The Varroa Problem: Part 6a – Bee Breeding for Dummies
Contents
It’s been thirty painful years. 2
Breeding is merely Human-directed evolution. 3
Natural and artificial selection. 4
The Bond method (you get what you wind up with) 5
the bond method, but without the Needless carnage. 8
Getting down to the nitty gritty. 9
A process of shuffling and elimination. 12
Randy’s K.I.S.S. RecipE for commercial queen producers. 13
OK, and what sort of progress might I expect?. 15
Maintenance of a developed stock. 17
The Varroa Problem Part 6a: Bee Breeding for Dummies
First published in ABJ March 2017
Randy Oliver
ScientificBeekeeping.com
Over a million queen bees are sold each season by commercial producers in the U.S. Yet as far as I can tell, few are sold with the claim of being “mite resistant,” despite the fact that varroa is our number one problem.
A note from Randy: I feel that this may be the most important article that I’ve yet written, as it has the potential to help return beekeeping to how it was during the “good old days” when we didn’t need to deal with The Varroa Problem.
In a recent scientific paper [[1]], Dr. John Kefuss and collaborators wrote two profound sentences with which I heartily concur:
We believe that it is the responsibility of everyone who breeds bees to try to select for mite resistance to reduce chemicals in hives. We owe this effort to the general public and to future generations of beekeepers.
It’s been thirty painful years
This year the varroa mite celebrates the 30th anniversary of its arrival to North America, and despite it being a decidedly unwelcome guest, it is thriving in its newfound home. That is a sad reflection upon our lack of will to adopt the obvious solution to The Varroa Problem.
It’s neither a secret nor rocket science as to how to breed for mite resistance. Danny Weaver was likely the first in the U.S., starting clear back in 1992. Then in 1997, Drs. John Harbo and Roger Hoopingarner demonstrated the feasibility of selecting for resistance in only ten weeks [[2]]. Three years later, in this very journal, Dr. Eric Erickson and collaborators spelled out the basic concepts for practical breeding [[3]]. What happened though is that the miracle of synthetic miticides made mite management so cheap and easy, that our queen producers simply had little incentive to make the effort (or take the risks) of developing such desirable stocks. But now, as amitraz begins to fail (and the link between miticide residues and queen failure becomes more evident), the astute observation of Samuel Johnson comes to mind:
The chains of habit are too weak to be felt until they are too strong to be broken.
I guess it’s pretty obvious that I feel that we need to break those chains of habit.
Practical application: there’s a change in the air–there are a couple of excellent books that I’d recommend that apply to the evolution of business [[4]]. I strongly suspect that industry demand for mite-resistant queens is about to shift. This will create new business opportunities for innovative queen producers.
Turning the fight over to the bee
Mite resistant bee stocks are no longer a matter of proof of concept—such stocks currently exist all over the world. The only issue is the lack of enough commercially available gentle, productive, mite-resistant queens to allow us to shift over to them as an industry.
During the past twenty years, we’ve had pioneers such as John Kefuss, Danny Weaver, Kirk Webster, and the Baton Rouge researchers continually reminding us that there are no insurmountable obstacles to breeding for mite resistance (with Kefuss recently publishing an excellent update [[5]]).
My goal: I’ve studied all the papers and recommendations, spoken with successful breeders, and will now try to lay out a practical “short version,” based upon what I, as a longtime California queen producer, feel is a realistic course of action for our transition from dependence upon miticides to turning the fight against the mite over to the bees themselves. I suggest a weaning process, rather than an abrupt change.
Breeding is merely Human-directed evolution
Let’s first define what the word “evolution” means to a biologist:
Evolution is change in the heritable characteristics of biological populations over successive generations.
Evolution has no goal or direction—it’s simply a matter of the relative proportions of various heritable traits (including novel mutations) in any natural population. It is driven by the relative degree of “fitness” conferred by those traits upon individual organisms in a breeding population in their specific environment. Fitness, in the biological sense, has nothing to do with six-pack abs or how many pushups one can do, but rather is the ability of an organism to successfully pass its genes into subsequent generations. It’s simple math—the offspring of the more successful individuals eventually make up a greater proportion of the breeding population. In time (provided that the selective pressure of the environment remains constant), the genes conferring the greatest degree of fitness become “fixed” in the population, and individuals tend to breed true.
But environmental selective pressure often changes. A heritable change (genetic or otherwise) that confers greater fitness is said to be “adaptive” to those changing circumstances. When varroa invades a population of the Western honey bee, it changes the selective pressure—a new aspect of “fitness” is introduced; any traits that reduce the chance of a colony being killed by the mite are now adaptive. In any breeding population not give the benefit of human-applied miticides (such as in South Africa, or in feral populations worldwide), the environment (in the form of the varroa/virus complex) quickly eliminates colonies that don’t display some degree of resistance to, or tolerance of, the mite (I’ll discuss the difference later on).
Biological clarification: Nature doesn’t breed for resistance—it simply dispassionately eliminates those that don’t exhibit it. Note: when I use the word “Nature,” I’m referring to the physical environment, not to some sort of intelligent entity with a mind or goal.
