Sick Bees – Part 18F2: Colony Collapse Revisited – Plant Allelochemicals
Sick Bees Part 18f2: Colony Collapse Revisited
Plant Allelochemicals
First published in ABJ February 2013
Randy Oliver
ScientificBeekeeping.com
Have You Ever Noticed Plant Toxic Effects?
What Doesn’t Kill You Makes You Stronger
An Unintended Effect on Varroa?
Bee Detoxification of Allelochemicals
Plant Allelochemicals
How do pesticides relate to colony collapse? That sounds like a simple question, but as I said in the last installment, it’s complicated (there are rarely simple answers in biology). In order to begin to understand the effects of manmade pesticides upon bee health, we must first back up and understand some of the complex biology involved in natural bee/plant/toxin interactions.
Nature Is Not Nice
Every form of life on Earth is a “survivor” in an unbroken lineage that traces back roughly four billion years. In some of those years, conditions were certainly not favorable, and each and every organism by necessity needed to have the ability to detoxify the numerous natural chemicals and toxic byproducts of volcanoes, natural radiation, ultraviolet light, and the free radicals created by exposure to oxygen. Things got even tougher as bacteria and fungi began to engage in chemical warfare—creating a slew of nasty toxins in order to kill off their hosts or competition. And once plants and their predators evolved, they developed an entire pharmacopeia of phytotoxins to repel, irritate, sicken, or kill anything that tried to eat them.
We humans tend to forget that plants are naturally full of toxic substances, but all one need do is to simply go outside and start randomly consuming wild plant leaves, fruit, and seeds to confirm that this is indeed the case! (Kids, do not try this experiment!). Plant breeders have intentionally bred some of the natural toxins out of our crops (or at least from the edible portions—e.g., breeding out the bitter alkaloids from potato tubers, while leaving them in the leaves to repel pests). One unfortunate effect of this sort of breeding is that it makes our crops more susceptible to herbivorous insects, which then forces farmers to spray synthetic insecticides to protect the crop [1].
Practical application: in order to reduce farmers’ reliance upon pesticides, plant scientists can breed for naturally insect-resistant plants. The tradeoff is in repelling insects without making the plant too toxic for humans.
Toxic Pollen and Nectar
My point is that both humans and honey bees evolved in a world awash in poisons, and both eat diets brimming with natural toxins. As Gold [2] points out, the vast bulk of toxins consumed by humans are the natural poisons in our food. Honey bees also consume substantial quantities of natural phytotoxins—flower nectars and pollens contain a vast array of poisons (Fig. 1).
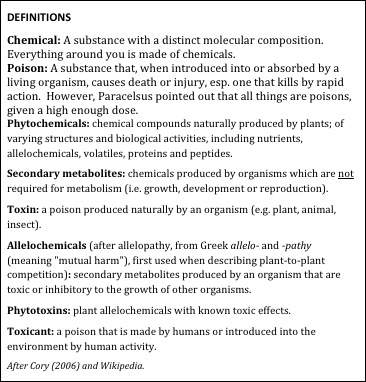
Figure 1. It helps to understand the vernacular used by toxicologists.
Although analysis of the bee genome indicates that for some reason honey bees appear to possess fewer detoxification genes than other insects, they are apparently adept at detoxifying or eliminating the plant toxins commonly found in nectar and pollen (Fig. 2).
Figure 2. A bee foraging on a species of Senecio. The pollen from this and a number of other related plants (comfrey, ragworts, and common groundsel) contain toxic pyrrolizidine alkaloids [[i]]—enough to raise concern by European health agencies about their levels in honey [[ii]]. Honey bees must metabolically detoxify these alkaloids [[iii]]. Photo courtesy Kathy Keatley Garvey.
[i] http://toxicology.usu.edu/endnote/Pyrrolizidine-alkaloids-in-food.pdf
[ii] http://www.efsa.europa.eu/en/efsajournal/pub/2406.htm
[iii] Reinhard, A, et al (2009) Feeding deterrence and detrimental effects of pyrrolizidine alkaloids fed to honey bees (Apis mellifera). J Chem Ecol. 35(9):1086-95.
The pollen and nectar from many bee-pollinated plant species contains toxic “allelochemicals” (alkaloids, cardenolides, essential oils, anthraquinones, flavonoids, saponins, coumarins, etc.). Some plants accumulate certain metals (e.g., selenium, manganese, copper) to levels that are toxic to plant-feeding insects [6], and onion flowers may concentrate enough potassium in the nectar that it discourages bee visitation [7].
