First published in: American Bee Journal, August 2017
The Varroa Problem: Part 9
Knowing Thine Enemy
First published in ABJ August 2017
Updated Dec 2021
Randy Oliver
ScientificBeekeeping.com
“If you know the enemy and know yourself, you need not fear the result of a hundred battles”– Sun Tzu. We are all beekeepers; we are also all varroa keepers (some of us better at the latter than the former). Varroa is the enemy of both bees and beekeepers. It would behoove us to know the strengths—and more importantly the weaknesses—of our enemy.
Evolution
I well remember listening to a well-known extension apiarist and columnist [[1]] back in the early days of Apistan®, tell an audience that the invasion of varroa had been a “good thing” for the bee industry—it had driven all the poor beekeepers out of business, and knocked out competition for resources from the feral bee population. At that time, Apistan strips had made beekeeping easy again. Unfortunately, the speaker was failing to account for evolution. He would have done well to remember the words of Heraclitus (500 B.C.):
“There is nothing permanent except change.”
Despite us all watching varroa demonstrate its ability to evolve resistance to one miticide after another, our industry has kept its collective head stuck in the sand, and we’re now facing a new varroa crisis. This has not gone unnoticed by the research community or its funders, resulting in new attention being paid to our unwanted hive guest (Fig. 1).

Fig. 1. Although varroa was a pretty hot topic upon its arrival in Europe and North America, scientific interest in the parasite was eclipsed post the CCD epidemic by the sexier neonics [[2]]. However, our growing pragmatic awareness of The Varroa Problem is again bringing scientific attention back to the parasite. Data from Science Direct.
As far as I can tell, beekeepers who pay attention to the nutritional needs of their colonies and keep varroa under control appear to have far fewer problems than those who are less diligent. It’s frustrating to me, that despite varroa being the #1 problem for most beekeepers worldwide, that so little basic research has been done in recent years on its reproductive biology. In Dr. Clarence Collison’s 2015 literature review on varroa reproduction [[3]], the majority of his cited studies were published prior to the year 2000. Keep in mind that Nature is not static—Varroa destructor and Apis mellifera are continually coevolving and adapting to one another—it’s a valid question as to whether the mite reproductive traits detailed in 1997 still apply two decades later [[4]].
The biology of varroa reproduction and its vulnerabilities
It is tedious field and microscopic work to study varroa reproduction in bee brood, and unfortunately such work doesn’t do much to polish a young researcher’s resume in this age of high-tech molecular biology. That said, I’ll attempt to briefly summarize what is known about varroa reproduction—based upon observations made during the 1990’s [[5]]. I’ll also point out weaknesses and vulnerabilities in our enemy that could be evolutionarily targeted by the bee to screw up the mite’s reproductive success.
Here a picture is worth a thousand words—thank you to Dr. Stephen Martin, who created the best image of varroa reproductive success that I’ve yet seen. Please take a minute or two to study his graphic (Fig. 2).:
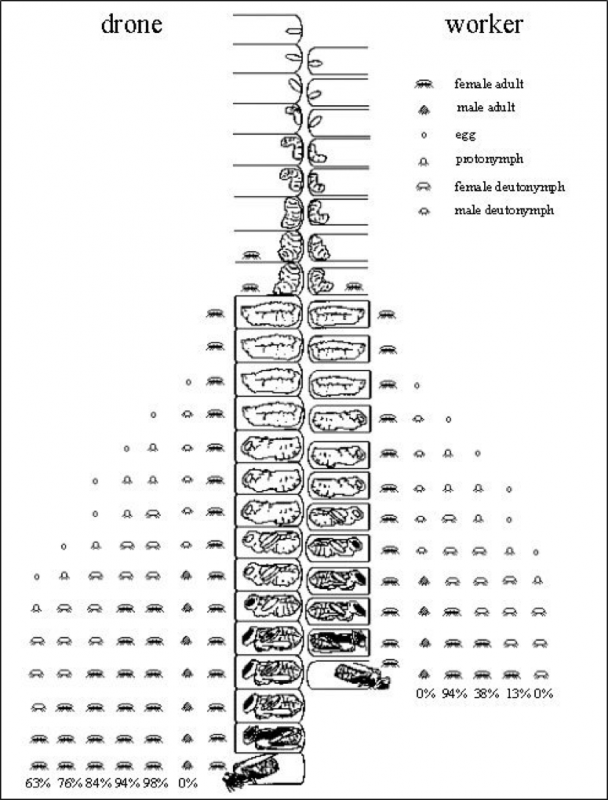
Figure 2. Varroa reproductive success in drone and worker brood, with the average survivorship of each offspring at bee emergence. Courtesy Dr. Stephen Martin (1997) [[6]].
Note that a foundress mite has the potential to produce 3 mated daughters per reproductive cycle in worker brood [[7]], and 5 in drone brood. Yet in study after study [[8]], fewer than half that many ever emerge. And in mite-resistant stock, even that percentage may be substantially lower [[9]].
Practical application: Although varroa may seem invincible, in actuality it never achieves its potential reproductive capacity in Apis mellifera (this is very fortunate for us, since if a mom mite’s fecundity were not constrained, varroa could increase its population in a hive by up to 8x per month). Conversely, if a bloodline of bees is able (by any combination of means) to reduce varroa’s current (in typical managed stock) rate of reproductive success by 50%, that would reduce the mite to mere nuisance status (you don’t need to kill a single mite to make varroa a non issue—you only need to reduce its rate of reproductive success).
Varroa sensory Perception
Try to imagine how the mite senses the world—it has no eyes, and is entirely dependent upon its acute senses of touch and smell. As described by Leonovich [[10]]:
Sensory organs form the interface between the environment and the behavior of an organism. All the information on the state of the environment and on its changes, necessary for the survival, reproduction, etc. reaches the central nervous system via this interface.
Practical application: Every behavioral and reproductive action of a varroa mite is triggered by specific (and sometimes complex) sensory cues. The take-home to me is that the battle between the bee and varroa takes place mainly at the olfactory level. This concept was first floated by Dr. Denis Anderson back in 2006 [[11]], after his observation that there were strain-specific varroa to each regional population of Apis cerana:
The signal (or signals) that triggers varroa mite reproduction will almost certainly be a chemical that interacts with a mite receptor. In isolated populations of A. cerana it is very likely that, through evolutionary time, both the signal and the receptor have changed slightly through the action of mutation and natural selection. For example, a beneficial mutation in an isolated population of A. cerana could produce a signal that is more difficult for the mites to recognise…Identifying this signal could present a new way of controlling the Korea and Japan haplotypes of V. destructor on A. mellifera. Once the signal has been found, then various approaches can be pursued to produce varroa-resistant bees.
Dr. Anderson nailed it—the varroa/bee battle is all about semiochemical [[12]] signaling and subterfuge. Even the brutal sacrifice of infested pupae via VSH is all about the specific chemical cues that trigger preexisting hygienic behaviors. The evolution of mite resistance in Apis mellifera appears to follow another observation by SunTzu:
The supreme art of war is to subdue the enemy without fighting.
When evolution is allowed to run its course, mite resistance generally appears to result more from “cyberwarfare” rather than from hand-to-hand combat. The bees mess with the semiochemical signals (or perhaps the within-cell environment) that the mites depend upon for successful reproduction. Building on the meticulous visual observations of mite behavior done in the 1990’s, researchers can now perform state-of-the-art chemical analysis to determine those semiochemical cues. What we’ve learned so far, though, is only a teaser to a more complete understanding, as well detailed in a fascinating review by Nazzi and Le Conte [[13]].
a walk through a varroa reproductive cycle
A typical varroa reproductive cycle during spring and summer takes about 17 days—with a bit over 12 of those days taking place in the capped brood cell. During the other 5 days, the mite is phoretic—hitching a ride on an adult worker (generally a nurse bee). So let’s start our walk with that phoretic female.
Update: as pointed out by Dr. Samuel Ramsey, the term “phoretic” has been misapplied to hitchhiking adult varroa mites, since they not only hitchhike, but also feed on the adult bees. Therefore, the proper term would be “dispersing” mites, since they are in a feeding dispersal phase, rather than a non-feeding phoretic phase.
Phoretic adult mites: The mite must avoid being groomed off the unwilling bee, and to feed it must locate the “sweet spot” on the bee, and dig its head between the abdominal plates and chew a hole through the soft integument to reach the fat bodies [[14]]. Not only that, but the mite must shift to a nurse bee if its ride ages to mid-age status, or it will have scant change of being carried to a prepupa about to be capped. Weak points—susceptibility to being dislodged by grooming/biting behavior, and the mite’s need to recognize specific olfactory cues for worker age and feeding site. Note, however, that varroa is exquisitely well adapted to avoid being dislodged by the bee, and on its native host can survive in the very hostile hive environment for a full year between drone rearing episodes.
Foundress invasion: in order to invade a brood cell at the proper time, the female mite must recognize a specific olfactory cue from the bee 5th-instar larva (perhaps the one used to signal the nurses to cap it over) [[15]]. Invading mites respond more strongly to the cue from drone prepupae than from that of workers. They may also prefer cells that have already been invaded by another female [[16]]. Weak point—again, the bees could modify their pheromonal or cuticular hydrocarbon scents to throw the mite off scent.
Pupation: The foundress mite then hides in the remaining jelly at the base of the cell, being freed as the larva consumes the remaining jelly after capping. At this time the mite must avoid being trapped in the silk as the larva spins its cocoon. From this point on, everything takes place in the close and crowded confines of the capped cell. Potential mechanisms—leaving excess jelly, trapping the mite in the cocoon.
Oogenesis: In order to synchronize its timing with the development of the pupa, the mite must respond to olfactory cues (as well as engage in a feeding on the prepupa) to initiate a sequence of egg laying beginning at about 60 hours after the cell is capped, and then a subsequent egg approximately every 30 hours thereafter [[17]]. But as found by Infitadis [16b], this eggl aying follows an unusual sequence: the foundress mite first lays a female egg, and then a male egg second, and then female eggs again thereafter (for a maximum of 6 female eggs in a worker cell, or 7 in a drone cell, of which only the first few ever reach maturity before emergence of the adult bee). Despite coming from the second egg, the male mite, due to its shorter development time, is sexually mature by the time that his older sister becomes receptive.
Weak point–failure to produce the critical male at precisely the right time appears to be a major Achille’s heel of varroa, and appears to be targeted by naturally-occurring lines of mite-resistant bees. Any olfactory tweaks that result in getting the foundress mite out of synch with pupal development can have a large impact on mite reproductive success.
Mite development: Varroa offspring emerge already legged from the egg, and then go through two developmental stages: protonymph and deutonymph before moulting into an adult (Figs.3 and 4).
Figure 3. A male varroa protonymph. Neither the nymphal stages nor the adult male can survive outside the capped cell. Thank you to Gilles San Martin for granting open access to these amazing photos.

Figure 4. A female varroa deutonymph. The tiny leglike structures in the center are pedipalps, which are part of the mite’s mouthparts. The first pair of true legs are used similarly to antennae in insects. There are tactile and chemosensory organs at the tips of the pedipalps and legs, as well as elsewhere on the mite. Photo by Gilles San Martin.
Male survival: it’s not easy to be a male mite (Fig. 5)—due to timing, it may not be able to feed for some time in drone cells, and its tiny larval stage must avoid being crushed by the movements of the pupating bee, and then make its way past the bees’ legs in the tight cell [[18]]. This is certainly a potential point in time to target the male mites by slight alterations in pupal morphology, behavior, or cuticular scents.

Figure 5. An adult male varroa walking over a bee about to emerge. By this stage of development, his male mite has done his job—mating dozens of times with one sister (or other young female) after the next [[19]]. Without a hard exoskeleton, the male quickly dies once the capping is removed [[20]]. Photo by Gilles San Martin.
Daughter survival: the first daughter mite has it the easiest (and enjoys a very high rate of survival), but her subsequent sisters often do not survive until adulthood (perhaps due to competition at the common feeding wound, or to thickening of the pupal cuticle)—refer back to Fig. 2. And of course no offspring survive should the pupa be sacrificed by nurse bees practicing varroa-sensitive hygiene (VSH). There are several potential defense mechanisms that the bees could use here—making it more difficult for the mites to feed, pupal altruistic suicide in response to wounding (as exhibited by Apis cerana pupae), olfactory signaling by the pupa that it is being parasitized, and of course, vigorous hygienic behavior by the nurses.
Mating: female mites must mate shortly after eclosure as adults or they remain forever sterile (thus being unable to contribute to further reproduction). Mating takes place on the fecal mound created by the foundress, and is dependent upon pheromonal cues (with the male mating solely with the most recently-emerged female) (Fig. 6).
Surprisingly, there are very few spermatozoa actually involved in the mating process, with a fully-mated female receiving only a couple of dozen spermatozoa after multiple matings [[21]]. Apparently the first daughter gets mated the best. Mites can get around the problem of lack of a male by multiply invading a cell. Even though competition reduces the number of daughters per foundress, at least some of those daughters get mated. Mating success is dependent upon pheromonal cues (and the success of the foundress at producing a son)—a possible resistance mechanism would be for hygienic bees to detect (or competitively overwhelm) the mite mating pheromone.

Figure 6. Mites engaged in mating. Surprisingly, very few spermatozoa are transferred in each mating, so multiple matings are required for good female fecundity. These matings must all take place before the next (and more pheromonally-attractive) female emerges. Photo credit: FAO TECA.
Developmental time, humidity, and temperature: varroa evolved to reproduce in the drone brood of Apis cerana. It does not have as much success in Apis mellifera worker brood, due to a number of factors. The Cape Bee greatly restricts varroa reproduction due to its extremely short post-capping duration, but knocking even a full day off our bees’ postcapping duration would only slightly reduce varroa’s overall reproductive success (refer back to Fig. 2). However, the uncapping of pupae (bald brood) may be a way to dessicate developing mites. Varroa also reproduces best at the lower brood temperature typical of Apis cerana drone brood—I’ll discuss this potential resistance mechanism further on.
Emergence (Fig. 7): mites can’t escape from a sealed cell, and are dependent upon either the adult bee to chew its way out of the cell, or for workers to open the cell during hygienic removal of the pupa. By entombing mites by thickening the cappings of infested cells, bees could conceivably trap varroa in the combs (as does Apis cerana in the drone brood).