Humans, on the other hand, can apply arbitrary selective pressure, provided that they can control which individuals pass their genes to the next generation. Thus, fitness becomes relative to the breeder’s arbitrary choices. For example, if the breeder can maintain an artificial minimal-varroa environment (by the application of miticides), the fitness of a queen might be determined by whether she is a pretty color, rather than by whether her colony can handle varroa. If the breeder’s customers suddenly demanded pure-black queens, then in this case, it would then suddenly be most adaptive in that breeding population for a queen to be black—and black queens would then exhibit greater fitness, since the breeder would not allow those of other color to pass on their genes.
Practical application: the traits that queen producers select for are those that their customers demand. If we want mite-resistant stock from our breeders, we only need to demand it.
Bees are still pretty wild
Humans, by applying selective pressure to promote specific traits, have in the last few hundred years “created” a wide diversity of domesticated breeds of animals. The tradeoff for maximum productivity in controlled feedlot environments is that the resulting breeds may no longer be adapted to life in the wild. For example, we’ve bred our lean native wild turkey into Broad Breasted White birds (Fig. 1), which can no longer fly, or mate without assistance, and are prone to health problems due to their excessive musculature.
Figure 1. The various races of the North American wild turkey were well-adapted to living in the wild in their respective ecoregions. Selective breeding has given us the Broad Breasted White turkey, which is well adapted to human husbandry and artificial selection, but would have little chance of surviving without our help[[6]].
Our managed bees are not bred to the extreme as are turkeys, since they still must do their foraging in the wild and mating on the wing. But their fitness is no longer determined by Nature, but rather by each breeder—since it is he or she that selects the queens from which the next generation is raised.
Natural and artificial selection
There are two main types of selective pressure:
Negative selection (also called purifying selection)—A purging process of deleterious genes. For example, Nature selects against colonies exhibiting poor winter survival or susceptibility to varroa/DWV– the less fit die without contributing their traits to the next generation. On the other hand, breeders typically select against the queens of colonies that send you running (although that trait may confer fitness in the wild). Over time, the characteristics of the species or stock shifts.
Positive selection—certain variants are favored. Examples in nature are when females prefer to mate with the showiest males, or in breeding programs when the beekeeper selects for high honey production or mite resistance (or queens of a certain color). You wind up with a population exhibiting traits selected for. Positive selection can rapidly shift the characteristics of a population.
Practical application: bee breeders are caught on the horns of a dilemma—selecting for productivity means selecting for robust broodrearing, which, frustratingly, is exactly the same as selecting for hives that produce the most varroa fodder.
Understand that selective breeding is simply a form of controlled genocide. From the entire gene pool of the metapopulation of honey bees in the U.S., a breeder can only cull genes from his breeding population—he can’t create new genes. However, he may be able to recognize and promote new combinations (or the rare mutation) of existing genetics (variants) that meet his criteria.
Practical application: one of the advantages of the bee haploid/diploid system is that combinations of genes that don’t work well together, as well as deleterious recessive genes, are “filtered” out by the haploid drones that must carry them to the next generation, since there don’t carry a second copy of that gene.
Assessment methods
The heart of any selective breeding program is the assay used in the selection process for the desired traits. That assay can be as simple as favoring yellow queens over black ones, or as complex as using cutting edge genomic or proteomic marker-assisted selection (MAS) [[7]]. As far as selecting for mite resistance, some assays are excellent but time consuming [[8]]; others involve the loss of colonies. I am not about to discount the value of those methods (there is huge potential in MAS), but as one who rears thousands of queens each season, I favor simplicity and minimal pain. That said, in this article I’m going to skip over MAS and any assay that requires a microscope or scientific skills, offer my two bits worth on the Bond Method, and then detail my K.I.S.S. method.
The Bond method (you get what you wind up with)
The simplest method for selecting for mite resistance would be to emulate Nature, in which environmental pressure selects who will live and who will die (Fig. 2).
Figure 2. Although the Bond method requires no particular breeding skills, it is neither “natural,” nor as cost effective as a more directed breeding program.
The term “Bond Test” was first coined at a meeting of the German Bee Research Institutes at Bremen to describe the principle of ‘Live and let Die’ for the testing they had been doing since 1993. The term was popularized by pioneer breeder Dr. John Kefuss [[9]], and was successfully applied by Danny Weaver in his Texas operation. The Bond Method is based upon rather brutal negative selection—allowing any colony that can’t handle mites to die. It is little different than natural selection, but with an important twist: the beekeeper maintains an artificial density of colonies in the apiary, which puts an unnaturally intense mite invasion pressure upon the surviving colonies.
The advantage of the Bond test is that it doesn’t require any particular skill in science, breeding, or beekeeping ability—if colonies survive, propagate from them.
Kefuss is the first to admit to some of the problems associated with the Bond method:
- It floods the environment with mites (to the great detriment to your neighbors)
- The extreme genetic bottlenecking may result in the loss of potential resistance mechanisms (Fig. 3).
