Sick Bees – Part 2: A Model of Colony Collapse
Sick Bees—Part 2
A Model of Colony Collapse
Randy Oliver
ScientificBeekeeping.com
First published in American Bee Journal September 2010
Over the past year, I have watched the experimentally-induced collapse of an apiary. The experience has helped me to understand the progression of colony collapse due to multiple parasite infection.
The California Trial
Last year I was approached by Nitzan Paldi, Chief Technology Officer of the Miami-based company Beeologics (and a beekeeper himself), to run a trial of their new product RemebeeTM—an antiviral drug that targets Israeli Acute Paralysis Virus (IAPV), and to some extent the closely-related Kashmir Bee Virus and Acute Bee Paralysis Virus. Remebee works by activating the bees’ natural antiviral immune response (via RNA interference; Maori 2009). The treatment had previously been demonstrated to help protect bees from IAPV in both cage and field trials (Maori 2009; Beeologics, unpublished), so the company funded two concurrent long-term controlled trials in Florida and California, following a strict FDA approved protocol.
I ran the California trial, assisted by local beekeepers and monitored by Dr. Eric Mussen, in the Sierra foothills at 2600 ft elevation, where we have a cold, wet, snowy winter (we intentionally wanted to cold stress the bees), followed by a hot, dry summer.
We started in late August by homogenizing, splitting, and then meticulously equalizing colonies at my Nosema ceranae test yard into 72 single deep hives. We requeened each hive with a fresh sister queen from a producer whose bees had not been exposed to varroa (in the expectation that they might lack resistance to viruses).
The trial site took place on an organic farm surrounded by wildlands, so that agricultural chemicals would not be a factor. I had not used synthetic miticides for 10 years, and most combs were of recent origin, so comb contamination was minimal. I purchased Apistan strips, and treated for two brood cycles prior to the start of the trial in order to eliminate most mites. For the next several weeks we equalized for strength and weight, fed syrup and pollen substitute, and replaced any poor queens with reserves from nucs in the yard, made from the same hives. Each colony received one dusting with Terramycin. The hives were color coded, and arranged into three circular groups of six sets of four hives, each group rotated 30 degrees, so that all treatments received equal sun/weather exposure.
The hives used had largely not been moved for two years (a few had gone to almond pollination), and had been largely untreated for nosema the past season except for a single feeding of fumagillin the previous fall. Average spore counts had run in the 2-10 million range (entrance bees) for about two years, but the colonies were thriving and had made a good crop of honey.
The trial began on September 30. We weighed each hive, and two inspectors graded every frame independently for bee coverage. All colonies were in good shape (one was removed due to queenlessness), with plenty of brood, stores, and fresh pollen. We then began a feeding program in which each colony received a half liter of 2:1 sugar syrup each week—one test group receiving Remebee each week, one every 4 weeks, and a control group fed only syrup. We fed for 20 treatments, with a break during the coldest weather when the colonies wouldn’t take syrup (for those of you doing the math, that means that we painstakingly mixed treatments for some 1400 feeder jars).
The California crew weighing and grading colonies for strength in the RemembeeTM virus treatment trial, prior to inoculating the hives with the virus cocktail. The two beekeepers in the far back are waiting for foragers to return so that they can take samples to test for Nosema ceranae. Photo by Eric Mussen.
Concurrent with the field trial to this point, I ran cage trials in an incubator in which I inoculated bees with a virus extract (cultured in pupae that we had hand injected with squashed bee juices from lagging colonies in my operation). The virus extract did not cause noticeable bee mortality in the cages, so collaborator Dr. Wayne Hunter supplied a purified virus “cocktail” (mostly IAPV) originating from sick colonies from beekeeper Jeff Anderson’s commercial operation, which had suffered CCD in California the previous year. This strain finally appeared to cause a sudden spike in mortality at about 9 days in some, but not all, cages (this incubation period was confirmed by Dr. Hunter in Florida). We now had a viable, virulent virus (say that three times real fast) inoculum on hand and were ready to roll!
So at this point we had 71 equalized, apparently healthy colonies at 6-frame minimum, still actively rearing brood in late November, with few of the common CCD suspects present:
- The bees were well fed, with natural pollen in the combs,
- Mite levels were low,
- Nosema ceranae was present, but the colonies had been building well.
- Each hive had a fresh young queen of good stock,
- There was no known exposure to insecticides or fungicides (notably no exposure to neonicotinoids),
- There was presumably no comb contamination from miticides, other than the small amount of fluvalinate from the Apistan strips,
- The hives had not been trucked, and the apiary was not crowded,
- Oh, I doubt that it is important, but all groups were equally exposed to any cell phone radiation.
