The Varroa Problem: Part 9- Knowing Thine Enemy
The Varroa Problem: Part 9
Knowing Thine Enemy
First published in ABJ August 2017
Updated Dec 2021
Randy Oliver
ScientificBeekeeping.com
“If you know the enemy and know yourself, you need not fear the result of a hundred battles”– Sun Tzu. We are all beekeepers; we are also all varroa keepers (some of us better at the latter than the former). Varroa is the enemy of both bees and beekeepers. It would behoove us to know the strengths—and more importantly the weaknesses—of our enemy.
Evolution
I well remember listening to a well-known extension apiarist and columnist [[1]] back in the early days of Apistan®, tell an audience that the invasion of varroa had been a “good thing” for the bee industry—it had driven all the poor beekeepers out of business, and knocked out competition for resources from the feral bee population. At that time, Apistan strips had made beekeeping easy again. Unfortunately, the speaker was failing to account for evolution. He would have done well to remember the words of Heraclitus (500 B.C.):
“There is nothing permanent except change.”
Despite us all watching varroa demonstrate its ability to evolve resistance to one miticide after another, our industry has kept its collective head stuck in the sand, and we’re now facing a new varroa crisis. This has not gone unnoticed by the research community or its funders, resulting in new attention being paid to our unwanted hive guest (Fig. 1).
Fig. 1. Although varroa was a pretty hot topic upon its arrival in Europe and North America, scientific interest in the parasite was eclipsed post the CCD epidemic by the sexier neonics [[2]]. However, our growing pragmatic awareness of The Varroa Problem is again bringing scientific attention back to the parasite. Data from Science Direct.
As far as I can tell, beekeepers who pay attention to the nutritional needs of their colonies and keep varroa under control appear to have far fewer problems than those who are less diligent. It’s frustrating to me, that despite varroa being the #1 problem for most beekeepers worldwide, that so little basic research has been done in recent years on its reproductive biology. In Dr. Clarence Collison’s 2015 literature review on varroa reproduction [[3]], the majority of his cited studies were published prior to the year 2000. Keep in mind that Nature is not static—Varroa destructor and Apis mellifera are continually coevolving and adapting to one another—it’s a valid question as to whether the mite reproductive traits detailed in 1997 still apply two decades later [[4]].
The biology of varroa reproduction and its vulnerabilities
It is tedious field and microscopic work to study varroa reproduction in bee brood, and unfortunately such work doesn’t do much to polish a young researcher’s resume in this age of high-tech molecular biology. That said, I’ll attempt to briefly summarize what is known about varroa reproduction—based upon observations made during the 1990’s [[5]]. I’ll also point out weaknesses and vulnerabilities in our enemy that could be evolutionarily targeted by the bee to screw up the mite’s reproductive success.
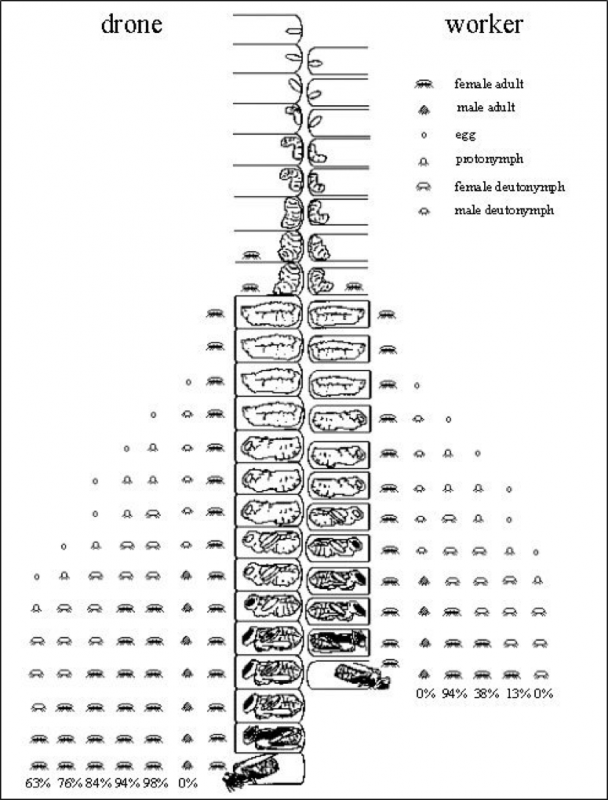
Here a picture is worth a thousand words—thank you to Dr. Stephen Martin, who created the best image of varroa reproductive success that I’ve yet seen. Please take a minute or two to study his graphic (Fig. 2).:
Figure 2. Varroa reproductive success in drone and worker brood, with the average survivorship of each offspring at bee emergence. Courtesy Dr. Stephen Martin (1997) [[6]].