- The initial high loss rate results in a reduction of working stock available for creating nucs for the next season’s testing of potential breeders
- You may wind up with an undesirable bee, such as happened in the Bond program on Gottland Island and in Le Conte’s program in France.
- Financial loss—this is the non-starter for most professional beekeepers—they’d go out of business if they had to accept major losses for the first few years.
Figure 3. In any breeding program, be careful about “throwing out the baby with the bathwater.” Applying strong selective pressure for any single trait can result in the loss of other desirable traits. In selecting for varroa resistance, if one wishes to end up with a saleable breed of bees, be sure to select only from gentle and productive colonies.
Practical application: the more traits that you select for, the more difficult any breeding program. A tip: forget about selecting for color—the “prettiness” of a queen goes only skin deep; her true beauty lies in the performance of the colony composed of her worker daughters.
the bond method, but without the Needless carnage
In a boxing match, you don’t need to see one boxer kill the other to determine the winner—the ref stops the fight when one begins to lose. The same applies to a bee breeding program. If a colony starts to lose its battle with varroa, treat it—but remove it from the breeder pool.
Practical application: Evolution in bees takes place only at the queen level—you don’t need to punish the workers.
I don’t understand the sick pleasure that some beekeepers seem to derive from allowing colonies of honey bees under their care to die a slow and ugly death from out-of-control mite-induced Parasitic Mite Syndrome. This is contrary to the ethics of both animal husbandry and scientific research.
Kefuss has a practical recommendation—the “Soft Bond” test. This involves positive (as opposed to only negative) selection. In the Soft Bond you positively select for indicators of mite resistance (and only from your more desirable colonies). Only the few that meet both goals are then not treated to control mites. Depending upon how your measure for resistance, the Soft Bond can also incorporate tedious testing (such as for VSH), but you don’t need to sacrifice any colonies.
Getting down to the nitty gritty
In writing this lengthy series, I realized that I if I didn’t skip ahead, that I wouldn’t be getting my suggestions published until after this spring’s queen rearing season was over. So I cut a bunch of important background information, and moved it to a later installment (please hold your criticisms ‘til then, thanks). Below I will outline plans of action, first for the large-scale breeder, and in my next article, for the sideline and hobby sector.
Practical application: what I am focusing upon are practical programs that are doable, and most importantly, that allow one to make the transition to mite resistance without incurring any avoidable losses of colonies.
Define the job description
You may have guessed by now that I’ve spent some time studying the mechanisms by which bees achieve resistance to varroa–which is fascinating to me as a biologist. But as a beekeeper, it really doesn’t make any practical difference to me how they do it, so long as they get the job done. That job is to prevent the varroa population in the hive from building up to damaging levels. And the easiest way for a beekeeper to assess how well a colony is doing that job is simply to use the alcohol wash to track the change in its mite infestation over time.
I’m hardly the first person to point this out—Drs. John Harbo and Jeff Harris published a paper in 1999 on how to use this approach for a breeding program [[10]]. Allow me skip to their key sentence, which is a rather profound no-brainer:
By comparing the growth of mite populations in each colony, one can determine which bees are more resistant to mites.
Practical application: you don’t need to understand how the bees do it, only that they do it (you can later leave it to the scientists to figure it out). What I suggest is not to tell the bees how to do the job, but rather to simply give them the job description, and fire all those that aren’t up to snuff.
The more that I learn about mite resistance mechanisms, the more I realize that natural selection can come up with ways for the bees to fight the mite that we’ve never even thought of. So the last thing that I want to do is to limit my bees by telling them how I expect them to solve The Varroa Problem. A quote from the famous scientist Norman Borlaug applies:
“Don’t tell me what can’t be done. Tell me what needs to be done, and let me do it.”
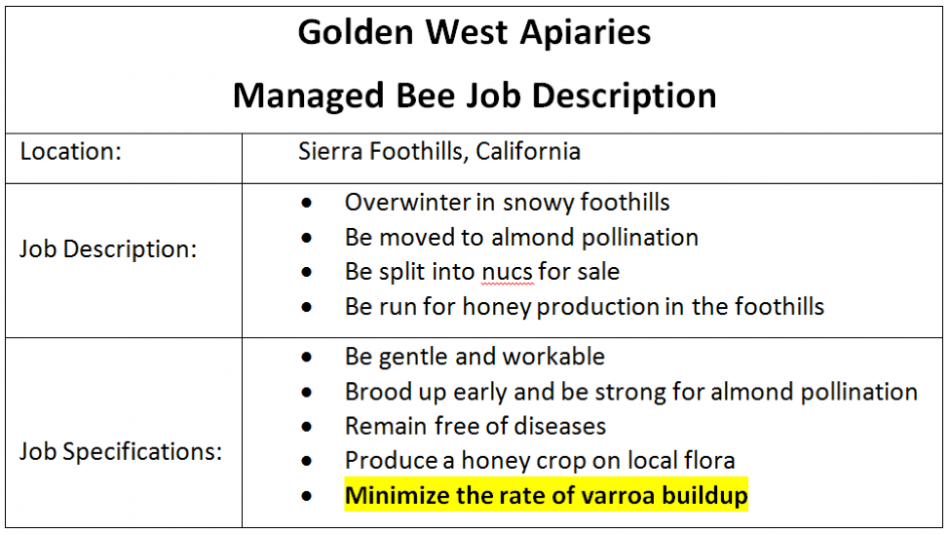
But since I’m going to kill a lot of queens, I feel that it’s only fair that I make it quite clear what I expect from them. So in my apiaries I post the following job description (Fig. 4):
Figure 4. Since I don’t want to hear any complaints or excuses from my queens, I let them know in advance exactly what I expect from them. If they’re not up to the job, I terminate their contract.
