The Varroa Problem: Part 16a Bee Drift and Mite Dispersal
Contents
Bee Drift and Mite Dispersal 1
Phoresy, grooming, and host preference by the mites. 3
The shifting of varroa’s preferred transport. 6
Our unnaturally close placement of hives in apiaries. 7
Measured rates of hive-to-hive worker and drone drift. 7
Are some hives more attractive to drifting bees?. 10
Are there other reasons that bees drift?. 10
Manipulation of host behavior. 12
Competition and the weaponization of infectious parasites. 14
The Varroa Problem: Part 16a
Bee Drift and Mite Dispersal
First Published in ABJ April 2018
Randy Oliver
ScientificBeekeeping.com
The success of any parasite is dependent upon its ability to disperse to new hosts in order to ensure the propagation of its bloodline. To do so, varroa has a number of tricks up its sleeve. Unfortunately, our beekeeping practices play right into the mite’s hands.
In my previous article, I relayed my suspicion that the bee drift from, or the act of robbing of collapsing colonies accounted for the sudden late-season spike in mite counts that I observed in a proportion of my potential breeder hives. Most frustratingly, since we had varroa well under control in all of our apiaries, I can only conclude that drift of mites must have come from feral or poorly-managed colonies outside of our control. This late-season onslaught of mites is one of today’s hottest topics among beekeepers struggling to keep their hives alive. So I’ve dug deep into the subject of the drifting of bees and mites from hive to hive.
Dispersal of Varroa
Varroa is considered to be a minor parasite of its native host Apis cerana [[1]]. It was a different story when it was introduced to Apis mellifera. But even then, an annual treatment with Apistan® was initially all it took to keep your colonies alive from year to year. Unfortunately, those days were not to last, since a previously ignored virus–deformed wing virus (DWV)–quickly evolved to form a mutually-beneficial (yet diabolical) symbiotic relationship with the mite. Here’s how their deadly game is played:
- The mite, which appears to be immune to the virus [[2]], transmits DWV to both adult and pupal bees. The virus in return, suppresses the pupal immune response, thus allowing the mite to be more successful at reproduction in pupae that are seriously infected by DWV [[3], [4]].
- Those DWV-infected worker bees that successfully emerge to engage in nursing, then transmit the virus to the larvae that they feed. Fortunately, those larvae are generally able to contain the infection, and the virus doesn’t seriously harm the bee colony until the mite reaches a very high infestation rate in the hive come late summer or autumn–at which point the virus gets out of control and causes the colony to collapse, just as robbing becomes prevalent.
- At this time the mites get carried from the collapsing hive to robbing colonies, thus successfully dispersing successful strains of both the mite and virus to new host hives.
What a perfect match—a mite and a virus unwittingly working in harmony to kill their host colony at just the right time for optimal dispersal!
Practical application: the above successful dispersal appears to be exactly what inundated my strong potential breeder hives with mites last fall—they must have robbed out collapsing colonies within flight range.
But it’s not only the mite and virus that are partners in this coevolutionary process—both the bee and the beekeeper are also involved, and the success of the above game is dependent upon us continuing to do two things. So long as beekeepers stubbornly restock their operations with non-resistant bee bloodlines and fail to control the mite, this problem will continue. And it is further exacerbated by our placing large numbers of colonies close together in apiaries.
Practical application: Biologically, this is a fascinating example of evolution taking place before my very eyes—the mindless process of natural selection has allowed a formerly obscure insect virus to rapidly evolve to take advantage of a recently-introduced vector (varroa) in an artificial situation created and sustained by beekeepers themselves.
Since I can’t imagine that we will ever dispense with keeping our hives in apiaries, as a biologist it’s clear to me that the only way out of this situation is for our beekeeping practices to also evolve–by switching to keeping resistant bee stocks, and learning to control varroa earlier in the season. Hence my being a cheerleader for the selective breeding for mite resistance, and for better understanding mite management.
| Side notes:
1. Many beekeepers nationwide experienced serious losses this winter, depleting the supply of strong colonies for almond pollination. The usual suspects—drought and poor varroa management—are likely to blame, but there is reason to suspect that a more virulent form of DWV may be involved, as it appears to be in the process of spreading throughout the country [[5]]. This variant has been associated with elevated colony mortality in Europe [[6]]; I hope to soon report on the analysis of the samples shipped to me in 2016 from across the U.S. From what I’m hearing, those beekeepers who got varroa under control early last season were less affected. This would make sense, since it would help to prevent their colonies from entering winter while their virus epidemic was still raging. Practical application: by waiting to control varroa until after you’ve pulled the honey, it may be a case of “too little, too late”—it takes a long time for the colony to get DWV back under control after a mite treatment, which may result in your colonies dwindling over the winter and perhaps struggling in spring [[7], [8]]. The other prime factor in drought years is nutrition—in order to thrive, a colony needs good nutrition to rear the generation of brood that will form its wintering cluster. Not only that, but during a warm winter, such as we experienced in California this season, a colony will need enough pollen or sub in January to get a round or two of brood reared prior to almond bloom. I had photos of brood combs sent to me at the beginning of bloom, looking pretty sick from what appears to be nutritional stress. 2. There’s been a lot of buzz about the serendipitous finding that lithium salts might be useful as a varroacide [[9]]. Unfortunately, the paper did not point out an important finding detailed in the authors’ patent application—that lithium is highly toxic to bee larvae. Please do not mess around with this chemical—qualified researchers will be running field trials this season. |
Phoresy, grooming, and host preference by the mites
Just when beekeepers are starting to learn to use the word “phoretic” to describe hitchhiking mites, Samuel Ramsey [[10]] points out that that is actually an improper use of the term, since the mites clearly feed upon the bee upon which they are riding (Figs. 1 & 2).
