Understanding Colony Buildup and Decline: Part 13d – The Impacts of Parasites and CO2
Understanding Colony Buildup and Decline: Part 13d
The Impacts of Parasites and CO2
Randy Oliver
ScientificBeekeeping.com
First published in ABJ October 2016
Winter is the most stressful time for the honey bee colony, and during times of stress, some parasites find opportunity in the hive. How can a beekeeper help his/her bees?
The honey bee is a tropical insect, and can only survive the winter by creating an artificial tropical environment within the hive. Not only does the cold cause stress, but since there is no fresh pollen coming in, the bees must survive on their body stores and a limited supply of aged beebread, which appears to decline in nutritional value with time [1].
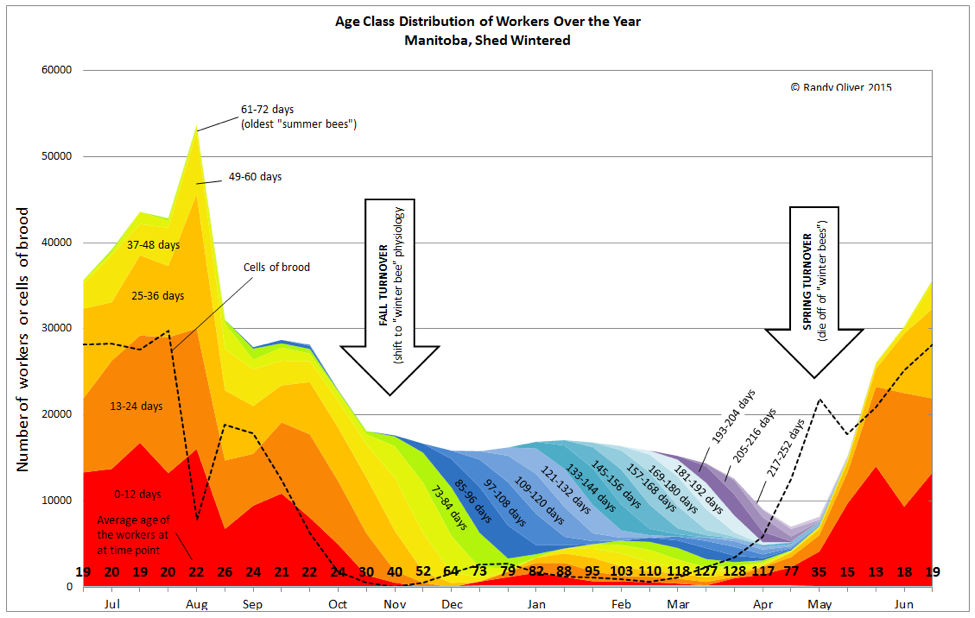
Perhaps even more important is the change in colony demography–refer to Fig. 1 below:
Figure 1. I’ve centered this graph over the winter. Take a look at the calculated average age of a worker bee at each time point (bold numbers at the bottom). From spring to fall, a colony enjoys a healthy, youthful population of workers. But during winter, the effects of parasites on the aging bee population can accumulate.
Bees fight parasites at both the individual level and at the colony level. The main defense at the colony level is to simply rear new workers faster than infected workers altruistically remove themselves from the hive [2]. During the spring and summer, the queen is laying perhaps 1500 eggs a day, and it’s relatively easy for the colony to replace aged or infirm workers. The turnover of the adult bee population is thus quite rapid, and the spring-through-fall workforce averages a youthful 24 days of age or less.
