Refining the Mite Wash: Part 3 – Dislodgement, Precipitation and Separation
Refining the Mite Wash: Part 3
Dislodgement, Precipitation, and Separation
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in September 2020
In my last article I showed how mites will quickly drop off the bees’ bodies if immersed in 91% alcohol. But there are still more steps remaining to separate the mites from the sample of bees.
I’m writing this series of articles as I’m doing the research, since I want to get my findings to you as quickly as possible. So please forgive me if this series seems disjointed. A number of my findings have surprised me, so I’m continually coming up with new questions, and thinking of ways to answer those questions.
Underside Inspection, Take 2
While working with volunteer beekeeper Michelle, we identified one of my test colonies with an extremely high mite infestation – around 100 mites per 300 bees. That works out to there being an average of 1 mite for every 3 bees. This presented a perfect opportunity for me to see whether the “clamshell method” of mite monitoring that I mentioned in my last article could be useful.
So we shook samples of those bees into plastic clamshell food containers, let them settle down for a minute, and took advantage of Michelle’s sharp young eyes (and my reading glasses) to see whether we could estimate the infestation rate by looking at the bees’ bellies (Fig. 1).
Figure 1. It was easy to spot mites on the undersides of the bees (two circled), but we were amazed at how few we saw – certainly not a mite on every third bee. We were lucky to see a single mite in 15 bees, and have no idea where the rest of the expected mites were. So although this technique initially looked promising, it needs to be validated, which will require first counting mites visually, then washing the bee sample to recover any unseen mites. I hope to collect that data soon.
Revisiting the Sugar Roll/Shake
Kamran Fakhimzadeh, from Helsinki, Finland, first brought sugar dusting to our attention back in the year 2000, in this very journal [[1]], with a photograph of how the sugar particles stuck to the mites’ empodia, causing them to lose their grip, and suggested it as a mite-control method [[2]]. Then in 2002, researchers in Dr. Marion Ellis’ lab tested using inert dusts to monitor for varroa [[3]], with the recommendation to use powdered sugar. This was followed by extension publications [[4]], where the term “sugar roll” was coined, due to rolling bees in a jar to coat them with powdered sugar. But Macedo and Ellis only suggested possible mechanisms for why the sugar roll worked.
As with many things in beekeeping, “common knowledge” takes on a life of its own, and nowadays, descriptions of the sugar roll technique often mention something to the effect that “The sugar acts as an irritant and bees will generate heat when trying the remove the sugar. It’s the heat that dislodges the Varroa mites from the bees” [[5]] (Fig. 2).
Figure 2. Rolling the bees in powdered sugar clearly bothers them, but does it bother them enough to make them heat up enough to make varroa mites abandon them?
The heating-up claim makes complete sense, until you think about it. Honey bees heat up their flight muscles to around 105°F, and return to the hive with thoracic temperatures above 102°F, and abdominal temps above 95°F [[6]], and can exhibit body temperatures on hot days of up to 113°F [[7]]. Thus, if a bit of heating was all that it took to get a mite to let go, a bee could lose attached mites by simply taking a flight on a hot day – an incredibly simple resistance mechanism.
It occurred to me that the “heating due to irritation” claim would be simple to test. So we scooped bees into a covered cup, rolled them in powdered sugar, and measured their temperature over 5 minutes as they struggled in a clump, using both a thermal probe and an infrared thermometer (Fig. 3).
Figure 3. To our great surprise, the temperature of the pile of bees slowly warmed from around 86-90°F to a maximum of only 95-96°F – right at broodnest temperature! We got the same results in a second replicate. These results do not support the claim that mites release due to the heating of the bees after rolling them in powdered sugar.
So, if it’s not the heating, do you really need to beat the bees up with a hard shaking? We made a bunch of shaker jars to see. For our first test, we rolled a half cup of mite-infested bees in enough powdered sugar to thoroughly coat them (Fig. 4), then inverted each screen-topped plastic jar over a cup to catch any mites that dropped off of their own accord as the bees clambered around grooming themselves (Fig. 5).