Figure 1. Two mites feeding upon a worker bee. For some unknown reason, the mites generally favor the left-hand side. It is difficult for a mite to gain safe access to the bee’s soft integument anywhere else on its body. Thanks to beekeeper Scott Koppa for permission to use this photomicrograph.
The definition of phoresy is: an association between two organisms in which one (e.g., a mite) travels on the body of another, without being a parasite. Ramsey points out that the mites are indeed feeding on the bee’s fat bodies, so technically they are in the dispersal phase. But rather than confusing things, I’ll just stick with the term phoretic to describe mites hitchhiking on adult bees until our good Editor tells me otherwise.
Figure 2. An even closer view. Dispersing female mites require both moisture and food while they are riding on a bee, waiting to find a prepupal cell to invade. In this position, tucked under an abdominal sternite, they can safely feed while avoiding being groomed off by their unwilling host. Thanks again to Scott Koppa for sharing these photos.
I hear a lot of optimism for selecting for bees that appear to exhibit better grooming or biting behavior against the mite. But keep in mind that Apis cerana has a long history with varroa, and shows it no mercy—fervently self- and allogrooming (one bee grooming a mite off another). Yet, as pointed out by Rath [[11]]:
Grooming workers have no chance to grab the parasite once an adult female V. jacobsoni reaches a position on the host where its concave ventrum fits closely to the rounded bee body. In this respect, the peculiar shape of V. jacobsoni is a morphological adaptation to the bees’ intensive grooming.
Indeed, varroa can successfully survive for a full year between bouts of drone brood rearing, all the while riding on hostile Apis cerana workers. This despite the fact that a mite can’t stay on the same bee indefinitely. Mites prefer to ride on nurse bees, likely for two reasons: (1) the nurses are more nutritious—having well-developed fat bodies, and (2) nurses are the only bees in the hive that stick their heads into a brood cell—a requisite behavior for a mite wanting to reproduce. But in a few weeks that nurse bee will graduate to foraging, and will then typically die in the field within another couple of weeks. That means that for a mite to survive in the hive, it will need to continually switch rides—thus exposing itself again and again to being groomed or bitten. Yet enough mites manage to survive in those inhospitable colonies to carry on.
In my modeling of mite population dynamics, I use the well-established figure of 0.5% of the mites dying per day from “natural” mortality (including grooming)–that’s only 1 mite out of 200 dying each day. If a colony were able to cause the death of only 2% of its mites each day, varroa would go extinct, since the mite death rate would exceed its reproductive rate. We haven’t seen that happen in any population of bees.
Practical application: although I strongly support selecting for bees that recognize and attack the mite, I’m not holding my breath that Apis mellifera is going to be able to become much better at grooming varroa than is A. cerana.
In support of the above, Kruitwagen [[12]] recently published a paper that found that the naturally-resistant Apis mellifera colonies that they studied did not groom any more intensely than did non-resistant bees.
The shifting of varroa’s preferred transport
Now here’s where it gets really interesting—back in 1997, Kuenen and Calderone [[13]] found that newly-emerged mites generally preferred to move off their newly-emerged worker (or drone) and onto a nurse bee. But a percentage of those mites appeared to be attracted to older workers. They pointed out that:
Colonies, like individuals, eventually die, thus mites must have some mechanism for transferring to new host colonies. Foragers leave the colony on a regular basis, thus mite presence on foragers may represent an avenue for mite movement from one hive to another. The transfer of mites among colonies likely occurs as foragers drift between colonies, and in temperate climates mite transfer rates are much higher during periods of nectar dearth, a time period during which robbing among colonies is more likely to occur…For reproduction, we would expect the mites to move onto bees that will most likely bring them near, or into contact with, brood susceptible to infestation. However, for dispersal to another bee colony we would expect mites to move onto foragers, perhaps foragers from a different colony (a forager that drifted into the colony or a robbing bee).