Note that a foundress mite has the potential to produce 3 mated daughters per reproductive cycle in worker brood [[7]], and 5 in drone brood. Yet in study after study [[8]], fewer than half that many ever emerge. And in mite-resistant stock, even that percentage may be substantially lower [[9]].
Practical application: Although varroa may seem invincible, in actuality it never achieves its potential reproductive capacity in Apis mellifera (this is very fortunate for us, since if a mom mite’s fecundity were not constrained, varroa could increase its population in a hive by up to 8x per month). Conversely, if a bloodline of bees is able (by any combination of means) to reduce varroa’s current (in typical managed stock) rate of reproductive success by 50%, that would reduce the mite to mere nuisance status (you don’t need to kill a single mite to make varroa a non issue—you only need to reduce its rate of reproductive success).
Varroa sensory Perception
Try to imagine how the mite senses the world—it has no eyes, and is entirely dependent upon its acute senses of touch and smell. As described by Leonovich [[10]]:
Sensory organs form the interface between the environment and the behavior of an organism. All the information on the state of the environment and on its changes, necessary for the survival, reproduction, etc. reaches the central nervous system via this interface.
Practical application: Every behavioral and reproductive action of a varroa mite is triggered by specific (and sometimes complex) sensory cues. The take-home to me is that the battle between the bee and varroa takes place mainly at the olfactory level. This concept was first floated by Dr. Denis Anderson back in 2006 [[11]], after his observation that there were strain-specific varroa to each regional population of Apis cerana:
The signal (or signals) that triggers varroa mite reproduction will almost certainly be a chemical that interacts with a mite receptor. In isolated populations of A. cerana it is very likely that, through evolutionary time, both the signal and the receptor have changed slightly through the action of mutation and natural selection. For example, a beneficial mutation in an isolated population of A. cerana could produce a signal that is more difficult for the mites to recognise…Identifying this signal could present a new way of controlling the Korea and Japan haplotypes of V. destructor on A. mellifera. Once the signal has been found, then various approaches can be pursued to produce varroa-resistant bees.
Dr. Anderson nailed it—the varroa/bee battle is all about semiochemical [[12]] signaling and subterfuge. Even the brutal sacrifice of infested pupae via VSH is all about the specific chemical cues that trigger preexisting hygienic behaviors. The evolution of mite resistance in Apis mellifera appears to follow another observation by SunTzu:
The supreme art of war is to subdue the enemy without fighting.
When evolution is allowed to run its course, mite resistance generally appears to result more from “cyberwarfare” rather than from hand-to-hand combat. The bees mess with the semiochemical signals (or perhaps the within-cell environment) that the mites depend upon for successful reproduction. Building on the meticulous visual observations of mite behavior done in the 1990’s, researchers can now perform state-of-the-art chemical analysis to determine those semiochemical cues. What we’ve learned so far, though, is only a teaser to a more complete understanding, as well detailed in a fascinating review by Nazzi and Le Conte [[13]].
a walk through a varroa reproductive cycle
A typical varroa reproductive cycle during spring and summer takes about 17 days—with a bit over 12 of those days taking place in the capped brood cell. During the other 5 days, the mite is phoretic—hitching a ride on an adult worker (generally a nurse bee). So let’s start our walk with that phoretic female.
