The “Nosema Twins” : Part 5- Alternative Treatments
The “Nosema Twins”
Part 5
Alternative Treatments and Prevention
© Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in April 2008
Update: see https://scientificbeekeeping.com/field-trial-of-several-nosema-treatments/
The more I learn about CCD and other colony maladies, the more I am impressed by the fact that honey bees are currently dealing with a complex of novel parasites, pathogens, vectors, stresses, and immunosuppressants. The nutrition/parasite/virus complex appears to me to be where the main action is, and viruses seem to be changing the rules of the game! (More on this in a later article). Unfortunately, at present, there isn’t much we can directly do about viruses ; but what we can do is feed our bees well, and take action against the parasites.
Healthy bees generally handle viruses fairly well until something starts punching holes through their integument. And that’s just what mites and nosema do—one pierces the skin, the other perforates the gut. As I speak to beekeepers who are successful at bringing strong colonies into the almonds, a common thread is that they’ve been diligent at keeping mite and nosema levels down—in the case of mites, way down. Many also swear by heavy feeding to supplement natural forage.
I’ve noticed that today’s successful beekeeper tends to be smart, diligent, hardworking, and willing to invest time and money into good bee husbandry.
Back to Nosema ceranae
The big question is: just how virulent is N. ceranae in this country? In Spain, Dr. Mariano Higes’ team found ceranae to be devastating to colonies and to individual bees in the lab. However, Dr. Steve Pernal of Alberta, in a small lab test, did not find it to be notably more virulent than N. apis. I’ve spoken to beekeepers who found high spore counts this fall, yet have thriving bees in almonds. Yet others, including myself, have seen colonies lag, or go downhill with moderate spore counts in summer. Others in the US report major collapses due to ceranae.
Researchers are trying to figure out the equation that makes ceranae the kiss of death in some colonies, and a mere nuisance in others (luckily, some serious talent from other fields (such as genomics and mycology) is stepping forward to lend a hand). Why do some yards go down, while other yards in the same operation thrive? As I mentioned in the forward, there are likely other aspects or players involved. In any case, it would certainly be prudent for any beekeeper to monitor nosema levels, and to treat if necessary. In the previous article, I discussed the very successful registered treatment for nosema–fumagillin. Now let’s take a look at other potential ways to deal with the problem.
Other Treatments
I certainly don’t want to give the impression that there is anything wrong with fumagillin! However, some folk have concerns that it (or any antibiotic) might inadvertently show up in honey. Others are simply loathe to pay the price for the treatment (although they may pay a higher price in lost colonies after putting their faith in home remedies). In any case, it would be prudent for us to have several treatments at our disposal, in order to customize or rotate treatments, and to avoid resistance to any single drug. As I mentioned in the previous article, hundreds of compounds have been tested to control nosema in honey bees and other insects (most failed).
I have come across several suggestions about adding vinegar (5% acetic acid) to sugar syrup. However, Forsgren and Fries (2003) found in both field and laboratory trials that adding acetic acid to sugar syrup was ineffective at controlling nosema. Their tests with syrup of up to 0.4% acetic acid (by my math that would be equivalent to 1 part vinegar added to 11.5 parts syrup) were meticulous and conclusive. They found that “acidification of the food of honey bees has no influence on Nosema prevalence or development.” The strong buffering capacity of the bee midgut may the parasite from effects of acid (Ostermann 2002). The same would likely apply to another home remedy that’s been suggested—sodium diacetate. This chemical would dissociate in water into essentially vinegar with a bit of sodium ions, and thus would be unlikely to have an effect upon nosema.
Here’s a wild idea: note that any treatment that kills infected bees before they transmit spores would be good for colony health. Rather than thinking of curing infected bees (according to Dr. Meana they are irreversibly injured by infection), instead just kill them off quickly to prevent transmission! Some of the “cures” may do just that—be the kiss of death for the sick bees.
Since plants produce numerous compounds in their sap and bark to resist fungal infection, the botanical kingdom may provide a pharmacopoeia of potential natural nosema treatments. Lodesani (2006) tested several natural compounds, and found both thymol and resveratrol (from red grape skins) to show promise. Others are testing various essential oils. A number of beekeepers swear by feeding HoneyBHealthy® (a solution of emulsified lemongrass and spearmint oils) in syrup, although the manufacturer makes no claims, and as yet has no data to support its efficacy. I am currently testing it. I’ve also been asked if copper gluconate would be effective—I’ve passed a request to test this on to those better funded than I.