A study by Cervo [[14]] supports the above reasoning. He found that at varroa infestation rates greater than 20 mites per hundred bees, the odor profile differences between nurses and foragers decreased, and that instead of 70% of the mites preferring to ride on nurses, they then split equally between nurses and foragers. Furthermore, although their results were not statistically significant, there did indeed appear to be a trend toward mites increasing their preference for “foreign” foragers over “home” foragers as the infestation rate increased.
And then Nolan [[15]] took it a bit further. He hypothesized that a mite that had already gotten one round of broodrearing under her belt might be more willing to take the risk of catching a ride outside of the hive. He found that although all mites, upon emergence, preferred young bees, those mites that had already reared a generation of offspring were more likely to hitch a ride on a forager (including bees from another hive) than were young mites emerging for the first time.
Practical application: it appears that as the mite level increases in a colony–and the mite population begins to age–that the mites become less risk averse. They then begin to act like rats ready to catch a ride off a sinking ship–which makes perfect evolutionary sense. And we beekeepers then make it sooo easy for those rats to successfully find a new ship nearby.
Our unnaturally close placement of hives in apiaries
Fries and Camazine [[16]] long ago pointed out that:
The drifting of bees into the wrong colony occurs frequently in apiaries where colony densities are greater than under natural conditions. In contrast, there is little or no evidence for disease transmission by drifting of individuals between feral colonies in the wild. In most cases, colonies are widely separated, precluding drifting of bees from one colony to another.
In nature, colonies are often widely separated, according to the availability of suitable nesting cavities and the carrying capacity of the landscape. For example, Seeley [[17]] counted a density of about 2.5 colonies per square mile in the Arnot Forest (both before and after the arrival of varroa). Under those conditions, even a foggy-headed errant forager could likely find its way home by smell, and there would be little expected drift or robbing between colonies.
According to Hepburn [[18]], the observed range of nest density of A. mellifera is from 1-20 per square mile. However, the number of managed hives has been increasing worldwide, so in areas of favorable forage, colony densities are more likely to be at the upper end of the above range. Jaffé [[19]], extrapolating from the genetic diversity of sampled drones, estimated that typical colony density in temperate regions nowadays is in the range of 15-20 colonies per square mile. A quick calculation of the number of hives that my sons and I maintain in the 85 sq. mi. area around my home supports this estimate. And that is not to mention those apiaries in other areas where I commonly see over a hundred, or even thousands, of hives in close proximity.
Practical application: at the above hive density (an average of 15 hives within a half-mile flight radius), it’s no wonder that there is a great deal of bee drift and robbing, making it easy for varroa to disperse from hive to hive.
Measured rates of hive-to-hive worker and drone drift
Beekeepers need to keep in mind the great amount of drifting of bees that typically occurs in apiaries. When we place numerous similar-looking hives all at ground level in an apiary, we create an unnatural situation to which bees are not evolutionarily adapted. A number of researchers have documented the occurrence of a substantial amount of drifting of workers from hive to hive, even before varroa entered the picture. Jay [[20]] found that in palletized hives some 5-10% of marked bees drifted within a pallet. And when non-palletized hives were placed in rows of three, he found [[21]] that up to 35% of marked bees introduced to the center hive drifted to a hive on either side.
In another study of hives placed in rows, Pfeiffer and Crailsheim [[22]] found that 50 – 90% of marked bees drifted out of their parental hive into other hives, and that approximately 15% of those drifting bees switched hives at least three times during their lifetimes. They calculated that up to 40% of the workers in a hive may not have been “born” in that hive.
Surprisingly, they found that there was greater amount of drifting of bees from hives with a high brood-to-worker ratio (typical of rapidly-growing colonies)—I have no explanation for this. However, they found no correlation between the amount of drifting and the number of varroa mites in a hive (but none of their colonies collapsed due to varroa).
J.B. Free [[23]] found that most drifting takes place during the early orientation flights of workers, and that bees tend to drift from weak colonies to strong ones. In support of the hypothesis that drifting is an artifact of confusion, studies by Pfeiffer [[24]] and Jay [[25]] indicate that most drifting is to adjacent hives. Another study using RFID tags [[26]] confirms a large amount of in-apiary drift, as well as the occasional bee drifting to a colony at least a half mile distant. Seeley [[27]] documented up to 50% drifting of drones between hives in an apiary with “normal” colony spacing, compared to scant drift when colonies were dispersed roughly 100 feet apart. Such drifting of drones was likely evolutionarily adaptive prior to varroa, since it allowed for the better dispersal of a colony’s genes (since drones could hopscotch from one colony to the next to reach congregation areas normally too far away to reach).
Practical application: it may no longer be adaptive for colonies to accept foreign drones.
On the other hand, Goodwin [[28]], using painted bees, observed only ~1% worker drift during summer from varroa-collapsing hives into adjacent hives. This result is especially surprising, since the same author had previously documented that at least 13% of painted bees could drift to adjacent hives during a two-day period [[29]].
