The Varroa Problem: Part 3 – A Creation of Our Own Doing
The Varroa Problem: Part 3
A Creation of Our Own Doing
Randy Oliver
ScientificBeekeeping.com
First published in ABJ in Dec 2016
The Varroa Problem didn’t just happen—we created it, and we unintentionally perpetuate it. And we will continue to prolong the agony until we, as a community, finally say “Enough!” and start to work together to solve it.
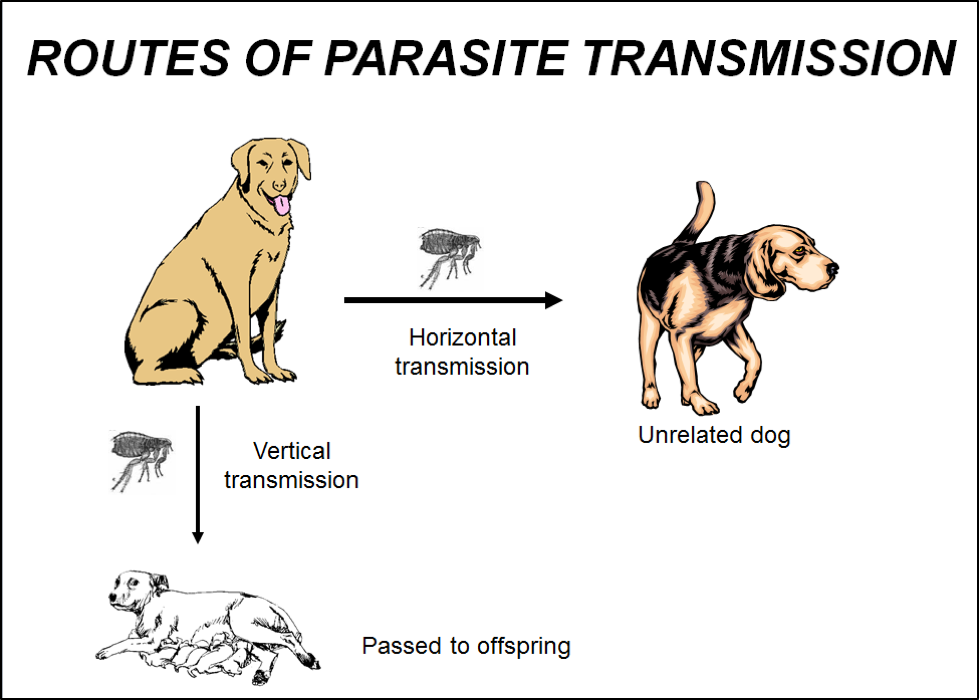
Routes Of Transmission
In order for a parasite species to survive, it needs to transmit from one generation of host to the next. There are two main modes of transmission—“horizontal” and “vertical.” An understanding of the implications of these two routes is critical for the understanding of The Varroa Problem.
Let me first remind you that we are dealing with two symbiotic parasites here—varroa and DWV. Both need to transmit from bee to bee, and from hive to hive. Bee-to-bee transmission within the hive, as well as that from hive to hive, is termed horizontal transmission (Fig. 3). Varroa readily transmits horizontally within the hive between adults, or from nurse bee to pupae. But for more distant horizontal transmission—to other hives—it depends upon vectoring by drifting bees or robbers. The most efficient horizontal transmission of DWV strains from hive to hive is by vectoring by varroa.
Practical application: so long as there are plenty of other colonies within flight range, it is to both the mites’ and the virus’ advantage to kill their host colony late in the season (but before winter), and thus be transmitted by robbing and drifting bees to new host colonies.
The other route of transmission is termed “vertical,” indicating from parent to offspring. With bees, that means from a queen to her daughters (in the case of DWV), or from a colony to its swarming offspring (for both varroa and DWV). Varroa gets transmitted vertically from every parent colony to a swarm (with a prime swarm carrying perhaps 20-25% of the mites in the hive) [1].
 As far as DWV, vertical transmission occurs via the egg or larval jelly, the oral-fecal route, via mating, and perhaps on flowers [2], but given adequate nutrition, the workers’ immune systems can generally keep the virus in check [3]. It is the wounding and introduction of active virus by varroa directly into the bee’s bloodstream that then turns a “latent” infection into an active infection, upon which it may spiral out of control within the infected pupa or adult [4].