Figure 7. A spectacular photograph of a fully-scelerotized female mite ready to emerge from a cell. Varroa is an exquisitely-adapted parasite of the bee, with every aspect of its anatomy and behavior fine-tuned by evolutionary trial and error for survival in the unfriendly (but resource-rich) environment of the bee colony. Photo by Gilles San Martin.
Practical application: there are a wide variety of targets for the bees to hit to reduce varroa reproductive success. As pointed out by Donzé:
Varroa’s population growth is…chiefly limited by the high number of sterile mites and by developmental mortality.
Knowing this, I’ll return to the wisdom of Sun Tzu:
So in war, the way is to avoid what is strong and to strike at what is weak… He who can modify his tactics in relation to his opponent can thereby succeed in winning.
It is clear that the Achille’s heel of varroa is the reproductive success of any foundress mite—it thus makes sense to focus upon varroa’s weak spot.
Practical application: VSH clearly reduces mite reproductive success. But of great interest to me is that despite the obvious utility of VSH, in survivor stock left to work it out by themselves, the most adaptive evolutionary responses appear to be targeted towards suppressing in-cell fecundity, rather than VSH or grooming behavior [[22]]. Why this is I don’t know, but when Nature talks, I listen!
A Recent finding
In a recent study, Oddie [[23]] compared the difference in fecundity between that in typical “mite-susceptible” Norwegian managed stock versus that in hives of “survivor stock” colonies. The difference was substantial—there were 1.24 presumably mated daughters (on average) per foundress in the susceptible hives vs. only 0.87 offspring per foundress in the “survivor” hives. The surprising thing about Oddie’s study was that the survivor hives were able to reduce the rate of reproductive success when given frames of already-sealed brood from another hive. If their findings prove to be true, this indicates that the colony can somehow affect the development of mites hidden under the cappings [[24]]! The authors conclude:
Our data support that a reduced V. destructor mite reproductive success seems to be a key factor for natural colony survival. However, grooming and VSH are unlikely for this Norwegian case. Instead, yet unidentified behavioral traits of worker bees seem sufficient to explain reduced mite reproductive success. Therefore, the underlying mechanisms remain elusive and should be a focus of future studies taking advantage of naturally selected survivors.
How in the heck, you may ask, could the workers possibly affect mite reproduction under the cappings? Glad you asked, since one answer is an amazing (and fascinating) example of how natural selection can come up with ways for the honey bee to fight varroa that we unimaginative humans might never have thought of, in this case, molecular warfare.
Varroa is so well adapted to its host that instead of digesting some honey bee proteins, it somehow absorbs them directly to use for its own egg production. Its direct use of those bee proteins allows varroa to reduce some of its metabolic pathways, and thus even its genome. We now understand that varroa can utilize not only bee vitellogenin for its rapid egg production, but also some critical enzymes in its ecdysone biosynthesis pathway. The steroid hormone ecdysone is associated with arthropod ecdysis (molting), but it is also a mater regulator for other developmental transitions, including egg formation (oogenesis).
And here’s another Achilles’ heel for varroa — it has given up its ability to produce some critical ecdysteroids, depending instead on obtaining them directly from ingested bee tissue. Conlon [24b] discovered that the varroa-resistant Norwegian bloodlines of bees had evolved to downregulate their expression of some of their ecdysteroid genes, thus disrupting the mite’s ability to produce eggs.
Who woulda thunk??? Would it have ever occurred to a bee breeder to select their breeder queens based upon their propensity to downregulate their expression of ecdysteroids?
Brood temperature and varroa
Varroa reproduces best at temps from 32.5-33.5°C, which reflects the normal temperature of drone brood in Apis cerana. I haven’t seen any recent research to check whether varroa has adapted to the higher brood nest temperatures of Apis mellifera (which the bees maintain within a narrow temperature range of 34.5±1.5°C, typically 35°C)—which is warmer than the optimal temperature for the mite. I’ve long been curious as to why no one has yet identified a bee population that uses elevated brood temperature as a mite-resistance mechanism
Practical application: Bees have the ability to create a “fever” in the brood, as previously reported by the Seeley lab [[25]], who found that when stimulated by chalkbrood, the workers would raise the brood temperature by half a degree centigrade. Could the “survivor” bees be doing this in response to varroa?
A half a degree C may not seem like much, but we’re talking about a parasite already at the edge of its ideal temperature range (Fig. 8). A slightly higher broodnest temperature could have a twofold effect upon mite reproductive success: (1) poorer foundress and offspring survivorship [[26]], as well as (2) accelerated development of the bee pupa (perhaps slightly reducing the number of mated daughters) [[27],[28]]. The combination of these two effects could plausibly result in decreased mite reproductive success.
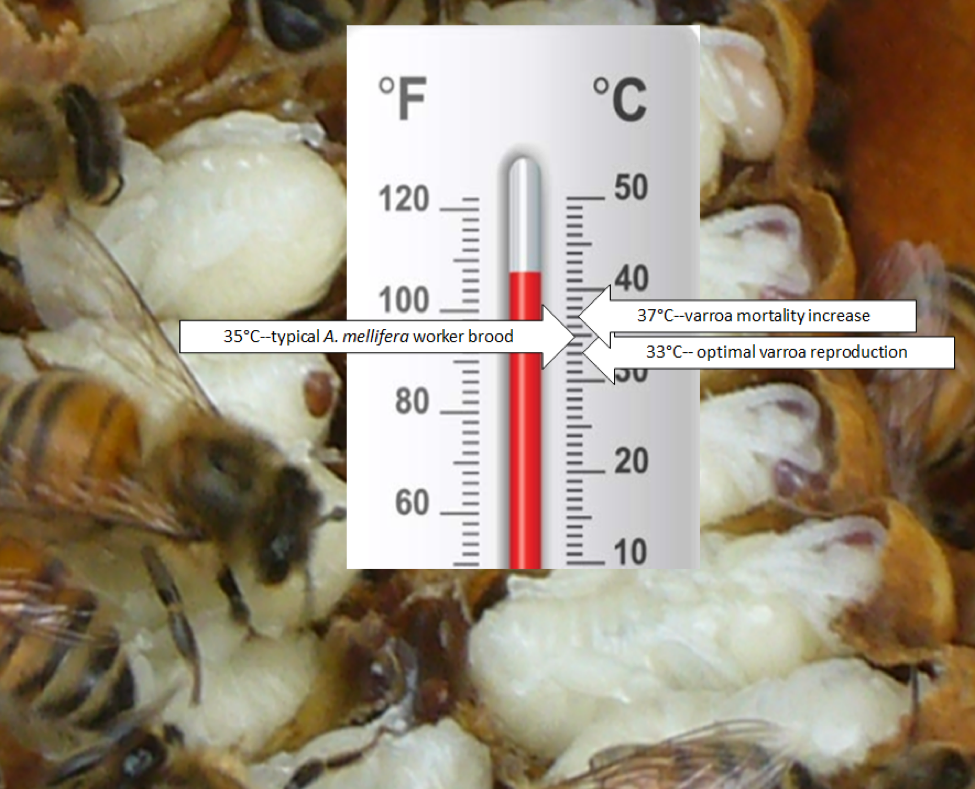
Figure 8. Varroa reproduces best in a very narrow temperature range (evolutionarily set by the temperature of Apis cerana drone brood). It wouldn’t take much of a “fever” in the worker brood to put the hurt to the mite.
I’ve reviewed Oddie’s experimental methodology, and it appears sound (although we need to see this remarkable finding replicated). So for the time being, I’m keeping my mind open to the possibility that the Norwegian survivor colonies might be elevating their broodnest (or individual brood cell) temperature to put the heat on the mites. I’ve corresponded with the senior author, who couldn’t yet tell me their own explanatory hypothesis (due to its being in review)—it’s not exactly as above, but apparently something along a similar line of reasoning.
Acknowledgments
Thanks to Pete Borst for his help in literature searching. To Dr. Stephen Martin and all the other dedicated researchers working on varroa. And to Gilles San Martin for sharing his amazing close-up photographs. Also a big thanks to Dr. Stephen Martin for his helpful comments as I was writing this article.
Notes and Citations
[1] Name withheld out of courtesy.
[2] Although it’s clear that neonics are vastly overused, can cause planting dust and some other unintentional bee kills, adversely affect some native pollinators and aquatic ecosystems, and exhibit sublethal effects in individual bees, I’ve yet to see convincing evidence that they are seriously affecting honey bees overall.
[3] Collison, C (2015) A closer look: varroa mite reproduction. http://www.beeculture.com/a-closer-look-varroa-mite-reproduction/
[4] There has been clear evolution regarding mite reproductive success in some bloodlines and races of Apis mellifera; refer to:
Strauss, U, et al (2015) Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology 143: 374–387.
Danka, RG, et al (2015) Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 47(3): 483–490.
[5] A few good references:
Donzé, G, et al (1996) The rate of infestation of brood cells and mating frequency affects the reproductive success of the honeybee parasite Varroa jacobsoni. Ecol. Ent. 21: 17-26.
Martin, S.J. (1994). Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honey bee Apis mellifera L. under natural conditions. Experimental and Applied Acarology, 18: 87-100.
Martin, S.J. (1995). Ontogenesis of the mite Varroa jacobsoni Oud in drone brood of the honey bee Apis mellifera L under natural conditions. Experimental and Applied Acarology, 19: 199-210.
[6] Martin, S.J. (1997a). Life and death of varroa. In Varroa! Fight the Mite (Ed. P. Munn & R. Jones), pp.3-10. International Bee Research Association, Cardiff.
[7] Note that Dr. Martin observed that development takes ~20 days, not the 21 commonly cited in textbooks. I don’t know the origin of the 21-day figure, but various researchers have confirmed the 19.5-20 day figure.
[8] Reviewed in Martin, SJ, et al (1997) Non-reproduction in the honeybee mite Varroa jacobsoni. Experimental & Applied Acarology 21: 539–549. Table 1 in Donzé (1996) is very instructive.
[9] Strauss, U, et al (2015) Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology 143: 374–387.
Oddie, M, et at (2017) Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ preprint.
[10] Leonovich, SA & MK Stanyukovich (2011) Sensory organs of mesostigmatic mites (Acarina, Mesostigmata) dwelling in body cavities of mammals and birds. Proceedings of the Zoological Institute RAS 315(3): 263–273. This open-access paper has beautiful electron micrographs of mite sensory structures.
[11] Anderson, D (2006) Clarification of aspects of Varroa reproduction—first stage of a possible new control method. RIRDC Publication No. 06/007. Unfortunately, this proposal did not get funded.
[12] Semiochemical–a pheromone or other chemical that conveys a signal from one organism to another so as to modify the behavior of the recipient organism.
[13] Nazzi, F & Y Le Conte (2016) Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu Rev Entomol. 61:417-32. This paper might better have been titled “Chemical ecology of varroa,” and details the state of the art of our knowledge of the chemical interplay between varroa and the bees.
[14] Ramey, Samuel, in prep.
[15] This kairomonal chemical was determined by the ARS: Carroll, MJ, A Duehl and PEA Teal (2010) Methods for attracting or repelling Varroa mites. U.S. Patent (pending).
[16] Donze and others have noted that it can be to varroa’s reproductive advantage for more than one female to invade a cell. Despite the fact that this would decrease the number of daughters per female (apparently due to competition at the feeding site), it increases the chance that the surviving daughters get adequately mated. I have no idea what cue an invading mite would use to recognize that another female is already at the other end of the cell.
[16b] M. D. Ifantidis (1983) Ontogenesis of the Mite Varroa�Jacobsoni in Worker and Drone Honeybee Brood Cells, Journal of Apicultural Research, 22:3, 200-206]
[17] Although the male winds up being haploid, the egg must first be fertilized in order to initiate development.
[18] The foundress mite actually moves the bee pupa’s legs to allow the male mite to pass. Although it’s an attractive conjecture, there is scant evidence in support of the hypothesis that smaller cell size decreases the survival rate of developing mites.
[19] Donzé (1996) observed that over the 50-100 hour mating period a male can complete 15 matings in a worker cell, and 30 in a drone cell. By his calculations, a male could fertilize some 3.75 females in a worker cell, and 7.5 in a drone cell.
[20] Presumably from desiccation, but I’m not sure.
[21] Donzé (1996) op cit.
[22] The VSH/SMR issue is tough to resolve, unless one protects the sealed brood from hygienic bees. That said, it appears that the USDA VSH line of bees may also exhibit some degree of SMR, as evidenced by the observation that in brood cells still intact at the purple-eyed stage, some 50% of foundresses had not reproduced at all, and another 10% did not produce a male. Danka, RG, et al (2015) Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 47(3): 483–490.
[23] Oddie, M, B Dahle, P Neumann (2017) Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. https://peerj.com/preprints/2976/
[24] There was scant different in VSH between the two groups.
[24b] Conlon, B, et al (2019). A gene for resistance to the Varroa mite (Acari) in honey bee (Apis mellifera) pupae. Molecular ecology, 28(12), 2958-2966.
[25] Starks, PT, CA Blackie, TD Seeley (2000) Fever in honeybee colonies. Naturwissenschaften 87: 229–231.
[26] Le Conte, Y, et al (1990) Influence of brood temperature and hygrometry variations on the development of the honey bee ectoparasite Varroa jacobsoni (Mesostigmata: Varroidae). Environ Entomol 19 (6): 1780-1785.
[27] Martin, SJ (1994) Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honeybee Apis mellifera L. under natural conditions. Experimental & Applied Acarology 18(2): 87–100.
[28] Donzé (1996) op cit. The third daughter to mature has only a few hours during which to mate with her brother.
First published in: American Bee Journal, June 2017
The Varroa Problem: Part 8
Regulatory Cascades, Varroa Tolerance, and a Moon Shot
Randy Oliver
ScientificBeekeeping.com
First published in ABJ June 2017
In writing this series, I skipped ahead over some details so that I could publish my suggestions for setting up a breeding program for mite resistance in time for this season’s queen rearing. I now return to pick up some of the pieces.
Do we need new genes for mite resistance?
In any selective breeding program, what you’ve got to work with is the innate amount of variability in heritable traits within the breeding population. And although we loosely speak of differences in “genes,” what we are really generally referring to are differences in the expression of genes. With regard to breeding for resistance to varroa, it is the expression of genes that result in phenotypic traits which we can observe and select for, such as morphology (the form and structure of the bees’ bodies), physiology (the biological functioning of the body, the immune system, pheromone production and olfactory sensing), and behavior (grooming, VSH, etc.).
It’s important to understand that there’s a big difference between genotype and phenotype. A bee’s inherited genes (the genotype) are like a library of instructions; the expression of those genes (the phenotype) is the choosing of which parts of the library to read, and then how those genes are used for the construction and function of the actual bee. This expression of the genetic material is done by genetic regulatory networks [[1]].
Think on this–any individual fertilized egg has the potential of developing into either a worker, a queen, or a diploid drone—there is absolutely no genetic difference (other than in the case of the females, which must exhibit slightly different alleles at only one single gene) [[2]]. By their looks and behavior, one might think that the three bees in Figure 1 below were different species entirely. Yet those differences in form and function were not due to any difference in their genetics whatsoever, but rather due to the initiation of different regulatory cascades of their identical genes.
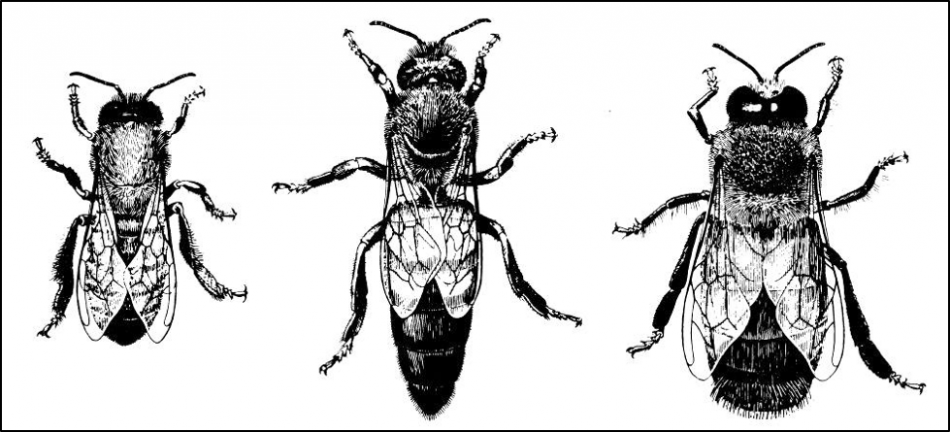
Fig. 1. If one didn’t know better, it would appear that we were looking at three different species of bees, rather that the two castes of females and the male of Apis mellifera. This is an example of how identical genetics can be expressed in very different forms as a result of flipping switches in the regulatory networks. This detailed illustration is taken from Norbert Kauffeld [[3]].
And that’s only the half of it—the same identical genes also coded for the each developmental stage of the above bees—the larva, the pupa, and the adult again each look like entirely different organisms. And then once a worker emerges as an adult, her behavior and physiology can shift from being a nurse, a wax producer, a forager, a laying worker, or a long-lived diutinus bee. Have I made it clear that despite possessing identical genetics, one can get all sorts of forms and functions out of an egg’s genes simply by how the regulatory networks express those genes?
What biologists say is that the honey bee has great “phenotypic plasticity”—from any inherited set of genetic “instructions,” it can grow into different forms, or express different biological functions or behaviors.
Practical application: The point that I’m trying to make is that we may have all the “genes” necessary for mite resistance already built into the North American bee population—we may need only to select for heritable changes in the bees’ genetic or epigenetic regulatory systems. I will return to this concept near the end of this article.
Resistance vs. tolerance
Another important detail that I’d like to cover is the difference between two often-confused terms—resistance and tolerance. These two terms have well-defined biological meanings [[4]]:
Tolerance: the ability of a host to limit the damage caused by a given parasite burden.
Resistance: the ability of a host to limit parasite burden (read that, the buildup of the mite level).
We don’t need to breed for varroa-tolerant bees—our bees were already quite tolerant when varroa first invaded. Back then, we could allow varroa to build up until the colony was literally crawling with mites, and notice no sign of harm so long as we hit the hive with a single treatment of Apistan each fall [[5]].
All that changed as the viruses evolved to take advantage of varroa as a vector. Dr. Stephen Martin was the first to deduce that it was an in-hive epidemic of virus, rather than the mite, that was the real problem, although he had a helluva time convincing the research community that this was true [[6]].
After a few years of evolution, in most every country (South Africa being an exception) we watched Deformed Wing Virus (DWV) evolve into a more virulent form [[7]], and colonies would collapse at mite levels that were previously well-tolerated [[8]].
Practical application: By definition, varroa-tolerant bees do not suppress mite buildup. So what we’re really talking about when we speak of tolerance is the ability to not be harmed by mite-vectored viruses.
Examples of tolerance
I observe signs of tolerance in the feral Africanized bees, in some colonies of Primorsky Russians, and in the occasional hive of my own Italian stock. Such tolerant colonies sometimes have quite high mite levels, but their brood patterns remain solid and healthy, and there is no sign of deformed wings or illness in the adult workers. Technically, although these bees might be called varroa tolerant, in fact such tolerance is likely due to their being virus-resistant (see [[9]]).
Alternatively, the bee might collaborate with a benign virus in order to confer resistance to a more virulent strain. Strains of virus compete against each other, and a recent study [[10]] suggests that by harboring a benign strain of DWV, that benign strain can outcompete the virulent strain (I’ve recently coordinated sampling from across the U.S. to determine whether this is happening here). Thus, a line of bees might be able to inherit tolerance to DWV by inheriting the protective benign strain transovarially from their queen.
Practical application: of course, breeding for tolerance of varroa-vectored virus(es) would be a good thing, but due to varroa’s exponential growth, the mite would eventually cause even tolerant colonies to collapse.
And here is where strict interpretation of definitions gets sticky. Nature (the environment) may select for some combination of both tolerance and resistance. As Sorci [[11]] points out:
The task of the immune system is not necessarily to clear the infection. In many cases, it might be more rewarding to coexist with the parasite instead of declaring the war.
A case in point is the host/parasite relationship between varroa and its natural host, Apis cerana. The bee apparently found it to be most adaptive to not waste any more energy in resistance than necessary to hold varroa to a tolerable level.
Keep in mind that Sorci also notes that:
An alternative view suggests that tolerance reduces the cost of virulence traits for highly exploitative parasite strains (infected hosts tolerate the infection and the parasite achieves its transmission). Therefore, tolerant hosts might actually select for more virulent parasites.
Practical application: although “varroa tolerance” has a nice ring to it, we must keep in mind that we want bees that tolerate varroa only at a very low level in the hive.
An inherent problem with managed bees
Dr. Tom Seeley, in two recent papers, has pointed out that there are substantial differences between wild and managed bee colonies [[12]]. Studying feral colonies, as well as unmanaged colonies maintained in a single Langstroth deep, Seeley and his team found that those colonies somehow manage to restrict varroa buildup—thus by definition, a form of resistance. Such suppression of varroa buildup appears to be due to those colonies’ restriction of broodrearing and frequent swarming [[13]]. Note that both the Africanized ferals and Primorsky Russian bees also swarm frequently, plus shut down broodrearing during pollen dearths, resulting in reduced mite buildup.