Update: as pointed out by Dr. Samuel Ramsey, the term “phoretic” has been misapplied to hitchhiking adult varroa mites, since they not only hitchhike, but also feed on the adult bees. Therefore, the proper term would be “dispersing” mites, since they are in a feeding dispersal phase, rather than a non-feeding phoretic phase.
Phoretic adult mites: The mite must avoid being groomed off the unwilling bee, and to feed it must locate the “sweet spot” on the bee, and dig its head between the abdominal plates and chew a hole through the soft integument to reach the fat bodies [[14]]. Not only that, but the mite must shift to a nurse bee if its ride ages to mid-age status, or it will have scant change of being carried to a prepupa about to be capped. Weak points—susceptibility to being dislodged by grooming/biting behavior, and the mite’s need to recognize specific olfactory cues for worker age and feeding site. Note, however, that varroa is exquisitely well adapted to avoid being dislodged by the bee, and on its native host can survive in the very hostile hive environment for a full year between drone rearing episodes.
Foundress invasion: in order to invade a brood cell at the proper time, the female mite must recognize a specific olfactory cue from the bee 5th-instar larva (perhaps the one used to signal the nurses to cap it over) [[15]]. Invading mites respond more strongly to the cue from drone prepupae than from that of workers. They may also prefer cells that have already been invaded by another female [[16]]. Weak point—again, the bees could modify their pheromonal or cuticular hydrocarbon scents to throw the mite off scent.
Pupation: The foundress mite then hides in the remaining jelly at the base of the cell, being freed as the larva consumes the remaining jelly after capping. At this time the mite must avoid being trapped in the silk as the larva spins its cocoon. From this point on, everything takes place in the close and crowded confines of the capped cell. Potential mechanisms—leaving excess jelly, trapping the mite in the cocoon.
Oogenesis: In order to synchronize its timing with the development of the pupa, the mite must respond to olfactory cues (as well as engage in a feeding on the prepupa) to initiate a sequence of egg laying beginning at about 60 hours after the cell is capped, and then a subsequent egg approximately every 30 hours thereafter [[17]]. But as found by Infitadis [16b], this eggl aying follows an unusual sequence: the foundress mite first lays a female egg, and then a male egg second, and then female eggs again thereafter (for a maximum of 6 female eggs in a worker cell, or 7 in a drone cell, of which only the first few ever reach maturity before emergence of the adult bee). Despite coming from the second egg, the male mite, due to its shorter development time, is sexually mature by the time that his older sister becomes receptive.
Weak point–failure to produce the critical male at precisely the right time appears to be a major Achille’s heel of varroa, and appears to be targeted by naturally-occurring lines of mite-resistant bees. Any olfactory tweaks that result in getting the foundress mite out of synch with pupal development can have a large impact on mite reproductive success.
Mite development: Varroa offspring emerge already legged from the egg, and then go through two developmental stages: protonymph and deutonymph before moulting into an adult (Figs.3 and 4).
Figure 3. A male varroa protonymph. Neither the nymphal stages nor the adult male can survive outside the capped cell. Thank you to Gilles San Martin for granting open access to these amazing photos.
Figure 4. A female varroa deutonymph. The tiny leglike structures in the center are pedipalps, which are part of the mite’s mouthparts. The first pair of true legs are used similarly to antennae in insects. There are tactile and chemosensory organs at the tips of the pedipalps and legs, as well as elsewhere on the mite. Photo by Gilles San Martin.
Male survival: it’s not easy to be a male mite (Fig. 5)—due to timing, it may not be able to feed for some time in drone cells, and its tiny larval stage must avoid being crushed by the movements of the pupating bee, and then make its way past the bees’ legs in the tight cell [[18]]. This is certainly a potential point in time to target the male mites by slight alterations in pupal morphology, behavior, or cuticular scents.
Figure 5. An adult male varroa walking over a bee about to emerge. By this stage of development, his male mite has done his job—mating dozens of times with one sister (or other young female) after the next [[19]]. Without a hard exoskeleton, the male quickly dies once the capping is removed [[20]]. Photo by Gilles San Martin.