In order to allow us to avoid excessive amounts of plant toxins, humans have extremely sensitive taste buds to detect the “bitterness” of typical allelochemicals. All you need do is to taste almond nectar to find that it is surprisingly bitter, or bee collected pollen (one color pellet at a time) to notice the bitter chemicals. Yet bees (and humans) readily consume, either by choice or necessity, such clearly toxic plant products. German researchers Detzel and Wink [8] tested 63 different plant allelochemicals for attractiveness, deterrence, and toxicity to adult bees. Nearly 40 showed some degree of feeding deterrence.
Surprisingly, they found that several were toxic at levels that didn’t cause feeding deterrence, meaning that bees might unwittingly consume a toxic dose! And remember that there is little chance for dilution of toxic nectar, since any individual nectar forager sticks to only one species of flower–which may be a good thing for the colony if an intoxicated bee is unable to return! But then those toxins are concentrated when bees process the nectar into honey.
And just how toxic were those natural allelochemicals? By my math, more than half of the tested compounds were acutely toxic to bees in the parts per million range–they would all have made Atkins’ [9] ranking as “highly toxic”—some of them approaching the toxicity of imidacloprid [10]!
Practical consideration: if one were to be admitted to the emergency room suffering from poisoning, the first thing that the doctor would ask would be, “What other drugs (toxins) had you taken?” Yet if a beekeeper submits a sample of beebread to the lab for the standard pesticide analysis of 170 toxicants, no one’s looking at the plant allelochemicals! When taken out of context of the bees’ total exposure to toxins both natural and synthetic, the typical pesticide analysis would give only part of the picture.
Beekeepers have long noticed that particular nectar flows may affect their bees—some nectar flows are said to make bees “pissy;” and the Australians refer to “hot” or “cold” honeyflows [11]. And bees may fare poorly if they get too much of a single nectar. For example, linden nectar (Tilia) contains the bee-toxic sugar mannose (as well as a bit of nicotine).
It makes one wonder why plants that depend upon insects to pollinate them would produce toxic pollen and nectar? Well, in the first place, pollen is precious to plants, and they only begrudgingly allow bees to eat it. Another theory is that toxins produced to deter herbivores from grazing on the foliage simply “wind up” in the flower products [12]. But it appears to be more complicated than that–in many plants the allelochemicals are either concentrated or diluted in the nectar and pollen (relative to the amount in the sap), or of different composition than in the leaves. So plants appear to be able to regulate their toxicity to pollinators.
Plants would then need to perform an evolutionary balancing act in being toxic enough to deter herbivores, yet keeping their nectar and pollen palatable to pollinators [13]. But nature abhors uniformity. The phytotoxins in plants differ in both mixture and concentration not only from species to species, but even from individual plant to plant (this is a common energy-saving strategy used by prey species to “train” predators). Linhart [14] studied wild thyme plants in the south of France, and found that individual plants varied in which of the six dominant monoterpenes that they produced. Thus, each individual plant exhibited a specific “chemotype.” Foraging bees may thus face a bewildering smorgasbord of plant chemotypes, even in a field of the same species of flowers! When given a choice, they may avoid those that are too potent (or maybe not!).
Practical application: during nectar dearth, or the failure of a particular bloom, foraging bees may find their choices to be limited, and be forced to bring back plant products that they normally would have avoided.
Other hypotheses [15] propose that plants toxify their nectar to favor specialized pollinators, or to deter “nectar thieves” such as ants, or generalists such as honey bees. One intriguing hypothesis is that plants serve up stimulants in their nectar to “hook” bees—bees apparently prefer a little caffeine or nicotine in their juice [16]. Such stimulants may also cause the bees to become more efficient pollinators by inducing them to move more rapidly from plant to plant, or not grooming the pollen off their bodies as efficiently. Nature is not constrained by any ethical rules of “fair play.”