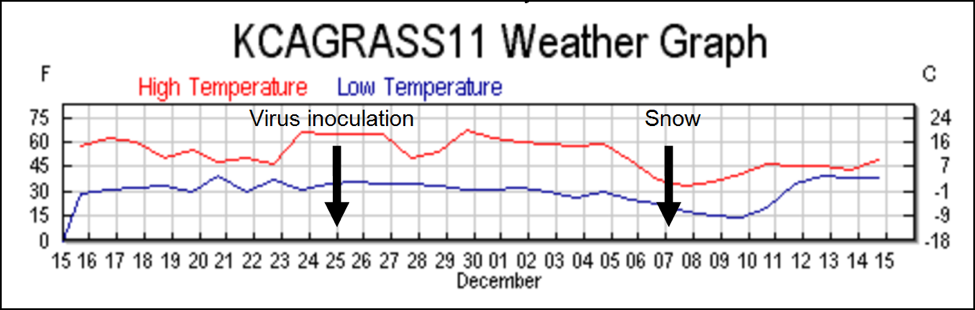
Things were about to get interesting! On November 25(Day 0) we inoculated all the hives with the virus cocktail in the syrup feeder jars. The last part of November was unseasonably warm, and the bees had been flying, gathering pollen. There was fresh egglaying in the three colonies that we checked on inoculation day.
The weather turned cold two weeks later, and snow fell the night of Day 12, which prevented noticeable bee flight for two days. On Day 14, there were handfuls of dead bees in the snow on the landing boards of many colonies—the dead bees were full of nosema spores. Upon inspection, these colonies appeared to have lost much of their population in the12-day period between virus inoculation and the start of the snow, since there were not enough dead bees present in the hives to account for the drop in population. This observation suggests that the bees had sickened quickly from the virus and abandoned the colony prior to the snowfall.
By Day 21, a number of colonies were in full collapse, and some were dead within a week. Since we didn’t have any formal uninoculated controls, I checked the few extra nucs that I had taken from the yard to a nearby location—they were not suffering mortality. Neither were any of my other several hundred colonies within the county. It was clear to me that things had started to go badly only for these colonies that received a virus inoculation. (I did not ask until the trial ended, but things went similarly in the concurrent Florida trial).
We discontinued Remebee treatments on February 24, which left all the remaining colonies on their own to fight the viruses. It continued to generally be cool and rainy, with weekly snowfalls until the end of May, but there were continuous nectar and pollen flows from the last week of February on. Colonies that could maintain enough field strength put on honey most weeks until the end of the trial in July. Despite the generally favorable conditions, many colonies were either unable to build up, dwindled, or collapsed suddenly despite having good sized broodnests (Fig.1).
The queens and nurse bees made heroic, but often futile efforts to expand the broodnests. It was heartbreaking to watch the dramatic losses occur in slow motion, despite conditions that afforded excellent forage, but it gave me valuable insights into the process of collapse.
Treatment with Remebee
This article is about colony collapse, not about the effect of Remebee, but as you are likely curious, I will give you a sneak preview. I was blinded until completion of the experiment (and have only recently received the final stats), but it wasn’t hard to tell during the trial which group did not receive treatment! (This observation strongly supports the hypothesis that the critical factor that took the colonies down was indeed the virus).
Although many of the colonies were unable to handle the combination of cold weather, N. ceranae, and the virus cocktail, those that received Remebee substantially outperformed those that didn’t receive treatment. The treated colonies were stronger and produced more honey. Unfortunately, I can’t yet tell you how well Remebee would have performed under “normal” circumstances, with stronger hives and a more realistic exposure to viruses, or had we treated for nosema. Had they started out as strong as I generally overwinter colonies in my climate, I suspect that more would have been able to shake the infection and rebound.
Figure 1. A hive showing evidence of recently having had five large frames of brood, but which suffered collapse in mid May (nearly five months after the virus inoculation). This colony reached the “starvation” stage due to its inability to muster a field force—there was little honey or pollen in the combs, despite plenty of forage available.
A Hypothetical Model
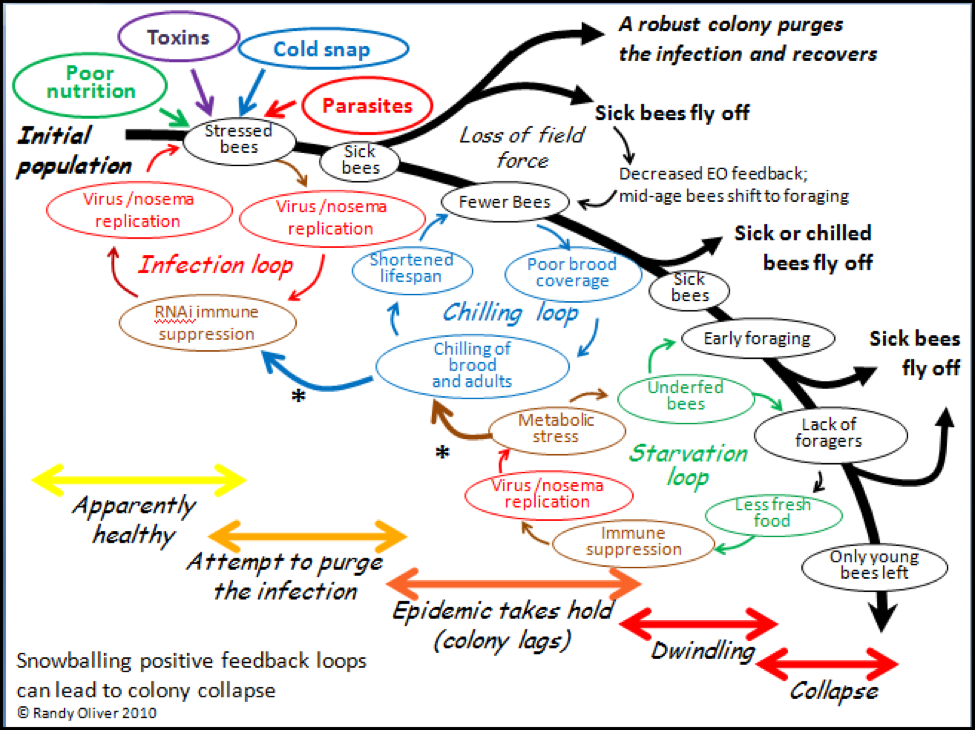
In order to make sense of a phenomenon, it often helps to attempt to create a model that explains your observations. I’ve attempted to do just that in the graphic below (Fig. 2). In the diagram I have tried to incorporate (as simply as possible) the major stages of colony collapse that I observed in this trial, in my previous experiences, and from reports of others. I also attempted to illustrate three major positive feedback loops that I feel are involved in the collapse process.