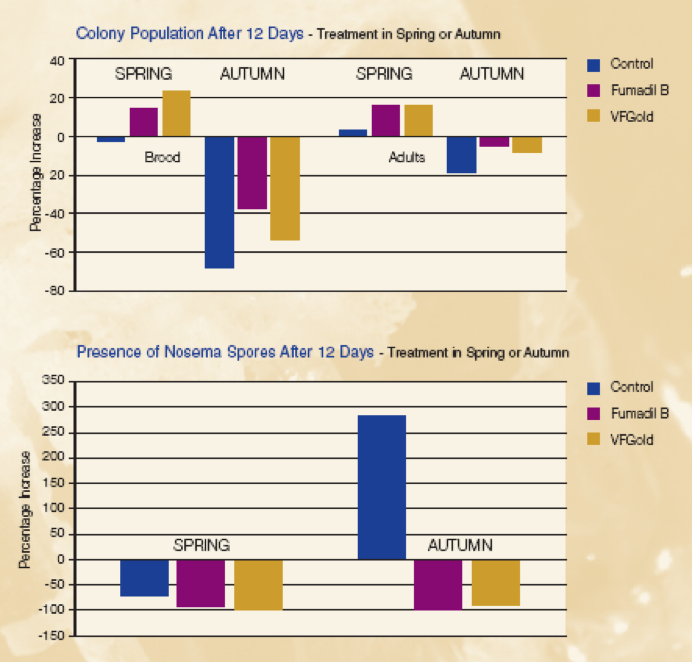
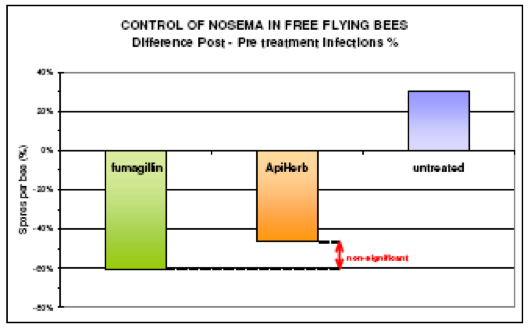
In Western Europe, two natural products are sold for nosema treatment—ApiHerb (Chemicals Laif) and Vita Feed Gold (Vita Europe)–both have data that demonstrate their efficacy (Unknown 2007).
Vitafeed Gold is a beet and molasses extract, rich in natural salicylic acid (the precursor to aspirin, also found in willow bark). This is dribbled over the bees, 5 times, every other day.
Data from the label of Vitafeed Gold. This product is sold as an “enhanced liquid feed for honeybees.” The company also sells Vitafeed Green–which contains essential oils and has been developed for fortifying colonies and retarding chalkbrood. Photos courtesy Dr. Max Watkins, Vita Europe.
ApiHerb is sold by Chemicals Laif—the same company that produces Apilife VAR. The above graph shows the results of a study by Dr. Antonio Nanetti (by permission). The results for treatment of winter clusters were less robust. For full results see http://www.beekeeping.com/articles/us/apiherb_nanetti.pdf
Another product, from Central Europe, is a traditional bark extract marketed as Nozevit. Dr. Joe Carson has found it to be effective during his long Alaska winters. He not only markets it (see References), but is also working with a chemist to analyze traditional cures.
Nosevit is a traditional bark extract from Central Europe. Dr. Joe Carmen uses it successfully to winter bees in Alaska! Joe says that it is the only product used in Croatia, Bosnia, Slovenia, Serbia and other close countries. Photo courtesy Joe Carson.
We clearly need additional research on alternatives to fumagillin. Drs. Robb Cramer, Steve Pernal, and Jeff Pettis are currently testing various compounds (Pernal, et al 2008). I, also, have recently received funding from the California State Beekeepers Association to trial various compounds for nosema control. It is sometimes difficult to test compounds in the field, since frequently the nosema spore counts of the control groups drop drastically without treatment, so the researcher’s hard work winds up being for naught (Dr. Pernal unfortunately experienced this problem in a recent large field trial).
In Chile, where nosema decimated colonies this past year, Juanse Barros and his associate Eduardo Moena, experimented with a bleach drench for nosema control. After some experimentation, they used 100cc of 10% sodium hypochlorite solution mixed into 250cc HFCS and 150cc distilled water (corrected 4/12/08), dribbling 5cc of the solution onto each seam of bees. Spore counts had dropped considerably in the test group when checked one week later. Juanse then went ahead and dribbled bleach solution twice again at weekly intervals. Winter survival was much better for treated colonies vs. the untreated controls. The numbers of colonies in each group was small, but the method appears to show promise.