Figure 2. A varroa restaurant. Those beautiful brood patterns that we so love can also be considered as all-you-can eat buffets for the varroa mite. This is why our strongest colonies often have the worst problems with mites come fall.
Practical application: As I’ve pointed out before, we beekeepers are part of the problem (Fig. 2). When we manage our hives for productivity (by encouraging early spring buildup, large broodnests, and by restricting swarming), we are also producing more varroa food (sealed brood) over a longer period of time.
On the other hand, as someone who makes his living from supplying large colonies to the almond orchards, by selling excess bees, and by producing honey, I would go broke if I kept only small, swarmy colonies. Thus, I definitely want to breed for resistance to varroa buildup, since so long as mite levels are low, there’s really no need for tolerance.
That then introduces another concept—intolerance. Intolerance of varroa (and viruses) at the individual bee level can result in resistance at the colony level.
intolerance as form of resistance
The flip side of tolerance would be “intolerance” (I’m using the term “intolerance” here in a way that has not yet been vetted by the research community). Intolerance can actually result in a very strong form of resistance. For example, let’s say that there was a line of bees exhibiting the trait of being completely intolerant of allowing a mite to hitchhike on their bodies—to the extent that such a bee would immediately rush to the hive entrance and commit “altruist self removal” (fly off and not return). Such an individual-level trait would, at the colony level, make it impossible for varroa to ever establish a population in the hive. End of The Varroa Problem.
An evolutionary point: as recently pointed out to me by Dr. David De Jong, the intolerance of drone pupae to damage by varroa (drones damaged by varroa during pupal development stand little chance of successfully mating), exerts very strong selective pressure for unmanaged bee populations to evolve resistance to varroa.
Returning to my hypothetical example of varroa-intolerant workers, most species do not have the option of exhibiting such extreme intolerance of parasites, since self sacrifice prior to reproduction would be an evolutionary dead end. Not so for social insects.
In the superorganism that we know as the honey bee “colony,” any single bee is somewhat analogous to a single cell of a multicellular organism. From an evolutionary perspective, the survival of that bee’s genetics (carried by its sister queens and brother drones) is more important than its own survival as an individual. Thus, any member can and will sacrifice itself for the good of the colony. We’re all well aware that a worker will not hesitate to sacrifice itself in defense of the colony by stinging, drones do it in the act of mating, and even a failing queen can give her workers a signal to supersede her. Even more regularly, any sick worker will commit altruistic self removal at the drop of a hat— should it start to feel sick, it simply flies out of the hive, never to return [[14]].
Anyway, the most effective mite-resistance trait yet identified, extreme varroa-sensitive hygiene (VSH), turns out to be based upon the intolerance of pupae to being bitten by varroa, as recently elucidated in a fascinating study. The discovery process of the details of the mechanisms involved in VSH makes for an interesting story.
VSH: A story of discovery
(For those interested in deeper information, refer to my end notes throughout the following discussion).
We’ve long known that varroa’s original host, Apis cerana (Fig. 3.), exhibits strong resistance to the mite—as evidence by its ability to prevent the mite from building up to damaging levels in the hive. The Asian bee utilizes a variety of behaviors to suppress mite buildup, such as fervent grooming behavior, frequent swarming, and absconding [[15]]. However, its main mechanism of resistance appears to be due to the fact that varroa rarely attempts to reproduce in its worker brood, thus limiting its reproduction to cerana’s limited amount of drone brood [[16]].

Figure 3. Queen and workers of the Asian bee, Apis cerana, the original host of varroa. Unlike Apis mellifera, the Asian bee coevolved with varroa, and exhibits strong resistance to the mite. Photograph by Azman [[17]].
Practical application: the Achille’s heel of varroa is its need for abundant brood cells in which it can successfully reproduce. The question then is why the mite does not normally attempt to reproduce in Apis cerana worker brood? There must be some evolutionary reason. If we could figure that reason out, we might be able to help our bees to conquer varroa.
It’s been clearly demonstrated that a foundress mite can differentiate between worker and drone prepupae by their pheromonal and cuticular odors [[18]], but why do they avoid cerana worker cells, and even if they enter one, tend not to ovulate? Evolutionarily, there must have been an adaptive reason for the mites not to do so. Could it be that because ceranae was able to make it futile for varroa to attempt to reproduce in worker brood, that it would then be nonadaptive for the mite to expose itself to the danger of doing so? A potential mechanism to achieve this futility might be the observed high degree of hygienic behavior (Fig. 4) [[19]] exhibited by cerana toward infested worker brood.

