Of particular concern is the long-term effect of the very persistent antibiotic tylosin, which when (illegally) fed in syrup, has an expected active effect upon bacteria in the hive for at least six months! I’m eager to see what the ARS Tucson lab discovers as they research this topic.
Practical application: beekeepers often ask, how can I tell when I should rotate out an older brood comb? Simple answers such as “by age” or “by color” do not directly take into account the actual reason for comb rotation—the removal of combs contaminated with pathogen spores, environmental toxins, or miticide residues. As such, combs from colonies exhibiting good health and/or minimal exposure to miticides should be “safe” to use for more years than those with obvious contamination.
One answer: look at the brood in that comb. If it is healthy and evenly aged as in Figure 2, that would indicate that nothing in the comb is causing harm to the larvae (although it could still carry nosema spores, which affect only adults). On the other hand, if the brood age pattern is uneven (as in Figure 3), then you can suspect that the comb should either be disinfected or discarded.
Figure 2. In this healthy frame of young brood you can see the solid, even age progression of larvae from the center out. There are few empty cells or larvae of the “wrong” age that might indicate that larval death had occurred.
Figure 3. In this broodnest, nearly every cell contains an apparently healthy larva, but note that the larval ages are scattered, rather than in an even progression. Something has likely been killing the larvae. The hygienic mid-aged workers then removed them, and the queen returned to fill in the empty cells. At first glance, one would see a frame of solid brood, but much of that brood is apparently dying young, although no sick larvae are to be seen! This phenomenon is likely a sign of excessive levels of miticides, bacterial or virus infection, or of the poisoning of larvae by fungicides, insecticides, or heavy metals.
I was recently demonstrating a midsummer hive inspection to a group of beekeepers, and pulled out several solid frames of brood from a strong colony. Each time, the group would say “ah, nice frame of brood.” But then I would point out that each frame contained a few pupae that were dying from what appeared to be a virus, and that there were bees with shriveled wings on the combs, indicating to me that that the strong, apparently thriving hive (with a moderate mite level) was actually on the edge of succumbing to a DWV epidemic!
Again, let me repeat that there is no particular evidence implicating bacteria in CCD. However, the case of EFB cited above gives pause for thought–even though M. pluton bacteria initiate the disease, it is generally other bacteria that take over (oddly, the “corn-yellow” brood that we’ve been noticing of late contain nearly pure culture of M. pluton, and in my experience, when they are evident, colonies simply do not grow). This leads us to the subject of what occurs when one or more pathogens take advantage of an infection by another pathogen.
When a bee is infected by more than one parasites things can get complicated: “direct competition between species or interactions through the host immune system may either increase or decrease the immediate virulence and transmission of one of the interacting parasites” (Rigoud 2010). In his recent review, Rigoud gives examples of stepwise infection by various parasites, and points out that there may be great differences in outcome dependent upon the specific strains involved, and the effects of environmental factors (which in the case of the bee would include the sublethal effects of miticides and other pesticides).
Pathogens may compete against each other, or more commonly, share the benefits of each other’s immune suppression. An infection by a combination of two relatively benign pathogens whose immune suppression mechanisms happen to synergize, can result in virulent disease, or an opening for other opportunistic pathogens to exploit. Oddly, though, infection by Acute Bee Paralysis Virus or varroa does not appear make larvae more susceptible to AFB (Brødsgaard 2000)!
I’ll allow three European giants in insect immunity to speak for themselves (Siva-Jothy, Moret and Rolff 2005):
“Given the omnipresence of pathogens and parasites in the natural world, the most likely scenario is that concomitant infections are prevalent. A recent study…shows that avirulent [normally not harmful] microorganisms out-compete virulent parasites in simultaneous infections once the virulent parasite breaks down the host’s immune defence. Therefore, the ‘mix’ of the pathogen cocktail will be crucial to the infection (and host response) outcome. This observation poses considerable challenges for studies of insect immunity. How are concomitant infections dealt with by the host? Can resources for defence (e.g. essential amino acids), be depleted during these complex insults?”