Something of interest is that Baker [17] found that the nectar of flowers pollinated by butterflies (which don’t eat pollen) tended to have a higher content of amino acids than did that from plants normally pollinated by bees. This certainly implies that the nectar of some plants may be more nutritious to bees than that of others. Of even greater interest is that when he surveyed plants from tropical lowlands up to alpine tundra, he found that the lower in elevation (or further south), the greater the concentrations of alkaloids or phenolics in the nectar (presumably due to the greater intensity of insect pressure)! As we will see later, this has implications as to the differences in tolerance to manmade insecticides by honey bees as compared to bumblebees.
It is energetically costly to plants (meaning that it slows their growth) to produce these allelochemicals, so many annuals and biennials hold back until they start to flower (presumably to protect their future “offspring”). Anyone who has tasted lettuce after it has started to bolt has surely noticed the sudden increase in bitter allelochemicals! Plants may also upregulate the production of toxins when stressed by drought or by insect predation, or if neighboring plants communicate a chemical signal [18].
Practical application: floral toxicity may vary from plant to plant, region to region, or year to year, and increase in time of plant stress due to heat, drought, or insect attack.
At the colony level, incoming toxic nectar and pollen may be diluted or mixed with other pollens. Foragers intentionally seek out a diverse mixture of pollens, which may help to keep them from poisoning the broodnest, since some nectars and pollens are quite toxic (Table 1):
Some Plants Acutely Toxic to Honey Bees
Largely from Atkins (1975), and Pellet (1920)
| Black Nightshade
California Buckeye Corn Lily Death Camas Dodder Henbane Horse Chestnut Locoweeds |
Mountain Laurel
Oleander? Rhododendron Seaside Arrowgrass Summer Titi Western False Hellebore Whorled Milkweed Yellow Jessamine |
Table 1. The above plants are not in any sort of class by themselves—they are simply those that beekeepers in North America have reported under some conditions to cause serious adult or larval bee mortality. Most other plants produce a continuum of phytotoxins which may cause sublethal or beneficial effects that go unnoticed by beekeepers.
For example, in California, bees readily gather nectar from Buckeye (Aesculus californica) (Fig. 3):
Symptoms of buckeye poisoning usually appear about a week after bees begin working the blossoms. Many young larvae die, giving the brood pattern an irregular appearance. The queen’s egg-laying rate decreases or stops, or she may lay only drone eggs; after a few weeks, an increasing number of eggs fail to hatch or a majority of young larvae die before they are 3 days old. Some adults emerge with crippled wings or malformed legs and bodies. Foraging bees feeding on buckeye blossoms may have dark, shiny bodies and paralysis-like symptoms. Affected colonies may be seriously weakened or may die [19].
Figure 3. Pretty, but deadly. This is the toxic plant with which I have the most experience—California Buckeye. In “normal” years my bees work it heavily for nectar, but I don’t see them collecting much pollen; the nectar is diluted by the concurrent nectar flow from wild blackberries. But in years in which the blooms are out of synch, Buckeye can really hammer an apiary!
Although researchers have shown that the larvae of various species of bees vary in their ability to handle different pollens [20], and that some pollens are toxic to honey bee larvae [21], I’m not quite sure as to exactly what happens during buckeye poisoning, since incoming pollen is generally fermented into beebread, then eaten and digested by nurse bees, and finally converted into jelly for feeding the larvae and queen (actual pollen normally only constitutes a tiny portion of a larva’s diet). So I’m unclear as to how the Buckeye nectar or pollen kills the brood (perhaps the toxin is passed via the jelly?).
Have You Ever Noticed Plant Toxic Effects?
The legendary bee toxicologist Larry Atkins [22] described the symptoms of natural plant toxicity to honey bees:
The substances in poisonous plants which are toxic to bees are specific in action and may be in both pollen and nectar or may be confined to the nectar or simply to the pollen. Symptoms of plant poisoning are sometimes difficult to recognize or to be substantiated by chemical or microscopical diagnosis…The presence of symptoms usually is limited to the blooming period of the plant if the nectar is poisonous…However, if the toxic substance is in the pollen, the symptoms may linger as long as the supply of pollen remains in the combs…When only the adult bees are affected, piles of them may be found dead in front of the hive entrance, and there may not be enough adults to care for the brood or cover the combs.
And this is not to mention bees simply getting drunk on fermenting nectar [23]. The nectar in flowers readily ferments into alcohol, and beekeepers have long noticed drunken foragers. Surprisingly, the alcohol in nectar appears to be the necessary substrate from which forager bees produce the primer pheromone ethyl oleate [24], by which the colony regulates the proportion of foragers to mid-aged bees [25]. So the presence of some amount of the toxin ethanol may be critical for the normal colony division of labor!