In positive feedback loops, an initial push away from equilibrium leads to a self-amplifying chain reaction (as with, for example, explosives). In colony collapse, the normal colony-level immune response to a parasite infection can go haywire if it is not successful at purging the infection, and then snowball into the rapid depopulation of the hive. The collapse can take place in a matter of days, but generally progresses over several weeks or even months, largely dependent upon weather and temperature.
Please refer back to the diagram as I go through each of the stages of collapse. The model begins in the upper left hand corner with a healthy colony being subjected to the stress of one or more of the “Four Horsemen of Bee Apocalypse” (see my previous article), and then progresses through the cascade of events that can result in either recovery, stagnation, or full collapse. The path to collapse could initiate with any of the stresses, and its resulting feedback loop, but it generally takes some combination of Horsemen to bring down a colony, and a multiple parasite infection to thwart the normal recovery of the colony from stress. I will go into more detail in subsequent articles.
Figure 2. A tentative general hypothetical model of what occurs during colony collapse due to infection by a parasite or combination of parasites. Note how the positive feedback loops play into one another (asterisks), and can result in either rapid depopulation or slow dwindling. The three feedback loops are shown separately for clarity, but in actuality overlap. I’ve color coded each of the factors to help illustrate their interactions.
The “Healthy” Colony
Every hive (and perhaps every bee) deals with parasites, especially viruses. As I mentioned in the last installment, our bees have not yet come to terms with all the new parasites that have invaded hives in the past few decades, and even the equilibrium with well established parasites is constantly changing. In general, though, a colony can simply outbreed the parasites—Lloyd Harris’ (pers comm.) data indicates that a healthy colony’s population can grow by 10,000 every 12 days during the spring growth phase!
With all those ready replacements, then the most important thing is for the bees to exhibit behaviors that minimize parasite transmission to the replacement bees. Hygienic bees actively remove sick larvae and pupae, and if they do so before the parasite generates infective spores or virions, they can often “shake off” the infection (unfortunately, varroa works against them by serving as an active vector of several parasites, notably viruses). It appears that if a nurse bee itself gets sick, then it quickly shifts to foraging duty, apparently so as not to infect the brood or queen with the parasite (Dr. Gro Amdam, pers comm).
How about older bees that get sick as adults (this includes those that became infected as larvae or pupae)? Just as bees fly outside to defecate in order to avoid fouling the hive, evolution has hard wired in a similar behavior called “altruistic suicide” in which sick or aged bees use their last bit of energy to fly (or crawl) out to die. I’m not sure that “suicide” is really the right word, since the bees appear to engage in foraging behavior up to the end, but with a vengeance—they’ll forage even in poor weather that would keep healthy foragers indoors.
There is a substantial adaptive advantage to such a behavior—by removing its sick or aging self from the hive, a bee saves its sister undertakers considerable work, plus more importantly, removes its pathogen-ridden corpse from the hive environment. This process can be very effective at “purging” a colony of infected bees. The self sacrifice of those bees allows the rest of the colony to recover and survive. This phenomenon takes place, largely unnoticed by the beekeeper, on a regular basis as the colony deals with the constant onslaught of the ever present parasites (mites, foulbrood, chalkbrood, nosema and viruses).
There are (at least) two important factors involved in the phenomenon—vitellogenin levels, and ethyl oleate inhibition of “aging”. The “aging” of bees has more to do with vitellogenin (Vg) levels and behavior than with chronological age. Bees that maintain high levels of Vg live for a long time, those with low levels die fairly quickly (see my Fat Bees articles). Such a shift occurs naturally as bees progress from wintering or nursing to foraging. But what is relevant to CCD is that bees that get sick accelerate the process, and immediately shift to “foraging” behavior (Tofilski 2009).
So far, so good. But what then happens when those sick foragers don’t return? The regulation of the house bee to forager ratio is dependent upon the inhibitory feedback from foragers to the mid-aged bees via the pheromone ethyl oleate (see my Primer Pheromones series). So when sick foragers die, younger bees quickly take their places. This works fine as long as there aren’t too many sick bees dying, and as long as there is plenty of emerging brood to take their places.
But if there is a parasite epidemic that knocks off the nurses and older bees faster than fresh bees can take their places (this is where the heavy black population arrow forks at the top of the diagram), then a vicious feedback loop can begin to take place, potentially leading to the depopulation of the hive! Any time that average bee longevity is decreased to the point that the colony can’t replace the workforce as quickly as it dies, then the population will inevitably plummet.
