A Study on Bee and Mite Drift: Part 2
Contents
Preparation of the Mite Donor Colonies. 3
Preparation of the Mite Receiver Hives 7
Acknowledgements And an Apology. 12
A Study on Bee Drift and Mite Immigration
Part 2
Beekeeper-funded applied research – by a beekeeper, for the benefit of beekeepers.
Randy Oliver
ScientificBeekeeping.com
First published in ABJ March 2023
Previous research indicates that varroa mites utilize drifting and robbing bees to get carried to other hives in the neighborhood. But there were still a number of outstanding questions about the details of mite immigration. Wanting more information, in 2018 I undertook an ambitious study to answer a few.
Back in 2018, beekeepers were talking about “mite bombs,” and blaming their problems on the immigration of mites from highly-infested neighboring colonies outside of their control. Last month I reviewed published studies regarding this phenomenon. But we must keep in mind that institutional researchers are often under pressure to “publish or perish.” In this competitive environment, I unfortunately see a lot of conclusions being drawn from datasets involving a relatively small number of marked bees and hives.
Wanting to gain a better understanding of bee drift and mite immigration in my own operation, I decided to run a larger-scale study, involving more colonies, more marked bees, and greater distances, in order to help answer the following eight questions:
Questions to Answer
- How much mite immigration actually takes place in late summer and fall?
- Is mite immigration steady or episodic?
- Does mite immigration correlate with robbing?
- Do all hives suffer equally from mite immigration, or are some more attractive to drifting bees?
- What proportion of workers from collapsing colonies drift to other hives?
- Do elevated varroa/virus levels increase bee drift from a hive?
- How far do bees from collapsing colonies drift?
- How does the drift of bees to nearby hives compare to that of those more distant?
Could I possibly get answers to all the above questions from a single field study? Since the devil is always in the details, in this article I’ll go over how we set up the study.
Materials and Methods
At my home in the dry Sierra Foothills [[1]], we set up ten “Donor hives” (containing tagged bees) to intentionally collapse from varroa (unfortunately one collapsed prematurely), and then twenty-four “Receiver hives” at various distances to determine the number of tagged bees drifting into them from the collapsing Donor colonies (Figures 1 & 2).
Placement of the Hives
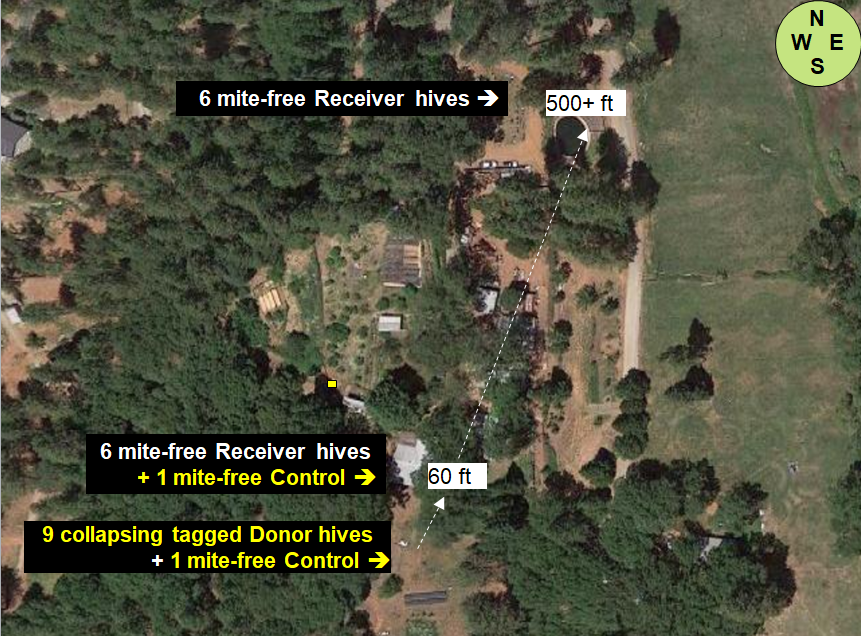
Fig. 1 The layout of the home yard, showing placement of the collapsing Donor hives, as well as the Receiver Hives from which data for bee drift and mite immigration was taken. We are surrounded by sloping mixed oak/pine forest, with irrigated pasture to the east. My garden and orchard are in the middle of the photo. The brown dry ground is how our non-irrigated land looks during the summer.