A promising alternative that really catches my attention is good old thymol—which is widely used for varroa treatment, and as a disinfectant and fungicide. Braga, et al (2007) found that its mode of action against the yeast Candida was to affect the “architecture of the [fungal] envelope,” thus hampering its infectivity. Well, it apparently also may control nosema when used in the form of thymolated syrup (doesn’t that term just roll off the tongue nicely?). I came across a reference to it in a state newsletter, which had picked it up from another, and yet another, and finally got to the original paper by Rice (2001) from Australia. What had piqued my curiosity was that all the references gave the recommended concentration as 0.44mM, which is a rather precise chemical term that no beekeeper would ever use. Rice apparently back-calculated that specific concentration from a recipe used by a beekeeper named Brown, who likely got it from another, Manley (1946, which I downloaded from the Australian library), who got it from a long letter from a Dr. Killick, who thought that the addition of thymol would make sugar syrup convert to a more natural “honey”—nosema wasn’t even mentioned. Rice then tested thymol against nosema, but used analogous lab surrogates–the Corn Earworm infected by Nosema vespula, which infects European wasps. For some reason, he also used weaker concentrations than 0.44mM—0.15 and 0.30mM. Anyway, he found that thymol killed N. vespula in caterpillars. He also discusses possible modes of action of thymol against nosema. It appears to me that the 0.44mM strength is based upon an old arbitrary concentration intended for another purpose, that serendipitously happened to also be effective against nosema in Australia.
Well folks, Rice’s was a great study, but I really couldn’t call it a slam dunk for our purposes. However, Yucel and Dogaroglu (2005), in Turkey, performed a controlled trial comparing thymol against fumagillin. The authors fed infected colonies 150ml of light (30%) syrup at weekly intervals for 4 weeks (for a total of 600ml, or approximately 2½ cups), both spring and fall. The treatment syrups contained either thymol at 0.44mM (they must’ve read Rice), or Fumidil-B at 1 gram per feeding (this is approximately the label rate for the small colonies they studied, but in less syrup); the controls were plain syrup. The treatments were continued for 3 years, in order to cleanse the colonies of residual nosema spores (the authors state that the infection was by N. apis, but had no reason to suspect that the colonies could actually have been additionally infected by N. ceranae).
The results were impressive for thymol! Over the term of the experiment, both fumagillin and thymol decreased colony losses, and increased number of bees, amount of brood, and honey yield, but thymol did the better job in every parameter measured! Plus, the thymolated colonies had fewer winter losses. Note that the study used only a small amount of very light syrup. The authors hypothesized that the syrup would be concentrated by the bees, thus effecting a stronger concentration of thymol once processed by the bees. Also note that it took three years of either treatment to really get nosema spore counts down!
With great hopes I fed several hundred gallons of thymolated syrup to my own infected colonies. Unfortunately, my results do not support the efficacy of thymol, nor do other short trials that I have seen from Europe and Canada. I am currently testing it at 3x strength. It may yet prove to be effective in the long term, but I’d consider it experimental for the time being.
The 0.44mM solution works out to be 1/4g of thymol per gallon of syrup—just enough that you can smell it, and barely taste it. The bees take it just fine. In fact, the feeding of thymolated syrup quickly trains foragers to seek out the odor of thymol anywhere in the vicinity (no surprise to Drs. Jerry Bromenshenk and Adrian Wenner). This makes for a pretty cool demonstration!
Be aware that you can’t just mix thymol crystals into water. But luckily, thymol is 1000 times more soluble in alcohol. So you simply dissolve it into alcohol first to make a premix, and then add the premix to syrup. I’ve fed a few hundred gallons now, and found an added benefit: thymol keeps light syrup from fermenting, and stops black gunk from growing in the jars. Since this article is already lengthy, I will post mixing details to my website. Note that thymol is not registered as a nosema treatment, and its use as such would technically be illegal. However, it is legal to use in order to prevent fermentation in syrup.
Thymolated syrup test. I inoculated both bottles with 5cc of fermenting syrup, and incubated at 80°F for a week. Note how microbial growth has made the control syrup cloudy, whereas the thymolated syrup remained fresh.
Management practices
Nosema is always present at some level, but appears to flourish under certain conditions. There are a number of management practices that you can utilize to minimize nosema infections. I will list them by the factor of infection that they may affect.
Here’s a tip: I’ve come across a number of studies that found a correlation between chalkbrood infection and either nosema or varroa infestation. Not saying that it’s causal, just associated. The point is that nosema and varroa buildup are easy to miss, but chalkbrood is an easy visual indicator that your bees that are under stress, and that the other bad guys may be there, too. I asked Dr Jerry Bromenshenk whether he found any correlation between chalkbrood and CCD in his survey of beekeepers. He didn’t, but he did note an interesting observation: “many of the post-CCD bee operations have had chalkbrood problems, even in mid-summer, when they’ve NEVER seen it before at that time of year.” I realize that I may digress from the subject of nosema, but these kinds of observations intrigue me!
Nutrition and Stress
Studies by Hirschfelder (1964) and Kleinschmidt (1988a) clearly demonstrated that bees well fed with pollen live far longer than nutritionally stressed bees, even if infested with nosema. A recent study by Eischen (2008) indicates that feeding pollen supplement during winter may be as effective as fumagillin treatment for promoting health of colonies with light infections! (Note, however, that the Spanish researchers do not feel that there is a nutritional connection with N. ceranae (A. Meana, pers comm)).