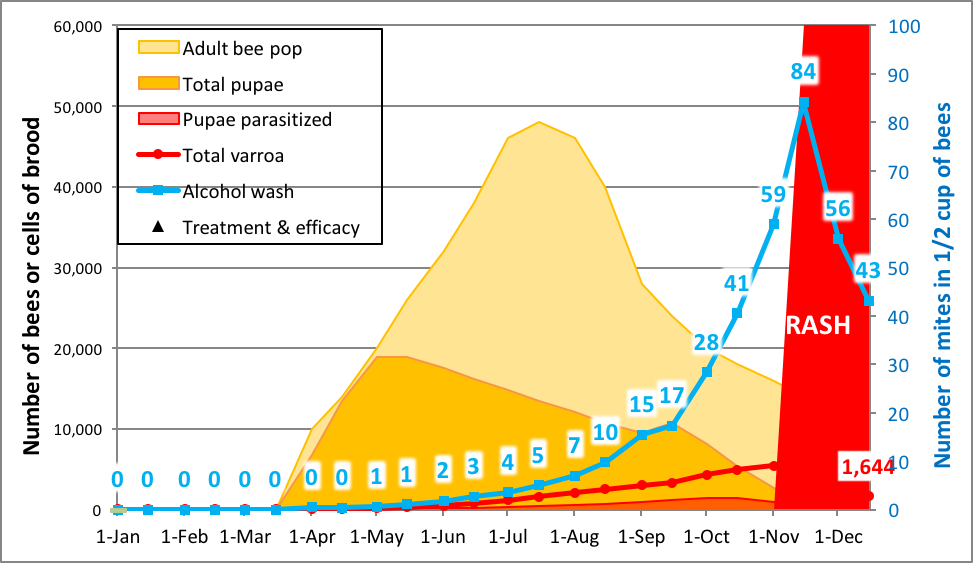
So how and when do you test for the rate of mite buildup? It’s actually quite straightforward. I’ve previously mentioned that during the period of broodrearing, the varroa population in a hive increases at a predictable rate (its r value). My modeling (confirmed by mite counts taken from my own hives) suggests that if you start a bunch of nucs with very low mite populations in April, that by mid to late July their mite populations would be expected to increase by a factor of about 30-35, and that their average alcohol wash count would go from half a mite average to around 8 (a 15x increase), after which it climbs rapidly (Fig. 5).
Practical application: mites build up quickly in rapidly-growing nucs (due to their high brood:bee ratio) [[11]]. What one wants to do is to track the trend, and then perform the mite assay right at the “sweet spot”—the time at which varroa counts reach a high enough level to easily differentiate resistant from susceptible colonies, yet before they “go exponential,” still leaving plenty of time to knock mite levels back before they reach damaging levels.
Figure 5. A simulation for a nuc started on April 1st with 50 mites. You can’t directly compare the slopes of the red mite pop line with the blue alcohol wash line, since alcohol wash reflects the rate of infestation of the nurse bees, rather than the absolute mite population. That said, the alcohol wash works just fine for comparing the rate of mite buildup from one colony to another.
Four of the world’s most knowledgeable researchers on mite resistance recently made the case for my proposed approach [[12]]:
There is a criticism of selecting “blindly” for resistance, i.e., by using an approach that simply targets low mite infestations. This has already, however, been documented to be a viable breeding approach that has led to honey bees that now are used by both small-scale and commercial beekeepers with no or minimal acaricide input…we suggest that beekeepers should first select their colonies for mite resistance to reduce colony mite populations. Then, select for tolerance to the damage caused by the mites and the diseases they vector.
Practical application: there is of course wide hive-to-hive variation in mite counts (which is critical for a selection process). When we perform mite washes in July on colonies equally-started as nucs in April, the counts range from zero to over 15 (mites/alcohol wash)—enough variation for a selection process. We mark the zeroes and ones as potential breeders, and treat the rest.
In a selective breeding program, no colonies need die. Keep in mind that heritable change is passed to the next generation solely by the queens and drones [[13]]. There is no benefit to allowing colonies to collapse from varroa, so long as the breeder propagates the next generation solely from queens and drones from colonies exhibiting the trait of resistance to mite buildup. This has major practical benefit, since during that invisible genetic transition, the operation can remain profitable.