The Cost of Immunity
Have you ever gotten a flu shot or other inoculation? Do you remember how sore your arm got, and how you felt a little under the weather the next day? The vaccine did not contain live pathogens—it simply upregulated your immune response. But by doing so your body paid a price—the cost of immunity. When your system is fighting an infection, much of what makes you feel “sick” is the effect of the cascade of your own immune response against the invading microorganism. Well, bees are no different. A bee carrying an infection is not the bee that it could be if it were uninfected. The bee must redirect resources toward fighting the infection (Laurenco 2009), resulting in depletion of its critical supply of the storage protein vitellogenin. Other immune proteins may result in “autoreactive self harm” to the bee (Sadd 2006). The immune response is energetically costly, and sick bees require more food and warmth (Moret 2000). This is a major point when we remember how critical thermoregulation is in maintaining colony health! I’ll return to this point soon when I discuss co-infection by nosema.
Of course, the major cost of infection to a colony is that bees which feel sick (Ruppell 2010) fly or crawl (in the case of nosema or DWV) off to die. The effect of the loss of these bees can rapidly change the size and organization of the colony population, resulting in chilling and poor nutrition (Fig. 5).
But there may be yet another mechanism at play. It has long been noted that bees will “pick” at nestmates infected with Chronic Paralysis Virus, resulting in the removal of their body hair, leading to the symptom of “hairless black disease.” But one must then ask the question, just how do the other bees determine that the infected bee is sick? Recent research suggests that bees may express an “I’m sick” pheromone in the form of a family of proteins called “pherokines” Dostert (2008). I’m very curious as to whether such chemical signals may help to explain the odd behavior of bees in collapsing colonies (Fig. 4), and the hygienic removal of sick or varroa-infested pupae (Salvy 2001).
 Figure 4. The broodnest of a colony in mid collapse in May. Note that the bees are not clustered normally over the brood, as was typical in the sick hives. Could this be due to chemical immune signals that disrupt normal behavior?
Figure 4. The broodnest of a colony in mid collapse in May. Note that the bees are not clustered normally over the brood, as was typical in the sick hives. Could this be due to chemical immune signals that disrupt normal behavior?
Opportunistic Pathogens
When a parasite gains a foothold in the bee, perhaps by taking advantage of some sort of environmental stressor, that initial infection may then open the door for other pathogens that normally aren’t virulent. I already mentioned that this occurs with EFB, which initiates the disease but may then be outcompeted by other bacteria.
An example of the above was illustrated by an experiment by Hughes and Boomsma (2004). They infected ants simultaneously with both a virulent and an avirulent (opportunistic) fungus. To their surprise, the normally harmless fungus took advantage of the immune suppression begotten by the virulent fungus, and then outcompeted it, eventually becoming the dominant infection which finally killed the ant! This sort of co-infection, in which a second parasite strain or species infects “on top of” another parasite and then supplants it is called a “superinfection” (Rigoud 2010).
The authors concluded: “Our results demonstrate that the outcome of within-host competition between parasites may not necessarily be as would be predicted from the virulence of parasites infecting in isolation, and that avirulent parasites may play a far greater role than is generally recognized.”
So what does this have to do with bees? Well, most bee pathogens (other than varroa) may well be considered to be relatively harmless at the colony level, so long as the colony is not “stressed.” But once the colony is stressed, then even a relatively harmless parasite can cause serious disease, or even colony collapse! In the end, the pathogen with the highest prevalence may have dealt the final blow, but it was actually the stress event or initial infection that started the snowballing downhill slide (Fig. 5).
Figure 5. A hypothetical model for the mechanics of colony collapse. Note the interrelationships of chilling, poor nutrition, toxins, and infection. Please refer to the September ABJ for more explanation.
Practical application: There is one critical concept to keep in mind with bee diseases–that so long as the colony is able to maintain productive broodrearing, the negative effects of a parasite infection may be largely overlooked by the beekeeper. Sick bees self purge themselves from the hive—generally disappearing unnoticed. Infections may not become noticeable except during pollen dearths, or in late summer when the colony shuts down broodrearing and there are no longer as many fresh recruits to take the places of the lost sick workers.