Daughter survival: the first daughter mite has it the easiest (and enjoys a very high rate of survival), but her subsequent sisters often do not survive until adulthood (perhaps due to competition at the common feeding wound, or to thickening of the pupal cuticle)—refer back to Fig. 2. And of course no offspring survive should the pupa be sacrificed by nurse bees practicing varroa-sensitive hygiene (VSH). There are several potential defense mechanisms that the bees could use here—making it more difficult for the mites to feed, pupal altruistic suicide in response to wounding (as exhibited by Apis cerana pupae), olfactory signaling by the pupa that it is being parasitized, and of course, vigorous hygienic behavior by the nurses.
Mating: female mites must mate shortly after eclosure as adults or they remain forever sterile (thus being unable to contribute to further reproduction). Mating takes place on the fecal mound created by the foundress, and is dependent upon pheromonal cues (with the male mating solely with the most recently-emerged female) (Fig. 6).
Surprisingly, there are very few spermatozoa actually involved in the mating process, with a fully-mated female receiving only a couple of dozen spermatozoa after multiple matings [[21]]. Apparently the first daughter gets mated the best. Mites can get around the problem of lack of a male by multiply invading a cell. Even though competition reduces the number of daughters per foundress, at least some of those daughters get mated. Mating success is dependent upon pheromonal cues (and the success of the foundress at producing a son)—a possible resistance mechanism would be for hygienic bees to detect (or competitively overwhelm) the mite mating pheromone.
Figure 6. Mites engaged in mating. Surprisingly, very few spermatozoa are transferred in each mating, so multiple matings are required for good female fecundity. These matings must all take place before the next (and more pheromonally-attractive) female emerges. Photo credit: FAO TECA.
Developmental time, humidity, and temperature: varroa evolved to reproduce in the drone brood of Apis cerana. It does not have as much success in Apis mellifera worker brood, due to a number of factors. The Cape Bee greatly restricts varroa reproduction due to its extremely short post-capping duration, but knocking even a full day off our bees’ postcapping duration would only slightly reduce varroa’s overall reproductive success (refer back to Fig. 2). However, the uncapping of pupae (bald brood) may be a way to dessicate developing mites. Varroa also reproduces best at the lower brood temperature typical of Apis cerana drone brood—I’ll discuss this potential resistance mechanism further on.
Emergence (Fig. 7): mites can’t escape from a sealed cell, and are dependent upon either the adult bee to chew its way out of the cell, or for workers to open the cell during hygienic removal of the pupa. By entombing mites by thickening the cappings of infested cells, bees could conceivably trap varroa in the combs (as does Apis cerana in the drone brood).
Figure 7. A spectacular photograph of a fully-scelerotized female mite ready to emerge from a cell. Varroa is an exquisitely-adapted parasite of the bee, with every aspect of its anatomy and behavior fine-tuned by evolutionary trial and error for survival in the unfriendly (but resource-rich) environment of the bee colony. Photo by Gilles San Martin.
Practical application: there are a wide variety of targets for the bees to hit to reduce varroa reproductive success. As pointed out by Donzé:
Varroa’s population growth is…chiefly limited by the high number of sterile mites and by developmental mortality.
Knowing this, I’ll return to the wisdom of Sun Tzu:
So in war, the way is to avoid what is strong and to strike at what is weak… He who can modify his tactics in relation to his opponent can thereby succeed in winning.
It is clear that the Achille’s heel of varroa is the reproductive success of any foundress mite—it thus makes sense to focus upon varroa’s weak spot.
Practical application: VSH clearly reduces mite reproductive success. But of great interest to me is that despite the obvious utility of VSH, in survivor stock left to work it out by themselves, the most adaptive evolutionary responses appear to be targeted towards suppressing in-cell fecundity, rather than VSH or grooming behavior [[22]]. Why this is I don’t know, but when Nature talks, I listen!