Costs involved
As pointed out by Bienfeld last year [[14]]:
The time-consuming performance testing of honey bee colonies is a fundamental requirement for the selection of breeding colonies. This has always been the case, and will remain unavoidable in the future.
Look, I make my living from my bees, and am every bit as strapped for time and money as any of you. I completely understand that you want to know how much extra labor it’s gonna take. The answer is, not as much as you might think:
Practical application: if you start 1000 test nucs each season, you don’t need to mite wash every one—prioritize productivity by selecting from only the only the top 50% of the hives after 3 months of buildup. That drops your number of alcohol washes in July to “only” 500. My two sons and I, using a portable shaker, can take bee samples and wash them at the rate of about 2 minutes per each—which works out (at $30/hr) to $3 per sample, or $1500. At this time you’d narrow your potential breeder pool down to perhaps 50-100 colonies to continue tracking, meaning that you’d only need to do relatively few washes over the rest of the season.
Starting stock
So where do you start? As Dr. Bob Danka pointed out at this year’s National Conference, the North American bee population already contains the tools necessary for mite resistance. In fact, if you run a large operation, it’s likely that there’s enough genetic diversity already in your own hives to begin a selection process, especially if your queens have been dallying with feral drones, or if you’ve introduced resistant breeder queens.
Since each queen breeder is justifiably proud of his or her own stock, it makes the most sense to begin by first assessing your own bees to determine whether a percentage of your colonies already exhibit some degree of resistance (I was surprised when I did this).
Practical application: I’ve been monitoring only a fraction of my hives for mite buildup, and then treating my entire operation based upon the trend. That approach worked well for mite management, but also meant that I was unlikely to identify any colonies that never needed treatment in the first place. Take home message: you’ll never know if you’ve already got a few mite-resistant colonies unless you do a heckuva lot of alcohol washes, and then withhold treatment from (and monitor) those colonies with the very lowest mite counts.
You can likely accelerate the process by starting with (or incorporating), outside stock exhibiting resistance, such VSH/Pol-line [[15]], Russian, or stock from other breeding programs that maintain their bloodlines without the application of any miticides. Alternatively, instead of starting with bloodlines bred for commercial use, you might experiment with locally-adapted feral survivor stock, or even (gasp) Africanized bees (better adapted to subtropical regions), either of which may require additional selection for gentleness and amenability to management.
A process of shuffling and elimination
You are not going to create any new genetics. All that you can do is create a relatively closed (by controlling the drones) interbreeding subpopulation of bees (starting with your own stock or introduced breeders), let them mix up their genes and epigenes each generation (honey bees are all about shuffling their genes), and then apply strong selective pressure to weed out the undesirables.
Practical application: honey bees are a polytypic species, with many races and breeds. What you’re going to create is a deme–a subdivision of the population consisting of closely related individuals typically breeding mainly within the group.
You’ll need to balance two aspects of selective breeding– the necessary genetic bottlenecking of your deme via the elimination of susceptible queen lines, against the inbreeding depression that can result from such bottlenecking. Luckily, due to the promiscuous matings of queens, you’re unlikely to run into problems so long as you breed off a number of breeders each season, and encourage drone production within your deme (I will elaborate upon this in a later article).
Randy’s K.I.S.S. Recipe for commercial queen producers
I know many of our commercial queen producers, and have no doubt that they have the ability to produce mite-resistant queen lines, should they put in the effort.
Practical application: if even a few of you get onboard, the number of hives in your selection programs would outnumber all the hives in the world involved in government-funded breeding programs, and we could make some serious progress.
If you’re already a queen breeder, the only major change would be to start prioritizing mite resistance up there with the traits of gentleness, productivity, and looks. I’m in the same boat as you, and am not going to suggest anything that I’m not already doing, or going to do myself. Tweak this recipe to fit your local seasonality. Small-scale breeders should also read this in order to grasp the concept; I’ll have more appropriate suggestions for you following.
- Grafting: as early as possible in the season, graft 1000+ queen cells from breeders exhibiting the highest degree of mite resistance (from last season’s selection process).
- Create an isolated mating yard: your problem the first season will be the drones; next year all the drones for matings in your breeding population will come from this season’s selected queens. Set up the most isolated mating yard (or yards) possible. If you use mini nucs, then stock the surrounding area with drone mother colonies of your most mite-resistant breeders.
- Mating: preferably, mate the virgins out in mini nucs (which may allow you to better control the drone pool). You can later transfer the mated queens to shook package colonies or full-sized nucs. Or use full sized mating nucs (thus dispensing with later caging and transfer), made only from hives headed by queens of last-season’s mite-resistant bloodlines (after almonds, the nucs themselves will contain enough mature drones for good mate out).
- Start 1000 equalized 5-frame nucs with those mated queens: if you use mini nucs, cage the queens and use them to start colonies with shook bees or treated nucs. If you used full-sized nucs for mating, treat them with an oxalic acid dribble 17-19 days after you make them up in order for them to start with low mite levels [[16]]. In either case, all nucs need to be roughly equal in strength and starting varroa counts, so that they all start the competition on equal footing. These nucs can be marked and then run for production in similar yards.