1. Make sure that your colonies have adequate pollen or protein supplement in fall, and at all times when broodrearing.
2. Do not allow colonies to run short on honey stores. Hungry colonies are stressed, have trouble maintaining broodnest temperatures, and are forced to forage in unfavorable weather.
Food for thought: I wonder if the massive collapses of French colonies in sunflowers (previously blamed on imidicloprid) could have been the result of nutritional stress due to the poor amino acid balance of sunflower pollen coupled with N. ceranae infection?
Transmission of spores
Both nosemas require bees to ingest spores in order to become infected. The beekeeper should make efforts to minimize any routes of transmission. This was easier with apis, since its spores were only found in bee feces. Unfortunately, Higes, et al (2007) found that ceranae spores can be transmitted in pollen. Chen (2008 Conf) detailed how ceranae infection spreads to the bees’ hypopharyngeal and salivary glands (specifically, nosema DNA was found there, Dr. Chen did not look for spores or the vegetative state (pers comm)). If indeed the vegetative state can make its way into the saliva or jelly, it could explain how foragers contaminate their pollen pellets with the spores, and would suggest that brood food, and perhaps any surface licked by bees may be contaminated (again, this is pure conjecture).
1. Overwinter bees with plenty of fully-cured quality honey or heavy sugar syrup. Honeys that promote dysentery increase the transmission of spores.
2. Minimize the crushing of bees during colony manipulations. Every bee that you crush is the equivalent of having a bee poop in the hive! Carelessly tossing in a pollen patty and crushing a hundred bees top and bottom smears any nosema spores that were in the crushed bees all over the patty that the colony will soon be eating. The resultant nosema infection may negate the benefit of feeding (Kleinschmidt 1988a).
3. Winter colonies in sunny locations to encourage cleansing flights.
4. Avoid allowing bees to drown or defecate in tanks of sugar syrup, insert feeders, or during barrel feeding, as this can spread spores.
5. Avoid having stagnant water sources close to the beeyard. These can become contaminated by dead bees or cleansing flights.
6. Minimize the amount of time that bees are confined during hauling. Not only are they stressed, but they may be forced to defecate within the hive.
7. Nick Calderone (2008 Conf) found nosema in some package bees. Test packages and nucs before you spread them into your operation!
8. Extreme: move the colony during daylight and kill the returning “drift” of infected field force so they can’t transmit spores to the emerging brood.
9. Finally, sterilize contaminated combs, or rotate them out.
Temperature
Nosema apis thrives in cool broodnests (Benecke 2003; Hornitzky 2005; Manning 2007), when bees are chilled (Kleinschmidt 1988b), and during periods of rainfall (Aydin, et al 2005). Other studies have demonstrated the elevating the temperature of the host kills off nosema (Cantwell & Shimanuki 1969, Booheme 2003, Manning 2007). Aydin (2006) also found a relationship between nosema, chalkbrood, and amoeba disease—indeed, they suggest using chalkbrood infection as an indicator for nosema infection! It is not yet clear if ceranae requires cool conditions, since it appears to flourish during summer as well as winter. Nonetheless, it may be wise to avoid cold, wet conditions. Especially avoid shady apiaries, since they are conducive to the above diseases, plus varroa and small hive beetle! In general, colonies placed in full sun have less parasite and disease problems than those in shade.
1. Locate colonies on high ground, in warm (or hot), dry, sunny areas.
2. Minimize disturbance to the winter cluster.
Stress
1. Minimize stress due to mite infection. Bees suffering from mites have reduced immunity to other pathogens.
2. Minimize disruption to the winter cluster—this may greatly stress the bees.
Breeding
Of considerable interest to me is the striking colony-to-colony variation in nosema spore counts that I’m finding. Also of interest is the apparent ability of some individual bees to resist infection. Webster, et al (2004) found that a large fraction of workers (40%) and queens (25%) remained uninfected despite having been given large doses of spores. It appears that nosema resistance is a heritable trait that can be amplfied in a few generations. A graph in the February issue of this journal illustrates the success of bee breeders in Denmark at selecting for nosema resistance over a period of 15 years (Traynor 2008). It would behoove us to do the same!
Comb Disinfection
OK, so what are you going to do with spore-contaminated combs from deadouts? The long-lived spores in the combs serve as a fresh inoculum as the colony cleans cells in order to prepare the brood nest. Higes (2008 Conf) feels that N. ceranae-contaminated combs will re-infest an introduced colony. This situation is similar to being stuck with AFB spore-contaminated combs—either burn ’em, or be forced to treat with antibiotics as long as you reuse them.
Comb replacement has been a hot item of discussion among beekeepers these last few years, and especially as afflicted beekeepers restocked CCD deadouts with bees, only to see the new colonies go downhill. The practice of rotating out older spore-infested combs is a time-tested method for control of N. apis, and will likely be even more important for control of ceranae. However, comb replacement is expensive, so finding methods of comb disinfection would be more economically practical.