Figure 4. Rolling bees in powdered sugar to get the mites to release and lose their grip. Note the cup and magnifying mirror holder in the background, which we use for counting mites washed using detergent.
Practical application: I often see beekeepers using heavy glass Mason jars for the sugar shake. It’s far easier on the arm to shake a lightweight plastic jar, such as the peanut butter jar pictured above. You can cut the center of the lid out with a hole saw (or by other means), and then use hot glue to attach 1/8” hardware cloth.
Figure 5. Once inverted, mites started dropping into the lower cups immediately. The photo above was taken after 15 minutes, by which time the bees had pretty well groomed the sugar dust off their bodies.
After allowing 15 minutes for the mites to drop, we washed the bees in detergent to recover any remaining mites (Table 1).
| Table 1. Mite drop from powdered sugar roll, no shaking. | ||||
| Run | 1 | 2 | 3 | 4 |
| Drop from sugar alone | 58 | 48 | 75 | 62 |
| Drop from detergent follow up | 31 | 3 | 14 | 36 |
| Total mites | 89 | 51 | 89 | 98 |
| % drop from sugar dusting without shaking | 65% | 94% | 84% | 63% |
Wow, we thought – perhaps there’s no need to beat up the bees, slamming them up and down by hard shaking. There is clear release and dislodgement of the mites without any shaking, so maybe all that you need to do to sift them out is to just bounce the bees gently up and down a bit.
So I performed 15 sugar roll shakes — 5 gentle, and 10 vigorous. I allowed 60 seconds of rest after rolling the bees in powdered sugar, then immediately followed with 60 seconds of shaking, then a detergent “clean up” wash. I alternated hard and soft shakes, but since I was short on high-mite bees, after five disappointing soft shakes, I focused upon getting more data on the hard shake. Results: mite recovery was substantially better with a “vigorous” shake: giving a median 88% mite recovery in 60 seconds of banging the bees up and down in the jar (n=10), as opposed to only 59% for a soft shake (n=5) (I’ll show the histogram in my next article).
Practical application: as much as I hate to see bees being beaten by a hard shake, it appears that with powdered sugar, it is necessary in order to obtain decent mite recovery.
Michelle was curious as to whether the hard-shaken bees actually survive for long once returned to the hive. I’ve often watched powdery-white bees walk back into the entrance. Unfortunately, during our experiments, there was a weak nectar flow on, and after a shake, their disgorged nectar often left the bees sticky with dissolved sugar. I caged some of these bees, plus a control group, into the incubator. There was some mortality of the sticky shook bees held overnight, as opposed to zero mortality of sugared bees not shaken.
We realized that for a meaningful test, the bees would need to be returned to their hive, where their nestmates could groom the sugar from their bodies. So we paint-marked 600 bees shaken from a hive, 300 with yellow, 300 with blue. I then sugar rolled the blue bees and shook them for 60 timed seconds, by which time, they had gotten wet and sticky, and a number looked as though they were dying (Fig. 6). We then returned both groups back into their hive.
Figure 6. Marked bees after rolling in powdered sugar, then shaking vigorously for 60 seconds. Due to disgorged nectar, these poor bees look beat up, sticky, and bedraggled.
We placed a dead bee trap in front of the entrance, and checked it and the bottom board for casualties the next morning. Surprisingly, other than the three yellow control bees that we expected to find dead due to their having stung me as I put them into their container, I found no dead blue-marked bees. This experiment needs to be repeated.
Practical application: I’ve long wondered whether the traumatized hard-shook bees survive after you’ve returned them to the hive. It appears that they can, at least overnight.
Back to Mite Washing with Liquid Release Agents
Review of the steps
Allow me to refresh you on the four steps involved in a mite wash or sugar roll.
Step 1: To cause the mites to release their grip on the bees.