So what are the factors that make the bee (at the individual or colony level) susceptible to pathogens? And why do normally benign parasite infections suddenly become virulent and cause colony morbidity (symptoms and disease)? The generic term thrown about would be “stress.” More specifically, the major stressors would be:
1. Cold, especially unexpected chilling due to sudden weather change or lack of stores for metabolic heat production. Here’s an interesting observation: bees taken from hives in winter and placed in an incubator are more susceptible to Israeli Acute Paralysis Virus than are bees taken from the same hives in summer (Nitzan Paldi, pers comm).
Practical application: winter bees in sunny, protected locations (or in sheds at constant temperature). Make sure that they have plenty of honey stores.
2. Poor nutrition; lack of honey stores is obvious; however, often unrecognized are the depressed vitellogenin/protein levels in a malnourished colony, often due to a lack of sufficient high-quality pollen intake. I’ve previously covered this topic in some detail.
Practical tip: A buddy asked me this fall how my bees coming back from Nevada looked. I said that they were really light in weight, and that I was throwing the feed to ‘em. He then said, yes, but how about the bees—were the bees OK? The short answer is that even if the hives were full of bees, a colony lacking in abundant stores in late summer/fall is not going to invest in rearing that last round of brood that is critical for forming the winter cluster. I could have boxes full of bees in late October, but those older bees generally abandon the hive at the beginning of winter. By that time it is too late to stimulate broodrearing. It’s not just about having bees in the box in late fall; it’s all about the age, nutrition, and health of those bees!
3. Toxins, whether natural plant products, environmental pollution, agricultural pesticides, or beekeeper-applied miticides. However, other than miticides, beekeepers have little control over which toxins their bees are exposed to.
Practical apps: minimize the use of miticides that leave persistent residues; avoid if possible areas with heavy pesticide use; rotate out contaminated combs.
4. Pre-infection by another parasite. At the top of this list is the varroa mite, followed by nosema, and then by viruses.
Practical application: In order for the beekeeper to effectively deal with bee disease in practice, it helps greatly to understand the how and why of how pathogens are able to initially overcome the bee immune system. Most importantly, the parasites that finally take a colony down can generally be considered to be opportunistic–that is, they require either an environmental stress or an initial infection by a more virulent parasite in order to be able to successfully reproduce within the colony.
This suggests a paradigm shift in beekeeping mentality. Instead of worrying about suppressing any specific pathogen, instead focus on promoting colony overall health via good husbandry. The most successful commercial beekeepers follow this model (I’ve been very impressed by ABF President Dave Mendes’ presentations about his successful management strategies, which focus on mite monitoring, good nutrition, pesticide avoidance, and comb rotation).
In this article, I want to focus on pre- and co-infections. When we say that a bee is “stressed” by an infection, what exactly do we mean?
Just as the bees fight infection (and attempt to “clear” themselves of the pathogen), pathogenic microbes fight back too–and they fight tricky, sneaky, and dirty. They do everything they can to evade, block, obstruct, or overpower the bee immune response. This becomes a very complex, mostly chemical, never ending game (well reviewed by Schmid-Hempel 2008). Any specific pathogen might use several mechanisms, and perhaps different ones at each stage of infection.
The most insidious thing that parasites do is to intentionally suppress the host immune response. A parasite may chemically interfere with the crucial phenoloxidase cascade, manipulate the host’s immune signaling network, disable the host’s antimicrobial peptides, tweak the RNAi response, or create “superantigens” that can overwhelm the host immune system. Unfortunately for the host, there may be other opportunistic pathogens waiting in the wings for just such an opportunity to invade when the immune system is impaired! (Fig. 6).