Fortunately, nosema spores are easier to kill than AFB spores, so that combs can realistically be disinfected for reuse. Indeed, a number of CCD-afflicted beekeepers report that even simple storage, or “airing out” of combs for a few months appears to improve restocking success. So we wonder, just what toxic or infectious agent degrades during storage—is it nosema spores, viruses, pesticides, or something else?
The next question is, “Can we accelerate that degradation?” A trial by Dr. Jeff Pettis demonstrated that some methods of comb disinfection improved restocking success, but not to the extent that one might hope. Since this article is about nosema, I will stick to disinfection methods for it alone. Therefore, an important question is, “Just how long do nosema spores remain viable on combs and in honey?”
Spores of N. apis can remain viable for years on the combs. A thorough study performed in New Zealand by Malone, et al (2001) tested the infectivity of spores stored either dried on glass, in sugar syrup, or in various honeys, and at different temperatures (the paper also has a thorough review of previous studies on spore longevity). In short, spores are dang tough. There is some loss of activity in some of the above conditions, but not much, other than by temperature. A portion of spores lose viability when stored in honey, but a substantial number remain infective. I have not yet found data to see if these findings apply to ceranae spores, but since this is the only data we have, let’s roll with it!
Higes also found ceranae spores in pollen, which suggests that stored pollen in the combs may be a source of nosema spores, as well as viruses (see Twins 1).
There are primarily three effective ways in which old combs can be disinfected (at least of nosema spores): irradiation, heat treatment (thermal sterilization), or fumigation. Irradiation appears to be most effective in the case of CCD combs (possibly since it kills a wide range of pathogens, plus may partially degrade some pesticides (Duarte, et al 2007)). Unfortunately, few have access to cobalt-60 irradiation facilities, and I strongly suggest that you don’t try this as a garage project!
More practically, spores can be killed by heating hive equipment or tools to a temperature of at least 140°F (60°C) for 15 minutes, but this temperature is too close to the melting temperature of beeswax (150°F) to treat combs. Combs can instead be sterilized (of N. apis) by heating to the lower temperature of 120°F (49°C) for a longer period of 24 hours (Cantwell & Shimanuki 1969), although if you don’t have a dedicated hot room, it can be damn tricky not to melt some combs. For unstated reasons, Shimanuki (1992) says that combs to be treated should “contain little or no honey or pollen,” which would definitely limit the utility of this treatment. However, data of White (1992) and LeBlanc (pers comm) indicate that toxic HMF in honey would not appreciably increase in a 24-hour exposure to that temperature. Even heating to 120°F may not be necessary. Malone found that storage of N. apis spores for even 5 days at 104°F (40°C) substantially decreased their infectivity!
Update: N ceranae spores appear to be more delicate than those of N apis. They are less resistant to either heating or freezing. Dr. Robert Cramer found that heating them to 120°F for only 90 minutes was sufficient to kill them. He also found that they are very susceptible to either bleach or lye solutions.
Another proven method to fumigate combs is with concentrated acetic acid (the “active ingredient” of vinegar). This is a time-honored practice that is mentioned in all the bee books, yet surprisingly, is illegal! No one has registered acetic acid as a pesticide for nosema. So if you do use it, make sure that you are applying it as a “comb freshener”– not as a nosema treatment!
Bailey (1957) notes that acetic fumigation also kills the spores of amoeba disease, and all stages of wax moth (and I imagine damn near anything else alive!). I tried acetic fumigation years ago, and found the acid fumes to be extremely corrosive to metal hive parts, and any metal nearby. I’ve found few others who have experience with the method. Various authors differ on suggested application methods, so here’s a summary. Stack your boxes in a warm place, with any cracks or holes sealed. Between each box, place an absorbent pad of some sort, and pour on ½ cup (125ml) of 60-100% acetic acid. In warm weather the fumes from 60% acid will kill spores within a few hours; stronger acid will kill them in minutes! And kill you, too, if you’re not careful! You must wear nitrile gloves, goggles, and avoid the fumes. Keep plenty of water on hand to wash off spills! Let the combs air out for a week or two before reuse.
Side notes: pure acetic acid is called “glacial” acetic acid because it freezes into ice-like crystals below 62°F. Be aware that the fumes can explode at temperatures above 102°F!
So how about using formic acid for disinfection (Fries 1991)? Anecdotal reports are that formic fumigation reduces nosema spore counts, but I could find only one supporting study: Underwood and Currie (2004), when using indoor formic fumigation to control varroa, found that formic vapors, even at that low concentration, had a significant effect upon nosema. Fries (1991) did not find it effective, but close reading of his bottom application method leaves me wondering. It might work to throw a MiteawayII pad between stacked supers (this would only be legal if you were trying to kill that last mite). I’ve spoken with the manufacturer about testing the product for this use.