Step 2: To then dislodge the mites from the bees’ bodies.
Step 3: To then agitate or wash the bee sample enough to allow for the precipitation of the mites through the tangle of bee bodies, and finally
Step 4: To separate the mite sample from the bee sample, typically by allowing the much-smaller mites to drop through a screen.
During a sugar shake, the mites precipitate through air, and thus fall rapidly. No so when they must sink through a liquid, such as alcohol or water.
The Precipitation (Sinking) Rate of the Mites
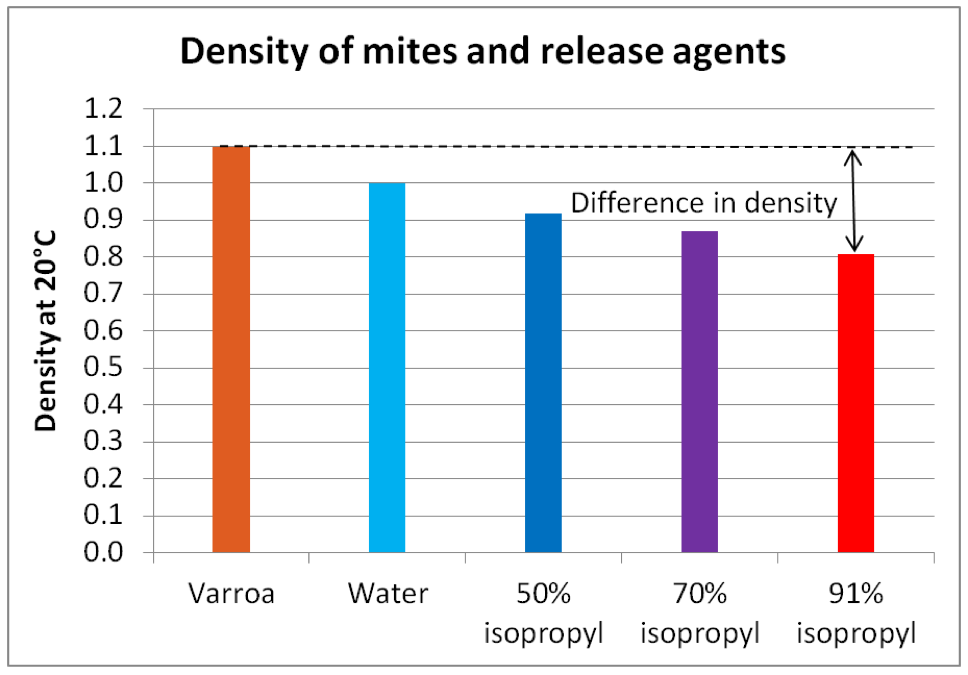
In the first installment of this series, I showed how we obtained much better mite recovery in mite washes by using 91% alcohol rather than 50%. Was this due to the greater toxicity of the 91% alcohol, or because of its lesser density? Since alcohol is less dense than water, we’d expect mites to sink more quickly in alcohol of higher concentration.
If you’ve shaken mites into water while performing a sugar shake, you likely noticed that they will float for a while, but that is not because they are less dense than water, but rather because of their hydrophobic (water-hating) cuticle. If you add a bit of detergent to the water, they will immediately begin sinking, albeit not as quickly as they sink in alcohol (ditto for bees, which turns out to be important).
As I was typing this article, I wondered just how dense mites actually are, compared to water or alcohol. So I did my usual thing – got up from the keyboard and performed a kitchen investigation to find out. I washed some mites off of bees using detergent in water, then quickly rinsed and drained them on a sieve before they could absorb any water. I then filled a tall jar with water and a bit of detergent, and checked its density (actually specific gravity) with a hydrometer, confirming the instrument’s calibration [[8]].
I dropped mites into the water and watched them sink to the bottom. Then I added salt bit by bit to increase the density of the solution, dropping mites in again at each 0.01 increase in density. They kept sinking, although slower and slower, until I reached a specific gravity of 1.1, at which point they stayed in suspension or floated (Fig. 7).