- Competitive colony buildup: allow the nucs to build up normally, working them into full hives, and managing them all equally for honey production (they can be in multiple yards). Don’t apply any mite treatments during this buildup period. You can cull any poor performers.
- Assay for mite buildup: in July (or after approximately 5 varroa reproductive cycles, when varroa counts begin to reach the treatment threshold of 3 mites/100), perform alcohol washes on only those colonies which were above average in buildup and productivity (your economic selection assay)–this would reduce the number of colonies to wash to around 500 (at a total labor and materials cost of roughly $1600). The other 500 hives that don’t make the grade can all be removed from the breeding program, treated, and used for production.
- Consolidate your potential breeders: based upon their alcohol wash counts, select the 50-100 colonies with the lowest mite counts, mark them as potential breeders, and consolidate them all into one yard for further evaluation. If you’re going to mate your virgins in full-sized nucs (containing drones) next season, then mark the other 400 (or so) colonies with the next lowest mite counts to use for making nucs next season (to recover the genes from their drones), and treat them. Use them for production, but keep track of them.
- Continue monthly assays: mite counts in late summer continue to increase, so take alcohol washes from the selected colonies again on month later. Labor cost would be approximately $320. At this time select the best 50 or so colonies, and treat the rest (if necessary), reserving them as drone mothers for next spring.
- Fall assay: in October, take washes from the remaining potential breeders, leaving only those with the lowest mite counts untreated for winter (unless none have low enough mite counts; if so, treat them so that they’ll survive, or consider another starting stock).
- Winter the untreated breeders: winter the selected colonies (marking the rest as reserve backups). Be sure to place a drone comb into each of the breeders (and the remaining 300 best and most mite-resistant hives if you’re not using mini nucs), so that next spring those queens will produce the drones for mating the next generation.
- Next spring: inspect all potential breeder colonies for strength and health, and remove any not suitable for breeding purposes. Perform mite washes on those remaining, for a labor cost of less than $150. Use the colonies with the lowest mite counts as breeders—perhaps 10 per queen line (if you bother to keep track), or ~25 if you don’t (in order to maintain genetic diversity).
- Maintenance of your stock: only half of the genes of the workers in a breeder queen’s colony come from the queen herself—the rest come from the drones that she mated with. The only way to recover those genetics is by having a large number of daughters from last season’s best breeders producing the drones for your mating yards. Otherwise, you risk losing critical genetic combinations, and suffer from inbreeding depression. And then continue to apply the same selective pressure for low mite counts each season.
A suggestion: if you ask your motivated buyers to track mite counts in outstanding colonies headed by your queens, they can expand your selection pool. Bring exceptional queens back for breeding purposes.
OK, and what sort of progress might I expect?
The expected rate of progress in a breeding program is a function of the degree of the selective pressure that you apply (provided that traits for resistance are present in your starting population). The trick is to be able to find that “golden” colony, and then to capture and recreate the crucial arrangement of genes and epigenetic factors that made that original colony golden.
Practical application: it should be apparent that there is a good deal of luck involved. But the more colonies that you can assess, the luckier you are likely to be. Most breeding programs are hampered by not having a large enough reproductive population (the deme) to which one can apply strong selective pressure without extreme bottlenecking of the overall gene pool (refer back to Step 12).
Remember, you’re not creating any new genes for mite resistance—by selection, you’re removing genetic combinations that don’t do the job. The question then is, how much variation is there in your starting genetic toolbox? Luckily, Nature has been selecting against the most susceptible bees for 30 years in the U.S., so there’s a good chance that you already have traits for resistance in your existing stock (especially if you have already brought in VSH, selected for hygienic behavior, or have feral drones mating with your queens).
Practical application: selective breeding is a process of the elimination of those colonies that don’t meet your job expectations. It is also promotion of any that come up with better ways of doing the job. The fervor of your firing and promotion is up to you.