There is one additional point that I’d like to make. It is of benefit to both the parasite and to the colony, to have its sick workers drift to other colonies. For the parasite, such drift is essential for transmission to other hives; for the bees, it is a chance to knock out the competition (other colonies)! So we would expect to see drifting of sick bees to other hives. This effect has been documented for nosema-infected bees (Kralj and Fuchs 2009), which may help to explain the observation that CCD appears to slowly spread across an apiary from sick colonies to apparently healthy ones (this slow spread is very different from what we would expect if the transmission were due to robbing).
The Infection Loop
All colonies are infected with various viruses and other parasites, but the combination of the individual bee immune system and the colony-level immune response generally keeps the colony relatively healthy. However, a combination of pathogens, a novel pathogen, or an exceptionally virulent strain of an established pathogen can sometimes get a toehold in the hive, especially if others of the Four Horsemen are involved—generally a chill event or poor nutrition that stresses the bees.
In the California trial, the viruses in the cocktail, and likely Nosema ceranae, were players. I’m still processing samples, and will let you know more later. There are certain aspects of the bee antiviral response via RNA interference and nosema-induced metabolic stress that come into play. Each virus produces “suppressors” of the bee antiviral response, and multiple virus infection becomes complicated by viruses competing against one another. We are only beginning to understand the complex virus/virus and virus/nosema interactions within the poor bee!
Cutting edge virologist Eyal Maori (2009) suggests that strains of bees previously infected with a virus may contain virus sequences in their genome that lie dormant like land mines, biding their time until they are triggered by infection by another pathogen. Or viruses may exist in the “latent” form, similar to the way that the herpes virus is present in virtually all adult humans, just waiting to be triggered to reproduce by either stress, or another virus infection (again, I will detail more on this fascinating subject later).
For now, let’s just say that it is common for collapsing colonies to be simultaneously infected with three or four viruses, varroa mites, Nosema (ceranae and especially apis), and trypanosomes (Evans 2010), and that there are interactions between the pathogens and bee immune function.
The colony response to infection is to get the sick bees the hell out of the hive! The adult bees that are sick fly off, and the nurse bees remove (and perhaps cannibalize) the sick larvae and pupae (which may lead to further infection of both nurses and brood by viruses). The result, if all goes well, is to purge the hive of the infection. But if the colony is unsuccessful at fighting the infection, things can start to go downhill as the colony is overwhelmed by opportunistic pathogens that take advantage of the stressed bees.
In this particular case, the inoculation with the virus cocktail was apparently a “tip point” that initiated the parasite infection cascade, and from which, either directly or indirectly, most colonies eventually succumbed.
This is the dwindling phase of collapse, which can progress very quickly in some cases. The lack of foragers can be stunning! I really noticed it when I tried to vacuum returning foragers from the entrances for nosema sampling—there just wasn’t any forager traffic. In collapsing colonies, there might be bees in the broodnest, but they are all busy trying to keep the brood alive—there just aren’t any foragers to speak of!
The progression of colony collapse was recently described by Dr. Jerry Bromenshenk and colleagues (Debnam 2009):
“Regardless of whether the condition expresses itself in the spring or summer, organization within the hive shows slight changes. Brood nests are slow to expand. Instead the colony shows a tendency to maintain a brood nest centered in a single hive body. After the adult bees emerge the brood cells are abandoned and not reused. A midday inspection will reveal that many bees are out foraging and that the remaining bees are widely dispersed throughout the hive. This symptom may vary depending on time of day and the ambient temperature. Moreover, the population stops increasing during the growing season.”
The above was typical in this trial of colonies attempting to purge the infection. I also saw the same in 2004 and 2005. This is a huge point—the mere presence of a colony in the hive does not mean that that hive is going to be a productive unit. We can’t simply categorize colonies as either “live” or “dead”—there are also “zombie” colonies that lie somewhere in the limbo between the two classifications.
“The bees may appear to be restless. When viewed from the outside, flight activity may appear to be normal, giving the illusion of a strong colony. Smaller hives often abandon the upper brood chambers and the bee population is completely contained within a few frames in the lower hive body. This is often the easiest and quickest sign of CCD for beekeepers to notice.”
That and the fact that the colony isn’t building normally. These signs appear as the epidemic takes hold of the colony. Also look for lack of forager flight and spotty brood. An indication of sick hives during a flow is that the bees don’t glue the lids down, and white wax is not present. The bees in collapsing colonies are often listless and nondefensive, and may cluster away from the brood (Fig. 3).
Figure 3. A colony at mid collapse, with listless, restless bees not properly covering the brood, which is suffering from neglect. Colonies can hang on in this sorry condition for many weeks.
“In the earliest stages the brood pattern may appear to be solid, but if the pupal caps are removed, it likely consists of brood of all ages, due to the replacement of dying brood. As this condition advances the lack of adult bees results in an inability to cover the brood. Capped brood cells may be abandoned and unattended. Removal of chilled brood is still obvious and abandonment and chilling can be seen on brood frames because of the ‘holes’ in the pattern. The removal of dead larvae and pupae results in a ‘shot gun’ pattern on brood frames. Healthy colonies can keep up with the removal of chilled brood, which makes the ‘shot gun’ pattern of CCD colonies a strong indicator.”