As far as DWV, vertical transmission occurs via the egg or larval jelly, the oral-fecal route, via mating, and perhaps on flowers [2], but given adequate nutrition, the workers’ immune systems can generally keep the virus in check [3]. It is the wounding and introduction of active virus by varroa directly into the bee’s bloodstream that then turns a “latent” infection into an active infection, upon which it may spiral out of control within the infected pupa or adult [4].
Practical application: Until the introduction of parasitic mites, viruses were rarely a noticeable problem in beekeeping, as bees had developed a strong immune response to viruses gaining entry orally (viruses can’t penetrate bee cuticle). The injection of virions directly into their bloodstream caught Apis mellifera completely off guard–their immune systems were completely unprepared for it. We are now witnessing the bees slowly coming to terms with this entirely new ballgame.
Host And Parasite Coevolution
For those interested, two of the best and brightest minds in bee research (Ingemar Fries and Scott Camazine) wrote a detailed (and readable) paper on this subject in 2001 [5], from which I drew heavily for the italicized snips below [6].
Once a novel parasite is introduced to a host population, the two parties need to work out some sort of arrangement in which each can survive (as a species; individual hosts may be killed). This “working out” is called coevolution, and we are lucky enough to be observers smack dab in the middle of the process of coevolution between the Western honey bee, the varroa mite, and DWV. The selective pressures that drive that process are based upon each player’s gain or loss in fitness.
Scientific terminology: “Fitness” is the probability that the line of descent from an individual with a specific trait will not die out. Nature ruthlessly selects for traits that increase the fitness of bloodlines. It’s important to remember, though, that Nature doesn’t have a plan, and the process of selection can be set back or go in surprising directions due to sheer chance. That’s why it’s important for us not to overload the evolving feral populations of bees with mite loads from collapsing managed hives.
It is clear that if a parasite lowers the fitness of its host, it will not survive unless there is also some degree of horizontal transmission.
If either varroa or DWV cause a colony to die, then it is imperative for their survival that they first manage to get transmitted to another colony.
Vector-borne pathogens are generally more virulent than directly-transmitted pathogens. In general we would expect the adverse effects of high virulence on the host to diminish opportunities for parasite transmission to new hosts. However, this effect can be offset by the vector, if it promotes parasite transmission. (An example is malaria. Though this disease may incapacitate its host, the mobility of the mosquito vector provides ample opportunities for the malaria parasite to reach new hosts).
In the case of DWV (which was formerly seldom noticed), the arrival of varroa as a vector increased the fitness of a more virulent strain, since drifting and robbing bees efficiently transported the virus from dying colonies to healthy ones.
Low host density favors low virulence whereas high host density favors high virulence.
This is the beekeepers’ dilemma: placing more than one hive in an apiary in the short term favors the most virulent parasites. However, in the long term, the increased opportunity for parasite transmission in an apiary (due to drift) could allow the parasite to evolve to become more benign, since there would be no need to weaken the host to effect transmission.
Practical application: during the early stages this coevolutionary process, crowded apiaries work against us. But in the long term, this is not necessarily the case. Eventually, it would be expected for benign strains of DWV to exhibit greater fitness in a dense bee population. That is good news for us.
In terms of fitness, the successful transfer of a parasite’s offspring to a new colony is a critical requirement in its life history. Without such transfer, the parasite will not achieve reproductive fitness, no matter how prolific it is in the original host colony.
Both varroa and DWV will always do everything they can to effect some sort of transmission from colony to colony. In the long term, it would be to the bees’ and beekeepers’ benefit if such transmission occurred either vertically or through drifting, rather than as a result of colonies being brought to death and being robbed out. This is a different situation than in feral populations, in which little drifting occurs between the widely-scattered colonies.
In feral populations, the virulence of varroa and DWV are kept in check by the fact that at low colony densities, transmission of either parasite must be vertical—through swarms. Thus, if either parasite (varroa or DWV) weakens its host colony to the extent that it isn’t healthy enough to produce swarms, those lines of virulent parasites will be lost with their hosts.
Practical application: our long-term goal is not to eliminate either varroa or DWV, but to select for strains of bees that resist excessive reproduction of varroa or DWV, and pass both parasites vertically (and relatively harmlessly) from one generation to the next.