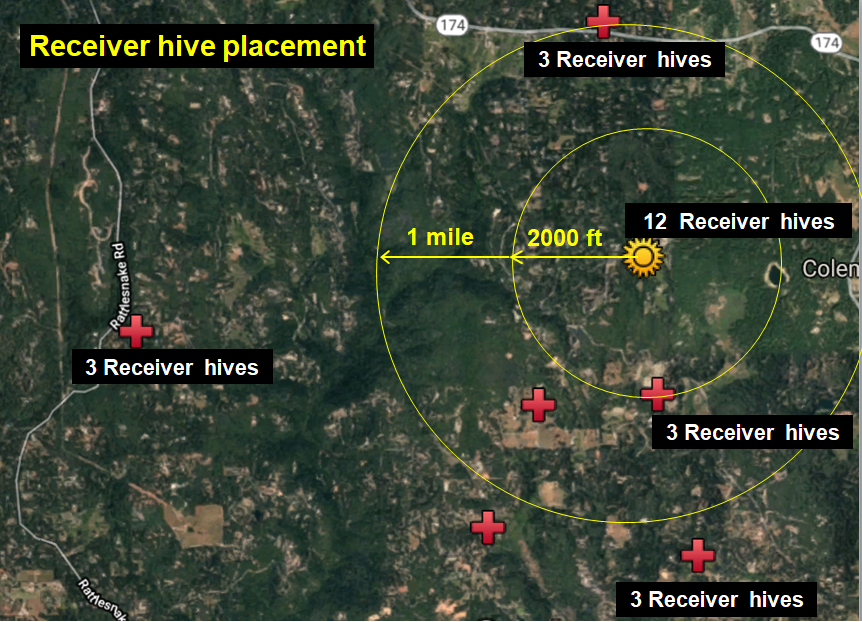
Fig. 2 Zooming out, with my home yard indicated by the golden star. We monitored six surrounding apiaries of ours (red crosses) for drifted bees. Each apiary held around 30 colonies, although only three vigorous colonies in each were prepared as “receivers.”
Study design: The above setup allowed us to answer the questions “How far do bees from collapsing colonies drift?” and “How does the drift of bees to nearby hives compare to that of those more distant?”
Preparation of the Mite Donor Colonies
Following a standard practice of using colonies of “black” or “yellow” bees to identify those that drift, in June we prepared two different sets of hives with bees of different colors. We requeened the Donor Hives (intended to collapse) with “yellow” cordovans, and the mite-free Receiver Hives with dark Carniolans (graciously provided by queen producers Frank Pendell and Valeri Severson of Strachan Apiaries respectively).
Over the course of July we fed the colonies to grow them, boosting them if appropriate with combs of high- or low-mite brood. At the end of July we took mite wash counts again — some of the Donors already had counts of 50-100 mites per half cup of bees! So we replaced some brood combs with those from low-mite colonies, and hit them with half a MAQS formic pad, in order to keep them from collapsing before we had the Receiver hives ready.
Tagging the Bees
It became apparent that due to the swapping of combs, that the black/yellow scoring method of bees for parent colony identification wasn’t going to work, so after reviewing various alternative methods for marking bees, I decided to instead modify a clever bee-tagging method developed years ago by the inventive Dr. Norm Gary [[2]], whom I’ve known for years, and who was happy to help me with details. My helper Brooke Molina and I spent the latter half of July and into August perfecting the technique, figuring out the best anesthetization method, holders for the bees, type of glue, etc. (Figure 3).