Practical application: although drifting due to confusion and misorientation can be substantial in an apiary, it’s not yet clear to what extent this contributes to the dispersal of phoretic mites from hive to hive. In any case, wider spacing between hives, landmarks, and distinct coloration of the boxes may help to reduce drift.
The Diffusion of Mites
The steady drifting of workers from hive to hive creates a ready transport system for varroa within a neighborhood. A study by the Tucson lab indicates that workers exiting a hive carry mites on their bodies at very close to the overall infestation rate of adult workers in the hive [[30]]. A couple of recent studies attempted to determine the effect of colony spacing upon mite buildup [[31], [32]]—both gave results that suggested some degree of mite drift was taking place, especially in late summer.
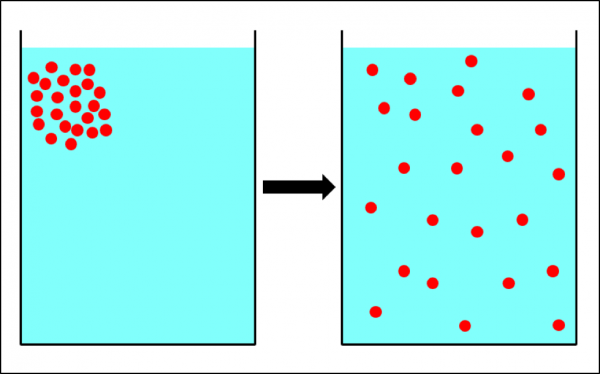
Similar to molecules, varroa mites would thus be expected to diffuse in an area’s bee population from hives of high “mite concentration” to hives with low concentration (Fig. 3).
Figure 3. The physical process of diffusion—the movement of particles from an area of high concentration into areas with a lower concentration. If the hive-to-hive drift of bees in an apiary worked the same way, it would tend to equalize the mite count between hives. It’s not clear to what extent this actually occurs.
If such drifting were random, then we’d expect the net result to trend towards an equalization of mite infestation rates throughout the apiary, since diffusion would favor the movement of mites from colonies with a high infestation rate to those with low infestation rates. The greater the “diffusion gradient” (between high-mite and low-mite hives), the greater the effect.
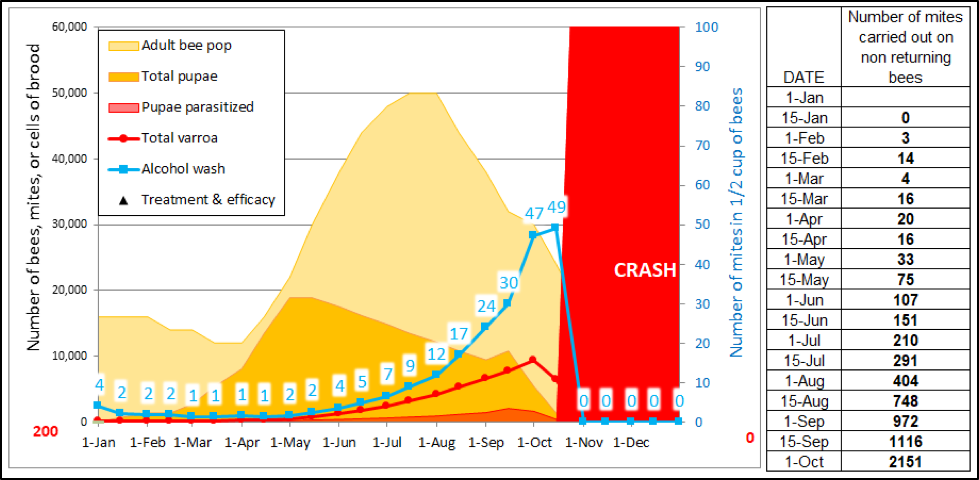
So what is the actual amount of mite transfer from high-mite hives to the rest of the colonies in an apiary? In order to get an idea, I ran a simulation, and looked at the number of mites that would be expected to be carried out of the hive each day on aging bees, but not expected to return to the hive (Fig. 4).
Figure 4. An example of a high-mite hive collapsing in the fall. Even before it collapses, it is spewing out mites every day on the bees that are lost from the hive due solely to aging. The figures to the right are independent of any hive-to-hive drifting, but indicate the sheer numbers of mites being lost to somewhere in the environment during each 15-day time period.
In reality, however, I’m not clear to what extent those emigrating mites make their way into other hives (I’ll show some measured numbers later in this article). There are far too many unknowns for me to attempt to calculate firm numbers, but my model suggests that even a daily immigration of 10 mites does not make an appreciable difference to a hive receiving at least one mite treatment per season.
Practical observation: in my monitoring for mites, I frequently find colonies with mite counts above 50 sitting right next to hives with counts close to zero, despite all colonies in that yard having been started from nearly identical nucs with sister queens earlier in the season. The question then, is whether those low-mite hives are somehow “dealing” with the mite drift, or driving off potential drifters, or something else.