There are additional proven or likely comb sterilization candidates. Ethylene oxide (ETO) will do it, but the chambers are expensive. Dr. Steve Pernal (2008 Conf) found that UV light quickly killed spores (apis was more susceptible than ceranae)—a simple device could be built to pass combs through individually on a track. Sunlight alone may do it, provided that care is taken not to melt the combs. Such UV sterilization would require the treating of individual combs, and thus would only be practical on small scale.
Commercial operations clearly need safe and inexpensive fumigants that can treat pallets of supers, yet leave no residue. Dr. Pernal found that isopropyl alcohol, Apilife VAR, paradichlorobenzene, and drugstore 3% hydrogen peroxide were fairly effective (but that there was variation in susceptibility between strains of spores). I’ve spoken with Dr. Rosalyn James about her experiments with using ozone as a sterilant against other pathogens, and am myself currently working on setting up a small trial to test its efficacy against nosema spores.
In summary, nosema spores are susceptible to a number of sterilants, several of which are inexpensive, and leave no residue. In general, vaporized sterilants work best in a warm chamber (too much heat could produce toxic HMF in residual honey). Currier, et al (2001), in studying ways to deactivate anthrax spores with ozone states that “it is known that the degree of re-hydration of dry biological spores influences the spore coat permeability and the deactivation rates.” He also found that too moist an environment may protect the spores from ozone action. This effect of humidity may apply to other fumigants. I would venture a guess that a heated comb sterilization chamber will become a common fixture next to the bee warehouse. I strongly suggest that researchers and beekeepers invest some effort into finding practicable methods for comb sterilization!
Update 4/22/2011
Recent research by Dr. Frank Eischen found that Phostoxin (a legal comb fumigant) was effective for disinfecting combs of nosema spores. However, other recent research by Dr. Steve Pernal indicates that their is little long-term benefit of comb sterilization with regard to subsequent spore counts of the bees.
Bottom Line
We have a great deal to learn about Nosema ceranae. The Spanish team is clearly the most knowledgeable about its epidemiology. Most of the world now has it (Australia just found it), and scientists everywhere are jumping on research.
What can you do?
1. Request your queen supplier to breed for (mite- and) nosema-resistant queens!
2. Rotate out, or disinfect, contaminated combs.
3. Keep your bees well fed, and keep your hives exposed to full sun.
4. Keep an eye open for chalkbrood infection as an indicator of stress.
5. Get a scope, since you can’t detect the disease without one.
6. If you detect spores, then treat with fumagillin as per the label instructions– at present, it’s the proven standby.
Dealing with this disease is new to me. I’m distressed by the levels of nosema that I’m seeing in some of my own bee yards. But some of the problems that I’ve been seeing are starting to make sense. As with varroa, the more you know about your enemy, the better you can fight him. I’m not sure by any means, but it looks like we’ll be fighting N. ceranae at least until we (I hope) breed resistant bees. Again, as with varroa, you will likely need to start monitoring for nosema, or if you already have been, start doing it more regularly, and throughout the year. I hope that this series of articles has been of use to you, and I wish you all good luck!
Oh, by the way, Paul van Westendorp (2007) recently reported finding a colony of honey bees in British Columbia infested with yet another nosema, Nosema bombi, previously only found in bumblebees!
Scroll to bottom for thymolated syrup recipe.
Feedback
Hi
The April ABJ has only just got to the UK and the first article I looked at was yours on nosema. Thank you for all the good work you are doing.
The reason for writing is to point out that Manley was an Englishman (the introduction to his Honey Farming gives a little on his background) and the Brown whom you mention is almost certainly another Englishman , Ron Brown who is quite well known in the UK as a lecturer and author but now in his 90s and retired. Both of Ron’s books mention adding thmol to syrup to prevent mould growth and he was also a friend of Norman Rice who stayed with the Browns in Torquay when he visited the UK many years ago.
When lecturing Ron commonly mentioned the suspicion that thymol in syrup helped to control nosema but noted that he had only anecdotal evidence for this.
Thymol is quite commonly added to syrup in the UK but there has been some concern about the additives in surgical spirit used to denature it since these may be found in honey later. It has been suggested that we use isopropanol (isopropyl alcohol) instead of the surgical spirit. I am not enough of a chemist to be able to comment on this.
Keep up the good work.
Best wishes
Brian Gant
Buckfast, Devon, UK
Acknowledgments
Thanks to Dr. Blaise LeBlanc for chemistry help, and the many researchers worldwide who have been generous with their correspondence. I appreciate the assistance of those who have sent me information, and of Chilean beekeeper Juanse Barros; also the technical assistance by my students at the Nevada County Science Center. I greatly wish to thank the California, Idaho, and Colorado State Beekeepers Associations, and Project Apis mellifera for their financial support.
References
Aydın, L., Çakmak, İ., Güleğen, E., and H. Wells (2005) Honey bee nosema disease in the Republic of Turkey”. Journal of Apicultural Research, 44(4):156-157.
