Nosema Ceranae: Additional Reports and Ramblings
Nosema Ceranae: Additional Reports and Ramblings
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in Oct. 2008
Allow me to swap hats, and now speak to you as a beekeeper. As I was writing this article, I was asked similar questions by a number of beekeepers. Here they are, with answers to the best of my knowledge:
Q: My bees tested at 2 x 106 spores per bee this summer—is that serious?
A: Hard to say. It looks like anything under about 5 million (5 x 106) may be OK if the bees have access to good nutrition.
Q: My bees are on a nectar flow and won’t take medicated syrup. Or, I’m afraid of contaminating my honey if I feed the colonies gallons of medicated syrup. Can I use the drench method?
A: Lots of commercial beekeepers are swearing by the drench method to solve the above problems, and because it saves time and money.
Q: I want to drench, but it will cost me over a buck a colony just for the Fumagilin-B. Can I cut the dose?
A: Apparently not. Commercial beekeepers are commonly using a large 9.5g bottle dissolved in 5-8 gallons of syrup, drenched at the rate of 1 cup per colony. As always, ask your state apiarist for recommendations.
Q: Can I just drench once or twice?
A: Looks like you need to apply treatment in the colony for at least four weeks to really break the infection cycle.
Q: What about feeding pollen supplement?
A: During winter, Dr. Frank Eischen’s data indicate that if spore counts are under 3 million, feeding pollen supplement may be as effective as treatment with fumagillin. Pollen supplement may also be a good idea if a strong honeyflow ends, leaving colonies with brood, but little natural pollen forage. It may also be helpful if the bees are on a poor pollen monoculture, such as corn or blueberries.
At this point I would like to include some other observations from my other nosema test yard (currently being analyzed by a collaborator). In this yard, I treated 64 infected colonies with various nosema treatments in early winter and early spring. About a quarter of the colonies died. Quick field sampling in the spring indicated that all the survivors were still infected with nosema. Of the survivors, most built up strongly, and several swarmed—the bees in the swarms were infected to the tune of about 3 million spores per bee average. These colonies and swarms continued to build strongly and put on great honey crops. However, a percentage of the colonies never built up, and stayed stuck all spring and summer at about 5-6 frames of bees (or less). They typically had brood problems—EFB-like symptoms, sacbrood, or unusual yellow sick larvae. I sent samples to Dr. Jay Evans, who found the usual viral and bacterial suspects, but nothing unusual. Other samples are currently being tested at Penn State by Dr. Nancy Ostiguy and Abby Kalkstein, and at U.C. Davis by Dr. Michelle Flenniken.
The big question is, are these colonies (now going into their second season with N. ceranae) going through Higes’ “false recovery” phase, soon to collapse? I took entrance samples from four of them August 21—they ran spore counts of about 5 million spores per bee, but the colonies appeared to be thriving. Time will tell.
The author at his makeshift lab bench. Preparing and counting multiple samples of nosema spores is a slow, tedious slog, about as much fun as counting mites on sticky boards, except that you are peering through a microscope. (When you close your eyes at night, you see visions of nosema spores.)
A big question to me (and others) is just how much a problem N. ceranae is when colonies receive adequate nutrition, and are otherwise healthy (read that, low virus and mite levels). Pajuelo (2008) tested the effects of fumagillin treatment with or without additional vitamin and protein supplementation of Spanish bee colonies: “we conclude that if the climatic conditions are good, with an availability of pollen and nectar, then the addition of vitamins and protein is not necessary. Their use only increases the cost of production. The addition of fumagillin as a preventative measure in hives where the asymptomatic presence of N. ceranae has been diagnosed, does not solve any problem, but merely increases costs, and risks unnecessarily contaminating hive products with this antibiotic or its subsequent metabolites. When there are no factors such as climate, inadequate nutrition, inadequate control of V. destructor or unsuitable management, causing immunosuppression in the bees, colonies do not collapse” [boldface mine].