Figure 6. The very air is full of opportunistic microorganisms just waiting for an unprotected place to proliferate. I inoculated this petri dish with a 5-second exposure to the dust in the air after slapping my sofa. Note the variety of molds (fuzzy), yeasts (slimy), and bacteria (small colonies); the air would also be full of virus particles, but they can’t grow in the agar medium. Consider the fact that I breathe in far more spores than settled on this dish in every breath I take! Without constant effort by our immune systems, we would all soon become mere heaps of diseased and rotting flesh.
Of note is that if a bee ramps up its defenses against one type of pathogen (say a virus), it often tends to decrease its defenses against others (like bacteria). So attack by the first pathogen may leave the back door open for invasion by another! Or, infection by one pathogen may provide a necessary product (at the expense of the host) for the reproduction of another opportunistic pathogen that may then become the major infection (Diggle 2007).
Numerous studies over the years have found that bees suffering from multiple infections exhibited more mortality than those that were (apparently) singly infected. Even co-infection by the closely related nosemas (apis and ceranae) appears to be more deadly than infection by either alone (Evans 2010; Zachary Huang, unpublished data). Unfortunately, nowadays most of our colonies are concurrently afflicted with the two major “prime” parasites, either of which, especially in conjunction with poor forage conditions, appear to be able to trigger multiple infection cascades:
Varroa and Nosema
Varroa is clearly the 800-pound gorilla in bee disease. Even the slightest varroa infestation depresses colony health and production, and every aspect of colony performance suffers as varroa levels go up. Plus varroa completely changed virus dynamics in the European honey bee. The mite often goes hand in hand with DWV, and opens the door for three nasty virus cousins–KBV, ABPV, and IAPV. I will devote a section to varroa soon.
Practical application: If you monitor for mites obsessively, and keep levels below 2 mites per 100 bees, most of your disease problems will go away.
The other prime parasite is nosema, formerly N. apis (which was generally only a serious problem in cold winter areas), and now N. ceranae (which may be prevalent at any time of the year). Again, most recent studies are finding N. ceranae alone to be relatively harmless to well-fed colonies, although it may suppress buildup and honey production (Dr. Frank Eischen, unpublished data), likely due to shortened forager longevity.
A more pernicious effect of nosema infection is that it usurps the bees’ most precious commodity—energy. Nosema is energy hungry, and depletes the blood storage sugar (trehalose) in the bee (Naug, Mayack 2010). This effect may have huge ramifications in an insect that is dependent upon having ample stores of trehalose to fuel its massive flight muscles, which are used not only for foraging, but even more importantly for generating the critical heat necessary for colony thermoregulation! Note how this effect plays into the “Chilling Loop” in Figure 5—nosema-infection impairs the ability of bees to generate heat, which leads to chilling, which further increases the bee’ susceptibility to nosema, toxins, and other pathogens.
The sixty-four thousand dollar question for N. ceranae is why it causes rampant and harmful infections in some operations, but seems much more benign in others. A very important clue was discovered by Judy Wu (working with Dr. Steve Sheppard). Their initial data (in prep) indicate that on combs contaminated by miticides, a greater proportion of bees become infected with nosema, and that the infected bees reach higher spore counts. This finding may help to explain why N. ceranae appears to be much less of a problem in my “clean” operation, and more of a problem in typical commercial operations that tend to have high levels of miticide residues in the combs (there are clearly other issues involved, however, as Hawaiian operations without a history of miticide use report substantial nosema infections).
Should nosema go rampant, then the entire colony can dwindle rapidly, and suffer from nutritional stress as the field force is decimated (Higes 2008). Another deleterious effect of nosema infection may be indirect, in that it ravages the lining of the gut, thus hampering digestion and breaching the gut defenses against viruses. Black Queen Cell Virus appears to depend upon nosema to gain entry into the bee (Bailey 1983); Kashmir Bee Virus is also normally harmless unless bees are infected with nosema (Anderson 1991).
This brings us to the topic of infection by viruses, with which I will continue in the next installment.
References
Alippi, AM (1999?) Bacterial diseases. http://www.culturaapicola.com.ar/apuntes/cursos/99600235.pdf
Anderson, DL (1991) Kashmir Bee Virus—a relatively harmless virus of honey bee colonies. ABJ Dec 1991: 767-770.