Aydin, L, E Gulegen, I Cakmak, AO Girisgin and H Wells (2006) Relationship between nosema and chalkbrood diseases, and its implication for an apirary management model. Bull Vet Inst Pulawy 50, 471-475.
Bailey, L. (1957) Comb fumigation for nosema disease. ABJ 97: 24-26.
Benecke, FS (2003) Commercial Beekeeping in Australia. RIRDC Publication No 03/037
Braga, PC, MD Sasso, M Culici, and M Alfieri (2007) Eugenol and thymol, alone or in combination, induce morphological alterations in the envelope of Candida albicans. Fitoterapia 78(6): 396-400.
C. K. Boohene1 C. J. Geden, andJ. J. Becnel (2003) Evaluation of Remediation Methods for Nosema Disease in Muscidifurax raptor (Hymenoptera: Pteromalidae). Environ. Entomol. 32(5): 1146-1153.
Cantwell, G. E., and H. Shimanuki (1969) Heat treatment as a means of eliminating Nosema and increasing production. Am. Bee J. 109: 52-54.
Currier, IP, et al (2001) Deactivation of clumped and dirty spores of Bacillus globigii. Ozone Science & Engineering 23:285-294.
Duarte , CL, , M.N. Moria, Y Kodamaa, H Oikawaa and M.H.O. Sampaa (2007) Decontamination of pesticide packing using ionizing radiation. Radiation Physics and Chemistry 76(11-12):1885-1889.
Forsgren & Fries (2003) Acidic food and nosema disease. Standing Commission of Bee Pathology (Broken Link!) http://www.apimondia.org/apiacta/slovenia/en/forsgren.pdf
Fries, I. 1991. Effect from treatment with formic acid for control of Varroa jacobsoni on viability of spores of Nosema apis. Proceedings of the International Symposium on Recent Research of Bee Pathology. Sept 5-7: 118-119.
Higes, Mariano , Raquel Martín-Hernández , Encarna Garrido-Bailón , Pilar García-Palencia , Aránzazu Meana (2007) Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J Invertebr Pathol. 2007 Jun 20; : 17651750
Hirschfelder H (1964) Investigations concerning the nutrition, length of life and the nosema disease of honey bees. In ‘Complete texts of lectures of the 19th congress of Apimondia in Prague, 1963’. (Ed. U Mzlvh) pp. 274–278. Apimondia Publishing: Prague
Hornitzky (2005) Nosema Disease in Honeybees. http://www.rirdc.gov.au/reports/HBE/05-055.pdf
Kleinschmidt, GJ (1988a) Influence of management on the effects of nosema disease in Queensland Agricultural College Apiculture Production and Research
Kleinschmidt, GJ (1988b) Banking of queen honey bees in Queensland Agricultural College Apiculture Production and Research
Lodesani, M, L Maistrello, C Costa, F Leonardi, G Marani (2006) Effects of natural compounds on Nosema diseased honeybees in laboratory conditions. Proceedings of the Second European Conference of Apidology EurBee Prague (Czech Republic) 10-16 September 2006
Manley, ROM (1946) Honey Farming http://www.soilandhealth.org/copyform.aspx?bookcode=030220
Manning, R, K Lancaster, A Rutkay and L Eaton (2007) Survey of feral honey bee (Apis mellifera) colonies for Nosema apis in Western Australia. Australian Journal of Experimental Agriculture, 2007, 47, 883–886
Malone, LA, HS Gatehouse, EL Tregidga (2001)Effects of Time, Temperature, and Honey on Nosema apis(Microsporidia: Nosematidae), a Parasite of the Honeybee, Apis mellifera (Hymenoptera: Apidae). Journal of Invertebrate Pathology 77, 258–268.
OME (2004) Rationale for the Development of Ontario Air Standards For Isopropanol http://www.ene.gov.on.ca/envision/env_reg/er/documents/2004/air standards/rationales/pa02e0014-r.pdf
Ostermann, D (2002) Interactions of varroa, Varroa destructor Anderson and Trueman, with chalkbrood, Ascosphaera apis (Maassen ex Claussen) Olive & Spiltoir, and nosema, Nosema apis Zander, in honey bee, Apis mellifera L., colonies treated with formic acid, and the influence o f hive and ambient conditions on formic acid concentration in the hive. Master’s Thesis, Univ of Manitoba
Pernal, S.F., Pettis, J. and A. P. Melathopoulos (2008) A preliminary evaluation of control methods for Nosema apis and Nosema ceranae. American Bee Journal 148: in press.
Rice, RN (2001) Nosema disease in honeybees. http://www.rirdc.gov.au/reports/HBE/01-046.pdf
Rogers, R.E.L and G.R. Williams (2007) Monitoring Nosema disease in honey bee colonies: Early detection and accurate spore counts are necessary. Bee Culture 135(12):19-21.