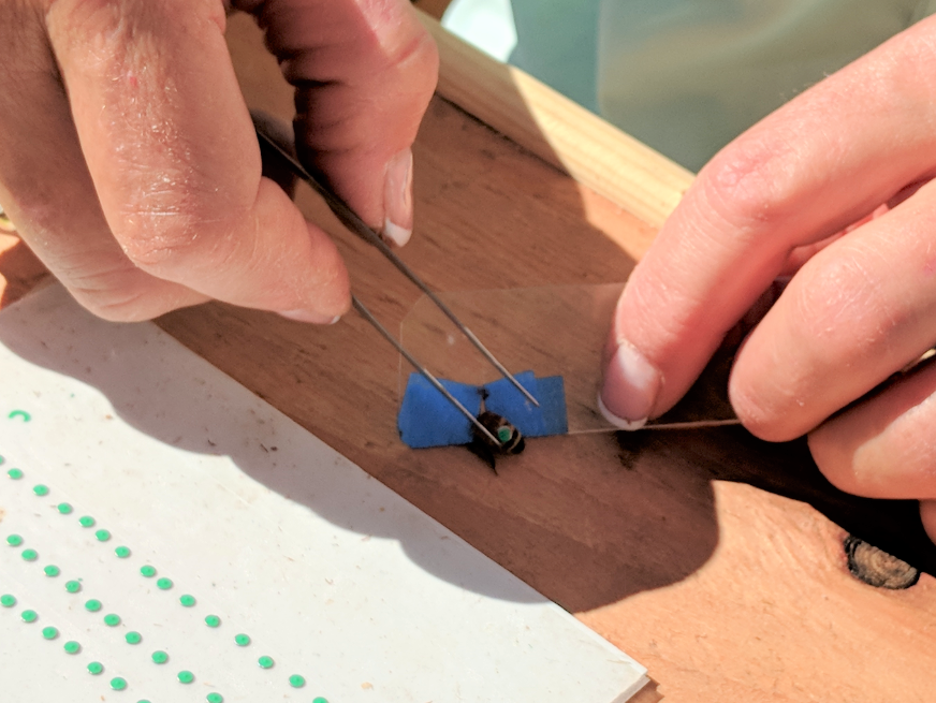
Fig. 3 We hand-punched over 6000 thin steel discs and Brooke hand-painted them with different colors for each Donor colony, placing them on cards covered with double stick tape. The tagging crew then glued them onto an average of 540 bees per colony during each Donor’s collapse. Here’s Alice Dunbar demonstrating how we used a piece of plastic with a V-notch (wrapped over with masking tape) to slide over a bee’s petiole, and harmlessly hold it still with its wings out of the way. We put a drop of Elmer’s quick-drying glue on the abdomen, then used forceps to drop and tap a disc into the glue.
Alice’s husband Brion filmed her tagging bees; you can watch her do it at https://youtu.be/2FEeL-O08qA.
Experimental note: After experimentally confirming Dr. Gary’s suggestions for disc thickness and diameter, we punched 0.09” diameter discs from 0.010” sheet steel. The painted discs weighed less than 10 mg — only a fraction of the weight of a load of nectar. We also followed his suggestion [[3]] that “Label attachment on the abdomen seems preferable to thoracic attachment for several reasons. There is less risk of accidentally fouling the wings with adhesive. Furthermore, labels tend to break free from the abdomen more easily because there are fewer cuticular setae. The abdomen is adapted for carrying nectar or water loads that constitute the heaviest weights transported by bees. Attachment of inflexible metal label to the thorax possibly may affect flight behavior, either by creating a balancing problem or by adversely affecting the scutum’s flexibility.” We were also careful to avoid magnetizing the discs, so as not to affect the bees’ magnetoreception during flight [[4]].
For tagging the bees, I custom built a chair with an adjustable desk to each of my helpers’ preferences. Some of the taggers just loved sitting outside and enjoying the scenery while they glued on tag after tag (Figure 4).

Fig. 4 Here’s Brooke sitting next to a hive, marking bees. We behaviorally-sorted young workers to tag by dumping shook bees into the black bottles — the older bees immediately flew out toward light, whereas young workers remained inside in the darkness. The tagger shook out as many bees as they could process in a few minutes into the clear plastic bottle, and then gave it a shot of CO2 (using a bicycle tire filler with a hollow needle) to briefly anesthetize them.
It takes a number of hands to tag 6000 bees, so in addition to Brooke and the Dunbars, we were helped by Anna Mudd. Some of us were quicker at it than others, so I paid my helpers by piece rate (Figure 5).

Fig. 5 Anna had been grafting queens for us so her fine-motor skills were well developed. I snapped this photo when she realized that she could earn more per hour tagging bees than she’d ever made in any previous job.
As we glued tags on the bees, we sat next to the hive, with the hive cover slid partially open, so that we could immediately return each tagged bee back to its colony (Figure 6).

Fig. 6 The taggers immediately placed the tagged bees onto the top bars of the hive from which they had been taken. The bees quickly recovered from the anesthetization, walked down onto the combs, and there acted normally.