Bees show clear preferences when offered various pollens [26], and produce more brood when fed some pollens compared to others [27]. Even when placed in fields of some popular row crops, foragers seek out other sources of pollen, sometimes to the near exclusion of the crop that they are supposed to be pollinating! But when we speak of the poor nutritional value of some pollen sources, we’re really not sure whether it is due to lack of nutrients, or to the presence of excessive toxins, or to some combination thereof.
Practical application: we really don’t know just how often bee colonies may be living on the “toxic edge.” We know that colonies build up (or maintain) better on some flows than others, but is the lack of buildup sometimes perhaps due to the bees being at the limit of their ability to detoxify the natural nectars and pollens that they are bringing in?
What Doesn’t Kill You Makes You Stronger
Paracelsus, regarded by many as the father of toxicology stated that, “All things are poison and not poison; only the dose makes a thing not a poison.” He recognized that something that can be toxic at a higher dose can be stimulatory or medicinal at a lower dose.
Early physicians carried strychnine in their bags and prescribed it as a tonic to their elderly patients—only in high doses was it considered to be harmful. Similarly, nicotine, caffeine, aspirin, and alcohol may be useful stimulants or have health benefits at low doses. The term for this effect is “hormesis” (from Greek hórmēsis ” to set in motion”) and is well described by Bniecki [28]:
If you haven’t yet heard of “hormesis” you probably soon will. A revolution is taking place in toxicology which will eventually change perspectives radically about the hazards or otherwise of pesticide traces in food. Hormesis is described as the paradoxical effect of toxins at low concentrations. The paradox is that although most chemicals are toxic at high concentrations (or dose), the majority are likely beneficial at low concentrations (or dose). The common regulatory assumption is that if a chemical is toxic at high dose it continues to be toxic but with diminishing toxicity as the dose is lowered. In contrast, hormesis indicates that many chemicals have the opposite effect at low doses to those at high doses.
The above words were written in 2003. You might have noticed that “hormesis” (or its implications) has not yet become part of the common lexicon. Baldwin [29] explains:
The hormetic perspective also turns upside down the strategies and tactics used for risk communication of toxic substances for the public. For the past 30 years, regulatory and/or public-health agencies in many countries have ‘educated’ — and in the process frightened — the public to expect that there may be no safe exposure level to many toxic agents… If the hormetic perspective were accepted, the risk-assessment message would have to change completely. Changing a dominant risk-communication paradigm is not as simple as flicking on a light switch. It changes beliefs, attitudes, and assumptions… It would certainly be resisted by many regulatory and public-health agencies…
Although the public has not yet resonated with hormesis, entomologists (who also refer to it as “hormogliosis”) are well aware of the effect [30, 31, 32]. Such hormetic effects would certainly be expected to apply to honey bees and plant alleleochemicals:
As with all toxins, carefully conducted dose response studies with allelochemicals generally results in the finding of hormetic responses [33].
So, in general it appears that sublethal doses of many toxins, instead of being harmful, may actually promote health! This concept is certainly contrary to what we’ve been led to believe, despite Paracelsus pointing out the obvious some 500 years ago. It’s funny that we haven’t made the connection when we speak of “chemicals” in the environment, since, if you think about it, hormesis is the basis of modern medicine, in which doctors prescribe toxins at low doses to cure our ills (Fig. 4).
Figure 4. There is no difference between a medicine or a toxin, other than the dosage. More than half of the world’s population still relies entirely on plants for medicines, and plants supply the active ingredients of most traditional medical products. Plants have also served as the starting point for countless drugs on the market today [[i]]. Photo credit [[ii]].
[i] http://publications.nigms.nih.gov/medbydesign/chapter3.html
[ii] http://www.cliparttop100.com/
I found a fascinating article on the problem of consumer acceptance of bitter fruits and vegetables, despite the healthful benefits of those bitter plant allelochemicals [36]. The authors point out that plant breeders and our cooking methods deliberately “debitter” our food, with the unintended effect of perhaps removing the most healthful (especially anti-cancer) components! The authors point out that, “When it comes to bitter phytonutrients, the demands of good taste and good health may be wholly incompatible.”
Perhaps the bees’ predilection toward bitter plant products may be telling us humans something important!