In my operation, I’m questioning whether N. ceranae is a serious problem in its own right. It is more prevalent in some yards more than others, and may reflect poor nutrition during our dry western summers. However, nosema is well known to stress bees, and to open up their gut for penetration by viruses. I do not discount the likely nosema/virus connection.
I am getting reports from other beekeepers, especially in the Midwest, of sky-high nosema counts (in the tens of millions of spores per bee). At these levels, I would certainly expect nosema to be a problem. However, in California this spring I saw colonies that had spore counts of over 50 million that appeared to be thriving! A question then, is why are they experiencing such a robust infection? One hypothesis is that since it has been well demonstrated that Nosema apis is clearly a disease of nutritionally-stressed bees, that N. ceranae may also be of the same ilk.
This again brings up my question as to whether bees foraging on monocultures that produce nutritionally-deficient pollens, such as corn, sunflowers,b blueberries, cranberries, white box (Australia), and alfalfa, are prone toward problems with nosema, and likely with opportunistic viruses following. The same could happen when low-protein colonies experience an intense nectar flow, or two buildups without recovery in the interim, or a pollen dearth after almonds, causing protein levels to drop even further. Any such nutritionally-stressed colonies would be sitting ducks for nosema and any associated viruses.
A number of commercial beekeepers are now adding fumagillin to their pollen patties as a relatively inexpensive way of getting some treatment into their colonies, especially when they were not taking syrup. One large operator told me that the effect was not nearly as good as with syrup. Although feeding fumagillin in a patty may reduce the infection, one must wonder whether it the fumagillin, or merely the additional protein, that is really helping the colony.
On a final note, I find the use of hemacytometer counts to be unnecessary for general beekeeping decisions. The blender method described in my article “The Nosema Twins 3” is quick, much easier to see spores, and adequately accurate. However, I suggest a slight revision from the method of Topolska (2005) that I originally detailed, since the half milliliter dilution does not allow for blender grinding of smaller samples, and the resulting fluid is full of debris. I now suggest following the standard dilution of 1ml per bee. I will revise my website to reflect the change. Go to “Nosema Twins—Part 3.”
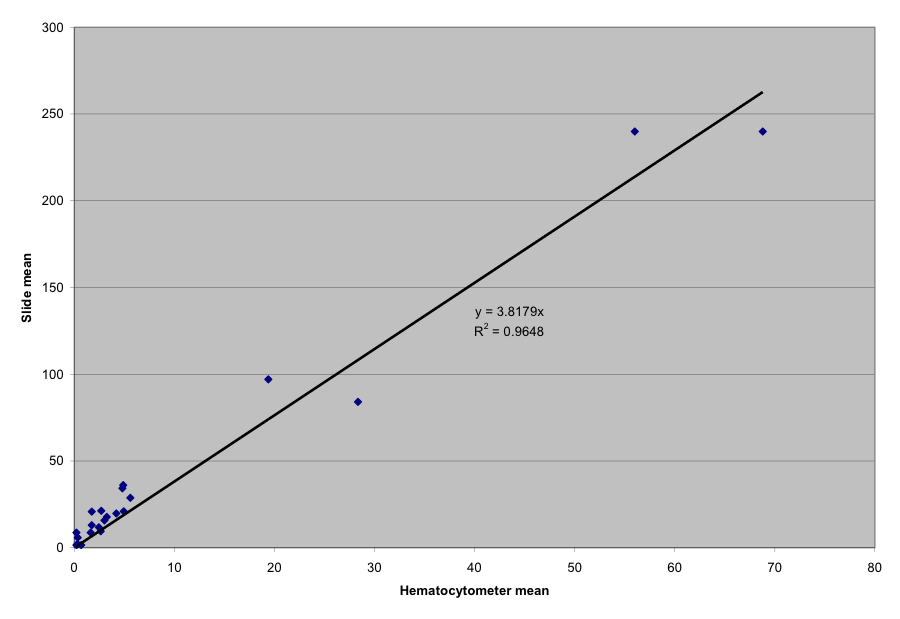
I wish to thank the California Beekeepers Association for a grant to calibrate the “field of view” count versus the Cantwell calculation of million spores per bee. The ratio is illustrated in Figure 6 and Table 4.