Update May 2019: In order to answer some of these questions about colony-to-colony bee and mite drift, I performed a large field trial during late summer of 2018. I hope to publish the surprising results soon. I’m also engaged in a citizen science project in 2019 to collect hard data on mite immigration in hives across the U.S.
Are some hives more attractive to drifting bees?
This brings to mind a conversation that I had years ago with Dr. Jerry Bromenshenk, as we discussed the results of his electronic entrance bee counters. He found that some hives in each yard consistently gained more bees from other hives than they lost, and suggested that the reason that some colonies grow so strong is that they are more attractive to returning foragers.
Practical application: prior to varroa, it may have been advantageous to a colony to pick up stray bees, but the table may have now turned. Could it be that a potential trait for mite resistance is a colony’s lack of pheromonal or other olfactory attractiveness to bees from other hives?
Are there other reasons that bees drift?
The ability of bees to return to their parent hive with pinpoint precision is well known. One can easily observe this by moving a hive a foot to the side, and then watching as returning bees hover where the entrance was previously located–before finally relocating their hive by smell. Without such incredible navigation ability, a colony would lose too many foragers each day to be able to survive.
Thus, when the discussion of bees “drifting” to other hives comes up, I wonder why it occurs. I can understand the propensity of drones to drift, since there would be an evolutionary advantage for them to disperse widely, but that would not apply to the sterile workers. If anything, one would think that honey bee colonies would do everything possible to avoid drift for two main reasons: (1) workers accidentally drifting away from their hive would be a lost investment of resources, and (2) foreign bees trying to get into a hive would be expected to be robbers, or if not, potential carriers of pathogens. That said, there are some reasons that such drifting may occur, other than simple confusion in a crowded apiary.
Altruistic self removal
Kralj and Fuchs [[33]] found that exiting bees carrying a mite spent more time on flights, exhibited poorer orientation ability, and were less likely to return than bees not carrying a mite. Additionally, a fifth of those that did return had somehow lost their mite (perhaps to another bee on a flower, or at another hive entrance). The authors hypothesized that this self-sacrifice of infested workers:
…could be a more general response to diseases, and might be a trait which could be enhanced in breeding programs to strengthen the behavioural defence against V. destructor and, possibly, to other honey bee diseases.
Practical application: I heartily agree. In creating my varroa model, I needed to factor in the loss of the thousands of mites on the outgoing bees that don’t return–which allows the colony to shed a lot of mites in late summer and fall when the colony is shrinking (Fig. 4). Keep in mind the concept of intolerance—if every bee responded to the feeling of a mite on its body by immediately flying out of the hive and sacrificing itself, varroa would no longer be a problem.
The same authors later found that nosema-infected bees also self sacrificed and/or oriented towards the wrong hive entrance [[34]].
Practical application: this misorientation to an adjacent hive entrance may help to explain why I observed that if a colony gets sick, its immediately-adjacent neighboring hives also tend to get sick. Kralj and Fuchs found that mite-infested bees were twice as likely to return to an adjacent “dummy” entrance than to the proper entrance to their hive—over 70% of mite-infested bees initially oriented to the wrong entrance.
Rueppel [[35]] followed up by elegantly demonstrating that bees that sense that they are sick readily sacrifice themselves for the good of the colony, terming the trait altruistic self-removal. Such suicidal altruism for the good of the colony is similar to a worker sacrificing herself by stinging in defense of her hive. Furthermore, sick bees tend to exhibit a different odor profile, which is recognized by their nestmates, often resulting in aggressive behavior towards the sick individuals that may result in them being driven out of the hive [[36]]. Baracchi [[37]] found that DWV changes the odor of infected bees, inducing aggressive behavior by other workers towards the infected bees.
There may, however, be additional factors at work…
Manipulation of host behavior
Note that Kralj and Fuch found that although some parasitized bees committed self-sacrifice, others attempted to return home, but had difficulty orienting to the right hive entrance. Could it be that the parasite was affecting their behavior?
It’s been well established that infective pathogens often manipulate the behavior of their hosts to the parasite’s advantage [[38]]. Rabies virus causes infected animals to bite others; Toxoplasma protozoans cause rats to be attracted to cat urine and to engage in risky behavior. And humans become temporarily more sociable when injected with a flu vaccine [[39]].
The coevolution between Tomato yellow leaf curl virus and its vectors—aphids and whiteflies—is especially of interest, since it is similar to that of DWV and varroa. The sapsucking insects normally prefer the odor of virus-infected plants, since the virus suppresses the immune system of the plant, thus allowing the insects to grow and reproduce more efficiently on infected plants (similar to how DWV enhances varroa reproduction on infected pupae). But when an insect itself becomes infected by the plant virus, the virus causes the insect to seek uninfected plants—thus assisting in the transmission of the virus to new hosts [[40]].