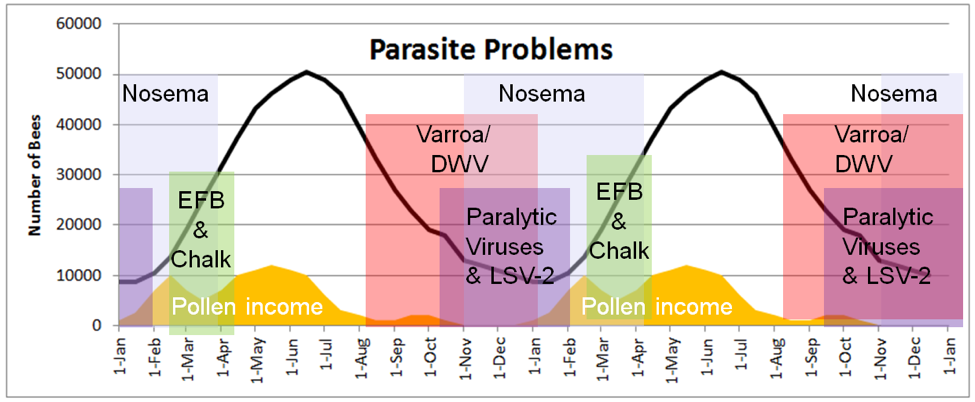
This completely changes once broodrearing is curtailed after the last fall pollen flows. Now the remaining “winter bees” must go into survival mode, and the colony’s immune response to parasites shifts to being completely dependent upon each individual bee. And those bees just keep aging and aging, depending upon equally aging lactic-acid-preserved beebread as their sole source of protein nutrition. As with humans, bees’ bodies suffer from age, and some parasites take advantage of this poorly-fed, chilled, geriatric population (Fig. 2).
Figure 2. The seasonality of parasite problems relative to pollen income, colony buildup and decline, and time of year in my California climate, over two seasons. These parasites are present at all times of the year, but tend to cause observable disease or colony morbidity at the indicated times. Other than AFB (not shown), which can build up over summer, summer bees enjoying abundant pollen resources are typically quite healthy. But things change come fall.
Colonies tend to be healthy from spring ‘til late summer. At that time, the beekeeper, by practicing proactive husbandry, can greatly help the colony to maximize its chance at successfully surviving the winter. Being proactive begins around mid August. We almond pollinators, who must supply strong, healthy hives in mid February, make a point stay on top of varroa and colony nutrition in late summer.
Keep Your Eyes on Varroa!
Most any professional beekeeper still in business has by now figured out the importance of late-summer varroa management. The buildup of varroa in the hive follows a predictable exponential function, and can double each month that the colony is seriously rearing brood. Since more brood means more mite reproduction, those colonies that build up to the greatest strength often end up with the highest mite levels.
If the colony begins the season with a low mite count, then two treatments that kill 95% of the mites (the entire mite population, including those in the brood), will hold varroa in check for the course of the season (I will be covering mite population dynamics in detail in an upcoming article). But if the efficacy of those treatments is only 70%, it will require three treatments (perhaps surprisingly, it makes little difference at what time of season the treatments are applied) [3].
As varroa develops resistance to the available miticides, the efficacies of those treatments tend to drop. Similarly, taking shortcuts in application of treatments may also result in low efficacy. And putting off mite treatment beyond the first of September may allow a virus epidemic to develop past the point of no return.
Practical application: my buddy Keith Jarrett told me of having a semi load of hives intended for almond pollination delivered in late fall from an out of state beekeeper. Before the driver could untie the load, Keith said, “Why’d you even bother to load these hives up—they’ll never make it into almonds.” The driver, incredulous, said “What are you talking about?” Keith lifted the bee net a bit to show an exodus of bees with deformed wings crawling out of the hives—“These colonies are already too far gone to try to save them.”
Even if the beekeeper manages to beat varroa into submission with a delayed late-season treatment, a colony going into fall with a raging DWV epidemic has little chance of surviving the winter. The last workers to emerge will be incurably handicapped, and never be able to become long-lived “winter bees.” The cold-stressed, aging bees in the colony will be unable to mount an effective immune response to the paralytic viruses and nosema. Coupled with the lack of incoming pollen, the combined effects of the parasites simply overwhelms the colony, really hitting hard during the difficult spring turnover.
LATE SUMMER and Winter Supplemental Feeding
In areas where goldenrod and asters provide abundant pollen in the fall, colonies typically go into winter in good nutritional shape, with plenty of reserves of beebread, and a fresh cohort of young bees that shift to wintering (diutinus) physiology. This is not the case in the arid West, unless you happen to have good Rabbitbrush locations.
Many of us Western almond pollinators feed our bees pollen sub in late summer and fall with great success. And if the bees are unable to get enough tree pollen in January, we feed them more sub at that time. Feedlot beekeeping, kinda [4]. Healthy, strong hives, yes.
