The Learning Curve – Part 4: The Synthetic Miticides
The Learning Curve: Part 4–The Synthetic Miticides
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in Sep. 2009
Paradise Lost
The overall impact of the varroa mite upon beekeeping was recently brought home when I spent time with beekeepers on the Big Island of Hawai’i. Until recently, these lucky beekeepers enjoyed a true beekeeping paradise—abundant nectar and pollen flows, minimal agricultural pesticides, and best of all, no loathsome varroa or tracheal mites! Unfortunately, this dream was shattered when varroa was discovered near the Hilo Airport. I was invited to speak to the beekeepers to prepare them for what to expect as varroa becomes established.
What struck me were the different situations and ramifications faced by individual bee operations, as illustrated by the two prominent businesses in the photos. Richard Spiegel lives a beekeeper’s dream. He lives in the heavenly Big Island highlands, where it is essentially spring- or fall-like weather year round. His business supports him and his lovely wife, and a crew of (currently) six women—all on the output of only 135 colonies! Richard is a premier marketer who has developed a worldwide demand for a unique organic honey that he produces from the Kiawe tree. Since his certified organic status forbids the use of synthetic miticides, it will be an extra challenge for him to deal with the mite.
Richard Spiegel of Volcano Island Honey, flanked by three of his happy crew–Kerry Ambrosio, Donelle Rodriguez, and Christina Neumann. Richard and the gals produce a world famous organic creamy-white honey.
On the other hand, queen producer Gus Rouse has painstakingly built a megascale queenrearing operation that supplies beekeepers throughout the world, and at any time of the year, with healthy non-Africanized queens from one of the last areas free of mites and miticides. Since he must stimulate his crowded colonies to produce drones year round, it will be a daunting challenge for him to deal with the onslaught of varroa, while at the same time avoiding miticide problems that might affect his queens and drones. I have the greatest empathy for Hawaiian beekeepers, and hope that they can benefit from our learning curve during their transition to beekeeping with mites.
Gus Rouse, owner of Kona Queens, showing the author boxes of queens ready for shipment in the morning. Gus has not yet had to deal with varroa.
Meanwhile, on the mainland, as fears of CCD subside, beekeepers are feeling more sanguine about the health of their colonies. A prominent beekeeper told me last season, “Randy, beekeeping has never been easier! With protein patties, nosema medication, and good mite control, it’s a no-brainer to keep strong colonies.” Unfortunately, this strategy has one very weak leg—the shakiness of chemical mite control.
The three main synthetic miticides used for varroa control in the U.S. have largely become ineffective, due to the mite developing resistances. Many large beekeepers are feeling a bit uneasy about how they are going to manage mites this year. Indeed, the current commercial beekeeping model is largely predicated upon the development of a novel miticide every several years.
Unfortunately, that is no easy task—killing a “bug on a bug” in a box full of human food product is a challenge. Since no one wants the miticide to contaminate honey, it must either degrade quickly to a harmless product, or not be water soluble. Since people are leery about “harmless” degradation products of synthetic chemicals, synthetic miticides are generally made to be lipid (oil) soluble. Unfortunately, that means that they soak into the beeswax, and if they are stable, build up over the years.
The Comb Contamination Issue
I visited the problem of the “toxic stew” of ag pesticides and miticides in a previous article. In fact, honey bees are so effective at inadvertently collecting pesticide residues, that two scientists from Greece state that “This study indicates that in agricultural areas with developed apiculture, useful information about the occurrence and the distribution of pesticide residues due to crop protection treatments can be derived from the analysis of randomly collected honey samples, used as bioindicators. It also shows that, very often, the chemicals used by apiculturists inside the hives in order to control disease are the main pollutants of the produced honey” (Balayiannis 2008).
The problem is worldwide, with coumaphos and fluvalinate being issues everywhere, and additional miticides in various countries. José Orantes Bermejo (2009) observes that in Spain, the industrial and liturgical demand for beeswax has decreased over the years, such that the beeswax market is now a “closed circuit” that recycles beeswax back to beekeepers as foundation. Since beekeepers in Spain have used miticides for some 24 years, some chemicals have built up to alarming levels, which his formal investigation found to be a contributing factor to the colony collapses in that country.