Figure 4. Bees exhibiting some degree of varroa-sensitive hygiene (VSH), as evidenced by the white pupae being chewed out (indicating that the pupae were being removed while still alive). However, as evidenced by the presence of at least five infested cells in this photo (as well as the mite on the bee’s thorax), this colony’s VSH behavior appears to have been too little, too late. Also note the small inspection hole chewed through the cell cap directly above the mite-carrying bee.
Perhaps the most important question of interest is, what cues mite-resistant bees to uncap and remove infested worker pupae? A great deal of meticulous research (sometimes with conflicting findings) has gone into answering this question. Aumeier [[20]] found that it wasn’t the movement of mites that triggered VSH, that Africanized bees often removed the mites without damaging the brood, and that the trigger appeared to be olfactory. They concluded that:
In the case of mite-infested brood in A. mellifera colonies, however, the scent or signs of life of the parasite appear to have low importance as recognition cues.
Shortly afterward, the Le Conte lab [[21]] found that there were differences in the volatile compounds within infested cells compared to uninfested cells, some of which bees could detect, and some of which resistant bees appeared to exhibit greater antennal response to. But this did not appear to be the odor of the mite itself, and his group later discovered that a mite quickly absorbs the odor of its host [[22]]. This “olfactory camouflage” helps prevent the bees from detecting a mite hidden on an adult body (preventing grooming response), or beneath the cell cap.
So the question remained whether the trigger for VSH was the actual detection of varroa (or varroa reproduction [[23]]), or a signal from the infested pupa, as suggested by Nazzi [[24]]:
[Some] olfactory cues coming from the infested larva may be involved in the recognition of infested cells by bees.
Then Schöning [[25]] demonstrated that the putative “varroa-sensitive” hygienic behavior was actually triggered by olfactory signals put off by “damaged” brood, including that damaged by an uncontrolled infection by DWV:
Our results suggest that bees show selective, damage-dependent hygienic behaviour, which may be an economic way for colonies to cope with mite infestation.
Recently, Mondet performed a brilliant study, from which she was able to elaborate on the olfactory mechanism involved in VSH [[26]]. She found that virus-infected and developmentally-delayed prepupae and pupa are sacrificed due to their emitting of altered brood ester pheromone (BEP)—more so when infected by Kashmir Bee Virus (KBV) than with DWV. Since she, despite being a scientist, is able to compose sentences comprehensible to the lay reader, I’ll allow her to speak for herself in some snips that I’ve lifted from her paper:
Here we show that varroa-infested brood produce uniquely identifiable cues that could be used by VSH-performing bees to identify with high specificity which brood cells to sacrifice. This selective elimination of mite-infested brood is a disease resistance strategy analogous to programmed cell death [remember this term], where young bees likely to be highly dysfunctional as adults are sacrificed for the greater good of the colony.
The results also imply that infested brood has a much better chance of escaping VSH detection with DWV infection than with KBV infection, thereby providing a mechanistic explanation for both the gradual disappearance of ABPV complex (i.e. KBV) and the long-term persistence of DWV in newly varroa-infested colonies.
A significant additional factor is that VSH behaviour appears also to be dependent on the sensory acuity of the detecting bees, which may be compromised by the same viruses facilitating the detection of varroa-infested brood. Thus, VSH behaviour itself may also break down through varroa-transmitted virus epidemics, placing limits on its ability to control mite infestation [refer to Fig. 4 above].
Practical application: by quick removal of any prepupae or pupa that emits an olfactory signal of being sick or developmentally delayed, a colony could prevent the reproduction of any mite that transmitted a virulent virus or otherwise “damaged” its pupal host. Due to the evolutionary penalty of non reproduction in such colonies of virus–intolerant pupae, varroa would never want to vector any virulent virus that sickened its pupa, since this would bring the mite’s reproductive success to zero (refer back to my second quote from Sorci).
Thus, those studying VSH figured out that it appears to mainly be an olfactory signal from a sick or damaged pupa that triggers VSH behavior, rather the bees detecting that a mite was in the cell, as opposed to “normal” hygienic behavior (“HYG,” as determined by the freeze- or prick-killed brood assay) in which the nurses detect the odor of decomposing brood [[27]].
Practical application: VSH is a composite behavior that involves a suite of traits. The most important is for a distressed prepupae or pupa to emit a “sacrifice me for the good of the colony” signal to the nurses. Then there must be a proportion of bees of nursing age that regularly chew holes in the cappings of occupied pupal cells to “sniff” inside (and another behavior to reseal them). Those hypersensitive bees must then possess the antennal receptors to detect the pupal olfactory signals, and for those odors to then cue the behavior of a hygienic removal response. The beauty of VSH is that not every bee in the hive needs to exhibit every trait—only a proportion of the population needs to exhibit each individual trait involving in serving as “hygiene police.”
A landmark study
This finally brings my story to the landmark paper published last year. It finally answered why varroa strains adapted to Apis cerana avoid reproducing in worker cells—it’s because the worker pupae are completely intolerant of being fed upon by a mite—whether they get infected by a virus or not.
Practical application: as pointed out by Mondet, viruses cleverly fight back with subterfuge. So simple emission of virus-infection-induced pheromones may not be enough.
This brings us back to the concept of programmed cellular death (apoptosis) mentioned above. This naturally occurs in any multicellular organism during the process of development or tissue rejuvenation. It is also used as an immune defense mechanism. Let’s say that a virus or nosema manages to invade a bee’s intestinal cell. That cell, in order to prevent the pathogen from multiplying, immediately sacrifices itself [[28]]. Thus, by such apoptotic cellular self sacrifice, the bee may avoid becoming seriously infected by the pathogen.
In the bee hive, in which any individual is expendable, the altruistic suicide of any individual for the good of the colony could be considered as a form of social apoptosis [[29]], a little-used term recently resurrected by Dr. Paul Page and collaborators [[30]]. They discovered that it is the social apoptosis exhibited by Apis cerana pupae that makes its varroa-sensitive hygiene so effective—so efficient that it’s not in the mite’s interest to even attempt to reproduce in worker brood. Page found that A. cerana worker pupae are completely intolerant of being fed upon by varroa. If a mite makes the mistake of feeding on a worker (rather than a drone) pupa, the pupa emits a “sacrifice me” signal, and then simply rolls over and dies, thus preventing the mite from reproducing in that cell. In contrast, A. mellifera pupae tolerate being fed upon by a mite, and generally go on to emerge as adults (so it is clearly to the benefit of varroa to parasitize mellifera worker brood).
Even more remarkable is that when Page experimented by piercing the skin of cerana and mellifera pupae with a sterile glass pipette drawn to the size of a mite’s mouthparts, that even though most cerana pupae, and nearly all mellifera pupa exhibited the ability to heal themselves, the cerana pupae nevertheless apparently emitted a “remove me” signal to the nurses. This sort of self sacrifice thus results in cerana’s well-known efficiency at VSH in their worker brood—they don’t need to detect the mite, their brood tells them if they need to be sacrificed.
Update March 2018: The study by Page mentioned above was only the first of what I hope will be a series by a collaboration of researchers whose names I’ve included in full for their open-access paper below [[31]]. I applaud these researchers for finally clarifying critical aspects of the methods by which Apis cerana has successfully achieved a workable host/parasite relationship with varroa. I feel that they have now presented perhaps the most important and applicable findings in over a decade towards our goal of coming to terms with the mite. The team, through well-designed experiments, has found that the Korean haplotype of Varroa destructor that now plagues beekeepers across the world can indeed successfully reproduce in the worker brood of Apis cerana, and that they main method by which cerana is able to control the mite is by the “susceptibility” or self-sacrifice of parasitized brood (I prefer the term “intolerance”). In the eusocial superorganism that we know as the honey bee colony, such self-sacrifice of the first individuals to be attacked by a parasite can allow the colony as a whole to avoid a damaging infestation. The team has now also described a relatively simple assay for us to select for “varroa proof” bees.
Practical application: by being completely intolerant of varroa parasitism (by not only emitting a “sacrifice me” signal, but also by then rolling over and dying), A. cerana worker pupae limit varroa reproduction to the drone brood only, where the colony can then further limit the mite’s success. The implications of this discovery are immense. If our bees exhibited a similar trait, varroa would also be forced to limit its reproduction to the drone brood. This would be a huge step toward mite-resistant bees (and counteract the evolutionary pressure for varroa to shift its preference to worker brood in Apis mellifera) [[32]].
Practical application: In order for altruistic apoptosis to be effective, not every single pupa needs to exhibit the trait. Similar to the “herd immunity” reached by vaccination of roughly 90% of a population, one can work out the math for what proportion of pupae would need to exhibit self sacrifice in order to bring overall varroa reproductive success to zero. By my modeling, cutting the overall reproductive success of the mite by 70% (of current “normal” values) in worker brood alone would lead to a net decline of the mite population.
Practical application: One might ask, “Can we import the “intolerance genes” from Apis cerana into Apis mellifera?” Please think back to my discussion of genetic regulation at the beginning of this article. Our North American bee population already possess all the traits necessary for the social apoptosis and VSH exhibited by the pupae of Apis cerana—all that we need to do is to select for those that upregulate those traits.
Some of Apis cerana’s other tricks
OK, so perhaps we’ve now figured out the last piece of the puzzle as to how Apis cerana evolutionarily forced varroa to eschew worker brood (which is apparently why varroa was not originally a problem when it was first introduced into Apis mellifera colonies in Asia).
So the question then is what steps cerana then takes to limit varroa’s reproduction in its drone brood (other than by simply only rearing drone brood on occasion). It may have to do with the fact that A. ceranae leaves an interesting ventilation hole in the center of the cap over a drone cell (Fig. 5), thus allowing the nurses to easily smell what’s happening inside [[33]].
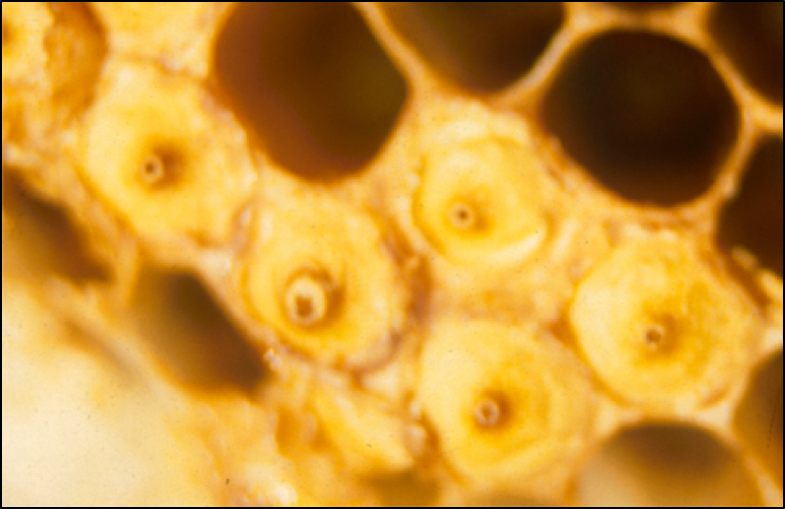
Figure 5. The pores in the cappings of Apis cerana drone cells. These pores allow both required ventilation through the extra-thick cap, as well as a means for the workers to get a whiff of what’s going on inside. Photo by Dr. Nikolaus Koeniger, by permission.
Should a drone pupa become sickened, that drone can then emit a pheromonal signal to the nurse bees to close the pore, resulting in the entombing both the sick pupa as well as any mites in that cell (thus putting an end to that specific mite bloodline). This puts strong evolutionary pressure upon the mites to not vector any viruses that might sicken the drone brood, nor to harm those drones in any other manner [[34]].
Practical application: a willingness by the drone pupae to perform self sacrifice again results in very strong selective pressure for varroa to be a benign parasite.
The “Moon Shot”
The groundbreaking finding by Paul Page and team (mentioned above) of what appears to be the main varroa-resistance mechanism employed by Apis cerana—“taking one for the team” to prevent oneself from being used as food for baby mites—opens my eyes to a “moonshot opportunity.” It occurs to me that we perhaps needn’t resign ourselves to being stuck with varroa at all! Natural selection may result in bees that resist varroa to the point that the damage caused by the mite is tolerable—since it might not be worth it to the bees to fight for total eradication. But that doesn’t mean that it wouldn’t be worth it for a breeder to do so.
What if we were able to select for a strain of bees in which 90% of both workers and drones exhibited self sacrifice if wounded by a mite during their pupal stage? This would absolutely arrest mite reproduction, and varroa would be completely purged from that bee population within a season—and even a stray mite could never be successful at reinvasion.
Practical application: I can see no biological reason that we couldn’t breed a bee with supersensitive pupae—pupae whose motto was, “We’re not gonna take it!” All that we’d need to screen for would be pupae that committed social apoptosis should a mite bite them.
Since such wounding of a pupae while protected by its cocoon would only occur in the case of invasion by a foundress mite, I can’t see any reason for there to be a downside for the pupae to have such a hair-trigger response to wounding (it doesn’t seem to hurt Apis cerana). So here’s my suggestion:
The Moon Shot Challenge: we could offer a $100,000 prize for the first team to breed a line of bees that exhibits complete intolerance of varroa due to pupal self sacrifice. The trait could be relatively easily selected for by the simple wounding technique used by the researchers. My pie-in-the-sky dream would be that we might be able to then cross those bees to other lines, and completely purge varroa from our bee population—forever [[35]] (hey, it doesn’t hurt to dream…).
Acknowledgements
Thanks to my research collaborator Peter Borst. And to all the dedicated bee researchers who have devoted their careers to helping to save our honey bee from the plight of varroa.
Notes and citations
[1] I’m not going to go any deeper into this fascinating subject. For an introduction, refer to https://en.wikipedia.org/wiki/Gene_regulatory_network
[2] Hold it, you say—diploid drones are inviable. Not exactly true; see
Woyke, J (1969) A method of rearing diploid drones in a honeybee colony. J. Apic. Res. 8(2) : 65-74.
As best I can tell, the default for any bee egg (fertilized or unfertilized) is to develop into a male. Only if that egg is fertilized by a sperm carrying an allele at the complimentary sex determination (csd) gene that differs from that of the egg itself will the egg develop into a female. It is the presence of two different alleles at the csd locus that triggers another gene—feminizer (fem)—to initiate the regulatory cascade for the larva to develop into a female. Without two different alleles at the csd locus, a fertilized egg will turn into a diploid drone, the larva of which is normally quickly consumed by a nurse.
[3] Kauffeld, NM (1980) Seasonal cycle of activities in honey bee colonies. In Beekeeping in the United States, Agricultural Handbook 335, USDA.
[4] Schneider, DS & JS Ayres (2008) Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases Nature Reviews/Immunology 8: 889-895.
Råberg, L, et al (2009) Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364: 37–49.
[5] During a visit to New Zealand in 2011, I was reminded of those days when I asked commercial beekeepers when they treated for mites. Their answer was invariably, “When we see mites crawling all over the bees.” It seemed like it had been ages since U.S. beekeepers had that luxury.
[6] Pers. comm., although he finally got it accepted for publication in his groundbreaking paper: Martin, SJ (2001) The role of Varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. Journal of Applied Ecology 53: 105–112. (This paper is what really kicked off my decision to start hitting the books and better educate myself about varroa). Open access. His modeling was further elaborated in:
Sumpter DJ & SJ Martin (2004) The dynamics of virus epidemics in Varroa-infested honey bee colonies. J Anim Ecol 73(1):51–63.
[7] Martin, SJ, et al (2012) Global honey bee viral landscape altered by a parasitic mite. Science 336(6086): 1304-1306.
[8] It’s still an open question as to why Apis mellifera scutellata colonies can tolerate high varroa counts. It appears to have something to do with lack of virulent DWV and other viruses (viruses are seldom detected in South African bees):
Mortensen, AN, et al (2016) Differences in Varroa destructor infestation rates of two indigenous subspecies of Apis mellifera in the Republic of South Africa. Exp Appl Acarol 68:509–515.
[9] Maori, E, et al (2007) Reciprocal sequence exchange between non-retro viruses and hosts leading to the appearance of new host phenotypes. Virology 362: 342 – 349. Short version: by incorporating a bit of viral genetic material into their own DNA, the bees can confer upon themselves a degree of resistance to that specific virus.
[10] Mordecai, GJ, et al (2015) Superinfection exclusion and the long-term survival of honey bees in Varroa-infested colonies. The ISME Journal 1 – 10. Open access
[11] Sorci, G (2013) Immunity, resistance and tolerance in bird–parasite interactions. Parasite Immunology 35(11): 350–361.
[12] Seeley, T (2017) Darwinian beekeeping: An evolutionary approach to apiculture. ABJ 157(3): 277–282.
Loftus JC, et al (2016) How Honey Bee Colonies Survive in the Wild: Testing the Importance of Small Nests and Frequent Swarming. PLoS ONE 11(3): e0150362. doi:10.1371/ journal.pone.0150362
[13] I’ve gone over the Seeley data with him and his team. In their experiment with commercial bee stock, the mite appeared to build up at a normal rate in the small hives, but is then got set back from time to time. Those setbacks did not occur in the larger hives.
[14] Rueppell, O, et al (2010) Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evol. Biol. 23: 1538–1546.
[15] Rath W (1999) Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie 30(2-3): 97-110.
[16] Reviewed in Boecking, O & M Spivak (1999) Behavioural defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30: 141–158.
[17] https://commons.wikimedia.org/wiki/File:Apis_cerana_queen_2010-04-30-_027.jpg
[18] Le Conte, Y, et al (1989) Attraction of the parasitic mite Varroa to the drone larvae of honey bees by simple aliphatic esters. Science 245: 638-639.
The late Dr. Peter Teal of ARS identified the pheromone that attracted varroa specifically to drone brood (I was fortunate to see his data and discuss this with him prior to his untimely death); unfortunately, his research was never published in a scientific journal. See: Luring varroa mites to their doom. https://agresearchmag.ars.usda.gov/AR/archive/2009/Jul/mites0709.pdf
[19] One of the pioneers in breeding for hygienic behavior and VSH was Dr. Marla Spivak: Spivak, M (1996) Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 27: 245-260.
[20] Aumeier, P & P Rosenkranz (2001) Scent or movement of Varroa destructor mites does not elicit hygienic behaviour by Africanized and Carniolan honey bees. Apidologie 32(3) :253-263.
[21] Martin, C, et al (2002) Potential mechanism for detection by Apis mellifera of the parasitic mite Varroa destructor inside sealed brood cells. Physiological Entomology 27: 175-188. Open access.
[22] Le Conte Y, et al (2015) Varroa destructor changes its cuticular hydrocarbons to mimic new hosts. Biol. Lett. 11: 20150233. Open access.
[23] It initially appeared that the bees detected when the foundress began to reproduce in the cell, but this doesn’t appear to be the case, although it’s possible that bees might be able to detect the odor of a pupal wound, mite feces, or mite mating pheromones.
Harbo, JR & JW Harris (2005) Suppressed mite reproduction explained by the behaviour of adult bees. Journal of Apicultural Research 44(1): 21–23.
[24] Nazzi, F, et al (2004) A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 35: 65–70. Nazzi was hardly the first to suggest this—it’s also mentioned by Rath and others.
[25] Schöning, C, et al (2012) Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. The Journal of Experimental Biology 215: 264-271.
[26] Mondet, F, et al (2016) Specific cues associated with honey bee social defence against Varroa destructor infested brood. Nature Scientific Reports 6:25444 DOI: 10.1038/srep25444. Open access.
[27] The discovery process of the differences between “normal” hygienic behavior (HYG), SMR, and VSH has been fascinating to follow.
Ibrahim, A & M Spivak (2006) The relationship between hygienic behavior and suppression of mite reproduction as honey bee (Apis mellifera) mechanisms of resistance to Varroa destructor. Apidologie 37: 31–40.
After the above paper, USDA dropped the term “SMR” and started using VSH. And although selecting for HYG in a mite-resistance breeding program does not appear to be as effective as selecting for VSH, it does appear to offer some benefit:
Al Toufailia, HM, et al (2014) Towards integrated control of varroa: effect of variation in hygienic behaviour among honey bee colonies on mite population increase and deformed wing virus incidence. Journal of Apicultural Research 53(5): 555-562.
As far as I can tell, the jury’s still out on the trait of SMR (suppression of mite reproduction)–which is also biologically plausible.
[28] The pathogens, not unexpectedly, have evolved to secrete proteins to suppress such apoptosis. This may be why infection by nosema (which suppresses apoptosis of the intestinal cells) may open the door for bees to become infected by Black Queen Cell Virus.
[29] “Social apoptosis” appears to be a relatively new term, with few hits in a Google search. It appears to have first been used in scientific literature (and in regard to honey bees) by:
Rueppell, O, et al (2004) From Genes to Societies. Sci. Aging Knowl. Environ. 2004(5): 4.
[30] Page, P, et al (2016) Social apoptosis in honey bee superorganisms. Nature Scientific Reports 6:27210 Open access at: http://www.nature.com/articles/srep27210 I feel that this may be one of the most important discoveries in our fight against varroa.
[31] Zheguang Lin, Yao Qin, Paul Page, Shuai Wang, Li Li, Zhengsheng Wen, Fuliang Hu, Peter Neumann, Huoqing Zheng, and Vincent Dietemann (2018) Reproduction of parasitic mites Varroa destructor in original and new honeybee hosts. Ecology and Evolution DOI: 10.1002/ece3.3802
[32] We may be seeing varroa evolve towards a preference for worker brood in Apis mellifera. I’m simply not seeing many mites in my drone brood trap frames. And a recent study from South Africa suggests that the mites there are shifting their preference away from drone brood to worker brood:
Strauss, U, et al (2016) Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite Varroa destructor. Parasitology 143: 374–387.
[33] Hänel, H & F Ruttner (1985) The origin of the pore in the drone cell capping of Apis cerana Fabr. Apidologie16(2): 157-164.
[34] It appears to actually be a bit more complicated, with variation between races of Apis cerana. See:
Boecking, O (1999) Sealing up and non-removal of diseased and Varroa jacobsoni infested drone brood cells is part of the hygienic behaviour in Apis cerana. Journal ofApicultural Research, 38(3-4):159-168,
[35] I’m fully aware that the evolutionary response by varroa would be to evolve chemicals to suppress the apoptosis response. And that once varroa pressure was off, that natural selection would back off on maintaining extreme pupal sensitivity. But I don’t see that either of these facts are reasons not to pursue this line of breeding.
First published in: American Bee Journal, May 2017
The Varroa Problem: Part 7
Walking the Walk
Randy Oliver
ScientificBeekeeping.com
First published in ABJ May 2017
I’m not one to tell any beekeeper what they “should” be doing—it’s up to nature, the market, personal preference, and history to determine what works. In my last two articles, I’ve discussed ways to go about breeding for mite resistance. Now it’s time for me to walk the walk.
Although I hardly qualify as a queen producer, I’ve bred all the queens for my own operation for decades (I currently mate out around 2500 queens a season). And although my selective breeding program would fully qualify as being half-assed, over the years I’ve found it to be surprisingly easy to breed for color, gentleness, productivity, and resistance to AFB, chalkbrood, and tracheal mite.
After my successes at breeding what I at the time considered to be “superbees,” I wasn’t concerned about the arrival of varroa in my operation (1993), figuring that I’d soon be showing off another notch in my belt. Boy was I wrong—varroa wiped me out, and although I was able to give up dependence upon synthetic miticides some 16 years ago, I still depend upon treatments to keep my bees alive.
This is not for lack of effort to breed a better bee. I’ve got nothing to hide as I walk the walk myself in attempting to breed mite resistance into my stock, and perhaps by relating what I’ve done, I might save others from repeating my mistakes, as well as sharing what I’m trying now, and, more importantly, the biological reasons for doing so.
Frankenbees
I first attempted to breed mite resistance based upon wishful thinking rather than biology. I bought all kinds of (often expensive) breeder queens from others who claimed mite resistance, with the grand vision that I’d just put them all in the same yard, let the drones mix up their genes, and abracadabra—pure magic would create The Perfect Bee in my very own yard!
In retrospect, it’s embarrassing that I could have been so naïve. That was like dreaming that I could disassemble a Ferrari, a Porsche, and a Ford, and then randomly reassemble their parts to create the ultimate racing machine. What I got instead was a bunch of worthless Frankenbees. Look, each of those cars was the result of many years of evolutionary trial and error, culminating in vehicles with perfectly-tuned interacting systems. Change one part randomly, and you wind up with junk. It’s generally the same with bees.
People often misunderstand the concept of “hybrid vigor,” thinking that any cross will be better than either parental type. Hybrid vigor (heterosis) may indeed occur when one crosses two artificially inbred strains of domesticated plants or animals (thus returning closer to the wild type), but crossing two wild types more often results in less fit hybrids. This is due to messing up the fine-tuning achieved by generations of natural selection acting upon the genes and epigenetic regulation so that they work well together as a system [[1]].
Allow me to give a simple example. Suppose that there are two regionally-adapted breeding populations of bees, different enough to be classified as separate races—each specialized for optimal fitness in their respective environments (Fig. 1).
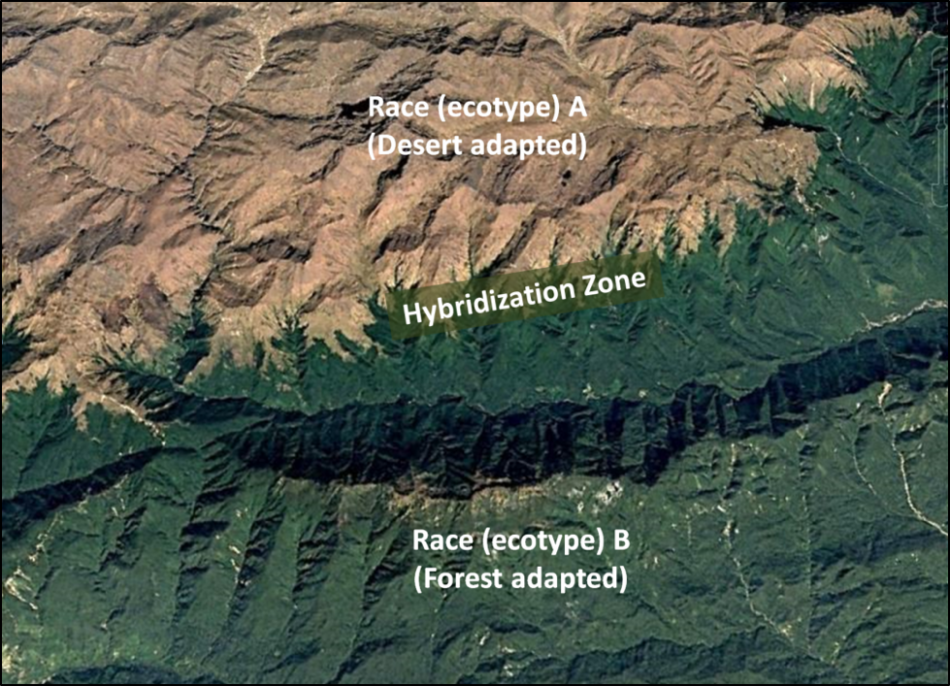
Figure 1. Two separated and regionally-specialized breeding populations (ecotypes) of bees may each be well adapted for their particular environments. But since all races of Apis mellifera are able to interbreed, a continuum of hybridization inevitably occurs between what taxonomists arbitrarily classify as separate “races” [[2]].
In between the ranges of the two races would be a “tension zone” of hybrids, with some degree of gene flow between the races [[3]],) but with the parental populations nonetheless retaining their genetic integrity. If any of those hybrids exhibited greater fitness in either of the parental races’ environments, the hybrid would quickly outcompete and displace the parental type, or it might develop into a race of its own. My point is that we generally don’t see bee races being rapidly replaced by hybrids resulting from human introductions, strongly suggesting that hybrids typically exhibit a lesser degree of “fitness” in that environment than does the naturally-evolved resident ecotype [[4]].
An exception and example: the hybrid Africanized bee clearly exhibited greater fitness than did the preexisting (but artificially introduced, and poorly adapted) European races in South and Central American tropical and subtropical regions. However, note that despite its rapid expansion, the hybrid has not displaced European types above about 30° latitude [[5]], suggesting that the Europeans are better fitted to those ecotypes (refer back to the ecoregion map in my previous article). Note also that the slow but steady flow of alleles and mutations between bee races allows for better evolutionary adaptation, even if you don’t notice a difference in how the bees look or behave.
This does not mean that a true-breeding hybrid miracle couldn’t occur in your breeding program, but the odds of it happening in the short term are not in your favor. Evolutionarily, time is not on the bee breeder’s side, since we typically only have one generation per year to select from, and it may take quite a few generations for a multi-hybrid breeding population to work out the bugs.
My Return to Basic Breeding
In my case, I decided to return to my old ways, and to work with what I already had—locally-adapted stock selected for characteristics that I found desirable. To that, I brought in the occasional instrumentally-inseminated VSH, Russian, and a few other promising breeder queens to produce drone mother colonies.
Practical application: by adding some of those drone mothers to my mating yards, I’d “offer” the new alleles (and genetic and epigenetic combinations) to my own breeding population without necessarily messing up the fine tuning that population already had going. If the new combinations proved to be adaptive, they’d make it into future generations; if not, they’d be bred out, since I wouldn’t select queens from second-rate colonies as breeders.
Then what I’ve done for the past 15 years is to mark potential breeders throughout the season, and to make my final selections each spring after almond bloom. By that time, the colonies had proven their mettle by making honey over the summer, then wintering successfully, and then building up early for almonds (all traits that make me money). I’d only choose the strongest colonies in March (having bees wall to wall), having put on honey in the almonds (as evidenced by weight gain and white wax), and exhibiting gentleness and solid brood patterns. Lastly, I’d perform an alcohol wash to see how much varroa had built up since the fall treatment. There was always enough colony-to-colony variation in mite counts to lead me to believe that this selection process was adequate, and over the years our cutoff for allowable mite counts for our breeders dropped from 4, to 3, to 2, to 1, to mostly zeroes. I’d then choose a minimum of 25 queens each year to use as breeders, in order to maintain some degree of genetic diversity (this may have been a well-intentioned mistake).
Practical application and reality check: although I could see what appeared to be progress towards mite resistance, when I then ran those breeders without treatment for their second season, nearly every one would allow the damn mites to build up to levels requiring treatment, year after frustrating year. That’s why I decided to step up my game, and go for the more formal selection process that I described earlier in this series.
It’s all about the team
Keep in mind that the queen herself can’t directly contribute that much to her colony’s performance, other than by laying a lot of eggs and producing the pheromones necessary for good colony morale. It’s up to her daughters to do the work of rearing that brood, foraging and honey processing, staying calm on the combs, and resisting mites.
A good baseball team consists of players each with specialties—you need a pitcher, a catcher, outfielders who can catch and throw, batters who can hit home runs (or be good runners if they can’t). A team of only batters who couldn’t throw or catch wouldn’t go far. It’s the same with bees. A queen mates with a diversity of drones, each of which then fathers a patriline of sister daughters. A queen that mated with twenty drones will head a colony that consists of twenty patrilines of workers—each patriline with different traits (analogous to each of the players on a baseball team). If the traits of all the players complement each other, then you’ve got a winning team or colony.
The beauty of the honey bee mating system is that in a natural situation, each queen is most likely to mate with the best drones from a cross section of all the best colonies in the vicinity. It seems to me that the honey bee reproductive system has evolved for each breeding population to be able to recover from decimation events, such as forest fire, drought, a severe winter, or the invasion of a new pathogen or predator. The few surviving colonies, due to the genetic diversity held in the spermatheca of the queens, allows for the recovery of much of that diversity as that population recovers, provided that those surviving colonies manage to swarm a number of times before the original queen dies (hence the high swarming rate of wild-type colonies).
Honey bees do everything in their power to avoid inbreeding. Their system of sex determination prevents incestuous breeding, since the female offspring of such matings are non-viable (they become diploid drones, which are generally removed by the nurses). Thus, when we attempt to bottleneck a bee population by selective breeding, we are forced to work against bee behavior. As I’ve stated before, unless we use instrumental insemination, the best that we can do is to breed a deme—a subpopulation of related bees, but with a requisite amount of genetic diversity to maintain good brood viability.
Practical application: unlike as with lab mice or livestock, it’s impossible to maintain a clonal (genetically identical) population of bees (since there would be only a single sex allele). Page and Laidlaw [[6]] calculated that the minimum number of reproducing breeders required for a sustainable “closed” breeding population is in the order of 35-50 each year.
Honey bees like to mix it up, and then filter
So let’s return to the genetic “magic” that might happen in your breeding program. Nature is generally pretty conservative genetically—we humans have roughly 44% of our genes in common with the honey bee [[7]]. But since the background mutation rate of genes is rather low, most species adopted sexual reproduction in order to allow for new combinations of heritable genetics in each generation, in order to allow for evolutionary adaptation to changing environments.
Honey bees take this “shuffling up” of genes to the extreme—a queen may mate with as many as 40 drones, meaning that any of her daughter queens will be likely to have been sired by a different father. Let’s say that you’ve got the dream colony—the success of that colony was due to the teamwork of say 20 different patrilines of daughters working as a team.
Practical application: you graft a bunch of daughters “from that dream queen.” Each of those daughters gets only half of her genes from the mother, and the other half from only one of the drones that the mother queen had mated with (so each virgin may carry only slightly more than 1/2 of the total alleles that contributed to the performance of the originally colony (any of which may have been critical for the success of the “team”).
You then mate that virgin out to a different population of drones. The workers of the resulting colony could now carry as little as 1/4th of the allelic genome of the original dream colony, and no telling whether the 3/4ths of the genes of the worker force consisting of those patrilines will work well together. And it’s the same for every daughter that you raise from that original breeder queen—each daughter colony will be genetically different. It’s little wonder why it is so difficult to replicate a “dream colony.”
Not only that, but in the process of meiosis that creates germ cells (eggs), a phenomenon called recombination occurs. When a queen splits her paired chromosomes in order to create haploid egg cells, some of the genes in each DNA strand (one coming from her father, one from her mother) swap places, resulting in novel combinations of alleles and epigenetics. Honey bees do this at a much greater rate than most other species—about 15 times as frequently as in humans [[8]].
OK, so it’s pretty obvious that honey bees really like to mix up their genes each generation, which allows for rapid adaptation to changing environments, but what about all the new combinations that don’t work well together? How do they purge deleterious mutations and combinations of alleles? Well, they’ve got a trick up their sleeve—a filtering/purifying mechanism.
It’s likely that every beekeeper reading these words has at least one genetic defect—some of you may indeed suffer from what we call “hereditary diseases” or “genetic disorders”. Serious genetic defects are often “masked” by the presence of a “good” copy of that gene inherited from your other parent [[9]]. Drone bees don’t have that luxury—since drones are haploid (only one copy of each gene) their genetics need to work perfectly if they are to survive and successfully mate with a queen.
Practical application: bees mix up their genes each generation, and then filter the new combinations through the drones (and virgin queens) to weed out those that don’t work. This out mating and mixing of genes plays hell on the queen breeder who purchases an instrumentally-inseminated queen, and then attempts to lock those genes into his breeding population. Unfortunately, this has left a bad taste in the mouths of many who tried to propagate mite-resistant stock.
I’ve been lucky to have seen a few colonies over the years that could hold varroa in check (mostly with VSH heritage). But I’ve never been diligent enough to lock that ability in past the second generation, and in each case watched that desirable trait disappear. But the fault was clearly mine, and I realized that it was time to step up my game and get serious about selective breeding.
The opportunity to test my mettle came two summers ago, when I needed to identify 70 hives with high mite counts for a late-summer test of miticides (you need high starting counts to obtain good efficacy calcs). We wound up needing to mite wash over 200 hives in order to find the required number of high-mite colonies. What caught our attention was one colony that had a mite count of zero—10 months after its last treatment. We tracked her for the rest of the season, and into the next spring, and never washed a single mite. She earned the nickname Queen Zero.
Queen zero
I had no idea how Queen Zero’s colony managed to keep the mite level so low, and didn’t want to disturb her to investigate, since for my breeding purposes it didn’t really matter. She was a large, healthy queen, with a nice brood pattern, and her colony was gentle and productive. The question was, was the apparent mite resistance of her colony heritable?
The only way to find out was to rear a bunch of daughters from her. So I grafted about 500 queen cells from her larvae (Fig. 2), and successfully mated out about 400 daughters in my various yards.