The Control Group
In order to answer the question “Do elevated varroa/virus levels increase bee drift from a hive?” I needed to run tagged mite-free Control colonies to compare their worker drift to that from collapsing colonies. I placed two treated mite-free Control hives within the high-mite Donor group of hives. Spoiler: As you’ll eventually see, I should have run as many Controls as Donors. (By good fortune I got lucky. Sometimes that happens).
Preparation of the Mite Receiver Hives
Eliminating the Mites
In order to quantify the amount of mite immigration into the Receiver hives, we copied the methodology of Sakofski [[5]], Greatti [[6]], and Frey [[7]], in which they used high-efficacy synthetic miticides to eliminate the mite population in receiver colonies. This allows the creation of virtually mite-free colonies (exhibiting daily mite drops and mite washes of zero). Continual treatment with slow-release miticide strips then causes any incoming mites (carried by bees) to drop within a day onto a stickyboard, providing a count for mite immigration.
Since we hadn’t used any synthetic miticides for over 15 years (and rotate a quarter of our brood combs each year), our mites exhibit no resistance to experimentally-applied amitraz or fluvalinate, so we applied those two active ingredients (Figure 7).

Fig. 7 We started the colonies in April 2018, treating them with oxalic acid, then in June with formic acid, followed by a combination of Apistan plus Apivar strips.
The prepared Receiver hives in the home yard thus were mite-free, continually treated, with stickyboards, scales, and magnetic entrance traps (Figures 8 & 9). We didn’t place stickyboards or scales on the outyard Receiver hives.

Fig. 8 A Receiver hive ready to go! I designed the entrance traps so when a drifted tagged bee entered the hive, its steel tag would snap onto the magnet very firmly. I set the entrance height so that the stuck bee could still grab the bottom board with its feet and pull itself free of the color-coded tag — leaving a clear record of the bee’s entry that we could then later record without having to be there to observe the actual event.
Magnetic Tag Recovery
After consultation with Norm Gary, I designed and fabricated magnetic tag retrieval entrance traps, improved by using super-strong neodymium magnets not available when he invented the method [[8]] — they worked great! A magnetic trap placed at the entrance of each Receiver hive removes and holds the tag of any drifted bee that attempts to enter it, allowing us to go out every couple of days and quickly remove and record any tags from drifted bees at each colony entrance. The beauty, for my purposes, of using Dr. Gary’s ferrous tagging technique is that it not only records the entrance of a drifted bee, but also its home colony.
Practical application: Although state-of-the art methods of tracking bees with RFID tags or video recording are wonderful, Norm’s tried-and-true “old school” technique works extremely well and is essentially foolproof, since it collects hard evidence in the form of painted discs that can be recovered at the investigator’s convenience, rather than by sitting in front of a hive (or a video) looking for returning bees, or opening the hive and searching for marked bees on the combs. I’m surprised that more researchers don’t use it!
The Stickyboards
We installed rear-access stickyboards under screened bottom boards in all the Receiver hives in the home yard (Figure 9). We recorded the number of dropped mites roughly every third day.
Study design: The stickyboard counts of the Receiver hives could help us to answer the following three questions:
How much mite immigration takes place in late summer and fall?
Is mite immigration steady or episodic?
Do all hives suffer equally from mite immigration, or are some more attractive to drifting bees?