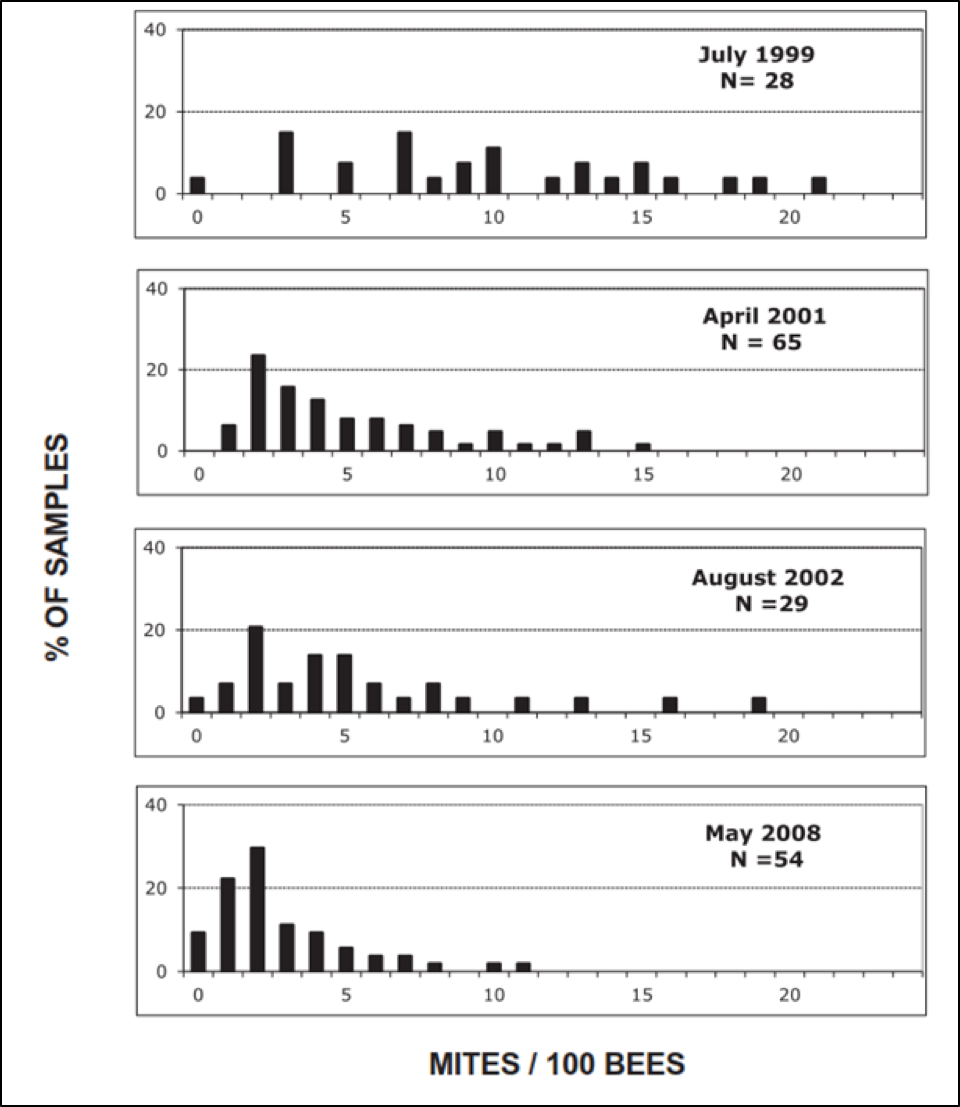
The degree of selective pressure is simple math. For example, in a Bond Live and Let Die breeding program, if 2/3rds of your hives collapsed each season, and you then bred from the 1/3rd that survived, you’d be applying a selective pressure of 3:1, which isn’t very strong. John Kefuss, on the other hand, applied a much stronger pressure at one of his test yards in France, running around 300-400 hives, from which he’d select 5-10 unrelated queens as breeders each season, which works out to a selective pressure of around 45:1. See the results of such selective pressure below (Fig. 6):
Figure 6. Note the great degree of variation in mite counts in 1999, and how over the years, the most susceptible bloodlines were largely eliminated. After Kefuss (2016), by permission.
Danny Weaver started selective breeding of his genetically-diverse Italian/Buckfast hybridized Texas stock in 1992, and was able to go entirely treatment free by 1999 (getting 175-lb average honey crop from 5000 colonies that hadn’t received any treatments in over four years)[[17]].
I asked Dr. Bob Danka about the degree of pressure applied at Baton Rouge in developing the Pol-line VSH strain—they selected roughly the best 15% of queens each season, for a selective pressure of around 7:1, and have achieved remarkable success.
Practical application (degree of selective pressure): the more hives in a selective breeding program, the greater the degree of selective pressure that you can apply. For example, if you start with 200 nucs in the spring (from an assortment of promising queen mothers), from which you select 25 to use as breeders the next year, you’d be applying an 8:1 selective pressure (the reason to select at least 25 breeders is to avoid extreme genetic bottlenecking). If, on the other hand, you increase your program to 1000 nucs each spring, and cull them down to 25 breeders, you would now have applied a 40:1 selective pressure (5 times stronger). Take home message: the more hives involved, the greater the potential for rapid progress (and the less the chance of deleterious inbreeding).
Maintenance of a developed stock
Many of us have seen how difficult it can be to hold traits for mite resistance in subsequent generations. This is because of the virgin daughters’ propensity to mate with unrelated drones. The more that a breeder can control the matings in his reproductive population, the better he can maintain purity of traits. Ideally, the breeder would maintain an isolated closed population (with only the occasional introduction of outside drones). However, if you’ve ever spent an afternoon in a large mating yard, you’d have seen that most of the queens get chased by a drone comet as soon as they get above head level (Fig. 7). Thus you can largely control matings by flooding the area around your mating yard with hives headed solely by selected breeders (acting s drone mother hives, or supplying mature drones in the newly-made mating nucs).
Figure 7. I was able to shoot this photo of a drone comet chasing a queen with a pocket camera, since such matings often occur only slightly above head level in a large mating yard [[18]]. If you’ve got sharp eyes, you can see the queen being mounted at the upper left, and the previous lucky drone dropping upside down in ecstasy a few bees below.