Orantes Bermejo points out that the lipophilic chemicals can leach out of the wax and into beebread as it ferments in the comb, especially if the pollen is naturally high in fatty acids (perhaps resulting in a delayed effect). Bee larvae are also exposed by contact with the cell walls, and by the leaching of miticides into the lipid-rich royal jelly.
Some beekeepers have questioned whether they should worry about miticide contamination in commercial foundation. Orantes Bermejo (pers comm) found that typical beeswax processing and filtration through diatomaceous earth does not adequately remove miticides; his Apinevada laboratory has patented a system to remove residues from beeswax. Jennifer Berry (2009) had trouble finding uncontaminated beeswax foundation in the U.S.
The manufacturers, of course, wish to supply a “clean” product, but have limited guidance as to tolerable levels. The U.S. tolerance level for coumaphos in beeswax was originally set at 100 mg/kg (ppm). However, Dr. Jeff Pettis (2006) found that bees reject roughly half of queen larvae grafted into wax cups containing 100 ppm of coumaphos! Please note that I’m not entirely clear as to whether there is indeed a current official tolerance level for beeswax. All I can find is a new tolerance level for “honeycomb” of 45 ppm.
A number of recent reports (unpublished) have also found deleterious effects of upon worker brood raised in combs contaminated with relatively low levels of fluvalinate, coumaphos, or amitraz. Unfortunately, there are few published test results of the actual amounts of residues in foundation (please send me your results if you have had foundation tested).
Please note that I do not wish to sound alarmist by hyping up the issue of miticide contamination of foundation wax. The levels of contamination that I generally see reported are well below those that would cause frank toxicity to bees or brood. Questions about foundation contamination have always been an issue—for AFB spores, and for the older generations of pesticides. Please realize that the technology of residue testing has advanced to the point that the limits of detection are often down to a single part per billion (1 drop in 22,000 gallons)—we didn’t worry previously about what we couldn’t see!
The bottom line is that beeswax has for many years been contaminated to some extent (even “organic” beeswax, since pesticides are ubiquitous in dust). It would be pretty evident if the contamination of foundation was an appreciable problem—you’d see spotty brood patterns on new combs drawn from foundation (deleterious effects should be most evident in freshly worked wax). I personally purchase several thousand preassembled frames with waxed foundation each season, yet haven’t seen problems, nor heard of them reported by other beekeepers.
Reality Check
I don’t want to play the part of Chicken Little. The reality is that many commercial beekeepers (excluding those who dumped in coumaphos dust) maintain apparently healthy, strong colonies on combs containing substantial levels of miticide residues—at least as long as weather and forage conditions are good. It appears that the incredible ability of colonies to produce upwards of 2000 new workers a day in times of good forage may overshadow any negative effects of comb contamination. Problems may only become noticeable during times of nutritional, parasite, or ag pesticide stress.
Then there is the nagging problem of continual reports of poor queen survival—a problem that could be related to the high susceptibility of queens, drones, and semen to common miticides. This was clearly the case several years ago when queen producers freely used coumaphos and fluvalinate, but those I’ve spoken with have moved away from these products. Any beekeeper who has used Checkmite+® or off-label coumaphos more than once should be aware of the chemical’s potential effect upon queen survival.
Although colonies may appear to thrive on tainted combs, several researchers (e.g., Martel 2007, Frazier 2008, Orantes Bermejo 2009) have pointed out that sublethal levels of miticides may act as continual stressors to the bees. Perhaps the biggest question is the epigenetic effects that the various miticides and pesticides exert upon the queen and the individual workers. The genetics of bees are only part of the story—more important may be just how the genes are expressed in response to environmental inputs. Just as two identical human twins may have different susceptibility to disease (Qiu 2006), two identical queen bees may produce quite different colonies depending upon their exposure to environmental cues—such as pesticides, parasites, or nutrition. It is an open question whether long-term exposure to miticides exerts a negative effect upon overall colony health and immune function.