Figure 2. Ian and I grafting daughters of Queen Zero in a dark room with headlamps (I brightened the photo so that you could see us). I really don’t know why more beekeepers don’t raise their own queens—it’s one of the most gratifying and cost effective things that we do.
Come July, it was time to start hitting the mites with a knockback treatment. We were crazy busy at that time of season, so I asked Eric and Ian to perform mite washes in each yard of Queen Zero’s daughters until they had identified at least two hives with mite counts not over 1, and then not to apply mite treatments to those hives. This typically took them washing about a half dozen hives (mite counts averaged around 5 for most hives at that time of year). In retrospect, I wish that we’d sampled every one of the 400 daughter hives, in order to have identified even more potential breeders. We then tracked those hives for the rest of the season, and marked any that still had mite counts of 1 or less in November (untreated hives in my area would typically have mite counts at that time of season of 30 or more, or be dead).
Earlier in the season, I’d moved the Queen Zero hive home, and placed her in a test yard chock full of colonies with mite counts averaging around 50-60. There was so much mite drift that Queen Zero’s count went up to 14, so I gave her hive a light oxalic dribble, and finally treated all the surrounding test hives. Her count eventually, and spontaneously, went down to 4 by fall.
Practical application: as noted by Dr. Tom Rinderer during his evaluation of Russian stock for mite resistance [[10]], mingling of mite-resistant hives in with infested hives in late summer can triple the mite count of the resistant bees due to the drifting of mites. Keep this in mind when evaluating your potential breeders—place them all in the same yard.
So, how did our selected Queen Zero daughters fare? We mite washed them all this week—counts ranged from 1 to 10, and Queen Zero was up to 7. I was disappointed by the higher counts (hoping for more zeroes), but none of the colonies had been treated in 11 months, and Queen Zero for nearly 3 years. My mistake of not identifying more potential breeders in July now comes back to bite me—we’ve only got a handful of breeders with really low mite counts—my quandary now is to decide how few queens I’m willing to graft from this season (my fear is too much genetic bottlenecking, and loss of sex alleles).
Practical application: those of you who live in the North, where your colonies get an extended brood break in the winter, have no idea how much more difficult mite management is in a subtropical region. Your alcohol wash counts may increase by perhaps 15x over the course of the season, whereas ours might increase by 50x, with colonies crashing in September (alcohol washes reflect roughly a third of the actual buildup of the mite population). Take home message—it takes a much greater degree of mite resistance for a colony to hold its own in areas not enjoying the benefit of an extended winter brood break.
Recapturing the Magic
I was now convinced that Queen Zero’s low mite counts were not merely a fluke, but an actual heritable trait. Apparently that trait was mostly (if not completely) due to her genetics (as opposed to the genes of the drones with which she had mated), since a good percentage of her crossbred daughters appeared to have inherited it. Her genes had come half from her mother and half from a single drone. But any of her daughters would carry only half the genes of that magical combination. My challenge would be to attempt to recapture that original genetic combination (including the genes of the drones with which Queen Zero had mated) that had proved so successful against the mite.
So here’s the problem. Instrumental insemination would be of little help in recovering the genes of the drones that sired Queen Zero’s workers [[11]]. The only way to recover those genetics would be from her daughters. Look, I make no claim of being any sort of geneticist or super queen breeder, so let me share my thinking out loud…O.K., I just deleted several paragraphs of explanation, realizing that a picture would be worth at least a thousand words (Fig. 3):
Figure 3. A pedigree schematic of the expected result of some serious inbreeding—crossing her daughters back to the drones of sister colonies started last season. I could recapture a lot of the genetics of Queen Zero herself (as indicated by red and orange), but at the expense of poor brood patterns—on average, at least an eighth of the worker eggs laid by those queens would become diploid drones due to having identical sex alleles.
OK, so what if I eased up a bit on the inbreeding? In Figure 4 I show what would happen if instead of grafting daughters from Queen Zero, I grafted granddaughters, and then mated them out as above.