A Recent finding
In a recent study, Oddie [[23]] compared the difference in fecundity between that in typical “mite-susceptible” Norwegian managed stock versus that in hives of “survivor stock” colonies. The difference was substantial—there were 1.24 presumably mated daughters (on average) per foundress in the susceptible hives vs. only 0.87 offspring per foundress in the “survivor” hives. The surprising thing about Oddie’s study was that the survivor hives were able to reduce the rate of reproductive success when given frames of already-sealed brood from another hive. If their findings prove to be true, this indicates that the colony can somehow affect the development of mites hidden under the cappings [[24]]! The authors conclude:
Our data support that a reduced V. destructor mite reproductive success seems to be a key factor for natural colony survival. However, grooming and VSH are unlikely for this Norwegian case. Instead, yet unidentified behavioral traits of worker bees seem sufficient to explain reduced mite reproductive success. Therefore, the underlying mechanisms remain elusive and should be a focus of future studies taking advantage of naturally selected survivors.
How in the heck, you may ask, could the workers possibly affect mite reproduction under the cappings? Glad you asked, since one answer is an amazing (and fascinating) example of how natural selection can come up with ways for the honey bee to fight varroa that we unimaginative humans might never have thought of, in this case, molecular warfare.
Varroa is so well adapted to its host that instead of digesting some honey bee proteins, it somehow absorbs them directly to use for its own egg production. Its direct use of those bee proteins allows varroa to reduce some of its metabolic pathways, and thus even its genome. We now understand that varroa can utilize not only bee vitellogenin for its rapid egg production, but also some critical enzymes in its ecdysone biosynthesis pathway. The steroid hormone ecdysone is associated with arthropod ecdysis (molting), but it is also a mater regulator for other developmental transitions, including egg formation (oogenesis).
And here’s another Achilles’ heel for varroa — it has given up its ability to produce some critical ecdysteroids, depending instead on obtaining them directly from ingested bee tissue. Conlon [24b] discovered that the varroa-resistant Norwegian bloodlines of bees had evolved to downregulate their expression of some of their ecdysteroid genes, thus disrupting the mite’s ability to produce eggs.
Who woulda thunk??? Would it have ever occurred to a bee breeder to select their breeder queens based upon their propensity to downregulate their expression of ecdysteroids?
Brood temperature and varroa
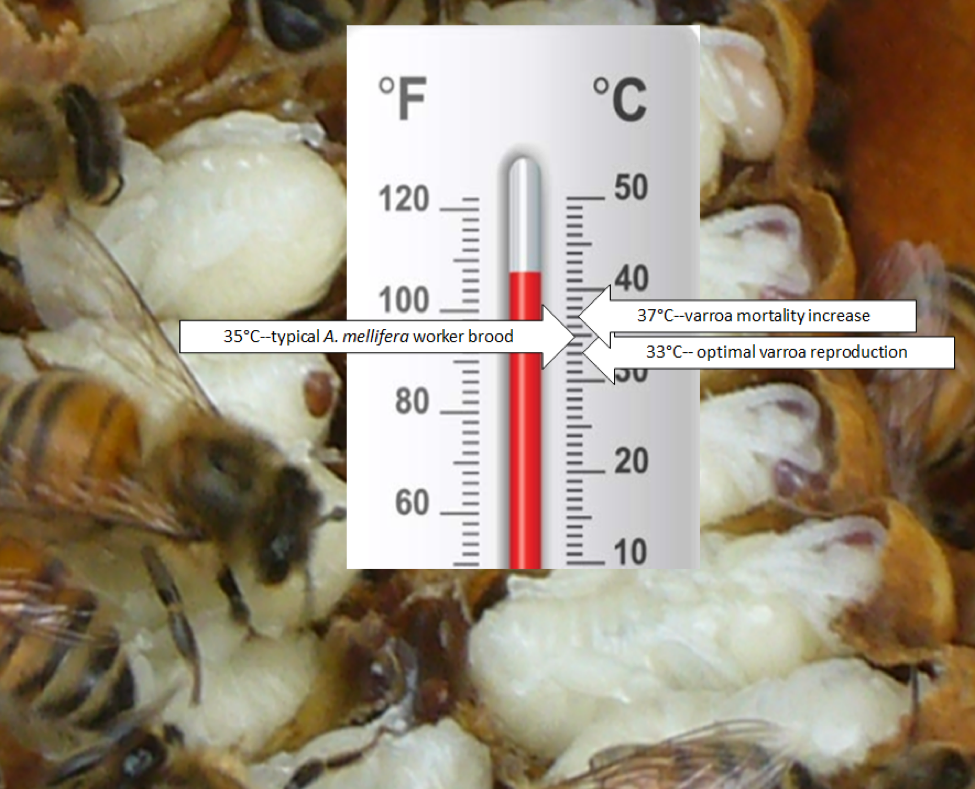
Varroa reproduces best at temps from 32.5-33.5°C, which reflects the normal temperature of drone brood in Apis cerana. I haven’t seen any recent research to check whether varroa has adapted to the higher brood nest temperatures of Apis mellifera (which the bees maintain within a narrow temperature range of 34.5±1.5°C, typically 35°C)—which is warmer than the optimal temperature for the mite. I’ve long been curious as to why no one has yet identified a bee population that uses elevated brood temperature as a mite-resistance mechanism