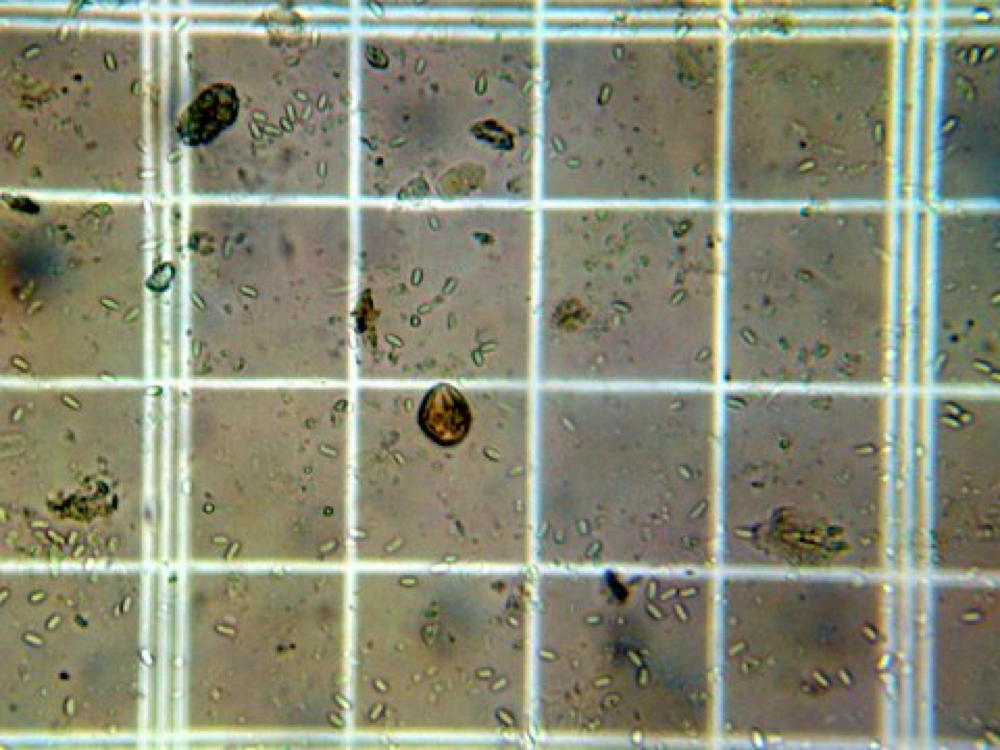
Nosema ceranae spores in the grid of a Bright-Line hemacytometer. At an average of over 5.5 spores per square, this bee contained more than 22 x 106 spores.