Bee breeding is a process, and will require continued deliberate selection year after year. It will be necessary to maintain selective pressure each and every year in order to produce the reliable and consistent stock that your buyers will demand.
Naysayers
The beekeeping community does not lack for critics, skeptics, and naysayers (see Letters to the Editor). Their negativity was realistically well responded to by Danka, Rinderer, Spivak and Kefuss [[19]]:
Honey bee strains that are resistant to varroa are a valuable resource that [many] beekeepers are [already] using successfully. Although these bees have not completely solved the problem, we are in fact moving toward the ideal of sustainable varroa control.
Practical point: we don’t need 100% bulletproof bees to make beekeeping much easier. Although our eventual goal is to have bees that need zero mite management, in the interim, running strains that simply reduce the need for treatment would be a big plus. Bees that exhibit only a moderate degree of mite resistance can allow beekeepers to dispense with the comb-contaminating synthetic miticides altogether.
I am wide open to constructive suggestions, as the transition to successful treatment-free beekeeping is going to take a lot of people working together. As explained by soccer legend Pele:
Success is no accident. It is hard work, perseverance, learning, studying, sacrifice and most of all, love of what you are doing or learning to do.
I’m not saying that it’s gonna be easy—but I am saying that it will be worth the effort.
Acknowledgements
I’m not about to publish an article such as this without checking my facts. I thank John Kefuss, Bob Danka, Jose Villa and Danny Weaver for their critiques and suggestions. And my hat is off to all the other dedicated bee breeders worldwide. And of course my appreciation to Peter Borst, for his assistance in research, as well as his challenging me on points.
Notes and citations
[1] Kefuss, J, J Vanpoucke, M Bolt and C Kefuss (2015) Selection for resistance to Varroa destructor under commercial beekeeping conditions, Journal of Apicultural Research, 54(5): 563-576. Open access at http://dx.doi.org/10.1080/00218839.2016.1160709
[2] Harbo, JR, & RA Hoopingarner (1997) Honey bees (Hymenoptera: Apidae) in the United States that express resistance to Varroa jacobsoni (Mesostigmata: Varroidae). Journal of Economic Entomology 90(4): 893 – 898.
[3] Erickson, EH, LH Hines, and AH Atmowidjojo (2000) Producing Varroa-Tolerant Honey Bees from Locally Adapted Stock: A Recipe. American Bee Journal 140(8):659-661. Download at: http://beesource.com/resources/usda/producing-varroa-tolerant-honey-bees-from-locally-adapted-stock-a-recipe/
[4] Johnson, S (1998) Who Moved my Cheese? Putnam Adult.
Gladwell, M (2000) The Tipping Point: How Little Things Can Make a Big Difference. Little Brown.
[5] Kefuss, J, et al (2015) Selection for resistance to Varroa destructor under commercial beekeeping conditions, Journal of Apicultural Research, 54(5): 563-576.
[6] Photo credit for wild turkeys–Andrea Westmoreland.
[7] The former used by Monsanto to develop their non-engineered germ lines; the latter successfully demonstrated in bee breeding by Dr. Leonard Foster.
[8] Villa, JD, et al (2009) Simplified methods of evaluating colonies for levels of varroa sensitive hygiene (VSH). Journal of Apicultural Research and Bee World 48(3): 162-167.
[9] Reviewed in: Kefuss,J, et al (2015) op cit
http://www.meamcneil.com/John%20Kefuss%20Keeping%20Bees%20That%20Keep%20Themselves.pdf
Kirk Webster http://kirkwebster.com/index.php/feral-bees
[10] Harbo, JR & JW Harris (1999) Selecting honey bees for resistance to Varroa jacobsoni. Apidologie 30: 183-196.
[11] The daily intrinsic rate of increase (r value) during this period is around 0.030, after which it drops considerably as the brood:bee ratio changes, and drone rearing is restricted.
[12] Danka, RJ, TE Rinderer, M Spivak & J Kefuss (2013) Comments on: “Varroa destructor, research avenues towards sustainable control”, Journal of Apicultural Research, 52(2): 69-71.
[13] OK, there may also be intergenerational transfer of bacteria and fungi, or something else carried by the workers. But in stock selection, we really only care about traits carried by queens.
[14] Bienefeld, K (2016) Breeding success or genetic diversity in honey bees? Bee World 93(2): 40-44.
[15] VSH or Pol breeders are available from Adam Finklestein at http://vpqueenbees.com/
[16] https://scientificbeekeeping.com/simple-early-treatment-of-nucs-against-varroa/
[17] His stock has apparently benefitted by the introgression of some African genes, which have likely helped to confer resistance to both varroa and viruses. Unfortunately, such genetic incursion also came at the expense of the occasional defensive hive—although many of his buyers are quite happy to deal with a bit of spiciness in return for varroa resistance. Others find it cause for complaint.
[18] I took this photo at the Olivarez Honey Bees mating yard in Northern California.
[19] Danka, RJ, TE Rinderer, M Spivak & J Kefuss (2013) op cit.