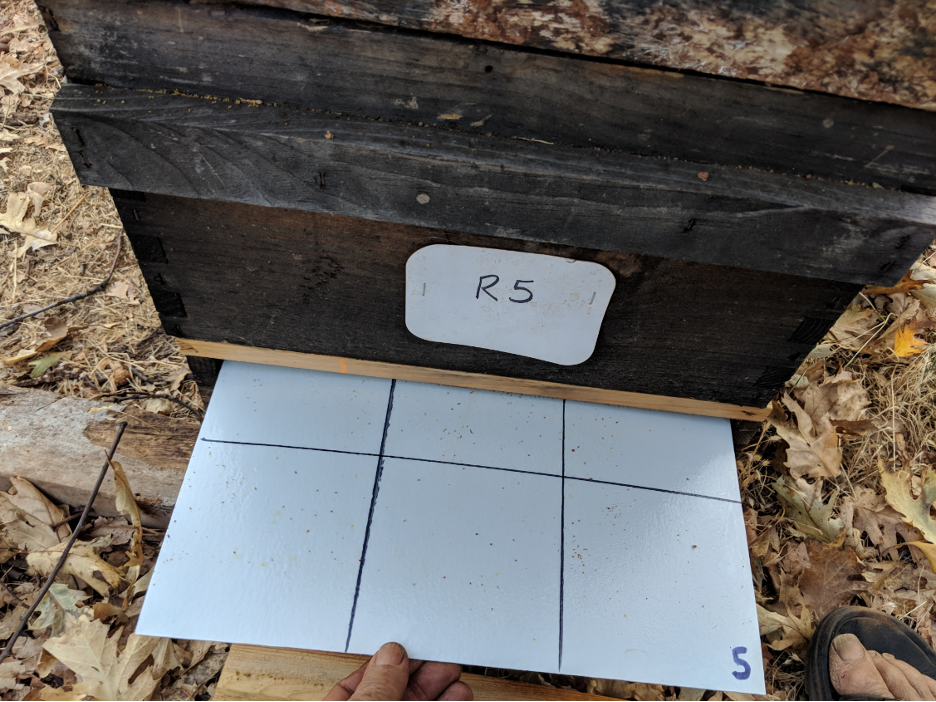
Fig. 9 I make sturdy stickyboards out of FRP (fiber-reinforced plastic) wall paneling, which can be used and reused for years. After counting and recording the number of mites, we scrape off the adhesive, mites, and hive trash with two strokes of a sheetrock knife. We then re-roll on a mineral oil/ petroleum jelly mixture with a small-diameter paint roller, and reinsert the stickyboard.
The Hive Scales
In order to track whether a Donor colony was being robbed, or a Receiver colony was robbing other hives, I put Broodminder electronic scales under both the Donor and Receiver hives in the home yard. Thanks to Rich Morris of Broodminder for cutting me a deal on the scales, and for his help with setup and data processing. I found the Broodminder scales to work very well for my purposes. I confirmed that placing a single scale under the front end of the bottom board accurately reflected total hive weight change.
Study design: In order to answer the question of whether mite immigration correlates with robbing (in our foothill environment), I intentionally performed this study during our late-season nectar dearth (when no weight gain would be taking place). A robbing colony would exhibit weight gain, whereas a colony being robbed would show a weight loss. The stickyboards would allow us to determine how many mites were getting rides into the hive on any day. We could then look for correlations.
Layout of the Donor hives
Figure 10 shows the layout of the donor and close Receiver hives.
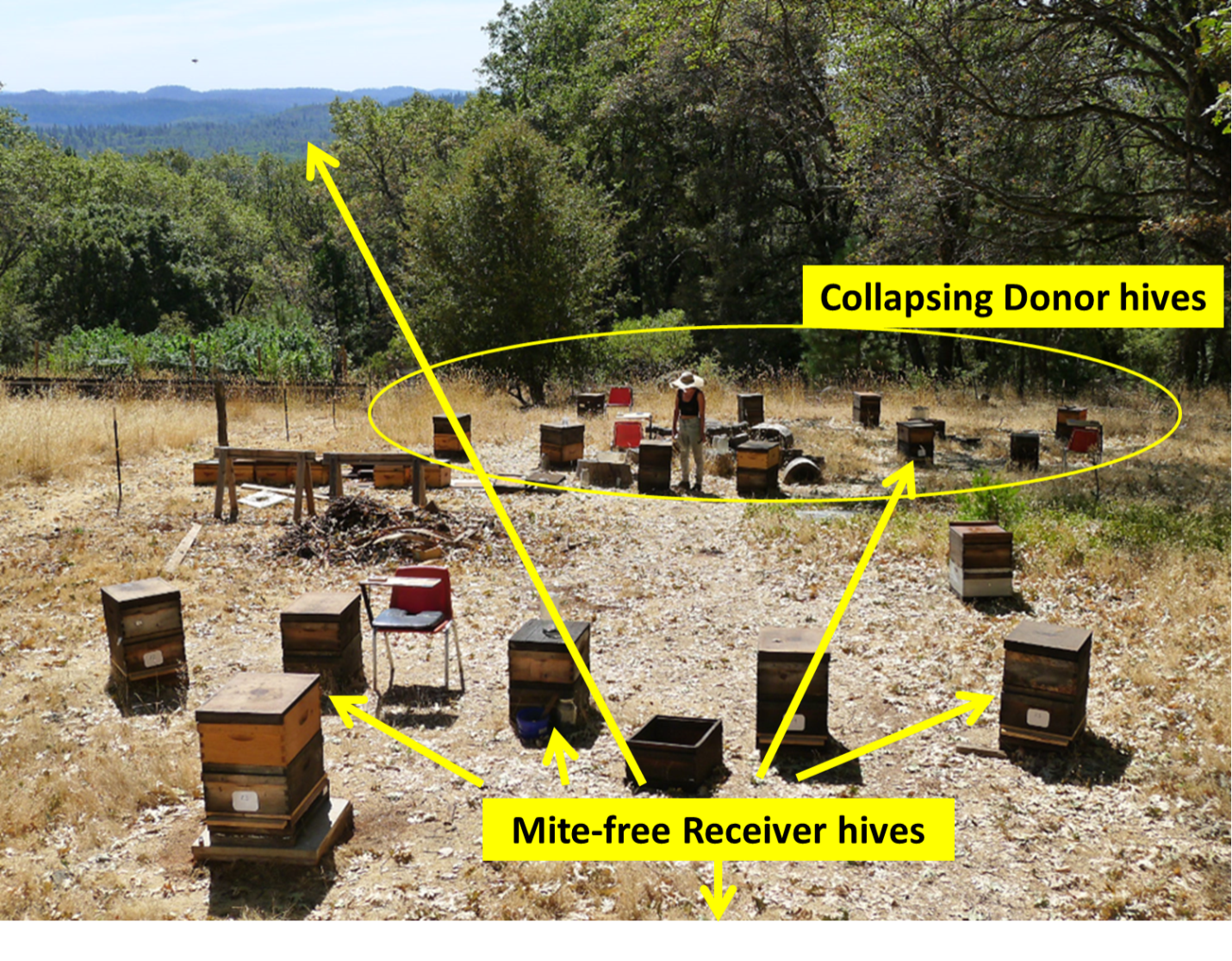
Fig. 10 The view looking south from my back porch. The long arrow points to our nearest apiary a half mile away. We’re in Northern California, so the requisite solar panels and cannabis garden are in the background. The down arrow points towards my house, with more Receiver hives some 500 feet away.