Figure 4. By mating F2 virgins (Queen Zero’s granddaughters) to drones from a number of sister/cousin colonies, I can still recapture a good deal of the genetics of the original Queen Zero colony, but at less expense (an expected minimum of 6% non-viable brood). I’m intentionally ignoring any problem with sex alleles from the blue or white drones (assuming adequate diversity), but in reality there would likely be some additional non-viability due to duplicate alleles.
Luckily, I now have about 400 marked hives to play with, all headed by daughters of Queen Zero, as well as several hundred unrelated colonies (which likely carry the blue and white genes indicated above). So I now have four main options for breeding, all of which I plan to use:
- I’ll graft some daughters from Queen Zero, and mate them in yards stocked with daughter colonies from last year. This will result in highly-inbred colonies as shown in Fig. 3, but may provide some really good breeding stock for next season.
- I’ll graft virgins from mite-resistant Queen Zero daughter colonies, and mate them as above, to obtain the results shown in Fig. 4).
- I’ll outbreed some Queen Zero daughters and granddaughters by mating them in yards of unrelated drones.
- And I’ll sorta “top cross” some unrelated virgins (from colonies with apparent mite resistance) by mating them in yards filled with drones from F1 daughters of Queen Zero (the drones will be her grandsons). This top crossing is described by Page, Laidlaw, and Ericson [[12]]:
In honeybee breeding, this is equivalent to selecting a single breeder queen (top-cross parent) to contribute all the drones (or a significant proportion) needed to inseminate the virgin queens of a closed population…This is a valuable method for rapidly increasing the frequency of desirable characteristics within breeding populations.
Practical application: let’s say that you wanted to introduce VSH genetics to your own breeding population, but wanted to maintain the traits that you like about your own stock. You could purchase some purebred instrumentally-inseminated VSH breeders. All the drones produced by those breeders would carry VSH genes, and could be used to top cross your existing lines of queens, and work the highly-desirable VSH trait into your queenlines.
I’m going to use the above strategies to severely bottleneck the genetics of my breeding population this generation, and shift it predominately to those of the Queen Zero colony. What, you say, won’t that result in too much inbreeding? After developing the schematics above, I have reason to believe that it won’t be too serious, and will attempt to subsequently rectify that problem by selecting only breeders with solid brood patterns.
Practical application: one method to maintain a diversity of sex alleles in a breeding population is to select only breeders having solid brood patterns—which indicates that the queen carries a diversity of sex alleles other than her own.
A closed population
Ideally, one would practice selective breeding in a “closed population,” which would require an island or a completely isolated mating yard. But I’m happy to have some introgression of “outside” alleles, either from locally-adapted survivor ferals, or from introduced drone mothers (such as VSH breeders). However, the genetics of my bees dominate the landscape, since I provide the majority of nucs and queen cells to the local beekeeping community. I’m also able to completely flood groups of mating yards with my own drones, by making up my nucs from chosen colonies chock full of drones after returning from almonds.
Practical application: unless you are really large scale, there’s slim chance of narrowing your pool of sex alleles too much, unless you only graft off a single queen line for more than one year in a row.
Walking the Walk
I’ll be the first to admit that I’d be a poor choice as a good example of a professional beekeeper. My beekeeping suffers from all my distractions—my experiments and field trials, my literature research, and the number of hours that I spend at writing and speaking engagements. But I do what I most love, and am able to squeak out a living at it.
We beekeepers need to move beyond varroa, and turn varroa management over to our bees. Breeding for mite resistance is indisputably the long term solution to The Varroa Problem. My half-assed breeding efforts to date have shown some success, but I’m as yet unable to dispense with mite management. It’s clearly time to step up my game.
There are others successfully keeping bees without needing mite treatments, and I want to be there too (but without going through the pain and cost of the Bond method). Perhaps by sharing my trials and tribulations in attempting to breed for mite resistance, I can further our collective progress.
Notes and citations
[1] Just to refresh terms—the bees all carry the same genes, but those genes may occur in many slightly different forms called alleles. It is allelic variation, as well as the genetic and epigenetic regulation of the expression of those genes, that causes differences in the phenotypes (the morphology, physiology, and behavior of the individual bee and its colony).
[2] The concept of species, subspecies, and divergent selection is well discussed by James Mallet in:
Mallet, J (2008) Hybridization, ecological races and the nature of species: empirical evidence for the ease of speciation. Philos Trans R Soc Lond B Biol Sci. 363(1506): 2971–2986. Open access.
Mallet (as did Darwin) points out that the concept of “species” is merely a convention useful in communication among biologists. What we call species and what we call populations actually blend imperceptibly into one another, with no clear natural dividing line.
[3] Well reviewed by Byatt MA, et al (2015) The genetic consequences of the anthropogenic movement of social bees. Insectes Sociaux 63:15–24.
[4] Coroian CO, et al (2014) Climate rather than geography separates two European honeybee subspecies. Mol Ecol 23:2353–2361.
[5] Nor 30° south latitude in South America.
[6] Page, RE Jr & HH Laidlaw Jr (1985) Closed population honeybee breeding. Bee World, 66(2): 63-72.
[7] http://ngm.nationalgeographic.com/2013/07/125-explore/shared-genes
[8] Wilfert, L, et al (2007) Variation in genomic recombination rates among animal taxa and the case of social insects. Heredity 98: 189–197.
[9] I’m really simplifying things here. Sometimes, as in the textbook case of sickle cell anemia, a recessive deleterious gene can confer fitness benefits.
[10] Rinderer, TE, L de Guzman, C Harper (2004) The effects of co-mingled Russian and Italian honey bee stocks and sunny or shaded apiaries on Varroa mite infestation level, worker bee population and honey production. Am. Bee J. 144: 481–485.
[11] Since those genes could only be directly recovered from the spermatozoa in her spermatheca.
[12] Page, RE Jr., HH Laidlaw Jr. & EH Erickson Jr. (1985) Closed population honeybee breeding 4. The distribution of sex alleles with top crossing. Journal of Apicultural Research 24(1): 38-42.
Open the link below to view the annotated pictorial presentation.
2017 KISS Breeding
and if you want to see us doing smokin’ hot mite washin’ in real time, Rachel surprised me by figuring out how to prop up her cell phone to take a video of us washing a yard–to see the 36-second video, click here :
Video
First published in: American Bee Journal, July 2017
Extended-Release Oxalic Acid Progress Report
Part 1
Randy Oliver
ScientificBeekeeping.com
First published in ABJ July 2017
In January I wrote about an exciting extended-release application method for oxalic acid [[1]]. I’m currently collaborating with the USDA Agricultural Research Service and the EPA to get this application method added to the current label for oxalic acid. In the interest of full transparency (and to show how I’ve been putting your donations to use), I’m submitting this progress report.
Introduction
Beekeepers worldwide are caught between a mite that has proven able to rapidly develop resistance to synthetic miticides, and the slow pace of our development and adoption of mite-resistant bee stock (the eventual solution to varroa). During the interim, by choice or lack of alternatives, beekeepers are shifting to the use of “natural” treatments. Oxalic acid (OA) is one of the most promising of those treatments [[2]], but its efficacy is limited unless applied during a broodless period. An extended-application method would free it from that limitation.
Practical application: although the natural treatments can certainly be effective [[3]], let us not forget that we should still consider them as stopgap measures to be used while we move forward on breeding for mite-resistant bee stocks.
The problem with oxalic acid is that either of the currently approved application methods (dribble or vaporization) kills mites for only about three days. So unless applied during a broodless period, or repeated at 4-day intervals, oxalic is not very effective over much of the season (due to a proportion of the mites being protected in the sealed brood). By creating an extended-release formulation, we may have found a treatment that is “natural,” suitable for organic approval, safe for the applicator, non-contaminating of honey, showing no noticeable adverse effects upon the colony, as well as being inexpensive and highly efficacious at reducing mite populations (I saved the best two for last).
Practical application: oxalic clearly kills mites, but its mode of action is not yet clear. It may have the additional effect of messing with their olfaction or tactile sense. This is purely speculative, but keep in mind that even a small reduction in the mites’ reproductive success can turn it from being an adversary into a mere annoyance.
Preliminary research
Oxalic acid efficacy when dribbled is improved by the addition of humectants such as sugar or glycerin. The technicians of the National Institute of Agricultural Technology in Argentina developed an oxalic acid/glycerin (OA/gly) product using cardboard strips, applied for patent in 2014, and brought a product to market called Aluen CAP. Although similar OA/gly cardboard strips worked in my test hives, the strips were simply too tedious to insert and later remove.
Experimenting with other cellulose matrices, I found the commercial beekeeper standby–the blue shop towel–laid across the top bars between the brood chambers to fit the bill, since it didn’t need to be inserted between the frames, and the bees themselves would remove the spent treatment. The problem was, the bees avoided glycerin-saturated cellulose; it was only when I reduced the degree of glycerin saturation of the towels that the bees would chew the towels, thus exposing themselves to the oxalic acid, and distributing it throughout the colony as they carried the glycerin-rich cellulose strands down through the combs. This took the typical colony roughly a month and a half to do—perfect for effective treatment, with minimal treatment residue left afterward in the hive.
Practical application: OA/gly has the potential to be of huge benefit to our industry—we just need to get it legal to use. It is currently just as illegal to use oxalic towels in a hive as it is to use Taktic or other unapproved chemicals.
Surprisingly, the process by which oxalic acid was federally registered occurred largely behind the scenes. I’m writing this article in part to bring more transparency to this important process.
Registration for use
Our industry is crying for effective varroacides. EPA is well aware of the desperation of beekeepers due to the failure of the previously-registered miticides (state lead agencies have been applying for an unprecedented number of Section 18 emergency registrations), and has been forced to turn a blind eye to our industry’s “off label” use of unapproved chemicals (including Taktic and oxalic acid).
Back in 2006, Dr. Marion Ellis and his student Nick Aliano confirmed European studies as to oxalic acid’s effectiveness as a varroacide [[4]], yet despite his best efforts, it took years to get it registered for use in the U.S.
The EPA is not responsible for bringing products to market—that’s up to the registrant. And the EPA doesn’t do the testing involved for registration–potential registrants need to submit the supportive data (generally generated by independent testing labs) to support the safety and efficacy of a product that they wish to register for sale, and to pay for such testing and subsequent review by the Agency. This process can be quite costly.
The problem with oxalic acid registration was that there was no incentive for a private chemical company to invest the substantial sum required to get it registered for sale to beekeepers—since it couldn’t be patented and was already readily and cheaply available at any hardware store, there would be little expected return on the investment.
As it became more and more evident that oxalic acid was an effective and safe mite treatment already registered for use in a number of other countries, pressure built for it to be “made legal” in the U.S. Then in 2014 President Obama highlighted specific instructions for EPA to review registration applications for new products targeting pests harmful to pollinators. Our Agricultural Research Service (ARS) stepped up to the plate and applied as the registrant for oxalic acid, and under an inter-agency cooperation agreement with EPA, was able to reduce the cost of registration.
EPA then expedited the registration process. This was done via a “work share” agreement with Canada’s Pest Management Regulatory Agency, which provided the necessary data reviews (Canada had registered oxalic in 2010). EPA issued a registration decision in spring of 2015 [[5]]. A distributor was sought for ARS to license for sale of oxalic acid as a varroa treatment; Brushy Mountain Bee Farm volunteered to play the role (not surprisingly, the amount of “approved” OA that they actually sell suggests that many beekeepers are using off-the-shelf unapproved oxalic acid).
Practical application: The current label allows only for trickle, vaporizer, or spray applications, and states, “Use only in late fall or early spring when little or no brood is present” and “Do not use when honey supers are in place to prevent contamination of marketable honey.” These last two restrictions prevent us from using oxalic acid for mite control when we most need it—in late summer before we’ve pulled the last of our honey, when mite counts are exploding.
So I approached EPA and ARS to see whether we could get the extended-release OA/gly application method approved. This would likely have been relatively easy, but I also said that we needed to get it approved for use when honey supers were on. This complicated things, since now we needed to deal with whether there would be residues in the honey, despite the fact that there is abundant data from Europe and Argentina that residues wouldn’t be expected; EPA needs to go by the book.
Although the EPA representatives in Risk Assessment and Registration, as well as ARS, have been extremely helpful, they’ve made it clear that they cannot shortcut the registration process, and must treat us like any other private registrant. Jay Evans, Research Leader of the USDA-ARS Bee Research Lab in Beltsville (who wasn’t involved in the original registration process) and I are on a learning curve for how to get a miticide through the registration process—unlike the chemical companies, we don’t have an established staff familiar with the process of the details involved in pesticide registration [[6]].
Our situation: the shop towel application method has to date been only cursorily tested—with scant hard supportive data. So we’re trying to kill several birds with one stone in our trials:
- To determine the efficacy of different doses of OA/gly at reducing mite populations,
- To look for adverse effects on the colony due to treatment, and
- To measuring whether treatment increases the amount of OA that naturally occurs in honey.
As you might imagine, we’ve gone through draft after draft of experimental protocols, and at this writing, are about to begin the first trials.
We are currently planning to run concurrent trials in low-humidity California (me) and high-humidity Georgia (with collaborators Jennifer Berry from University of Georgia and Geoffrey Williams from Auburn University), at three different doses of OA, with solvent (glycerin-only towels) and sham (dry paper towels) control groups. I have received a Pesticide Research Authorization from California Department of Pesticide Registration to perform such testing.
Practical experience: it is indeed interesting to be required to jump through the same EPA hoops as do the pesticide companies when they register a new product. And although it seems to me that the OA/gly application method should be a slam-dunk, EPA has made it clear that despite guidance to expedite the registration of safe varroa-control products, that they aren’t about to shortcut their requirements for good supportive data. I thank the EPA personnel with whom I’m communicating for being helpful, and greatly respect them for their diligence at doing their job properly.
Distributors
The status of oxalic acid is that the only entity currently authorized to sell it as a varroa control product is Brushy Mountain Bee Farm. The small quantities offered by Brushy won’t fill the needs of commercial operators (although they will special order 55-lb bags for those who request them). If demand grows (should this application method be approved), Brushy or other distributors will make “legal” bulk oxalic available.
Chemical terminology: Although anhydrous oxalic acid is available as a chemical reagent, with regard to this article, every mention of oxalic acid refers to common oxalic acid dihydrate (the acid bonded to two water molecules) commercially sold as a cleaning and bleaching agent.
The preparation of OA/gly towels requires some mixing and pouring of a heated acidic solution—something that the EPA risk assessors aren’t crazy about. But since the towels are so inexpensive to make (they cost me about 25¢ ea to prepare), I’m hoping that a manufacturer will follow up and offer a preformulated product to the market at a reasonable cost.
OA/Gly is still not an approved use
At this point I want to make perfectly clear that this article is an informational progress report only, in the interest providing full transparency of my approved experimentation and involvement in the registration process.
Disclaimer and legality: I do not encourage nor condone the illegal application of any pesticide, including mite treatments to bee hives. The information in this article is solely to report on my progress (working in conjunction with ARS) towards getting this application method approved by EPA, and is not intended to promote illegal use in any way. I neither approve of, nor encourage, applications not sanctioned by local authorities [[7]]. If you wish to experiment yourself, please check with your State Lead Agency for pesticide regulation to see whether you need to obtain an experimental use permit [[8]].
Progress on the application method
There has been considerable interest worldwide on OA/gly application, and my shop towel article has already been translated and reprinted in other countries [[9]]. Since publication, I’ve done quite a bit of further experimentation, and have received field feedback from other beekeepers (whom I presume have obtained use permits for experimentation). You can follow our progress at https://scientificbeekeeping.com/oxalic-shop-towel-updates/
Adverse Effects: although I received a couple of reports from beekeepers that OA/gly applications appeared to harm their test hives, neither I nor several large-scale beekeepers have noticed any such adverse effects. One sent me data from 50 hives treated in spring with shop towels compared to 50 treated with Apivar strips—after 46 days there was no statistical difference between buildup or increase in number of frames of brood between the two. I’ve applied towels to colonies midwinter during rain and snow, and to nucs building up in the spring (Fig. 1), and have yet to see a problem.