An Unintended Effect On Varroa?
In researching hormesis, I came across an item of interest—that low doses of some insecticides have the hormetic effect of increasing the fecundity of the insect that the chemical was intended to kill [37, 38]! What especially piqued my interest was a study that found that the same applies to mites [39]. Could it be that miticide or insecticide residues in brood combs might increase varroa fecundity?
Self Medication
We humans (at least some of us) intentionally eat plants rich in potentially toxic phytochemicals for their beneficial hormetic effects—think of the aromatic flavors of herbs, the tingling burn of spices, strongly flavored fruits, and “healthful” bitter salad greens. Bees may well do the same in seeking out allelochemical-laced pollen and nectar for its healthful benefits.
Some plant toxins help bees to fight parasites—Laurentz [40] found that despite the fact that it was metabolically costly to detoxify plant toxins, it may be of benefit to the insect in that those toxins help to defend it from parasites! Amazingly, there is even evidence that insects may actually self medicate with plant products when they are sick—Singer [41] found that parasitized caterpillars consume plant toxins to excess in order to kill their parasites. And how about the toxic antimicrobial, ant-repelling, and mite-killing tree resins that we call “propolis”? Mike Simone-Finstrom [42] recently found that honey bee colonies experimentally challenged with chalkbrood went out of their way to collect extra propolis.
But it gets even more complex than that…
Tritrophic Interactions
Just in case you’re not yet overwhelmed by the complexity of nature, let’s now move on to on “tritrophic interactions” (taking place at three levels):
Many insects live in close association with microorganisms (e.g., bacterial endosymbionts). Given the many enzymatic activities known to occur in bacteria and fungi, their role in detoxifying secondary plant compounds has been suspected but not yet clearly demonstrated. Further research will involve evaluating the role of endosymbionts in the detoxification of plant toxins [43].
Unfortunately, we don’t know squat about how important the honey bee gut- or beebread symbiotic bacteria and fungi are in detoxifying harmful chemicals! But it’s not just in detoxification that we have tritrophic interactions. In addition, bees are generally infected by one or more parasites (bacteria, fungi, viruses, mites, or nosema). There is evidence that any or all of these parasites may be affected by both the plant allelochemicals that bees consume, as well as any synthetic pesticides (or beekeeper-applied miticides) that they are exposed to.
So what’s a “tritrophic interaction”? Let’s say that a plant is preyed upon by grasshoppers, and that the grasshopper population is largely controlled by the presence of a gut parasite (say Nosema locustae). All that the plant need do is to produce allelochemicals that favor the gut parasite (perhaps by suppressing the grasshopper immune response), so that any grasshoppers that eat that particular plant would suffer greater parasitism. This is a common strategy in biocontrol using insect parasites—the parasite spores are introduced along with a synthetic insecticide that weakens the insects’ defenses.
It also works the other way—a plant can favor a pollinator or animal that distributes its seeds by producing healthful allelochemicals. Can this phenomenon occur in bees? You bet!
Diet has a significant effect on pathogen infections in animals and the consumption of secondary metabolites can either enhance or mitigate infection intensity. Secondary metabolites, which are commonly associated with herbivore defense, are also frequently found in floral nectar. One hypothesized function of this so-called toxic nectar is that it has antimicrobial properties, which may benefit insect pollinators by reducing the intensity of pathogen infections. We tested whether gelsemine, a nectar alkaloid of the bee-pollinated plant Gelsemium sempervirens, could reduce pathogen loads in bumble bees infected with the gut protozoan Crithidia bombi…Gelsemine significantly reduced the fecal intensity of C. bombi 7 days after infection when it was consumed continuously by infected bees…Lighter pathogen loads may relieve bees from the behavioral impairments associated with the infection, thereby improving their foraging efficiency. If the collection of nectar secondary metabolites by pollinators is done as a means of self-medication, pollinators may selectively maintain secondary metabolites in the nectar of plants in natural populations [44].
Just as humans eat certain strongly-flavored plants to ward off disease, could plant allelochemicals protect bees from viruses and nosema? Cory, in the best paper on tritrophic interactions that I’ve seen [45], states that, “Thus far, only the tip of the pyramid of complex multitrophic interactions has been exposed.” But she explains several mechanisms by which plant allelochemicals can protect bees from pathogens:
- The lining of the bee gut (the peritrophic matrix) is a key barrier to viruses and nosema. Phytochemicals can affect its structure, permeability, and physiology.