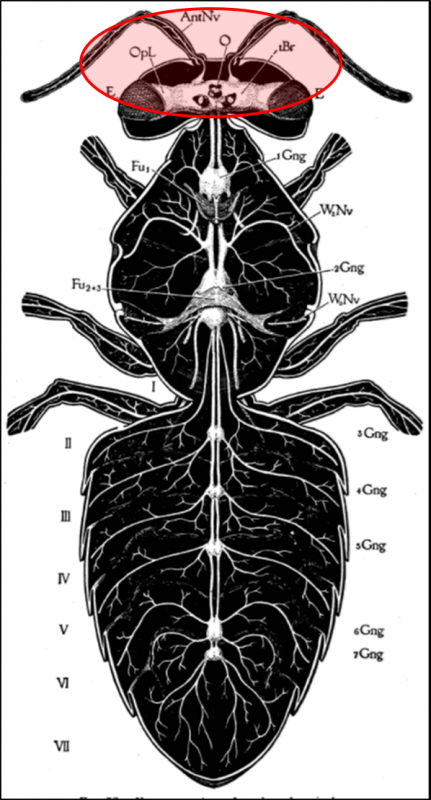
So how about the bee viruses? Some of them replicate in the bees’ brains and appear to affect homing ability or other behaviors [[41]]. DWV replicates in the parts of the brain responsible for vision and olfaction (Fig. 5) [[42], [43]], and one strain has been identified as causing changes in bee defensive behavior [[44]]. I wouldn’t be the least bit surprised if some bee viruses modify workers’ behavior to drift to other hives.
Figure 5. Viruses display tissue tropism, i.e., the adaptation to infect specific tissues. DWV replicates in various tissues of the bee, including those of the brain, suggesting that it is to the virus’s advantage to do so. Could it be that such infection causes poorer orientation and homing ability, perhaps leading to increased drifting to other colonies? Drawing from Snodgras [[45]].
It also occurs to me that there could be a dark trait of the honey bee that I have yet to hear being discussed in scientific circles:
Competition and the weaponization of infectious parasites
I can see an evolutionary advantage to the colony when sick workers fly away to die. But I observe that something else also occurs. In my field trials, I time and again observe that when a colony sickens, its immediately-adjacent neighbors tend to sicken and fail too. I can only assume that this is due to sick bees entering a neighboring hive [[46]], rather than simply flying away to die. It occurs to me that this may not be accidental.
Try visualizing a natural landscape of bee colonies as a bunch of small permanent “tribes” of sterile soldiers, each protecting the single fertile female of their bloodline. All tribes would be competing against nearby tribes for resources, and avoid each other’s foraging areas [[47]], robbing from each other at every chance (think of the relentless robbing pressure when hives are stocked beyond the carrying capacity of the landscape), and whose greatest threat is starvation due to competition for food.
It would be adaptive for any individual soldier, should it feel sick, to commit altruistic self-removal for the good of its tribe. At that point it could no longer help to promote the genes of its parent tribe– unless at that point that soldier took the further step of invading and dying in a different tribe, thus transmitting whatever pathogen it was suffering from to the competing tribe. Since its parent tribe had already been exposed to that pathogen, sharing the infection would have no downside to its bloodline, but would have the upside that the competing soldier tribe would now be equally exposed to the pathogen that the parent tribe was already forced to deal with. Such action would help to level the evolutionary playing field, and perhaps even knock out the competition—to the benefit of its parent tribe. It appears to me that such Trojan Horse behavior would favor the evolutionary success of a colony, even if it doesn’t seem like “fair play” to us humans. It would also explain why tribes set guards to prevent such Trojan Horses from entering.
A scientific challenge: so my question is, is there any reason to think that bees wouldn’t weaponize their parasites? It would be of huge evolutionary advantage to any colony exhibiting some degree of genetic resistance to that parasite to make sure that the competition was also having to deal with the disease. Some bright grad student could test this hypothesis by following the method used by Rueppell [[48]].
Update from the 2018 American Bee Research Conference:
Parasite manipulating hosts? Nosema ceranae infected honey bee workers are more easily accepted by non-nestmates
Yujie Li1, ZacharyY. Huang2
Parasites have been known to manipulate host physiology or behavior so that their own fitness is increased. Richard Dawkins cites many examples of this in his book, The Extended Phenotype. One of the examples he cited was that honey bees infected with Nosema apis will become foragers earlier and this might be beneficial to the parasite. We have previously shown that even though Nosema infection increases juvenile hormone biosynthesis and results in higher hormone titers (Huang & Lin, 2004 Eighth International Conference on the Juvenile Hormones, Scientific Program Abstracts p. 12), this is most likely not host manipulation because infected bees also showed increased higher metabolism.