Practical application: if, in late summer, you see that the nurse bees are cutting back on the amount of jelly that they are feeding to the young larvae, feeding patties of a high-quality pollen sub can work wonders. It allows the colony to ramp up its broodrearing, and gives that generation of recruits the protein that they require for optimum performance. I personally allow my colonies to cut back on broodrearing in August, and use that opportunity to get varroa under control while there is less brood for the mites to hide in, and afterward stimulate the colonies with protein for fall buildup [5].
Similarly, if there are not enough beebread reserves for midwinter broodrearing in January, pollen sub again can work wonders. Those not going to almonds can wait a bit later to help with late winter broodrearing.
As far as varroa problems, the worst case scenario is a warm winter, a pollen-rich spring and early summer, followed by a dry late summer. The warm winter allows early broodrearing, in which varroa then gets a head start. A pollen-rich spring and summer then creates ideal varroa breeding conditions, after which the late-summer dearth crowds those mites onto fewer and fewer worker bees and pupae, resulting in a stressed colony suffering from a ugly virus epidemic.
Viruses and Nosema
During the depth of winter, Acute Bee Paralysis Virus, Kashmir Bee Virus, and Lake Sinai Virus 2 can run rampant in the hive, as can Deformed Wing Virus if varroa was not controlled. Nosema ceranae can also build up in the aging bees, and go epidemic if the workers also suffer from dysentery [6]. If cold weather restricts cleansing and self-removal flights of sick bees, things can go downhill quickly.
Practical application: U.S. beekeepers could learn from our Canadian brethren, who, despite dealing with severe winters, have recently managed to enjoy a lower rate of winter loss (16.8%) [7] than U.S. beekeepers (28.1%). Some snips from the recent CAPA [8] report:
- These results demonstrate that Canadian beekeepers recognize the value of surveillance and monitoring of Varroa [9] mites. During the 2015 season, a large majority of surveyed beekeepers monitored Varroa mite infestations. The alcohol wash of a sample of 300 bees per colony was the most preferred technique in [most] provinces.
- Most beekeepers in Canada manage Varroa mites using a combination of non-chemical and chemical control measures. Oxalic acid was one of the most favored fall treatments.
- Fewer than half of the surveyed beekeepers treated in fall with fumagillin. Interestingly, when I worked the data at the provincial scale [10], there was no correlation between fumagillin treatment and winter loss rate. I’m not saying that fumagillin didn’t help during their long, cold winters, but nosema was ranked by the beekeepers as a major cause of colony loss only in Alberta and Saskatchewan, despite 92% and 68%, respectively, of the beekeepers in those provinces applying fumagillin in fall [11].
Back to the U.S., proactive preparation for wintering consists mainly of knocking varroa back to very low levels prior to the last rounds of broodrearing, and ensuring good colony nutrition. Any iffy colonies should be culled or combined (“taking one’s winter losses in the fall”). There’s nothing else you can do about viruses, and there is no scientific consensus on what to do about nosema [12]. There is no clear economic threshold spore count that indicates a need for treatment, and no treatment other than fumagillin with strong supporting evidence of its efficacy (and there are questions about the cost/benefit ratio of fumagillin, its legacy effects, and its potential to contaminate honey). However, a fall oxalic acid dribble for varroa may have the additional benefit of knocking back Nosema ceranae [13].
Once the colony ceases broodrearing in the fall, the best that the beekeeper can do is to provide a warm, dry, well-provisioned hive, treat the colony with an oxalic acid dribble [14], and perhaps offer the bees pollen sub once they initiate midwinter broodrearing.
Recent Findings
There are a couple of recently published studies worth discussing. Traynor [15] summarized the findings of the US National Honey Bee Disease Surveys from 2009-2014. Points of interest:
- Beekeepers overall are doing a rather poor job of managing varroa, with migratory doing a better job than stationary beekeepers. Negligent beekeepers, by allowing their hives to collapse from varroa, flood the environment with mites, to the great detriment of surrounding managed or feral colonies.
- Nosema ceranae prevalence has steadily increased, as this new parasite completed its invasion of the U.S. bee population [16].
- But nosema spore counts did not appear to be alarming, with average counts in the 1 million per bee range during the April peak.
- The team’s data suggests that the recently-discovered Lake Sinai Virus 2 appears to be associated with Nosema ceranae, although an unpublished data set from my own colonies does not exhibit that correlation.