This is when the chilling feedback loop starts to come into play.
The Chilling Loop
An observation by vanEnglesdorp (2009) is important:
“The premature loss of forager bees, the older cohort in a colony, results in younger bees prematurely becoming forager bees. If these replacement bees die at a rate that exceeds the colony’s ability to replace them, the result would be rapid depopulation, a reduction in the bee-to-brood ratio, and eventually colony failure.”
“Eventually” can be in a matter of days! But the term that I want to bring to your attention in the above quote was something that I first heard in a presentation by Dr. Frank Eischen—the “bee-to-brood ratio.” This is something that I really noticed in the trial—that in collapsing colonies, there simply weren’t the expected number of bees that should be there to cover the amount of brood present (Fig. 4).
Figure 4. A rapidly-collapsing colony with an obviously low bee-to-brood ratio. Note that the brood is still alive and white, but that there are no longer enough bees left in the hive to tend to it.
Although the altruistic suicide of the sick adult bees is of great benefit to the colony, the downside is that unless they can be quickly replaced, the bee-to-brood ratio will drop, and in cool weather, the bees may then simply not be able to keep the brood warm. We are all familiar with the phenomenon of “chilled brood,” in which we see dead brood on the landing board the morning after a cold snap; the colony quickly recovers. The effects of chilling in collapsing colonies are more subtle, but much more devastating.
As I wrote in my “Old Bees, Cold Bees” series, bees are warm-blooded animals, and chilling causes them great stress. There is suggestive evidence that their antiviral immune response is temperature dependent, meaning that when they are chilled, they may not be able to fight off virus infections. Ditto for chalkbrood and nosema, both of which thrive in slightly chilled bees.
Brood that is slightly chilled may appear normal the next day, but if the chilling allows the ever present viruses to explode in their bodies, the removal of their corpses may spread that virus into the young workers that chew them out—a positive feedback to the nascent epidemic (Figure 5).
Nosema infection also comes into play here. Two recent papers from Dr. Dhruba Naug’s lab offer some intriguing suggestions. They found that bees infected with N. ceranae preferred warmer temperatures, and were not able to generate body heat as well, especially if not satiated with nectar (Campbell 2010). And Mayack (2010) found that even relatively low levels of N. ceranae infection put forager bees under energetic stress, and kept them from maintaining their normal levels of the storage sugar trehalose in their haemolymph (blood). Trehalose is the major “blood” sugar of bees, and critical for the effective use of their flight muscles for both flight and the heating of their bodies (and thereby thermoregulation of the hive).
Figure 5. Chilled brood in a collapsing colony. The bees will uncap and remove the chilled or virus-killed larvae and pupae, resulting in a spotty pattern of uneven-aged brood. Unfortunately, the process of removal is likely to further transmit the viruses.
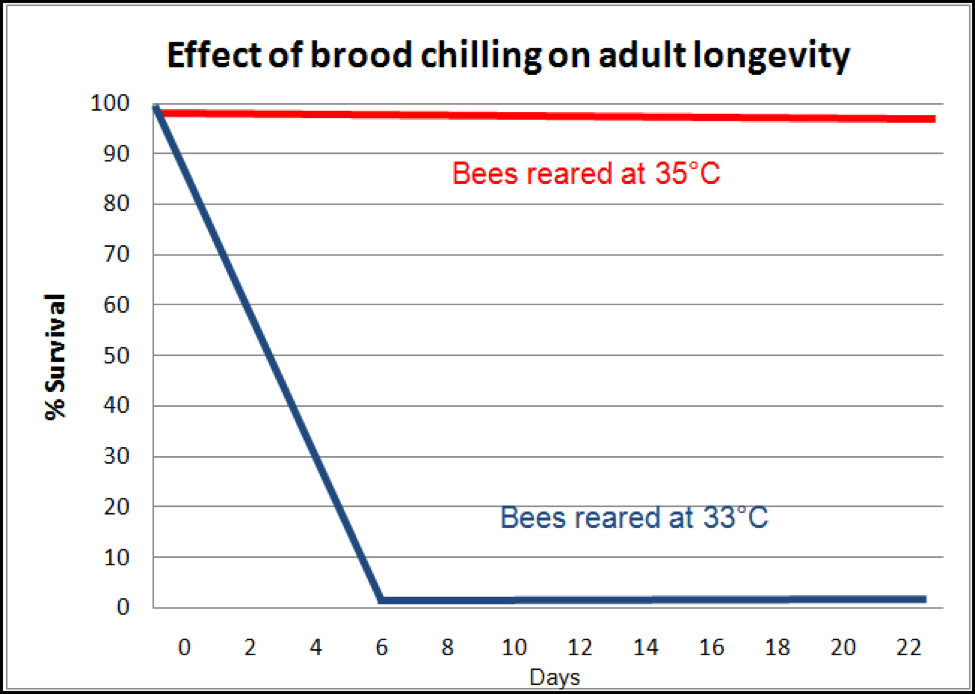
Even more insidious is the effect upon the adults which later successfully emerge from brood that was slightly chilled. Researchers (Tautz 2003, 2008; Jones 2005) found that bees that were chilled even slightly as pupae emerge as adults lacking in short-term memory. And that ain’t the half of it! They may not even live long enough to make it to foraging age! Medrzycki (2010) found that “Our results showed that lower rearing temperature had no significant effects on larval mortality and adult emergence, but adult bee mortality was strongly affected” (Fig. 6). Again, we have a nasty positive feedback loop at play—loss of adult bees causes chilling of the brood, which then leads to shortened lives for emerging bees, and even quicker adult bee loss!