Honey bee workers show guarding behavior with specialized “guards,” with an average age of ~14 days (Huang et al., 1994 J Comp Physiol A 174: 731–739). They “smell” each incoming forager and reject those that do not belong to the same colony. It is possible that Nosema might change host “smell” such that infected bees will be more easily accepted by non-nestmates, so that the pathogen may gain entry to a new colony to increase its fitness. The aim of this work is to determine whether honey bee workers infected with Nosema ceranae will become more easily accepted by non-nestmates. If so, it will more likely be a case of Nosemamanipulating the honey bee hosts.
We infected newly emerged worker bees with 50,000 fresh spores by feeding then with 2 microliters of 50% sugar syrup. Control bees were fed with syrup alone. The bees were then isolated in glass vials for 30 min to ensure all food was consumed to reduce Nosema spore transmission among bees. The bees were then caged for 7 days in the laboratory. On the 8th day, they were presented to a non-nestmate guard bee using a standard nest-recognition assay (Breed et al., 2004 Animal Behaviour 68: 921–928). We repeated the experiment with bees from 3 different colonies, each of them presented to four different recipient colonies.
There was a significant reduction in the percentage of bees rejected by non-nestmates when bees were infected with Nosema ceranae, compared to those not infected. We also found a significant colony effect for the colonies that provided the guard bees; the interactions between infection status and guard colony source were also significant. Source of infected bees did not differ significantly in their acceptance. These results suggest that besides other changes made by the microsporidian parasite, Nosema ceranae can also reduce aggression toward their host, perhaps to increase their spread to other colonies. The most likely mechanism is a change of hydrocarbons in the infected bees which will be investigated in a follow up study.
Next Month
I plan to cover why colonies allow bees to drift in and how those escaped swarms come back to bite you in the butt.
Acknowledgements
Thanks as always to Pete Borst for research assistance, and to all the dedicated and hard-working bee researchers from whose publications I draw useful information.
Notes and Citations
[1] Surprisingly, as pointed out by Rath in 1999 [[1]]: The important issue of mite dispersal among A. cerana colonies has not yet been studied.
[2] DWV virus has never been found in any varroa tissue other than the gut, and even then it’s not completely clear whether it is reproducing in varroa tissue. This question is far from resolved, and a paper currently in press claims to have detected DWV replication within the mite. One informative paper is: Erban, T, et al (2015) In-depth proteomic analysis of Varroa destructor: Detection of DWV-complex, ABPV, VdMLV and honeybee proteins in the mite. Scientific Reports doi:10.1038/srep13907
[3] Di Prisco et al (2016) A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. PNAS 113(12): 3203–3208. See Fig. 3 in the Supplemental Material, in which the mites exhibited nearly twice the reproductive success in pupae that later emerged with deformed wings.
[4] Piou V, et al (2016) Impact of the phoretic phase on reproduction and damage caused by Varroa destructor (Anderson and Trueman) to its host, the European honey bee (Apis mellifera L.). PLoS ONE 11(4): e0153482. Similar to above, the mites infesting pupae that developed deformed wings had more offspring.
[5] Ryabov, EV, et al (2017) Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Scientific Reports 7: 17447. Open access.
[6] Natsopoulou, ME, et al (2017) The virulent, emerging genotype B of Deformed wing virus is closely linked to overwinter honeybee worker loss. Scientific Reports 7: 5242. Open access.
[7] Locke B, et al (2017) Persistence of subclinical deformed wing virus infections in honeybees following Varroa mite removal and a bee population turnover. PLoS ONE 12(7): e0180910.
[8] Wu, Y, et al (2017) Characterization of the copy number and variants of Deformed Wing Virus (DWV) in the pairs of honey bee pupa and infesting Varroa destructor or Tropilaelaps mercedesae. Front. Microbiol., https://doi.org/10.3389/fmicb.2017.01558 Open access. A good review of our current knowledge.
[9] Ziegelmann, B, et al (2017) Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action. Scientific Reports 8:683.
[10] In prep.
[11] Rath (1999) op cit.
[12] Kruitwagen, A, et al(2017) Naturally selected honey bee (Apis mellifera) colonies resistant to Varroa destructor do not groom more intensively, Journal of Apicultural Research 56(4): 354-365.
[13] Kuenen, L & N Calderone (1997) Transfers of Varroa mites from newly emerged bees: preferences for age-and function-specific adult bees (Hymenoptera: Apidae). J. Insect Behav. 10(2), 213-228.
[14] Cervo R, et al (2014) High Varroa mite abundance influences chemical profiles of worker bees and mite-host preferences. J Exp Biol 217: 2998–3001.
[15] Nolan, MP IV (2016) Impacts of inter-colony distance, mite host choice, and colony polyandry on the host/parasite relationship between Apis mellifera and Varroa destructor. Dissertation, University of Georgia.
[16] Fries, I & S Camazine (2001) Implications of horizontal and vertical pathogen transmission in honey bees infested by Varroa destructor mites. Apidologie 38: 525–533.
[17] Seeley, TD, et al (2015). A survivor population of wild colonies of European honeybees in the northeastern United States: investigating its genetic structure. Apidologie, 46(5), 654-666.