- Of considerable interest is the apparent increase in prevalence in recent years of Chronic Bee Paralysis Virus (CBPV) (Figs. 3 and 4). This finding is worrisome, since the presence of CBPV increased the likelihood of finding several other viruses in the same bees [17].
Figure 3. I’ve long seen the occasional bee with signs of Chronic Bee Paralysis Virus—Type 2 syndrome, also called “Hairless Black Syndrome.” The infected bees quiver, and their coworkers pick at their wings and pluck the “hairs” from their bodies, revealing the dark exoskeleton.
Figure 4. In Type 2 syndrome, anywhere from a handful to thousands of bees drop quivering to the bottom of the hive. This has all the appearances of a pesticide kill, but I see it from time to time in areas free of pesticide exposure, usually in only a hive or two in the yard. Such kills can occur at any time of the season.
Evolution
Our beloved honey bees are being forced to adapt to a number of environmental and biological changes. In recent years we’ve introduced a number of new parasites, with varroa and Nosema ceranae appearing to have the most lasting effects. Varroa completely changed the virus dynamics of the hive, and DWV is now our most serious problem.
Our rapidly-warming climate is resulting in shorter winters, and earlier flowering of many bee forage plants. The resultant compression of the spring bloom period shortens the time available for colonies to build up to full strength (which is forcing me to change my long established management practices). Perhaps most scary are the invisible changes due to the increased concentration of CO2 in the atmosphere. Studies are showing that as we humans increase the CO2 level of the atmosphere, plant tissue contains less of the critical nutrients iron, zinc, and protein. This may have major implications for pollinators that depend upon pollen as their sole source of nutrients, especially since zinc is a necessary component of the all-important vitellogenin–used by bees for nutrient storage and transfer, and in immune function.
In a recent eye-opening study [18] the investigators studied the effect of rising CO2 levels on the amount of protein in one of the major sources of autumn pollen—Goldenrod (Fig. 5). They found that over my lifetime the protein content of Goldenrod pollen has decreased by roughly a third. Think of the consequences of this.
Figure 5. Due to rising CO2 levels, this bee needs to gather half again as much Goldenrod pollen as did a bee 65 years ago in order to obtain the same amount of protein. Credit for this lovely photo to Steve Burt.
The reduced protein content means that pollen foragers need to collect half again as much pollen to provide the same amount of protein. That extra energetically-expensive [19] pollen collection diverts the forager force from nectar collection. Nurse bees need to eat half again as much pollen, and may not be able to feed as many larvae. They’d also need to take half again as many cleansing flights. Since many nurses never return from their cleansing flights, this may decrease the overall survivability curve of the workers. Colonies need to store half again as much beebread for winter, and, oh yes, that increased winter beebread consumption means a greater need for winter defecation flights. The reduced nutritive value of pollen also makes it more difficult for individual bees to maintain a robust immune system [20], and for a colony to recover from exposure to insecticides. The cumulative result of the above effects could have a profound negative impact upon colony buildup and productivity, yet occur entirely invisible to us, with no trace of residues or other identifiable signs.
Practical application: many of us long-time beekeepers complain that bees just don’t seem to be as robust and productive as they used to be. Could part of the cause be the invisible effect of increased carbon dioxide on the nutritional quality of pollens? Although the above study was only of Goldenrod pollen, it is likely that increasing CO2 affects the nutritional quality of all pollen sources [21]. Couple this with the varroa/virus complex, Nosema ceranae, changes in plant phenology, and higher summer temperatures and increased drought in many areas, and one can easily see that bees are clearly facing serious evolutionary pressure to adapt to this changing environment [22]. One thing that I can say for sure is that beekeeping is sure a lot harder for my sons than it was when I first started.
Note To My Readers
This article wraps up my series Understanding Colony Buildup and Decline. I will post all installments to ScientificBeekeeping and keep them updated with new findings. I appreciate all the positive feedback, and hope that this series helps you to better understand bees as much as researching it did me. I give special thanks to Lloyd Harris for sharing his incredible data set, and to Pete Borst for his great assistance in literary research.
Notes and Citations
[1] A study by Patrick Maes, currently in review.
[2] I like the recently-coined term “social apoptosis.” Similar to the larval death described in the paper, sick adult bees “take one for the team” by flying out of the hive to die before they transmit the infection to their nestmates. Page, P, et al (2016) Social apoptosis in honey bee superorganisms. Nature Scientific Reports DOI: 10.1038/srep27210.