Practical application: Bees have the ability to create a “fever” in the brood, as previously reported by the Seeley lab [[25]], who found that when stimulated by chalkbrood, the workers would raise the brood temperature by half a degree centigrade. Could the “survivor” bees be doing this in response to varroa?
A half a degree C may not seem like much, but we’re talking about a parasite already at the edge of its ideal temperature range (Fig. 8). A slightly higher broodnest temperature could have a twofold effect upon mite reproductive success: (1) poorer foundress and offspring survivorship [[26]], as well as (2) accelerated development of the bee pupa (perhaps slightly reducing the number of mated daughters) [[27],[28]]. The combination of these two effects could plausibly result in decreased mite reproductive success.
Figure 8. Varroa reproduces best in a very narrow temperature range (evolutionarily set by the temperature of Apis cerana drone brood). It wouldn’t take much of a “fever” in the worker brood to put the hurt to the mite.
I’ve reviewed Oddie’s experimental methodology, and it appears sound (although we need to see this remarkable finding replicated). So for the time being, I’m keeping my mind open to the possibility that the Norwegian survivor colonies might be elevating their broodnest (or individual brood cell) temperature to put the heat on the mites. I’ve corresponded with the senior author, who couldn’t yet tell me their own explanatory hypothesis (due to its being in review)—it’s not exactly as above, but apparently something along a similar line of reasoning.
Acknowledgments
Thanks to Pete Borst for his help in literature searching. To Dr. Stephen Martin and all the other dedicated researchers working on varroa. And to Gilles San Martin for sharing his amazing close-up photographs. Also a big thanks to Dr. Stephen Martin for his helpful comments as I was writing this article.
Notes and Citations
[1] Name withheld out of courtesy.
[2] Although it’s clear that neonics are vastly overused, can cause planting dust and some other unintentional bee kills, adversely affect some native pollinators and aquatic ecosystems, and exhibit sublethal effects in individual bees, I’ve yet to see convincing evidence that they are seriously affecting honey bees overall.
[3] Collison, C (2015) A closer look: varroa mite reproduction. http://www.beeculture.com/a-closer-look-varroa-mite-reproduction/
[4] There has been clear evolution regarding mite reproductive success in some bloodlines and races of Apis mellifera; refer to:
Strauss, U, et al (2015) Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology 143: 374–387.
Danka, RG, et al (2015) Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 47(3): 483–490.
[5] A few good references:
Donzé, G, et al (1996) The rate of infestation of brood cells and mating frequency affects the reproductive success of the honeybee parasite Varroa jacobsoni. Ecol. Ent. 21: 17-26.
Martin, S.J. (1994). Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honey bee Apis mellifera L. under natural conditions. Experimental and Applied Acarology, 18: 87-100.
Martin, S.J. (1995). Ontogenesis of the mite Varroa jacobsoni Oud in drone brood of the honey bee Apis mellifera L under natural conditions. Experimental and Applied Acarology, 19: 199-210.
[6] Martin, S.J. (1997a). Life and death of varroa. In Varroa! Fight the Mite (Ed. P. Munn & R. Jones), pp.3-10. International Bee Research Association, Cardiff.