Honey bees are able to detoxify small amounts of natural or synthetic poisons. For example, Kevan (2005) notes that the natural amygdalin in almond nectar and pollen is toxic to bees, yet California beekeepers took their bees to almonds even before they were paid for pollination, since colonies build up so well on the “toxic” pollen and nectar! The bottom line is that there are often tradeoffs—colonies may thrive on somewhat toxic pollen if the benefit gained by the richness of the protein outweighs the cost of detoxification. Ditto for miticides. Despite the deleterious effects of miticide contamination of combs, such contamination may be of less harm than the alternative of a terminal varroa infestation.
Might I suggest that those who are concerned about the sublethal effects of miticides or pesticides read the review by Thompson and Maus (2007) in which they put the results of lab testing into perspective: “It is also an open question whether the results of tests on sublethal effects other than under field (or semi-field) conditions, especially when only effects on individuals are measured, can really be extrapolated
to a realistic field situation with bee colonies. For instance, a behavioural effect seen in individuals will not necessarily have a potential to cause harm at the colony level.”
Honey bee colonies are remarkably tough and resilient, despite having to deal with a scary list of modern day contaminants in the combs. For example, Chauzat (2009) monitored colonies in France for a 3-year period, and could find no correlation between pesticide residues and colony health (imidacloprid, coumaphos and fluvalinate were common, at relatively low levels).
The fact is that episodic colony collapse events have occurred for as long as records exist, and the described symptoms virtually mirror those described for recent collapse events—the workforce of colonies suddenly “disappearing,” leaving only the queen and a handful of young workers. Please note that these collapses happened prior to modern pesticides or in-hive miticides.
The most likely culprits for such historical collapses are likely due to a combination of weather-induced nutritional stress and/or the emergence of virulent strains of new or existing parasites—notably nosema and viruses.
My point is, miticides and pesticides are certainly harmful to bee colonies, but since they weren’t present for previous colony collapse events, I’m not convinced that they are the sole cause for the present spate (other than the clear cases of colonies overdosed with coumaphos or chlorfenvinphos). However, the toxic impact of chemical contamination of combs cannot be ignored as a likely contributory factor whenever colonies are stressed by crowding, poor weather, inadequate nutrition, or high parasite loads.