- Plant allelochemicals can damage midgut cells so that they are sloughed off before viruses or nosema can replicate in them.
- Plant-derived chemicals can cross the midgut and initiate signaling cascades to bring about cellular immune responses (hormesis).
- By improving the nutritional benefit of the bee diet, phytochemicals could indirectly reduce bee mortality due to infection by pathogens.
- Many phytochemicals, especially allelochemicals and nutrients, can modify the physiology and growth of the bee, affecting its susceptibility to infection.
Practical application: a number of researchers have found that sublethal doses of pesticides may make bees more susceptible to nosema or other pathogens. On the other hand, some may actually help to protect them. Perhaps the reason that bees do better on certain forage is because the phytochemicals in those plants help the bees to fight pathogens! I am especially excited by the prospect that there are likely natural plant products that we could supplementally feed to colonies to help protect them from viruses and nosema (I currently have several at hand that I plan to test against nosema).
Bee Detoxification of Allelochemicals
In the first place, bees may not need to detoxify allelochemicals if they simply don’t absorb them in the first place–the bee gut is pretty efficient at simply shepherding some toxins straight through. Suchail [46] found that only a small percentage of ingested radioactively-tagged imidacloprid (an alkaloid) ever makes it in any form into the bees’ haemolymph or thoracic muscles.
If absorbed, insects then depend largely upon three groups of detoxification enzymes to metabolize the toxins: the cytochrome P450 monooxygenases (P450s), the carboxylesterases (COEs), and the glutathione S-transferases (GSTs) [47]. The production of these enzymes is normally up- or down-regulated by the presence of allelochemicals in the diet. Bees use the same enzymes to metabolize synthetic pesticides.
Older texts gave credit to the insect fat bodies as being the site of that enzymatic detoxification, but recent research [48] suggests that even more important are the Malpighian tubules (Fig. 5), which play a major role in metabolism and detoxification of insecticides, as well as secreting antimicrobial peptides in response to infection [49].
Figure 5. Left to right, the bee midgut (or intestine), where most digestion takes place; the ileum (narrower tube) in the middle; and the head of the rectum to the right. The slender Malpighian tubules are attached at the junction of the midgut and the ileum. The Malpighian tubules and ileum perform detoxification, immune, and excretory functions analogous to the human liver and kidney. (I took this photo with a pocket digital camera held to the scope, illuminated by a flashlight clamped between my teeth).
Something of interest is that some toxins or toxicants only affect either the adult bees or the larvae. For example, bee larvae are apparently completely immune to neonicotinoids [[i]]; yet the nectar of summer titi appears to only poison the brood [[ii]].
Another oddity is that there may be instances of a “paradoxical effect,” in which it may take a sufficiently high dose of a chemical to initiate the detoxification response [[iii]], at which point the organism starts to exhibit “immunity” to that toxin. However, I have yet to come across any instances where this has been demonstrated in bees.
Summary
- Nectar, pollen, and propolis are chock full of plant allelochemicals, many of which may be toxic to bees at the levels found in natural settings.
- When other forage is unavailable, bees may be forced to work toxic plants that they would normally avoid.
- Sublethal doses of those allelochemicals may have beneficial hormetic effects upon bees.
- Some plant allelochemicals may help bees to fight parasites, and bees may self medicate.
- The coevolution of plants and bees involves complex chemical interactions that we are only beginning to understand.
- Bees must first detoxify any plant allelochemicals in their diet before they can begin to deal with any additional manmade chemicals.
- We cannot fully understand the impact of synthetic pesticides upon bee health unless we also take into consideration the above fact.
To be continued
In the next installment, I will explore the interactions between natural allelochemicals and synthetic pesticides.
Acknowledgments
As always, I’m indebted to Peter Borst for his assistance in research.
References
Atkins, EL (1975) Injury to honey bees by poisoning. In The Hive and the Honey Bee, Dadant.
Pellet, FC (1920) American Honey Plants. http://archive.org/stream/americanhoneypla00pell/americanhoneypla00pell_djvu.txt
[1] Wink, M (1991) Plant Breeding: High or low alkaloid levels? Proc. 6. Intl. Lupin Conf. Temuco, ILA, 326-334. http://www.uni-heidelberg.de/institute/fak14/ipmb/phazb/pubwink/1991/11.%201991.pdf
[2] Gold 2002 Misconceptions about the causes of cancer http://potency.berkeley.edu/pdfs/Gold_Misconceptions.pdf I highly recommend!