Figure 6. The effect of slight chilling of the brood upon adult bee survival. Compare the survival of normally-reared workers (which would live an average of about 56 days), to that of those that were slightly chilled as pupae (which in this particular experiment all died by day 6; in two other experiments, death came at day 10 or 14). Note how such an effect can cause worker depletion in a hive to snowball! After Medrzycki (2010).
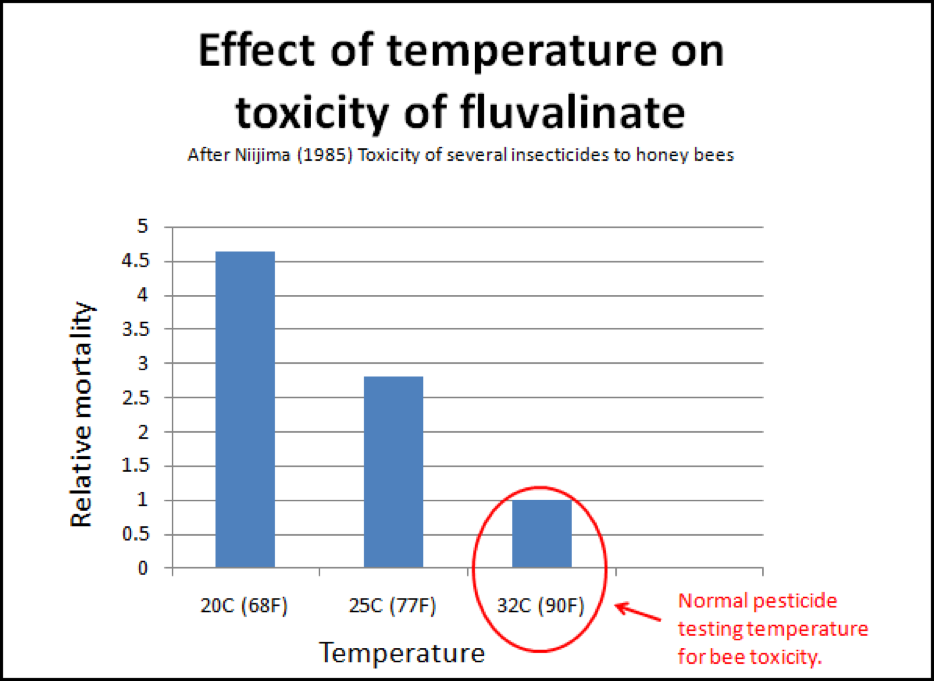
But we’re not done with chilling yet! Medryzcki also found that chilling increased the bees’ susceptibility to pesticide poisoning! This should be of special interest to those whose combs are contaminated with miticides. I found an interesting piece of old data regarding the most commonly used miticide, fluvalinate. In general, pesticide and miticide testing on bees is done in an incubator held at about 32°C, which would simulate the temperature within a hive. But on a cool night, or during winter, the temperature of the bees may drop to as low as 10°C. Niijima (1985) found that fluvalinate was 4-1/2 times more toxic to bees at 20°C than at 32°C (Fig. 7)! Another potential case of unexpected positive feedback!
Figure 7. This is a little-addressed phenomenon when we speak of pesticide, or in this case, miticide toxicity to bees. The normal testing of pesticides at broodnest temperatures may not truly indicate their toxicity to the older bees at the outside of the cluster. Note that the common miticide fluvalinate is four and a half times more toxic to bees if they are chilled. It is possible that the chilling that occurs in collapsing colonies could enhance pesticide toxicities that would not normally be noticed.
Pesticide and miticide exposure were not factors in the collapses that I observed (nor in historical collapse events), so are certainly not necessary components. However, there are many operations today in which they could well be contributing factors.
The Starvation Loop
The most surprising observation that we made was how strongly the premature loss of foragers could affect the colony’s nutritional intake! Dr. Mussen and I looked at two side-by-side colonies—one with a broodnest overflowing with fresh pollen and nectar, making white wax, and putting on honey, while the struggling colony right next to it was starving in the midst of abundance because it simply could not maintain a forager force (Figs. 8 and 9)!
Figure 8. An apparently healthy colony in mid May. Note the solid brood and plenty of pollen stores (there was freshly-stored honey in the super above—not shown). Note also the normal bee-to-brood ratio. Compare this photo to the one below.
Figure 9. A brood frame from a hive adjacent to the colony shown above. This colony is in the final stage of collapse. The still-living (white) brood is clear evidence that collapse came rapidly. Although the area of brood is similar to that in Figure 8, note the lack of pollen stores due to the depleted forager force that preceded the final collapse.
I watched a few colonies starve to death on nights when there was an overnight cold snap, during a substantial honey flow! I wouldn’t have believed it if I hadn’t seen it with my own eyes (Fig. 10). Even supplemental feeding of these sick colonies may not help.