[7] Note that Dr. Martin observed that development takes ~20 days, not the 21 commonly cited in textbooks. I don’t know the origin of the 21-day figure, but various researchers have confirmed the 19.5-20 day figure.
[8] Reviewed in Martin, SJ, et al (1997) Non-reproduction in the honeybee mite Varroa jacobsoni. Experimental & Applied Acarology 21: 539–549. Table 1 in Donzé (1996) is very instructive.
[9] Strauss, U, et al (2015) Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology 143: 374–387.
Oddie, M, et at (2017) Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ preprint.
[10] Leonovich, SA & MK Stanyukovich (2011) Sensory organs of mesostigmatic mites (Acarina, Mesostigmata) dwelling in body cavities of mammals and birds. Proceedings of the Zoological Institute RAS 315(3): 263–273. This open-access paper has beautiful electron micrographs of mite sensory structures.
[11] Anderson, D (2006) Clarification of aspects of Varroa reproduction—first stage of a possible new control method. RIRDC Publication No. 06/007. Unfortunately, this proposal did not get funded.
[12] Semiochemical–a pheromone or other chemical that conveys a signal from one organism to another so as to modify the behavior of the recipient organism.
[13] Nazzi, F & Y Le Conte (2016) Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu Rev Entomol. 61:417-32. This paper might better have been titled “Chemical ecology of varroa,” and details the state of the art of our knowledge of the chemical interplay between varroa and the bees.
[14] Ramey, Samuel, in prep.
[15] This kairomonal chemical was determined by the ARS: Carroll, MJ, A Duehl and PEA Teal (2010) Methods for attracting or repelling Varroa mites. U.S. Patent (pending).
[16] Donze and others have noted that it can be to varroa’s reproductive advantage for more than one female to invade a cell. Despite the fact that this would decrease the number of daughters per female (apparently due to competition at the feeding site), it increases the chance that the surviving daughters get adequately mated. I have no idea what cue an invading mite would use to recognize that another female is already at the other end of the cell.
[17] Although the male winds up being haploid, the egg must first be fertilized in order to initiate development.
[18] The foundress mite actually moves the bee pupa’s legs to allow the male mite to pass. Although it’s an attractive conjecture, there is scant evidence in support of the hypothesis that smaller cell size decreases the survival rate of developing mites.
[19] Donzé (1996) observed that over the 50-100 hour mating period a male can complete 15 matings in a worker cell, and 30 in a drone cell. By his calculations, a male could fertilize some 3.75 females in a worker cell, and 7.5 in a drone cell.
[20] Presumably from desiccation, but I’m not sure.
[21] Donzé (1996) op cit.
[22] The VSH/SMR issue is tough to resolve, unless one protects the sealed brood from hygienic bees. That said, it appears that the USDA VSH line of bees may also exhibit some degree of SMR, as evidenced by the observation that in brood cells still intact at the purple-eyed stage, some 50% of foundresses had not reproduced at all, and another 10% did not produce a male. Danka, RG, et al (2015) Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 47(3): 483–490.
[23] Oddie, M, B Dahle, P Neumann (2017) Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. https://peerj.com/preprints/2976/
[24] There was scant different in VSH between the two groups.
[24b] Conlon, B, et al (2019). A gene for resistance to the Varroa mite (Acari) in honey bee (Apis mellifera) pupae. Molecular ecology, 28(12), 2958-2966.
[25] Starks, PT, CA Blackie, TD Seeley (2000) Fever in honeybee colonies. Naturwissenschaften 87: 229–231.
[26] Le Conte, Y, et al (1990) Influence of brood temperature and hygrometry variations on the development of the honey bee ectoparasite Varroa jacobsoni (Mesostigmata: Varroidae). Environ Entomol 19 (6): 1780-1785.
[27] Martin, SJ (1994) Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honeybee Apis mellifera L. under natural conditions. Experimental & Applied Acarology 18(2): 87–100.
[28] Donzé (1996) op cit. The third daughter to mature has only a few hours during which to mate with her brother.