Figure 7. Ignore the tiny bit of foam at the top – it’s an artifact of dissolving salt in water. Once mites stopped sinking (you can see some floating, others hung suspended in the water column), I had a figure for their density which I could compare to that of water and alcohol (Fig. 8).
Figure 8. The greater the difference in density, the faster something will sink. Note that mites are not much denser than water, but considerably more dense than 91% alcohol [[9]]. This is confirmed by my field observations that mites sink more slowly in a detergent wash than in an alcohol wash.
But would the small differences in densities between the alcohols make much difference in the sinking rate of mites? Besides density, there is also the molecular attraction of mite cuticle to the alcohol or water, as well as viscosity (liquid “friction”). I needed to run a test. I first measured the maximum distance that a mite might need to precipitate through the alcohol (Fig. 9).
Figure 9. I live in California’s Gold Country, and have plenty of experience in precipitating gold flakes through gravel when panning or sluicing. The same principles apply to dropping varroa through bee bodies. In the mite wash cups that we use, a mite may need to sink as much as 2½ inches. I want them to do so in less than 60 seconds.
Assisted by my assistant Brooke Molina, I shook live mites from bees using powdered sugar. Using soft forceps, I dropped them one at a time into a graduated cylinder filled with a concentration of alcohol to be tested. We watched as the mite began to sink, the sugar dissolved, and the mite reached its terminal sinking velocity. Brooke would then hit the stopwatch as the mite passed the marked start and finish lines (Fig. 10).
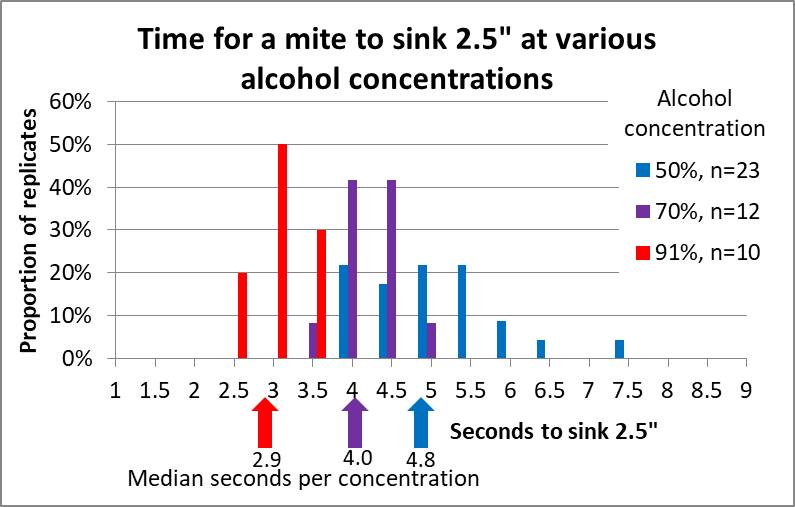
Figure 10. We timed the sinking rate of 51 mites in all. There was some variation, but a clear difference between alcohol concentrations.
Rather than showing you a bunch of calculated means and statistics, I feel that a picture is worth a thousand numbers. For this sort of information, I like histograms (frequency distributions), which allow your own brain to look for patterns (Fig. 11).
Figure 11. It only takes a mite around 3 seconds to sink 2½” in 91% alcohol (the clump of red columns), compared to nearly 5 seconds (on average) in 50% alcohol (with wider clump of blue columns).
Since I my agitators run for 60-seconds, the difference between sinking for 3 or 5 seconds is likely not going to make a huge difference – so long as there is no vertical agitation stirring them back up.
Practical application: When washing mites, swirl rather than shake up and down – up-and-down shaking simply keeps stirring the mites back up into the bees. When using 91% alcohol (or Dawn detergent), there is actually little need to agitate to achieve mite detachment – you only need to keep the bees in motion in the solution so that the mites can precipitate down through their bodies.