Figure 1. A frame from a nuc this spring, halfway through treatment, with a half-chewed OA/gly towel pulled to the side. Note the solid brood pattern. To my surprise, these “acidified” hives do not appear to exhibit noticeable adverse effects so far as buildup, brood development, or queen problems.
Dosage and efficacy: Although I obtained good efficacy in late summer with a single towel treatment per double deep hive, I wasn’t impressed by the degree of mite knockback from spring treatment. The amount of OA is limited to 12 g per towel (more further on), so I’m experimenting with stacking two or three towels one atop the other to increase the OA dose per hive.
I have yet to determine the optimal dosage rates for growing colonies in the springtime, for treatment during the honey flow, or for late summer treatment in dry or humid climates. We hope to answer these questions with further testing, and from beekeeper feedback. Luckily, there appears to be room to increase the dose– neither Maggi [[10]], nor I in my trial last summer, observed any adverse effects from applying up to 80 g of OA per hive in cardboard strips—the equivalent to that in a total of 7 shop towels.
Duration of treatment: A typical varroa reproductive cycle takes around 17 days, so a 35-45-day exposure would cover two generations. It’s not yet clear whether a 35-day exposure is optimal, or whether it needs to be for a longer duration. However, since the presence of the towel hampers hive inspection, I’d prefer to increase the dose if necessary, rather than prolonging how long there needs to be a towel in the hive.
The cellulose substrate and saturation: In the first place, I’m not hung up on shop towels—other biodegradable cellulose substrates could serve just as well. What we want is a substrate that is fibrous, so that the bees wind up distributing the OA/gly throughout the hive during the process of removal, and that also stimulates the bees to perform such removal. Beekeepers already know that blue shop towels meet those criteria.
With shop towels, the key issue appears to be the degree of saturation of the towel with glycerin—too “wet” and the bees simply avoid it. But at about 50% saturation, the bees chew and remove the towel with vigor, effecting near complete removal in little over a month. The trick is to get the towels to evenly wick to half saturation, so that the applied dosage in each towel is consistent.
Initially, I pressed half the liquid back out of the towels after they were saturated. But this was a pain, and it was difficult to get the towels to evenly absorb the solution. So I looked for another way to prepare half-saturated towels…
Chemistry, solubility, and saturated solutions: for the registration process, I needed to come up with an exact formulation to test. That involved a goodly amount of kitchen chemistry experimentation—making batch after batch of solutions and towels in order to arrive at an appropriate formulated product that we could test in controlled field trials.
Here are some of the considerations:
- The main limiting factor is the maximum amount of glycerin that can be added to each towel and still result in good chewing and removal by the bees—this appears to be about 13 mL.
- Then there is the matter of the maximum amount of OA dihydrate that can remain in solution in that 13 mL of glycerin at broodnest temperature—this appears to be about 12 g. At this degree of saturation, some oxalic acid will precipitate out (recrystallize) at room temperature–which actually makes the towels a bit easier to handle and apply (they become “stiffer” and less messy).
- Glycerin is hygroscopic and will absorb moisture (water) from the air at high humidity. I used my queen cell incubator (Fig. 2), which is held at broodnest temperature and humidity, to test how prepared towels would react to those conditions—the towels rehydrate and the oxalic crystals redissolve.
- Anhydrous laboratory OA will react with glycerin to form oxalate esters, especially at high temperatures. Less clear is to what degree the commonly sold OA dihydrate (wood bleach) will do so, or whether the presence of added water inhibits or reverses such esterification [[11]]. Bottom line—low heat and added water are likely good.
- The viscous glycerin solution does not wick well through a roll of towels. A hot solution wicks better. But a cold roll of towels may cause the OA to crystallize out before even saturation of the roll is obtained. Adding water to the solution greatly improves wicking.
- Oxalic acid is relatively insoluble in room-temperature water (13.3 g/100 mL at 68°F), but highly soluble in hot water (168 g/100 mL at 194°F).
- Besides rolled towels, I experimented with interfolded or hand-stacked towels. The rolled towels were the easiest to dispense in the field, as the interfolded or stacked towels were difficult to peel apart.