[3] http://toxicology.usu.edu/endnote/Pyrrolizidine-alkaloids-in-food.pdf
[4] http://www.efsa.europa.eu/en/efsajournal/pub/2406.htm
[5] Reinhard, A, et al (2009) Feeding deterrence and detrimental effects of pyrrolizidine alkaloids fed to honey bees (Apis mellifera). J Chem Ecol. 35(9):1086-95.
[6] Trumble, J and M Sorensen (2008) Selenium and the elemental defense hypothesis. New Phytologist 177: 569–572.
[7] Baker , H.G . ( 1977 ) Non-sugar chemical constituents of nectar . Apidologie, 8 , 349 – 356.
[8] Detzel, A and M Wink (1993) Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 4(1): 8-18.
[9] Atkins, EL, et al (1981) Reducing pesticide hazards to honey bees: Mortality prediction and integrated management strategies. Univ. Calif. Div. Agric. Sci. Leafl. 2883.
[10] In Detzel’s experiments, he fed the allelochemicals to the bees in 1:1 sugar syrup. The midrange of toxicity was at about 0.3%. Since a caged bee typically consumes about 30µL of syrup a day, that works out to be about 0.1µg of alleleochemical consumed. Atkins ranked any toxicant with an LD50 of less than 1.0 μg a.i./bee as highly toxic. Some of the allelochemicals were 100x more toxic than that average.
[11] Sommerville, D (2005) Fat bees, skinny bees. RIRDC Publication No 05/054.
[12] Adler, LS and RE Irwin. 2005. Ecological costs and benefits of defenses in nectar. Ecology 86(11): 2968-2978. http://people.umass.edu/lsadler/adlersite/adler/Adler%20and%20Irwin%202005%20Ecology.pdf
[13] Strauss, SY (1999) Ecological costs of plant resistance to herbivores in the currency of pollination. Evolution 53(4):1105-1113
[14] Linhart, et al (2005) A chemical polymorphism in a multitrophic setting: thyme monoterpene composition and food web structure. The American Naturalist 166(4): 517-529.
[15] Adler, L. S. 2001. The ecological significance of toxic nectar Oikos 91: 409-420. http://people.umass.edu/lsadler/adlersite/adler/Oikos00.pdf. Dr. Adler has a substantial body of research on this subject: [http://www.bio.umass.edu/biology/about/directories/faculty/lynn-adler; http://people.umass.edu/lsadler/adlersite/adler/adlerpublications.html]
[16] Singaravelan, N, et al (2005) Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectar. J. Chem. Ecol. 31:2791–2804. http://web.oranim.ac.il/teachers/ido/My%20reprints/Journal%20of%20Chemical%20Ecology%2031%20(2005).pdf
[17] Baker, HG (1977) Non-sugar chemical constituents of nectar. Apidologie 8(4): 349-356. Free access.
[18] Karban, R, et al (2000) Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125: 66-71.
[19] Mussen, EC, et al (1987) Beekeeping in California. http://www.beeguild.org
[20] Sedivy, C, et al (2011) Closely related pollen generalist bees differ in their ability to develop on the same pollen diet: evidence for physiological adaptations to digest pollen. Functional Ecology 25: 718–725.
[21] Hendriksma HP, et al (2011) Testing pollen of single and stacked insect-resistant Bt-maize on in vitro reared honey bee larvae. PLoS ONE 6(12): e28174
[22] Ibid.
[23] Bozic J., et al (2006) Reduced ability of ethanol drinkers for social communication in honeybees (Apis mellifera carnica Poll.)”. Alcohol 38(3): 179–183.
[24] Castillo, C, et al (2012) Biosynthesis of ethyl oleate, a primer pheromone, in the honey bee (Apis mellifera L.). Insect Biochemistry and Molecular Biology 42: 404-416.
[25] https://scientificbeekeeping.com/the-primer-pheromones-and-managing-the-labor-pool-part-3/
[26] Schmidt, JO and BE Johnson (1984) Pollen feeding preference of Apis mellifera, a polylectic bee. The Southwestern Entomologist 9(1): 41-47.