Figure 10. This colony starved overnight, despite having honey stored on the periphery. Note the green grass, as this happened during spring weather under good foraging conditions with an ample nectar and pollen flow. There simply weren’t enough foragers to restock the stores each day!
As I noted in my “Primer Pheromones” series, the presence of fresh pollen around the broodnest is of paramount importance to nurse bee function and regulation. Young bees that go hungry start foraging prematurely (Toth 2005), and the absence of fresh incoming pollen appears to induce a rapid loss of the forager force (Mattila 2007).
Dr. Jürgen Tautz explains that the presence of cells of nectar in the middle of the broodnest is critical to thermoregulation of the broodnest temperature by “heater bees.” So the lack of fresh nectar and pollen due to lack of foragers has severe consequences upon the health of the colony and the aging of the bees; again an example of positive feedback that can lead to rapid collapse.
And let’s return again to the Naug group’s recent studies of the effects of Nosema ceranae infection. They found that unless the infected forager bees that they studied were fed sugar solution to satiation, that they could not maintain the necessary sugar levels in their “blood” necessary to produce heat and forage for extended periods. So it would not be surprising if nosema infection contributes to the demise of collapsing colonies simply by thwarting their ability to generate heat.
In this final stage of collapse, when foragers are unable to keep the broodnest stocked with adequate fresh nectar and pollen, “starvation” starts to have an effect—even in the midst of plenty! The lack of proper provisioning of the brood area has the effect of accelerating the “aging” of young bees into foragers, which, coupled with the shortening of the lives of bees due to chilling and disease, can finally topple the colony, despite heroic efforts of the queen and few remaining young workers.
Figure 11. The end result of a dwindling collapse. This was a common sight—a colony that had dwindled down to a healthy queen heroically laying eggs (see multiple eggs in some cells), a handful of young bees, and a patch of brood about the size of a silver dollar. In warm weather, colonies could hang on like this for weeks!
Practical Applications
It is clear that colony collapse can be initiated, and continue for months until it eventually decimates an apiary, simply by the introduction of one or more virulent strains of virus. The presence of nosema is likely contributory, but at least in this trial, the virus was key, as evidenced by the observation that those colonies treated against the virus were noticeably stronger. As I write this article, some eight months after inoculation, the difference between the treated and untreated colonies is unmistakable!
Beekeepers everywhere ask, what can we do to prevent or cure CCD? I’ve spoken to a number who have recovered, or have avoided it (so far) while others suffered. Their management suggestions are pretty much common sense:
- Learn to recognize the early signs of colony collapse: lack of normal buildup, low bee-to-brood ratio, spotty brood, lack of stored nectar and white wax when expected. You may wish to move sick colonies to an isolated yard.
- Make sure that your colonies are well nourished—plenty of honey reserves, and adequate pollen forage. If not, the feeding of several pounds of high quality pollen supplement during times when colonies are nutritionally stressed can make the difference between boomers and deadouts. (Although in this case, colonies succumbed despite good nutrition).
- Keep mite levels low. Varroa acts as a vector for viruses, as well as causing major stress to bees. (Again, mite levels were low in this trial).
- Treat for nosema if levels get high. Just how high is the question! The colonies in my yard did just fine with N. ceranae levels in the few millions for two years, until I added the virus inoculum.
- Avoid the added “Horseman” of toxins by minimizing the exposure of your hives to pesticides, including beekeeper-applied miticides, which have been strongly implicated in several studies with colony losses. (Again, not a factor in this trial).
- It’s pretty clear that the one factor that is most difficult to specifically control for is viruses, to which your bees will be exposed either through transport to almonds, or even from visiting flowers (Shen 2005). RemebeeTM is the only specific antiviral product that I know of, and it could prove to be a godsend to those with stressed bees; the company hopes to have it commercially available in a few months (followed by a multivirus product). There are also some indications that certain essential oils may also help to control viruses—Dave Wick is currently setting up trials to get some actual data.
- Start yards of fresh colonies with broodless shook (or package) bees, treated to eliminate most mites. This practice serves to break both the varroa and virus epidemics in the hive. The resulting colonies are generally very healthy for the first year, and Dr. Jerry Bromenshenk has documented that virus levels generally remain very low.
- Don’t combine collapsing colonies with healthy colonies! Collapsed deadout equipment appears to become less infective after “aging” in a dry environment for a month or so, or after sterilization with bleach, formic acid, or radiation. I’m currently setting up a continuation of this trial in which I am going to restock the deadout equipment from the untreated control group.
- Propagation of resistant stock. It’s equally clear that bees (at either the colony or population level) are able to develop resistance to any virus that comes along (one, but only one, of the unmedicated control colonies in my test yard is thriving, despite having been inoculated). Breeding from survivors is our best long-term hope for dealing with colony collapse, no matter what the cause(es).
Closing Thoughts
All colonies of bees are forced to deal with the Four Horsemen (parasites, chill, toxins, and poor nutrition) on a regular basis, yet they generally don’t collapse. In my California trial, collapse was clearly initiated by the introduction of virus(es). The other Horsemen may stress a colony severely, or even kill it outright (like a pesticide kill, or winter starvation), but unless a parasite epidemic is raging within the hive, any challenge would be unlikely to cause the type of progressive collapse observed in CCD.