Mite Resistance and Chemical Persistence
A further problem with the persistence of certain miticides in the combs is that it continually exposes the surviving mites to the chemical. This effect contributes not only to the development and successful spread of resistant mites, but also suppresses any rebound of nonresistant mites—the residues actually help the resistant mites, since they eliminate the competition.
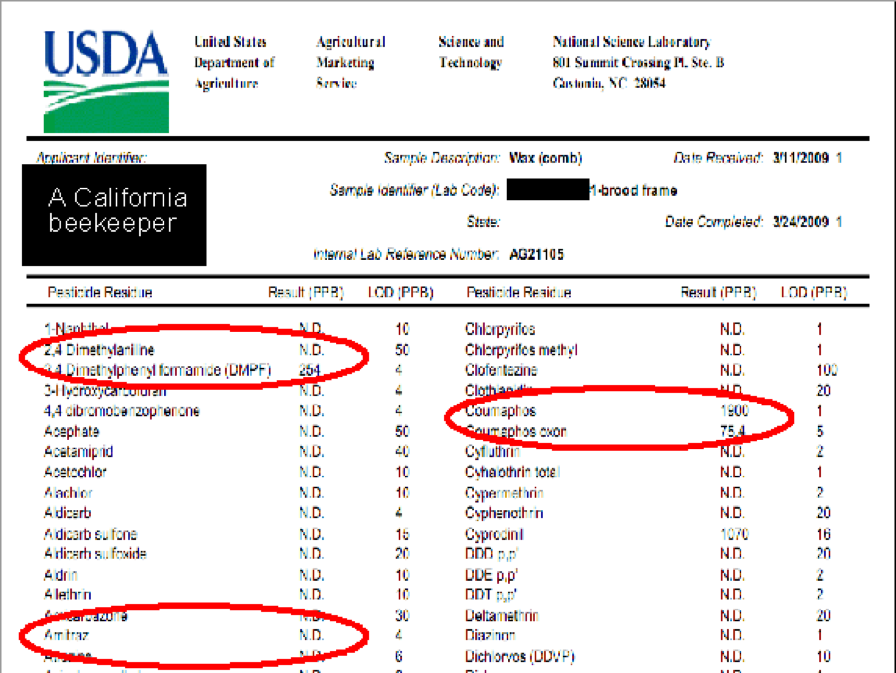
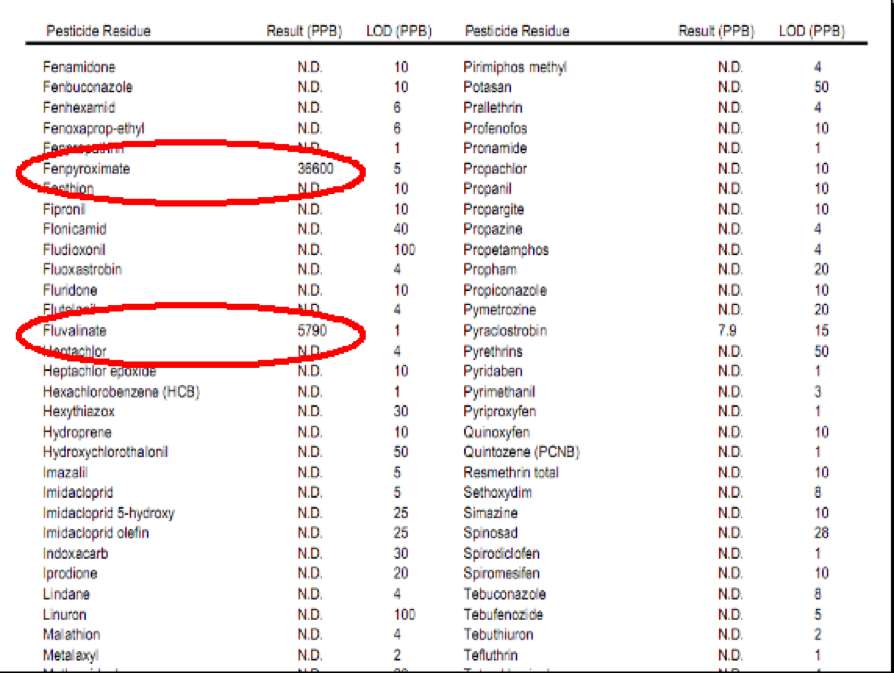
When I refer to miticide “persistence,” I really mean it! I recently was shown the testing results for a brood comb from a beekeeper who last used Checkmite+ six years ago, and fluvalinate (as Mavrik) three years ago. Even after that substantial elapsed time, his comb still contained nearly 2 ppm coumaphos and 6 ppm fluvalinate. Reality check: neither of these amounts are high enough to cause frank toxicity, but even several years after being last used, concentrations were still high enough to suspect the potential to affect queens and drones (Pettis 2006, Rinderer 1999).
Partial test results from a California beekeeper’s brood comb. This beekeeper hadn’t used coumaphos for 6 years, nor fluvalinate for 3 years, yet residues of both products persist. DMPF is a persistent degradation product of amitraz (last year’s treatment). The colony was treated with fenpyroximate shortly before the test. Note that most plant protection products are at the not detectable (N.D.) level.
As more pesticides and miticides are added to the combs, the synergy of toxins may reach the point that they affect colony health or productivity. This point should be considered by beekeepers whose bees are taken to agricultural areas where pesticides are used—if your colonies are going to be exposed to ag pesticides, you may wish to both avoid persistent miticides and to closely monitor nosema levels.
The Rundown on Available Synthetic Miticides
Fluvalinate
Fluvalinate is the most renowned varroacide—widely used in the form of Apistan® strips, which were an improvement on the early pieces of wood or cardboard dipped into the agricultural formulation Mavrik®. It was truly a wonder chemical, which was safe to handle, didn’t get into the honey, had no noticeable effect upon the bees, and knocked the snot out of mites (99+% kill).