[27] Campana, BJ and FE Moeller (1977) Honey bees: preference for and nutritive value of pollen from five plant sources. J Econ Ent 70(1): 39-41.
[28] Baniecki, JF (2003) Changes in toxicological dogma. http://www.wvu.edu/~agexten/lookwhat/lwot203.pdf
[29] Calabrese, EJ and LA Baldwin (2003) Toxicology rethinks its central belief. Nature 421: 691-692.
[30] Joseph G Morse (1998) Agricultural implications of pesticide-induced hormesis of insects and mites. Hum Exp Toxicol 17(5): 266-269.
[31] Guedes, RNC, et al (2009) Stimulatory sublethal response of a generalist predator to permethrin: hormesis, hormoligosis, or homeostatic regulation? Journal of Econ Ent 102(1): 170-176.
[32] Guedes, NMP, et al (2010), Insecticide-induced hormesis in an insecticide-resistant strain of the maize weevil, Sitophilus zeamais. J of Appl Ent 134: 142–148.
[33] Duke, SO (2011) Phytotchemical phytotoxins and hormesis – a commentary. Dose Response 9(1): 76–78.
[34] http://publications.nigms.nih.gov/medbydesign/chapter3.html
[35] http://www.cliparttop100.com/
[36] Drewnowski, A and C Gomez-Carneros (2000) Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr 72:1424–35. http://ajcn.nutrition.org/content/72/6/1424.full.pdf
[37] Dutcher, JD (2003) A Review of resurgence and replacement causing pest outbreaks in IPM. In A. Ciancio and K. G. Mukerji, eds. General Concepts In Integrated Pest And Disease Management. Springer.
[38] Guedes (2009) Op. cit.
[39] James, DG and TS Price (2002) Fecundity in twospotted spider mite (Acari: Tetranychidae) is increased by direct and systemic exposure to imidacloprid. J. Econ. Entomol. 95(4): 729-732.
[40] Laurentz, M , et al (2012) Diet quality can play a critical role in defense efficacy against parasitoids and pathogens in the Glanville Fritillary (Melitaea cinxia), J Chem Ecol 38:116–125
[41] Singer MS, et al (2009) Self-medication as adaptive plasticity: increased ingestion of plant toxins by parasitized caterpillars. PLoS ONE 4(3): e4796.
[42] Simone-Finstrom, MD, and M Spivak (2012) Increased resin collection after parasite challenge: a case of self-medication in honey bees? PLoS ONE 7(3): e34601.
[43] Després, L., et al (2007) The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 22: 298-307. http://www-leca.ujf-grenoble.fr/membres/fichiersPdf/despres/despresDavidGallet2007.pdf
[44] Manson, JS, et al (2009) Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia 162(1): 81-89. http://labs.eeb.utoronto.ca/thomson/publications/Manson%20et%20al.%20nectar%20alkaloid%20reduces%20pathogen%20load%202009%20Oecologia.pdf
[45] Cory JS, Hoover K. (2006) Plant-mediated effects in insect-pathogen interactions. Trends Ecol Evol. 21(5):278-86. http://cals.arizona.edu/ento/courses/ento446_546/readings/Cory_2006.pdf I highly recommend!
[46] Suchail, S, et al (2004) In vivo distribution and metabolisation of 14C-imidacloprid in different compartments of Apis mellifera L. Pest Manag Sci 60:1056–1062.
[47] Johnson, RM, et al. (2006) Mediation of pyrethroid insecticide toxicity to honey bees (Hymenoptera: Apidae) by cytochrome P450 monooxygenases. J Econ Entomol. 99(4):1046-50.
[48] Yang, J, et al (2007) A Drosophila systems approach to xenobiotic metabolism. Physiol. Genomics 30(3): 223-231. http://physiolgenomics.physiology.org/content/30/3/223.full.pdf+html
[49] Dow, JAT and SA Davies (2006) The Malpighian tubule: Rapid insights from post-genomic biology. Journal of Insect Physiology 52(4): 365-378.
[50] Lodesani, Marco, pers comm
[51] Atkins, EL (1992) Injury to honey bees by poisoning. In Graham, JM, ed, The Hive and the Honey Bee.
[52] Stevens, DA, et al (2004) Paradoxical effect of caspofungin: Reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48(9): 3407-3411.