Will CCD “go away” as have the other instances of “disappearing disease“? Most viral epidemics do eventually either disappear, or morph into opportunistic background infections. We are in the midst of the Deformed Wing Virus epidemic, which started shortly after varroa arrived, but shows no signs yet of abating. As pointed out earlier in this series, bees are currently coming to terms with an onslaught of novel parasites and their interactions, so we may be seeing sick bees for a while.
Complete collapse is not inevitable. Some colonies may collapse quickly, seemingly overnight. Others may stay “stuck” at just a few frames of healthy-appearing bees and brood, for weeks or even months. And others may dwindle to a couple of frames of bees, and then, if they manage to purge the infection, spontaneously recover (especially if they are on a good pollen and nectar flow in warm weather).
A few things surprised me during the California trial, like our success at inoculating the colonies with virus by the feeding in sugar syrup. It is unlikely that each bee in the hive fed directly from the feeder jars (which each had only one small hole), so I must assume that the virus was passed throughout the hive via trophallaxis, and that it was infective via the gut. We tend to associate virus infections with varroa, but in this case, varroa levels were still quite low when we checked in May, so either the virus alone, or in combination with N. ceranae, was able to increase to lethal epidemic proportions.
I was also surprised by how quickly ostensibly healthy colonies collapsed after the virus inoculation, even before cold weather hit. It was not so surprising that the remainder struggled during the cold, wet winter. As beekeeper Zac Browning told me after he suffered major losses last winter, “Bees don’t get better over the winter.” However, they generally do get better once they are on a good spring nectar and pollen flow. But in this case, most didn’t. This was the most unexpected observation of all—that many colonies were not able to rebound during the spring, and continued to collapse even during excellent foraging conditions in early summer!
I would have expected the virus, or virus/nosema infections to go “away” after the initial kills, and for the surviving colonies to recover. Jeff Anderson (from whose bees we obtained the virus cocktail) tells me that many of his colonies dwindled to about two frames, and then spontaneously recovered. So my questions are, why were so many of my colonies unable to recover during spring, and what were the pathogen dynamics during this time? I currently have samples in for testing, and am slowly slogging through other samples to determine how much of a role nosema played.
To be continued…
Acknowledgements
I’d like to thank those beekeepers who assisted me in the hard work of the field trial–my sons Eric and Ian, my partner Stephanie Hughes, and local beekeepers Rob Slay, Steven Kauffman, Jerry van Heerington, Aaren Bryars, Larry Merrit, and Thom Staser. Phil Carville of Loma Rica Ranch generously provided us the location on the organic farm.
I’m also grateful for the scientific assistance of researchers Nitzan Paldi, Eyal Maori, Denis Anderson, Jay Evans, Wayne Hunter, and especially Jerry Bromenshenk and Scott Debnam, whose observations of a vast number of collapsing colonies were critical to the formation of this model.
I’d also like to tip my hat to the heroic efforts of Dennis vanEnglesdorp, Jeff Pettis, Diana Cox-Foster and Nancy Ostiguy and the rest of the Penn State group, and to all the others who are working so hard at finding the causes of Colony Collapse Disorder.
A special thanks to my friend and colleague in this experiment, California Extension Apiculturist Eric Mussen, with whom I bandied thoughts and observations in the field.
References
Cox-Foster, DL, et al. (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318(5848): 283-287.
Debnam, S, D Westervelt, J Bromenshenk, R Oliver (2009) Colony Collapse Disorder Symptoms. Bee Culture Feb 2009 https://www.beeculture.com/storycms/index.cfm?cat=Story&recordID=629
Jones J, P Helliwell, M Beekman, RJ Maleszka, BP Oldroyd (2005) The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J Comp Physiol A 191: 1121–1129.
Kralj, J, and S Fuchs (2009) Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 41: 21–28
Maori, E, et al (2009) IAPV, a bee-affecting virus associated with Colony Collapse Disorder can be silenced by dsRNA ingestion. Insect Molecular Biology 18(1):55-60.
Mattila, HR and GW Otis (2007) Dwindling pollen resources trigger the transition to broodless populations of long-lived honeybees each autumn Ecological Entomology 32 (5): 496-505.
Medrzycki, P, et al (2010) Influence of brood rearing temperature on honey bee development and susceptibility to poisoning by pesticides. Journal of Apicultural Research 49(1): 52-59
Shen, M L Cui, N Ostiguy and D Cox-Foster (2005) Intricate transmission routes and interactions between picorna-like viruses (Kashmir bee virus and sacbrood virus) with the honeybee host and the parasitic varroa mite. Journal of General Virology 86: 2281–2289.
Tautz, J, et al (2003) Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Natl Acad Sci 100(12): 7343–7347.
Tautz, J (2008) The Buzz about Bees: Biology of a Superorganism. Springer.
Toth, A.L. et al., (2005). Nutritional status influences socially regulated foraging ontogeny in honey bees. Journal of Experimental Biology 208, 4641–4649.
vanEngelsdorp, D, et al. (2009) Colony Collapse Disorder: A Descriptive Study. PLoS ONE 4(8): e6481. (This is an excellent study, and is a free download)