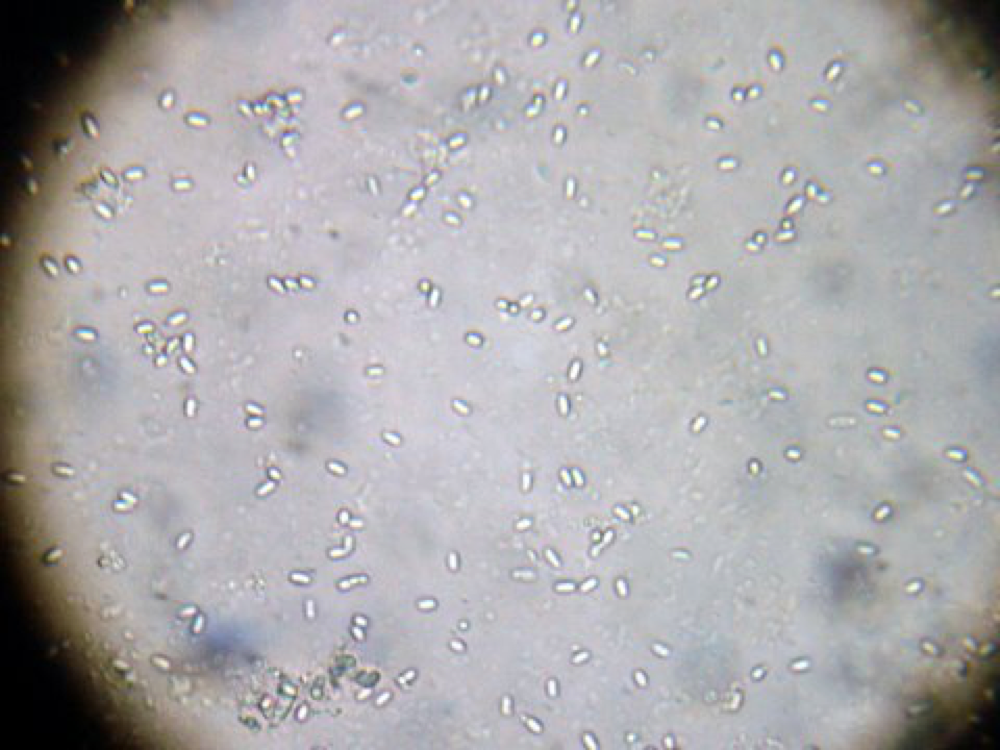
Nosema spores on a simple glass slide, single bee, crushed in 1ml of water. This concentration indicates an infection level for this bee of about 40 million spores.
Figure 6. A calibration of the “field of view” count at 400x, at the dilution rate of 1ml of water per bee in the sample, bees blended at “chop” setting. Each data point represents the means of thirteen separate measurements, but please note that there is great variability in counts. Note that the equation tends to underestimate infection level somewhat at field of view spore counts of under 50 (I don’t know why—I just count the spores).
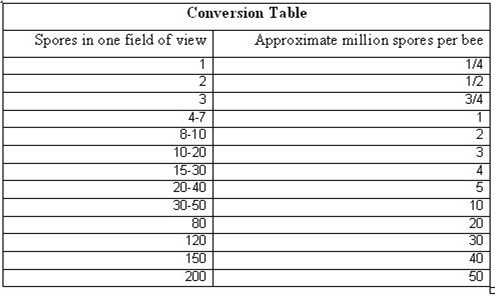
Update: Forget the table above! For general estimates, figure that for every 5 spores in a field of view that you are seeing an infection rate of about a million spores per bee. I.e., if you see 10 spores, figure an infection of 2 million spores per bee.
Table 4. Conversion of numbers of spores in one field of view to approximate millions of spores per bee by the Cantwell method (close enough for management decisions). It is difficult to count more than 40 spores per field of view accurately, but that really doesn’t matter by the time your bees reach that level of infection. (At 400x magnification, at the dilution rate of 1ml of water per bee, one drop of milky solution (no bee pieces) under a plastic cover slip, lightly tapped down once with your finger tip.)
So what exactly does such and such a spore count actually mean? I’ve spoken to several large commercial beekeepers who are tracking spore numbers, as well as watched my own colonies. The best that I can tell, under good nutritional conditions, spore counts of bees from the entrance under 5 million (5 x 106 spores per bee, average) are not unusual, and the colonies do not appear to suffer. How can this be, you say? The key word is “average.” That number does not mean that all the foragers suffer to that level. As an example, I took spore counts today from 25 individual bees whose colony ran at 5 million spores per bee average. Out of 25 foragers, 20 had zero to only a few spores. The remaining 5 had counts of up to 50 million spores per bee! Averaged out, 20 bees with zero spores, 3 bees with 8 million, and 2 with 50 million each, would give an average count of 5 million spores per bee. But note that 80% of the foragers had no nosema infection at all!