Aubert, M (2008) Virology and the Honey Bee. European Commission. Free download—Google the title.
Bailey L., BV Ball, JN Perry (1983) Association of viruses with two protozoal pathogens of the honey bee, Ann. Appl. Biol. 103, 13-20.
Brødsgaard, CJ, et al (2000) Interactions among Varroa jacobsoni mites, acute paralysis virus, and Paenibacillus larvae larvae and their influence on mortality of larval honeybees in vitro. Apidologie 31: 543–554.
Dostert, C, et al (2008) Innate immunity of insects to infection in Virology and the Honey Bee.
Evans, J (2010) The role of multiple pathogens in colony collapse disorder. 110th General Meeting of the American Society for Microbiology.
Feldlaufer, MF, WR Lusby, DA Knox and H Shimanuki (1993) Isolation and identification of linoleic acid as an antimicrobial agent from the chalkbrood fungus, Ascosphaera apis. Apidologie 24: 89-94.
Forsgren, E, TC Olofsson, A Vasquez, I Fries (2010) Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie 41: 99–108.
Genersch, E., Ashiralieva, A., Fries, I., 2005. Strain- and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing American foulbrood disease in honeybees. Applied and Environmental Microbiology 71: 7551–7555.
Higes, M, et al (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10: 2659–2669.
Holst, EC (1946) Process of preparing an antibiotic substance from Bacillus larvae. US Patent 2,442,006.
Hughes, W. and J Boomsma (2004). Let your enemy do the work: within-host interactions between two fungal parasites of leaf-cutting ants. Proc. R. Soc. Lond. B 3 (Suppl), S104–S106.
Kleerebezum, M (1997) Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Molecular Microbiology 24(5): 895-904.
Mayack, C and D Naug (2010) Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. doi:10.1016
Moret, Y., and P. Schmid-Hempel. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290:1166–1168.
Nayudu, M and S Khan (2009) Anti-fungal Bacterial Symbionts of Bees. RIRDC Publication No 09/120.
Reynaldi, FJ, MR De Giusti, and AM Alippi (2004) Inhibition of the growth of Ascosphaera apis by Bacillus and Paenibacillus strains isolated from honey. Revista Argentina de Microbiologia 36: 52-55.
Ribière, M, B Ball and MF Aubert (2008) Natural history and geographical distribution of honey bee viruses in Virology and the Honey Bee.
Rigaud, T, Marie-Jeanne Perrot-Minnot and Mark J. F. Brown (2010) Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc. R. Soc. B published online 28 July 2010
Rueppell, O, MK Hayworth, NP Ross (2010) Altruistic self-removal of health-compromised honey bee workers from their hive. Journal of Evolutionary Biology 23(7):1538–1546.
Sadd, BM and MT Siva-Jothy (2006) Self-harm caused by an insect’s innate immunity. Proc. R. Soc. B 273: 2571–2574.
Salvy, M, et al (2001) Modifcations of the cuticular hydrocarbon profile of Apis mellifera worker bees in the presence of the ectoparasitic mite Varroa jacobsoni in brood cells. Parasitology 122: 145-159.
Schmid-Hempel, P (2008) Parasite immune evasion: a momentous molecular war. Trends in Ecology and Evolution Vol.23 No.6: 318-326.
Shimanuki H, Knox DA, Feldlaufer MF (1992) Honey bee disease interactions: the impact of chalkbrood on other honey bee brood diseases. Am Bee J 132, 735-736
Siva-Jothy, MT, Y Moret and J Rolff (2005) Insect Immunity: An Evolutionary Ecology Perspective. Advances in Insect Physiology 32: 1-48.
Smith-Sommerville, H (1995) Journey into the cell. http://www.rockefeller.edu/rucal/journey/journey.html
Travis, J (2009) On the Origin of The Immune System. Science 324:580-582.
West, A, et al (2007) The Social Lives of Microbes. Annu. Rev. Ecol. Evol. Syst. 2007. 38:53–77.