Fries and Camazine summarize the situation nicely:
The hypothesis that the mode of transmission molds pathogen virulence predicts that a parasite such as the varroa mite will be transmitted horizontally between colonies during the initial phase before the infestation is well established. Later, the vertical spread will be the main source of transfer to new colonies as the parasite has become widespread. Over time, the most susceptible colonies die and the selective pressure on the parasite will select against effects on colonies that inhibit reproductive swarming. Thus, a benign host parasite relationship should evolve.
And the above is exactly what has happened in feral populations of bees. Unfortunately…
Beekeeping Creates An Unnatural Situation
Fries and Camazine continue:
In the case of Varroa, we believe that apicultural practices are responsible for maintaining virulent forms of the parasite…
Those are strong words. But the authors explain the problem:
Honey bees swarm to reproduce. Swarming behavior results in vertical pathogen transmission. Since bees that swarm produce less honey, beekeepers generally restrict swarming through a variety of management practices. To increase the number of colonies or to replace losses, beekeepers make nuclei from old colonies. Normally these nuclei receive a developing queen cell or a mated queen from pre-selected stock and, thus, prevent the formation of daughter colonies genetically related to the colonies from which nuclei were formed. In this system, parasites may be transferred to daughter colonies on adult bees and with brood combs. However, this is not equivalent to vertical pathogen transmission since daughter colonies will, for the most part, be unrelated to the colonies from which they were formed. On the contrary, from a selective perspective, the forming of new colonies under managed conditions will promote horizontal transmission of pathogens to new colonies rather than the vertical transmission that occurs under natural swarming conditions.
Note To “Treatment Free” Beekeepers
Practical application: all “treatment free” beekeepers need to take the above paragraph to heart. This is really important!
There is a common misunderstanding among hobby “treatment free” beekeepers. By allowing untreated hives containing commercial stocks to collapse from varroa, they flood the surrounding feral population with mites, thus overwhelming any colonies exhibiting some degree of mite resistance, causing the loss of those hard-won genes. Thus, rather than helping, such beekeepers may actually set back the natural process of evolution. For treatment-free beekeeping to be of benefit to the evolutionary process, you can’t start or replace colonies with varroa-susceptible commercial stock. Just because you wear the “treatment free” hat, that doesn’t suddenly make the commercial stock in your hive any more resistant to mites or DWV. Treatment free can work, but your hives need to be started with stock that has a fighting chance against varroa!
Practical application: allowing untreated colonies of commercial stock to die from varroa year after year benefits no one, and hurts both your neighboring beekeepers, as well as the evolutionary process. Think about it—please!
The Varroa Problem Is A Creation Of Our Own Doing
Unmanaged honey bee populations worldwide have dealt with varroa and DWV, and have often recovered to pre-varroa levels. But it’s generally a different situation in managed apiaries.
Practical application: So long as beekeepers continually replace varroa-killed colonies with fresh colonies of similar bloodlines, we artificially maintain the situation of an initial invasion, favoring the most virulent strains of the parasites. This makes it nearly impossible for any colonies with genes for resistance to survive, due to their being overwhelmed with mites from their collapsing neighbors. And any that do are outbred with drones from artificially-supported susceptible stocks. Thus, we’re only seeing the natural evolution of mite resistance occurring in unmanaged (or minimally managed) stocks that were reduced to low density [7].
The Solution To The Varroa Problem
We beekeepers will continue to perpetuate The Varroa Problem until we grasp and embrace the fact that if we’re going to maintain an unnatural density of colonies (by keeping them in an apiary), then we must stop thwarting the natural evolutionary process.
Scientific terminology: the evolutionary process can be simply defined as the change in the heritable characteristics of biological populations over successive generations.
Those heritable changes are shifts in the overall genetic structure of a breeding population (all the colonies within mating range). Eventually, the genes (alleles) for mite resistance will become “fixed” in that breeding population, and at that point, varroa and DWV will be only nuisances, not our major problem.
Since the essence of the problem is that so long as we keep replacing our varroa/DWV-killed colonies with fresh colonies from the same susceptible stocks (isn’t that someone’s definition of insanity?), the situation is only going to get worse as the mite and the virus continue to evolve.
Practical application: eventually, the mites will develop resistance to every miticide, and DWV will evolve to become more virulent.
The solution is for us to stop fighting the evolutionary process, and instead work with nature, rather than against it. We need to strike a deal with varroa and DWV [8]. We beekeepers can then stop doing the fighting, turning that job over to our bees. We will only breed from colonies that exhibit traits that restrict mite reproduction (yet allow the mite and virus to survive at benign levels and transmit vertically from parent colony to daughter).