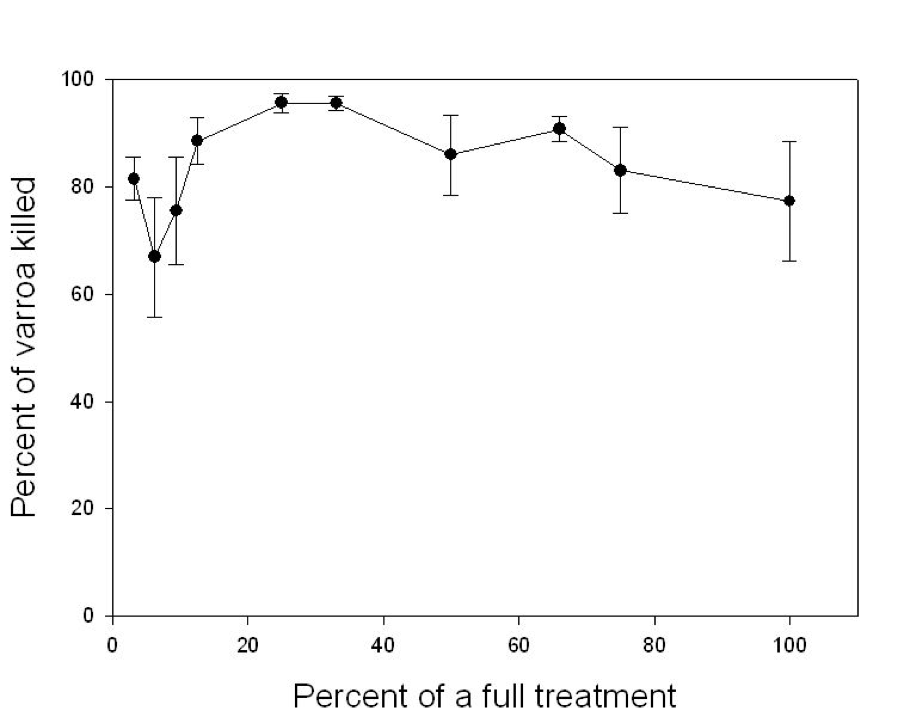
Surprisingly, when Dr. Mark Goodwin (pers comm) first tested Apistan strips in New Zealand, he found that with as little as 3.1% of a full treatment provided adequate mite control! The manufacturer simply started with a relatively high dose that would ensure a kill of virtually every single mite. Beekeepers thus came to expect mite control products to provide near total eradication of mites with each use. In retrospect, the initial excessively high dose of fluvalinate likely helped to accelerate the development of resistant mites. In the case of Apistan, it wasn’t long before such strong selective pressure created mite populations that started rebounding sooner, so beekeepers started using more strips, or treating more often.
Plot of percentage of mites killed vs. percent of the recommended dose of Apistan applied (to the original nonresistant mites in New Zealand). Note that only a small fraction of a single strip was as effective as a full dose (100%–consisting of 2 strips). Courtesy of Dr. Mark Goodwin.
Commercial beekeepers, in their desire to cut costs, stuck with the popsicle stick dipped in Mavrik (and later, various other forms of application), and before long were using extremely high doses. We’ve all heard how this has led to widespread mite resistance to fluvalinate, and the worldwide contamination of beeswax.
Dr. Maryann Frasier (2008), after analyzing colonies suffering from CCD, found that “The most significant difference in pesticide levels relative to bee health was that fluvalinate residues tended to be higher in pollen, wax and brood of weak, dead and recovering colonies relative to strong colonies. Highest levels of pesticides were found in the wax, followed by the pollen and brood, but levels in wax were much more variable than in pollen or brood. The fluvalinate levels found in brood are within a lethal range for honey bees.”
Recently, it has been suggested that the fluvalinate sold today is more toxic than of old, since it contains only the two “active” forms of the molecule (named tau-fluvalinate). However, when I asked the Wellmark representative, Doug VanGundy, about this, he explained the only change was in name, as the company had always used the tau-fluvalinate form of the product.
Apistan strips and Mavrik are still used by beekeepers, with their success dependent upon the degree of resistance of the local mites, and whether the product is rotated with other mite control measures. Resistance comes at a cost to the mites—nonresistant mites will out compete the resistant ones if they are not continually treated with the chemical. Unfortunately, residues in the combs have a similar effect as nonstop treatment.