Shimanuki , H, et al (1992) Nosema Disease in The Hive and the Honey Bee, Dadant
Traynor, K (2008) Bee breeding around the world. ABJ 148(2):135-139.
Underwood, R. and Currie R.W. (2004) Indoor winter fumigation of Apis mellifera (Hymenoptera:Apidae) colonies infested with Varroa destructor (Acari:Varroidae) with formic acid is a potential control alternative in northern climates. Journal of Economic Entomology. 97(2):177-186.
Unknown (2007)(Broken Link!) http://www.ulss22.ven.it/UploadDocs/2107_diapo3_Nosema.pdf
van Westendorp, P (2007) Beelines in Bee Scene 24(4): 7-9.
Webster, TC, KW Pomper, G Hunt, EM Thacker and S Jones (2004) Nosema apis Apis infection in worker and queen mellifera. Apidologie 35: 49-54.
White, JW Jr et al (1992) cited in Honey. The Hive and the Honey Bee. Dadant & Sons. P 889.
Williams, G., A. Shafer, D. Shutler, R. Rogers, and D. Stewart (2008) (abstract). Nosema and bees have incongruent phylogenies: how, why, and what might this portend? Proceedings of the 2008 American Bee Research Conference, Sacramento California, 9-11 January 2008. American Bee Journal (submitted).
Yucel, B & M Dogaroglu (2005) The impact of Nosema apis Z. infestation of honey bee (Apis mellifera L.) colonies after using different treatment methods and their effects on the population levels of workers and honey production on consecutive years. Pakistan Journal of Biological Sciences 8(8): 1142-1145.
Alternative treatments
Api Herb from Chemicals Laif:
http://www.beekeeping.com/articles/us/apiherb_nanetti.pdf
http://www.beekeeping.com/articles/us/apiherb_dublin.pdf
http://www.beekeeping.com/articles/us/apiherb_07_melbourne.pdf
Vita Feed Gold: http://www.vita-europe.com/
Nozevit– A traditional bark extract, widely used in Central Europe: http://www.apivita.hr/index.php?option=com_content&task=view&id=11&Itemid=3
Inexpensive (30¢ per treatment), available from Joe Carson, AlaskaHeavenlyHoney@hotmail.comThis e-mail address is being protected from spam bots, you need JavaScript enabled to view it , (907) 727-8200.
Thymolated syrup formula
Update: this strength of thymolated syrup does not appear to control nosema! I am currently undertaking a trial at 3x strength. My current recommendation is to use FumagilB in syrup per label directions.
OK, here’s how you make the premix. You can use either ethyl or isopropyl alcohol. The cheapest and easiest is 91% isopropyl alcohol from the drug store (70% will also work, but won’t dissolve as much thymol). Isopropyl alcohol is relatively nontoxic to insects, and the small amount won’t hurt your bees. I wouldn’t use denatured ethanol, since they add nasty “ingestion deterrents.” Theoretically, you can dissolve 1g of thymol in 1ml of pure alcohol, but in practice this is difficult, and the solution doesn’t mix well with water.
What works well is to add 12.5 grams of thymol crystals 88ml of of 91% isopropyl alcohol (total volume = 100ml) (from the drug store, isopropyl alcohol is not harmful to bees at this concentration; 70% works, but not as well; 151 proof Everclear grain alcohol or 160 proof vodka works great, and is less toxic to bees than isopropyl).
Add 2ml of premix per gallon of syrup (2 tsp per 5 gallons). Slowly pour the premix into the hot water that you’re using to mix syrup (watch the fumes) and stir vigorously until thoroughly mixed. Note that thymol sinks in alcohol, but floats in water, and will rise to the surface of the syrup until it is thoroughly dissolved by stirring. The premix can also be added to the bottom of a tank as you fill it with warm HFCS. Caution: you should always wear nitrile gloves when handling thymol, if for nothing else, to remind you not to rub your eyes! If you do get it on your skin, wash it off with warm water—cold water won’t dissolve it.
OK, OK, I’ll do even more math for you, in order to help you to avoid mistakes. Here’s a really simple way of making the premix. Take a 1 pint (473ml) bottle of 91% alcohol, mark the fill level, then pour the alcohol out. Then weigh 59g of thymol crystals, and pour them carefully into the empty bottle. Then pour about three quarters of the alcohol back in, recap, and shake until the alcohol’s dissolved. Then top off back the the original fill level with alcohol. You now have a bottle of premix that will thymolate (dontcha just love the sound of that word?) 236 gallons of syrup. Similarly for a liter bottle, put in 125g thymol, and top off to 1 liter with alcohol. For God’s sake, immediately relabel the bottle so that no one rubs it on their skin (I guarantee you, they’re not gonna drink it)! Any of these premixes can be added at the rate of 2ml per gallon of syrup.