[18] Hepburn, HR, et al (2014) Honeybee Nests: Composition, Structure, Function. Springer Science & Business Media.
[19] Jaffé, R, et al (2009) Estimating the density of honeybee colonies across their natural range to fill the gap in pollinator decline censuses. Conservation Biology, Volume 24, No. 2, 583–593.
[20] Jay, SC & D Dixon (1988) Drifting behaviour and honey production of honeybee colonies maintained on pallets, Journal of Apicultural Research 27(4): 213-218.
[21] Jay, SC (1965) Drifting of honeybees in commercial apiaries 1. Effect of various environmental factors. Journal of Apicultural Research, 4(3): 167-175.
[22] Pfeiffer, KJ & K Crailsheim (1998) Drifting of honeybees. Insectes Soc. 45: 151 – 167.
[23] Free J.B. (1958) The drifting of honey-bees, J. Agric. Sci. 51, 294–306.
[24] Pfeiffer, KJ & K Crailsheim (1998) Drifting of honeybees. Insectes Soc. 45: 151 – 167.
[25] Jay, SC (1965) Drifting of honeybees in commercial apiaries 1. Effect of various environmental factors. Journal of Apicultural Research, 4(3): 167-175.
[26] Thompson, H, et al. (2016), Thiamethoxam: Assessing flight activity of honeybees foraging on treated oilseed rape using radio frequency identification technology. Environ Toxicol Chem, 35: 385–393.
[27] Seeley, TD & ML Smith (2015) Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie 46:716–727.
[28] Goodwin, RM, et al (2006) Drift of Varroa destructor-infested worker honey bees to neighbouring colonies, Journal of Apicultural Research, 45:3, 155-156
[29] Goodwin, RM et al (1994) The effect of drifting honey bees on the spread of American foulbrood infections. Journal of Apicultural Research 33(4): 209-212.
[30] DeGrandi-Hoffman, G, et al (2016) Population growth of Varroa destructor (Acari: Varroidae) in honey bee colonies is affected by the number of foragers with mites. Exp Appl Acarol 69:21–34.
Compare the late-season mite infestation rates of bees in the hive in their Fig. 3 with the infestation rates of exiting bees in Fig. 4.
[31] Seeley TD, Smith ML. (2015) Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie 46: 1–12.
[32] Nolan, MP IV & KS Delaplane (2017) Distance between honey bee Apis mellifera colonies regulates populations of Varroa destructor at a landscape scale. Apidologie 48(1,): 8–16.
[33] Kralj, J. & Fuchs, S. 2006. Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie 37: 577–587
[34] Kralj, J. & Fuchs, S. (2010) Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 41(1): 21 – 28.
[35] Rueppell, O, et al (2010) Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evol. Bio. 23 (7): 1538–1546.
[36] Drum, NH & WC. Rothenbuhler (1983) Non-stinging aggressive responses of worker honeybees to hivemates, intruder bees and bees affected with Chronic Bee Paralysis. Jour Apic Res (22:4): 256-260.
[37] Baracchi, D, et al (2012) Evidence for antiseptic behaviour towards sick adult bees in honey bee colonies. Journal of Insect Physiology 58: 1589–1596.
[38] Poulin, R (2010) Parasite manipulation of host behavior: an update and frequently asked questions. In H. Jane rockmann, editor: Advances in the Study of Behavior, Vol. 41, Burlington: Academic Press. https://pdfs.semanticscholar.org/6135/71a29484f3e4be59233f01edf46a82bd6eae.pdf
[39] Reiber, C, et al (2010) Change in human social behavior in response to a common vaccine. Annals of Epidemiology 20(10): 729–733.
[40] Rajabaskar, D, et al (2014) Preference by a virus vector for infected plants is reversed after virus acquisition. Virus Res 186:32–37
Moreno-Delafuente, A, et al (2013) A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS ONE8(4): e61543.
[41] Li, Z, et al. (2013) Viral infection affects sucrose responsiveness and homing ability of forager honey bees, Apis mellifera L. PLoS ONE8(10): e77354.
[42] Yue, C and E Genersch (2005) RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). Journal of General Virology 86: 3419–3424.
[43] Shah, KS, et al (2009) Localization of deformed wing virus (DWV) in the brains of the honeybee, Apis mellifera Linnaeus. Virol J. 6:182.
[44] Fujiyuki, T, et al (2005) Kakugo virus from brains of aggressive worker honeybees. Adv Virus Res 65:1–27.
[45] Snodgrass, RE (1910) The Anatomy of the honey bee. US Government Printing Office.
[46] Pfeiffer (1998) op cit. also found that most bee drift went to an adjacent hive.
[47] Gary, NE, et al (1978) Distribution and foraging activities of honeybees during almond pollination. J. Apic. Res. 17(4): 188-194.
[48] Rueppell (2010) op cit.