Curiously, the author of the EPA registration of the product (EPA 2005) did not appear to understand just how Apistan strips were to be used, as evidenced by the following paragraph:
“However, an appropriate label statement is required to protect foraging honeybees when the LD50 is less than 11µg/bee. For tau-fluvalinate, the acute contact toxicity study to honeybees indicates that the LD50 is 0.2µg/bee. This classifies tau-fluvalinate as highly toxic to honeybees. The impregnated strip formulation [Apistan] is used in beehives to treat Varroa mites when bees are not present.”
Apparently the author was unable to entertain the notion that beekeepers would actually put the product into a hive of live bees! LD50’s aside, the reality is that I used the strips for several years, and sure didn’t notice them hurting the bees. However, it is rough on drones and queens, plus its toxicity is amplified if used in colonies already containing coumaphos residues (Johnson 2009).
Fluvalinate has enjoyed a long run. For many beekeepers, Apistan strips provided effective mite control for about six years (although toward the end we needed to treat more frequently). The product is still sold, and continues to work in some areas. Commercial beekeepers were able to extend the life of fluvalinate by increasing the dose of Mavrik and/or rotating it with other products—extending its effective life for nearly twenty years!
Fluvalinate may indeed be able to be used in future miticide rotations, especially if beekeepers practice some degree of comb rotation to avoid residue buildup, thus allowing nonresistant mites to return. This is a bit complicated, since nonresistant mites, unhampered by resistance mechanisms, may be more virulent than resistant mites!
Fluvalinate should be used carefully, if at all, by queen producers as it can result in queen supersedure (Currie 1999), early drone mortality (Rinderer 1999), and reduced sperm viability (Burley 2007).
Coumaphos
I remember well the year when Apistan was failing, and we got a Section 18 to use Checkmite+ strips. When a friend and I opened the first foil package of Checkmite, and took one whiff of the coumaphos, we were immediately convinced that using an organophosphate nerve gas analogue was not a part of our idea of beekeeping!
I used it anyway; but my friend declined, and used Apistan again. My bees came through the winter in fine shape; hers all died from mites. It was simply amazing to see a chemical control that had been so effective fail so thoroughly, while the new one decimated the mites!
Coumaphos is nasty stuff—there’s apparently enough active ingredient (a.i.) in a single strip to kill an adult human (the coumaphos LD50rat of 13mg/kg x 70kg for an adult man = 0.91g estimated dose to kill a man; there are 1.36g per strip). For bees, the math is also interesting: the LD50 for bees (depending on the source) is in the range of 2-15µg/bee (Anderson 1968). That means that there is enough coumaphos in the two-strip dose to theoretically kill all the bees in a strong colony! Obviously, this does not happen, but it does illustrate that I would hardly call the product harmless to bees.
One Bayer website [1] for the product is all warm and fuzzy, and amazingly makes the claim, “CheckMite+®: No chance for resistance.” The website has no date, but surely someone must have noticed that mites developed resistance in a few years, and that the product is now ineffective in many areas.
In the U.S., the only coumaphos product registered for bee hives (for either varroa or small hive beetle control) is Checkmite+. However, in Europe Bayer sells a liquid formulation (Perizin®), which is dribbled onto the bees with a syringe (anecdotal reports from beekeepers who save time by using a trigger sprayer say that they feel sick after spraying, and need to take the rest of the day off).
However, beekeepers in some parts of the world have used the ag or veterinary coumaphos powders Co-Ral® or Asuntol® in hives—sometimes with deleterious results to the bees and combs (Martel 2007). Coumaphos residues are really tough on queens and on bee larvae, plus they synergize with fluvalinate. Worldwide, the degree of coumaphos contamination of combs is of considerable concern.
Dennis vanEnglesdorp notes that coumaphos residues have a positive correlation with CCD. I find it strangely ironic that beekeepers in Europe are blaming (with scant scientific evidence) the Bayer neonicotinoids for their sick colonies, while at the same time they continue to dose those same colonies with yet another Bayer product for which there exists abundant evidence demonstrating its harmfulness bees!
Coumaphos should be definitely avoided by anyone producing queens, as it can suppress queen development and survivability, and sperm viability (Burley 1999). Lest I be accused of disparaging a registered mite-control product, let me state that Checkmite+ appears to be safe to use at least once, and is effective on nonresistant mites.