What I’ve learned so far
This has been quite a learning experience for me. I’ve now covered release and dislodgement of the mites, and their precipitation. The sugar shake requires hard agitation, whereas some of the liquid release agents require scant agitation. I’ve learned that with the better methods, that you can recover 95% of the mites in 60 seconds.
In my next article, I’m going to focus upon the liquid release agents, to try to figure out why some work well, and some don’t.
A Spoiler
Since alcohol is in short supply, I’ve been asked by many beekeepers what they can use for monitoring varroa, and I don’t want to make you wait until I publish my full results next month.
Practical application: I’ve switched to using Dawn Ultra dishwashing liquid (my data is from using the clear “Lemon essence” product), which works very well for mite washes, on par with 91% isopropyl, and better than any other release agent I’ve tested. Disclaimer: I have no connection with, or interest in, the manufacturer of any products. I’ve tested Dawn dishwashing liquid and two other detergents (which weren’t impressive); but other foaming detergents may work as well.
Dilute the Dawn at the rate of 1-2 tablespoons per gallon of water. A weaker solution is less efficacious, and there is no benefit to making it stronger.
Don’t agitate immediately – instead allow the bees to soak in the solution for a full minute before agitation, by which time most of the mites will have already dropped to the bottom of their own accord. Final agitation should be a swirl action, with no up and down shaking. Little agitation is required for basic mite monitoring.
For counting after agitation, a wonderful trick I’ve found is to build a stand to hold the mite wash cup 4 inches above the face of a 6-inch diameter, 10x magnifying mirror (makeup mirror) placed horizontally below the cup. 10x is the best magnification, and 6″ diameter the best size. Looking down, this gives you a greatly enlarged view of the mites (you can see their legs) and makes counting a piece of cake (Figs. 4 & 12).
Figure x. Viewing from the bottom up means that the foam from the detergent is not a problem. Since your treatment threshold should be no more than 6 mites, counting is a breeze. Since I often count in the 50’s to 70’s for testing, I’ve found that it helps to close one eye for counting, and use a pointer.
Warning: a magnifying mirror, casually placed, can easily start a fire should the sun hit it. The focus point is a few inches from the mirror, and sunlight through a truck window is enough to start a fire within seconds (practical experience by my sons). ALWAYS place the mirror into an opaque holder when not in use!
Next month: the result of testing various solutions, and trying to understand why some work better than others.
Acknowledgements
My research is supported by donations from beekeepers. If you find it to be of benefit, I appreciate donations at ScientificBeekeeping.com. I also thank Peter Borst, Brooke Molina, and Michelle for their assistance.
References
[1] Fakhimzadeh K. (2000) Potential of super-fine ground, plain white sugar dusting as an ecological tool for the control of Varroasis in the honey bee (Apis mellifera), Am. Bee J. 140: 487–491.
[2] https://scientificbeekeeping.com/powdered-sugar-dusting-sweet-and-safe-but-does-it-really-work-part-1/
[3] Macedo, P, J Wu & M Ellis (2002) Using inert dusts to detect and assess varroa infestations in honey bee colonies, Journal of Apicultural Research, 41(1-2): 3-7.
[4] https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=2174&context=extensionhist
[5] https://gpmb.unl.edu/varroa-sugar-roll-sampling
[6] Cooper, P, et al (1985) Temperature regulation of honey bees (Apis mellifera) foraging in the Sonoran Desert. J. exp. Biol. 114: 1-15.
[7] Bernd Heinrich (1996) How the honey bee regulates its body temperature, Bee World, 77:3, 130-137.
[8] Before any nitpickers write to correct me, I converting all the specific gravity figures to densities with regard to temperature.
[9] Lebo, R (1921) Properties of mixtures of isopropyl alcohol and water. J. Am. Chem. Soc. 43(5): 1005–1011.