[3] It’s a common misconception that an early treatment has a multiplier effect, based upon the argument that killing a mite in April prevents it from producing dozens of mites by late summer. This seems to make perfect sense, but it doesn’t work that way (I’ll explain in an upcoming article). The benefit of early treatment is simply that it reduces the impact of viruses on the colony during its buildup. A benefit of late treatment is that it hits the incoming mites from nearby collapsing hives. It’s best for the bees to keep mite levels low all season long.
[4] Not really feedlot, since the bees continue to free forage. The pollen sub is actually a supplemental protein source, augmenting the scant natural late summer pollen available in my area.
[5] Any colony that does not quickly consume their pollen sub patties has something wrong with it—it’s either queenless or sick.
[6] From being forced to survive on poorly-digested honeydew honey, poor beebread reserves, or low-quality pollen sub.
[7] http://www.capabees.com/shared/2015/07/2016-CAPA-Statement-on-Colony-Losses-July-19.pdf Open access.
[8] https://beeinformed.org/2016/05/10/nations-beekeepers-lost-44-percent-of-bees-in-2015-16/
[9] Sic. For the nitpickers, I’m aware that if “varroa” is capitalized, that it should be put into italics.
[10] Plotting the percent of beekeepers in a province who applied fumagillin in fall against the colony loss rate for that province.
[11] Dr. Ingemar Fries in Sweden noted that Nosema apis was mainly a serious problem only at the extreme northern range of the honey bee. This could well be the same for N. ceranae, as in Alberta and Saskatewan.
[12] Holt, HL & CM Grozinger (2016) Approaches and challenges to managing Nosema (microspora: nosematidae) parasites in honey bee (Hymenoptera: Apidae) colonies. Journal of Economic Entomology 2016: 1–17. doi: 10.1093/jee/tow103
[13] Nanetti, A, et al (2015) Effect of oxalic acid on Nosema ceranae infection. Research in Veterinary Science 102: 167–172.
[14] Vaporized (sublimated) oxalic acid works very well, but I haven’t seen a study that shows that it has the same effect upon nosema as dribbled OA/sugar solution.
Toufailia, H, et al (2015) Towards integrated control of varroa: 2) comparing application methods and doses of oxalic acid on the mortality of phoretic Varroa destructor mites and their honey bee hosts. Journal of Apicultural Research http://dx.doi.org/10.1080/00218839.2015.1106777 Open access.
[15] Traynor, KS, et al (2016) Multiyear survey targeting disease incidence in US honey bees. Apidologie DOI: 10.1007/s13592-016-0431-0 Open access.
[16] See Fig. 3 at https://scientificbeekeeping.com/sick-bees-17a-nosema-the-smoldering-epidemic/
[17] ABPV, IAPV, KBV, LSV-2, and especially BQCV.
[18] Ziska, LH, et al (2016) Rising atmospheric CO2 is reducing the protein concentration of a floral pollen source essential for North American bees. Proc. R. Soc. B 283: 20160414. http://dx.doi.org/10.1098/rspb.2016.0414
[19] Feuerbacher, E, et al (2003) Effects of load type (pollen or nectar) and load mass on hovering metabolic rate and mechanical power output in the honey bee Apis mellifera. The Journal of Experimental Biology 206:1855-1865.
[20] CO2-enriched plants produce more carbohydrates, at the expense of critical trace elements (such as zinc) which are critical for immune function.
[21] Bloom, AJ, et al (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328(5980): 899-903. DOI: 10.1126/science.1186440 Open access.
Weigel, HJ (2014) Crops and Climate Change: Plant quality declines as CO2 levels rise. eLife 3:e03233 DOI: http://dx.doi.org/10.7554/eLife.03233 Open access.
Meyers, SS (2014) Increasing CO2 threatens human nutrition. Nature 510: 139-142. Doi:10.1038/nature13179 Open access.
Feng, Z, et al (2015) Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Global Change Biology 21: 3152–3168. doi: 10.1111/gcb.12938 Open access.
[22] Such natural adaptation is likely hampered in our managed bee population, due to its restricted gene pool resulting from the genetic bottlenecking caused by the selective breeding for a limited number of desirable traits.