Chlorfenvinphos
Chlorfenvinphos is another nasty organophosphate, which is no longer available in the U.S. However, the product Supona® is widely used illegally in Spain and some other countries. Chlorfenvinphos is about 50 times more toxic to varroa than coumaphos (Milani 2009)! Similarly to the disasters caused by off-label dusting with coumaphos products, colonies in Spain suffer from serious comb contamination from chlorfenvinphos (Orantes-Bermejo 2009).
Amitraz
Amitraz was one of the first varroacides to be registered in the U.S., and is effective against both the varroa and tracheal mites. It was formulated in a plastic strip as Miticur, but the registration for use in bee hives was withdrawn after some lawsuits. However, beekeepers worldwide commonly use the ag products Tactik® and Ovasyn® off label. In Czechoslovakia amitraz was used for many years as a fumigant, by burning two drops of Tactik on a piece of treated filter paper.
In the western U.S., Tactik in oil or grease quickly became the darling of commercial beekeepers when coumaphos failed. However, it was no surprise a couple of years ago when I started hearing reports that mites were showing resistance to the product, and by this year, beekeepers in some areas could no longer count on amitraz to consistently kill mites.
Apivar® is a French formulation of amitraz widely used in Europe, and also registered in New Zealand and Canada (but unfortunately not in the U.S.).
Canada recently registered an amitraz strip—Apivar®—for emergency use (I get confused by all the “api’s” and “vars,” too!). This product has a long successful history in Europe (although mites have developed resistance where it has been used for many years).
Amitraz has the desirable quality that it degrades fairly quickly in honey and wax (although its degradation products are persistent, and may have health issues). Of all the synthetic miticides, it is perhaps the most benign in the hive.
Hivastan
The newest synthetic miticide on the U.S. market is fenpyroximate, marketed as Hivastan®. It’s been a hard slog for Wellmark to work out the bugs—mainly some initial adult bee mortality, but it’s now registered for use, provided that you sign a liability release.
As I mentioned in my last article, there were issues this spring with the product was applied early in the season—there will need to be cold weather trials run to determine the problem.
However, beekeepers who have used the product in warm weather have been quite pleased with it, other than the large amount of goop in the recommended dose. Several beekeepers are using only a fraction of the dose, and are happy with the results. I spoke with the sales rep, Doug VanGundy, about the dosage issue. He said that the eight-ounce dose was found to be optimal for good mite control in their tests, but that the full amount may not be necessary under some circumstances.
The biggest question with Hivastan is probably whether there will be yet another “hangover” in the form of residues building up in the beeswax. Once again, beekeepers are acting as guinea pigs and won’t know the answer for a few years.
As with Apistan and Checkmite+, commercial beekeepers wasted no time in figuring out how to pinch pennies by whipping up homebrew formulations of fenpyroximate. Not all got everything right, and some wiped out their colonies.
Bottom Line—Synthetic Miticides
When varroa first hit North American apiaries, we could control it with a once a year treatment with Apistan. Nowadays, few commercial beekeepers can survive without at least two mite treatments a season (and some up to a dozen!), and they aren’t sure that the treatment will even work from year to year. Clearly, we are losing the battle, as the mite laughs in our faces, and says “Is that your best shot?” (I will speak to this issue in an upcoming series on breeding bees for general parasite resistance).
There are now three synthetic miticides registered for use in the U.S.: Apistan strips, Checkmite+ strips, and Hivastan “patties.” These three, along with (illegal) amitraz provide commercial beekeepers with the possibility of chemical “rotation” to avoid mite resistance, especially if alternated with “natural treatments” such as thymol, formic acid, or (technically illegal) oxalic acid. The key idea is to “mix ‘em up”—if you’re going to use miticides, don’t just keep using the same one until it fails!
Unfortunately, with mite treatments, there’s no free lunch. All miticides, whether synthetic or natural, have negative effects upon the colony—such as developmental effects upon the brood (coumaphos, amitraz), overt brood toxicity (coumaphos, formic acid, thymol), adult bee toxicity (Hivastan), immune suppression, behavioral changes (such as fanning of fumigants or queen shutdown), or effects upon queens and drones (fluvalinate, coumaphos, formic, thymol). These facts suggest three tactical points:
1. Use treatments as seldom as needed,
2. Introduce as little miticide as necessary, and
3. Avoid miticides that leave persistent residues in the combs. This last point applies especially to coumaphos and fluvalinate (time will tell if it is an issue with Hivastan).