Small-scale beekeepers can do this by simply making increase only from colonies healthy enough to swarm in their second year. As Fries and Camazine acknowledge, it would be impractical to suggest that large-scale beekeepers do so. In their words:.
However, a similar effect may be accomplished by paying attention to pathogen impact when breeding stock is selected. Beekeepers should continually select strains of bees that show disease resistance.
How simple is that? There is no need to allow any colonies to die—we can continue to use mite treatments during the transition. But we gotta kill any queen whose hive gets sick from mites, just as we kill the queens of “hot” hives. We’ll need to stop rearing or buying queens that are not selected for mite resistance. Unfortunately, unless you’re isolated from other beekeepers, you won’t be able to make much progress alone. We need to do this as a community. It won’t happen overnight, but it won’t require any change in our practices until we’ve shifted the genetics of our local managed bee populations (I suggest using stock selected for adaptation to each region, but that’s not absolutely necessary, so long as they are mite resistant).
Luckily, there are those who are ahead of the curve (both beekeepers and scientists), who have made substantial progress towards developing mite-resistant stocks, plus a large contingent of beekeepers who are working with survivor/feral bloodlines [9]. Following Everett Rogers’ categories in Diffusion of Innovations, I see the Innovators as having already opened the door for the Early Adopters (generally working on their own). I suspect that there’s an Early Majority getting tired of rolling that Sisyphean boulder, ready to create a willing market for bees that can handle varroa on their own.
Unfortunately, our commercial queen producers are unlikely to change their stocks until their customers demand it. I suspect that the hobby market for queens will lead the way, and if the hobbyists create a demand, breeders and producers will respond. Eventually, the market will reach a tip point, and treatment-free beekeeping will become the norm. How long this will take is entirely up to us.
In the interim, I will continue this series so that we can better understand varroa, its management, and how to set up a biologically-based breeding program.
Acknowledgements
Thanks to all the scientists who have done the research upon which this article is based. And to those Early Innovators who have demonstrated that treatment-free beekeeping is possible. And as always, my thanks to Pete Borst for his help in research.
Notes and Citations
[1] At the time of swarming, the colony is full of sealed brood, which contains roughly 70% of the mite population—leaving 30% on the adult bees. If 70% of the adults leave with the swarm, that means that only 30% x 70% = 21% of the mites would go with the swarm (and that doesn’t count the developing mites in the brood).
[2] Yue, C & E Genersch (2005) RT-PCR analysis of Deformed Wing Virus in honeybees (Apis mellifera) and mites (Varroa destructor). J General Virology 86: 3419–3424.
Chen, Y, et al (2006) Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. Journal of Invertebrate Pathology 92: 152–159.
Yañez,O, et al (2012) Deformed wing virus and drone mating flights in the honey bee (Apis mellifera): implications for sexual transmission of a major honey bee virus. Apidologie 43:17-30.
Graystock P, et al (2015) Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. B 282: 20151371.
[3] DeGrandi-Hoffman, G, et al (2010) The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). Journal of Insect Physiology 56: 1184–1191.
[4] This phenomenon is well described by Nazzi: in the absence of any additional immunological strain on the host (such as mite feeding), DWV can be effectively regulated to low copy number, so long as DWV is kept below a high and critical threshold. However, any factor that depletes the immune system will cause a gradual increase in the stable set-point until a critical transition occurs and uncontrolled viral replication ensues. The sudden transition to explosive viral growth results directly from the non-linear immuno-suppressive behaviour of DWV, potentially allowing the virus to function as an opportunistic pathogen, sensing and exploiting host weakness with escalating immuno-suppression and explosive growth.
Nazzi, F et al. (2012) Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog 8(6): e1002735.
[5] Fries, I and S Camazine (2001) Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 32: 199–214. Open access.
[6] I took the liberty of editing and paraphrasing their sentences for readability.
[7] The exceptions are in Bond yards restocked only from offspring of survivors, or in directed selective breeding programs, subjects to which I will return.
[8] I will go more deeply into the “deal” arrived at by Apis cerana and varroa when I get into selective breeding.
[9] https://scientificbeekeeping.com/whats-happening-to-the-bees-part-5-is-there-a-difference-between-domesticated-and-feral-bees/