So how about the bees inside the hive? I took a sample of 100 bees from the periphery of the brood nest. I sampled them 25 at a time. Two samples had zero spore counts, and the other two only had a few spores. It appears that only two house bees out of a hundred had any nosema infection at all—and this is in a colony that came out of winter with a spore count of over 1 million spores per bee, has gone untreated through the end of August, and now tests at 5 million spores per bee! Sure I’d like to see this colony to be free of spores, but it appears to be doing just fine. In colonies with spore counts under 5 million, enjoying good nutrition, it appears that very few bees in the colony are actually infected to any level!
I strongly suggest that no beekeeper make management decisions based upon spore counts obtained from only a few dozen bees. The most reliable and comparable spore counts are likely those obtained from large samples of bees collected (vacuumed) from the entrances of many colonies at mid day, and processed as a batch, in order to determine an overall average spore count for the foragers. Do not include “crawlers” in the count, as they will skew your numbers sky high! Samples taken from inside the bee nets each time you move would be a good idea.
I’ve been trying to get DNA confirmation of a pure N. apis spore sample so that I could compare it visually with my confirmed N. ceranae samples. Thanks to collaborators Ruary Rudd, who sent me a sample of apis from Ireland, and Dr. Robert Cramer of Montana State University, who has been helping me greatly with my nosema research, and who has generously performed PCR DNA analysis for me, I can now show you the two species of nosema side by side (I placed a droplet of each on a slide, and photographed where they met).
As you can see, they are hard to tell apart unless you’ve looked at an awful lot of nosema spores. N. apis spores are a smidgen longer, more oval, and noticeably “fatter,” with curved sides. N. ceranae spores are generally a tad shorter than apis, and more “slender. They are more the shape of Good n Plenty candy, with nearly parallel sides. Ceranae can be much more variable in shape and size than apis–they can be banana or wedge shape, and sometimes stretched long (but the same width).
These descriptions are for general information, not for diagnosis! They are also subject to revision as I obtain more information.
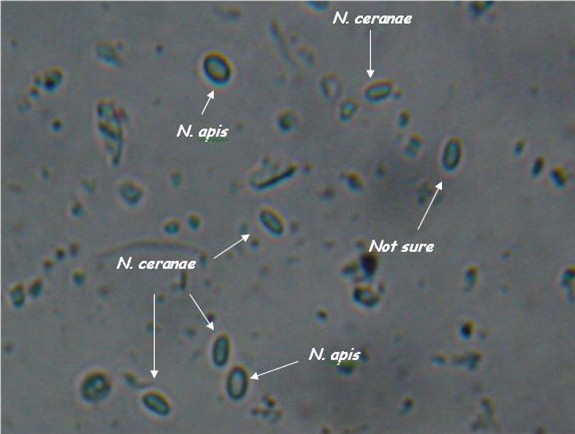
Nosema apis and ceranae spores side by side. Note that apis is slightly longer, and “fatter.” The apis spores are courtesy of Ruary Rudd from Ireland, ceranae from my California test yard. PCR confirmation courtesy of Dr. Robert Cramer, Montana State University. Photo by the author, enlarged from 400x.
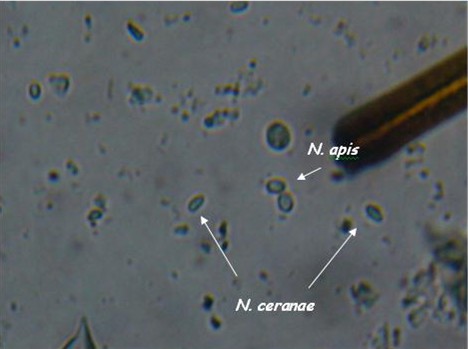
Another view, enlarged only slightly from 400x.