Miticides should not be viewed as the sole means of varroa management. In fact, they should ideally be used only as a last recourse. Mites can be managed effectively with a combination of mite-resistant queenstock, spring or fall splits, and perhaps drone trap frames, backed up with a rotation of some combination of (synthetic and/or natural) miticides as needed.
Coming Up
A report on the Apis cerana eradication effort in Australia. Then, a look to the future—miticides in development, avoiding resistance, advances in bee breeding, and the potential effects of climate change.
References
[1] www.animalhealth.bayerhealthcare.com/fileadmin/media/ah/BPC_Bro_engl.pdf
Anderson, LD (1968) Relative toxicity of pesticides to honeybees as determined by laboratory and field tests in California (1950–1966). Department of Entomology, University of California, Riverside in: Pesticide Usage in Relation to Beekeeping.
Balayiannis, G and P Balayiannis (2008) Bee Honey as an Environmental Bioindicator of Pesticides’ Occurrence in Six Agricultural Areas of Greece. Arch Environ Contam Toxicol 55:462–470
Berry, J (2009) Pesticides, Bees And Wax. Bee Culture 137 (1):
Bloom, A (2009) As carbon dioxide rises, food quality will decline without careful nitrogen management. California Agriculture 63(2): 67-72.
Burley, LM (2007) The Effects of Miticides on the Reproductive Physiology of Honey Bee (Apis mellifera L.) Queens and Drones scholar.lib.vt.edu/theses/available/etd-08162007-092313/unrestricted/lmburley.pdf
Chauzat, MP, P Carpentier, AC Martel, S Bougeard, N Cougoule, P Porta, J Lachaize, F Madec, M Aubert, JP Faucon (2009) Influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environmental Entomology 38 (3): 514-523.
Currie, R.W. 1999. Fluvalinate queen tabs for use against Varroa jacobsoni Oud.: efficacy and impact on honey bee, Apis mellifera L., queen and colony performance. Am. Bee J. 139(11): 871- 876.
EPA (2005) Reregistration Eligibility Decision (RED) for Tau-fluvalinate, List A, Case No. 2295
Frazier, M, C Mullin, J Frazier (2008) Hazardous working zone. Georgia Bee Letter, April 2008.
Kevan, P.G. and T. Ebert (2005) Can almond nectar & pollen poison honey bees? American Bee Journal 145(6): 507-509.
Martel, A-C, S Zeggane, C Aurières, P Drajnudel, J-P Faucon and M Aubert (2007) Acaricide residues in honey and wax after treatment of honey bee colonies with Apivar® or Asuntol®50. Apidologie 38: 534-544.
Milani, N, GD Vedova, M Lodesani (2009): Determination of the LC50 of chlorfenvinphos in Varroa destructor. Journal of Apicultural Research and Bee World 48 (2): 140-141.
Orantes Bermejo, Fco. José., Gómez Pajuelo, A., Megías Megías, M. and Torres Fernández-Piñar, C. (2009). Pesticide residues in beeswax and breed pollen samples collected from honey bee colonies (Apis mellifera L.) in Spain. The role of bee losses (in press)
Orantes Bermejo, Fco. J (2009). Estudio de la prevalencia y estacionalidad de seis virus en Apis mellifera iberiensis y Varroa destructor. Evaluación de factores asociados a su presencia. Proyecto API 06/002. Ministerio de Agricultura y Pesca (Gobierno de España)
Pettis J.S., Collins A.M.,Wilbanks R., Feldlaufer M.F. (2004) Effects of coumaphos on queen rearing in the honey bee, Apis mellifera, Apidologie 35, 605–610.
Rinderer, TE, L de Guzman, V Lancaster, G Delatte & J Stelzer (1999) Varroa in the mating yard: I. The effects of Varroa jacobsoni and Apistan® on drone honey bees. ABJ 139(2): 134-139.
Thompson, HM and C Maus (2007) Perspective: The relevance of sublethal effects in honey bee testing for pesticide risk assessment. Pest Manag Sci 63:1058–1061.