OK, I’ve shown how we set up a study to attempt to obtain answers to all eight our original questions. I’ll continue with our findings next month.
Acknowledgements And an Apology
I greatly appreciate the help from my crew — Brooke Molina, Anna Mudd, and Brion and Alice Dunbar, without whose help I could never have pulled this study off. Also a shout out to Dr. Norman Gary, whose impressive body of research has been instrumental to mine.
I again thank Valerie Severson and Frank Pendell for the queens, and Rich Morris for help with the Broodminder scales.
Last but not least I thank those beekeepers whose donations help me cover the costs of my applied research projects. That said, I apologize for waiting so long to publish my results, but at the time of this project I was chastised by a university lab leader who was upset that I kept getting the jump on their own research projects. So out of courtesy I’ve delayed presenting my findings until they published theirs.
Citations and Notes
[1] GPS coordinates 39.17702, -120.99153, 2880 ft elevation, yellow pine/black oak woodlands.
[2] Gary, N (1971) Magnetic retrieval of ferrous labels in a capture-recapture system for honey bees and other insects. J. Econ. Ent. 64: 961-965.
[3] Ibid.
[4] Liang, CH, et al (2016) Magnetic sensing through the abdomen of the honey bee. Sci. Rep. 6: 23657.
[5] Sakofski, F., Koeniger, N., & Fuchs, S. (1990). Seasonality of honey bee colony invasion by Varroa jacobsoni Oud. Apidologie, 21(6), 547-550.
[6] Greatti, M, N Milani & F Nazzi (1992) Reinfestation of an acaricide-treated apiary by Varroa jacobsoni Oud. Experimental & Applied Acarology 16(4): 279-286.
[7] Frey, E., H Schnell & P Rosenkranz (2011) Invasion of Varroa destructor mites into mite-free honey bee colonies under the controlled conditions of a military training area. Journal of Apicultural Research 50(2): 138-144.
[8] Magnet size 3 x 6 x 60 mm, set in line in a groove.