Figure 2. Testing formulations at broodnest temperature and humidity in my queen cell incubator. An air-dried towel which feels “dry” at room temperature and low humidity, quickly rehydrates and softens under broodnest conditions of 95°F and 65% RH.
OK, after a lot of experimentation, I’ve arrived at a working formula (as of this writing) that is easy and safe to prepare, that wicks quickly and uniformly into a roll of towels, and produces a towel with the right attributes for handling in the field and dispensing of the treatment within the hive:
| My working formula for OA/gly towels |
Per towel* |
Per roll of 55 towels** |
Per half roll*** |
| Oxalic acid dihydrate (99.6% purity) |
12 g |
672 g |
336 g |
| Water |
10 mL |
560 mL |
280 mL |
| Vegetable glycerin (food grade) |
13 mL |
728 mL |
364 mL |
| *For a Scott Shop Towel—I haven’t experimented with other towels that may differ in absorption.
**The per-towel formula x 56 to account for the amount of solution absorbed by the center cardboard roll.
***Cut in half crosswise with a kitchen knife.
|
My preparation of towels for experimentation
THIS IS A PROGRESS REPORT ONLY OF MY LEGAL EXPERIMENTATION; THIS APPLICATION METHOD HAS NOT YET BEEN REGISTERED WITH THE EPA.
Safety
Oxalic acid is commonly used for bleaching decks and cleaning metal, and has a long history of being safely used, with standard precautions. However, it’s easy to forget that you’re still working with a strong acid. Although oxalic acid is not nearly as corrosive as battery acid, it’s still an acid, and any stray drops of the solution remain caustic indefinitely. And in the processes of weighing and mixing, tiny crystals of acid and droplets of solution invariably spill onto work surfaces.
I make a point of wearing safety glasses (in case of splashes) and nitrile or kitchen gloves (the label calls for the wearing of 14 mil gloves). Wearing gloves reminds me not to touch anything, and to clean up any tiny splashes or spills.
Since the OA/gly solution doesn’t cause any sort of immediate burning sensation if it gets on the skin (unless it enters a cut), it’s easy not to notice if I’ve gotten acid on my fingers, and even worse, the glycerin makes the solution stick to absolutely everything. I find that this makes it especially important for me to be conscientious, meticulous, and fastidious, since I sure don’t want to accidentally rub my eye, or get the corrosive solution onto my cell phone or computer, or (heaven forbid) touch my wife’s…er…face with acidified fingertips. Luckily, the solution is easily washed off with warm water, and a solution of baking soda in water neutralizes any residual acid on work surfaces.
I wash my gloves before I pull them off, and then wash my hands. I’ve gotten into the habit of tasting my fingers after I’ve washed them, to double check that I’ve gotten off all the acid, since one’s taste buds are exquisitely sensitive to acids, and even a trace of oxalic acid makes your fingers taste like tart rhubarb pie.
Practical application: Although oxalic acid is a common component of vegetables, and can be considered as being “food grade,” it is nevertheless a strong acid, and in the concentrated form used in OA/gly towels, it packs a lot of acid wallop into every tiny drop. I find that I need to continually remind myself to exercise basic lab safety precautions to protect my skin, and to make sure that I pay attention to the thin film of solution that sticks to my gloves, and to not touch anything else after I’ve touched a prepared towel.
I prepare the solutions in my kitchen (a dedicated work area with a sink would be better), after warning my wife to stay clear. I keep a spoon rest next to the pot, so that I have a dedicated place to set the spoon between stirrings. I do any pouring in the sink, or over a catch tray, and have running water handy so that I can immediately wash off any spills, especially should I get it into my eyes. That said, I find that with the above precautions, preparing OA/gly shop towels can easily be done quickly and safely.
Measuring devices
I use a triple-beam balance to weigh the acid crystals, using a 32-oz plastic yogurt container (after checking the tare weight), which holds enough oxalic to make one batch of towels. I use a 1000 mL plastic graduated cylinder to measure the water and glycerin.
Preparation
I’ve been mixing the solutions in a dedicated stainless steel saucepan (which immediately discolored). I found that it was safest to first place the acid crystals into the pan before adding any liquid, since this greatly reduces the chance of splashing. I then gently pour in hot water (Fig. 3).

Figure 3. In order to minimize the chance of splashes, I first place the oxalic acid crystals into a dedicated stainless steel saucepan, and then gently pour in the measured amount of hot water. It then requires some further heating to fully dissolve the OA in the water.
I then turn on the burner, and gently stir the solution as it heats. I found that the lumps of oxalic acid crystals break apart and dissolve far more easily in hot water than in hot glycerin [[12]]. I continue stirring until the crystals are fully dissolved, and the solution is crystal clear (Fig. 4).

Figure 4. I stir until the solution is crystal clear (the brown color is discoloration of the pan itself, not the solution). I don’t heat to anywhere near boiling. Note the glass spoon rest bowl in which to place the spoon between stirrings (I use a plastic spoon). At this point, I turn off the heat.
I then carefully move the pan to the sink or pouring tray, and gently pour in the measured amount of glycerin, then mix thoroughly, so that each towel will end up with the same amount of active ingredient. Then I prepare a roll of shop towels (Fig. 5).
Figure 5. To save time later fumbling in the field, I lift the corner of the first towel and mark it with tape, since it is tricky to locate the free edge once the towels have been soaked. To date, I’ve experimented only with Scott brand Shop Towels.
If I’m preparing only a few towels, I simply fold them and place them into a large Ziploc bag, into which I can pour the appropriate amount of solution. I place the bag into a bowl, with the edges folded back for easy pouring. For a full roll, I found that a 1/3 x 6” stainless steel steam table insert pan (from any restaurant supply) holds a full roll of shop towels very nicely. I first line the insert pan with a transparent (so that I can watch the absorption process) plastic turkey roasting bag, again folding it back to provide a clear opening.
At this point, I generally pour the still-hot solution over the roll of towels (Fig. 6).

Figure 6. I’m pouring hot OA/glycerin solution evenly over a test roll of towels. After pouring, I lift the bag of towels out of the pan and rotate the towel roll in the bag to allow absorption into any dry areas. Full absorption occurs within about 2 minutes. .
After the solution is fully absorbed, I stroke any wrinkles out of the roll of wet towels (to make for easier unrolling of the towels in the field). I then open the bag (or remove the roll and place it into a plastic tray) in order to allow the excess water to evaporate. This makes the towels less slippery when we later apply them in the field.
Avoiding a hot pour: I also tested to see whether a roll will absorb a cooler solution. The problem to overcome is that as a cooler solution wicks into the roll, the cold layers of towels cool the solution, causing the OA to crystallize out of solution before it reaches the center, thus resulting in uneven distribution.
In this experiment, I allowed the solution to cool ‘til it was warm to the touch (110°F). Then I prewarmed the roll of towels for 30 seconds in a microwave in order to prevent crystallization during absorption.
Caution: microwave heating for over 30 seconds may cause the towels to overheat and start to smell like they are about to catch fire!
Result: The solution wicked into the prewarmed roll very nicely (but more slowly).
Practical application: other than saving time, there is apparently no need to heat the solution, nor the roll of towels, above 110°F at any time during the process of preparation (so long as you’d added the glycerin along with the water). But I haven’t yet had the patience to try this [[13]].
Shelf life
I have some rolls of towels that I made months ago and stored at room temperature, that still peel easily and look (and taste) essentially the same as the day that I made them. That said, I suggest that towels be prepared shortly before use in order to avoid recrystallization of OA at the edges.
Field application
I find the towels difficult to apply by myself, since I don’t want to get the sticky acidic solution on my smoker or hive tool—so I work with a helper. We carry the towels to the field in a plastic bag, and choose one of us to be the designated Towel Applicator [[14]]—he/she wears 14 mil gloves and is the only one who ever touches a towel. The other crew member tips open the hives and smokes the bees. The Towel Applicator carries the roll of formulated towels, and peels individual towels from the roll to apply to the hives (Fig. 7).

Figure 7. Note how if the excess water is allowed to evaporate off, the towels get a bit stiff and easy to handle. With a 2-person crew, it’s a simple matter to tear them off one at a time and apply them to the hives.
We make a point to carry wash water and plastic disposal bags to the yards, and don’t allow the Towel Applicator to touch anything other than the towels themselves. After application, any remaining towels are safely secured, and then the Towel Applicator can wash and remove the gloves.
Practical experience: the towels look so benign, that I must regularly point out to helpers that they can’t touch a towel and then touch something else, without first rinsing their fingers. It would be very easy for the careless helper to acidify their hive tool, smoker, the steering wheel of our truck, or whatever else they next touched! Always keep wash water on hand (a labeled jug of water and baking soda for neutralization is handy).
I’m currently testing by laying a full towel (or stacked towels) over the top bars between two brood chambers, centered over the cluster.
- There may be benefit to using two half towels instead, placed crossways across the frames with a slight space between them, so that the bees have a passageway up and down, but I haven’t yet formally tested.
- The towels do not work well if placed at the top of the hive directly under the cover—the bees don’t tend to chew them unless they have access from both top and bottom. Using a spacer rim (to allow bees top access to the towels) appears to help when applying to singles.
With a single layer of towel, the chewing by the bees is noticeable within a few days (as are fallen mites on top of the towels) (Fig.8).

Figure 8. A typical towel after about 20 days in a moderately-strong hive. Nearly all of the towel will be completely gone in 30-45 days. We see blue towel fibers on the ground in front of the hives, where they will harmlessly biodegrade after the next rain.
A caution: when I need to pull frames from below a towel during treatment, I flip the towel aside with my hive tool. I make a point to not forget that any towel residue is still corrosive, and that I need to rinse my hive tool immediately afterward. It would be better yet to keep a dedicated set of kitchen tongs handy.
Development of resistant mites
I’m often asked the question; won’t varroa develop some degree of resistance to oxalic acid? The biological answer is that one would certainly expect so if oxalic acid were applied over many generations without rotation of treatments. But that does not appear to be the case. In a recent study, Dr. Matías Maggi [[15]] compared the susceptibility to oxalic acid between two Argentinian mite populations:
- One that had been exposed to 64 consecutive treatments of oxalic acid dribble (8 times a year for 8 years) as the sole mite treatment, vs.
- A control population that had never been exposed to beekeeper-applied organic acids (the beekeeper had used coumaphos, flumethrin, or amitraz in a rotation scheme).
He found that the oxalic-exposed mite population didn’t exhibit any sign of resistance—in fact, it surprisingly appeared to actually be more susceptible to oxalic than the oxalic-naïve population. This is good news, since it suggests that whatever the mode of action of oxalic acid is against mites, that it’s not easy for them to develop resistance.
Update 20 May 2017
Research Update on the Oxalic/glycerin application method
We now have two formal field trials underway for registration of OA/glycerin towels as an approved application method. What I want everyone to keep in mind is that this research is in the very preliminary stages. I’m getting (and hearing of) mixed results on efficacy during spring buildup, and clearly see that efficacy is largely dependent upon the removal rate of the towels—unless the bees chew and remove the towels, there is little reduction in the mite population.
I’m also finding that we could use some consistency in terminology, so I’ll use that from a patent application filed in 2005 by Stuart Volby [[16]] for a degradable miticide strip. Stuart used the terms “active ingredient,” “carrier,” and “matrix.” In the case of the oxalic shop towel, the applied formulated towel is the delivery vehicle, with oxalic acid being the active ingredient, glycerin the carrier, and the cellulose shop towel the matrix. The active ingredient is distributed throughout the hive by the action of the bees rubbing against and removing the matrix. Ideally, the entire delivery vehicle is entirely removed from the hive by the bees by the end of the treatment period (thus eliminating the problem with plastic miticide strips, which continue to release active ingredient if not physically removed from the hive by the beekeeper, thus facilitating the evolution of miticide-resistant mites).
I chose the shop towel for my initial trial due to its familiarity to commercial beekeepers, as well as for its absorptive, fibrous, and biodegradable nature. But shop towels may well not be the ideal delivery vehicle—the most oxalic acid that I can get into solution in the amount of glycerin that is not repellent to bees is about 12 grams. In my test hives, when I stacked towels to increase the total dosage, the bees did not chew the towels adequately. So since my last article I’ve changed the protocol, and am formally testing the application of three half-towel strips.
I plan to experiment with other matrices, especially those that can hold more oxalic acid.
Update 29 June 2017
We just finished our midpoint grading of the Calif early-season trial. A heat wave quashed our honey flow, so the colonies did not build up and remove the towels very well in some of the test hives. That said, at 3 weeks into the trial, mite suppression was erratic. I haven’t yet worked the data, but it appears that the towel application method may not be adequate for mite control early in the season.
Acknowledgements
Thank you to all the beekeepers who have donated to ScientificBeekeeping, which allows me to fund this research—thanks for your support! I’d also like to express my appreciation to Dr. Matías Maggi and his dedicated group of collaborators in Argentina for their groundbreaking research. And to retired chemist and beekeeper Dick Cryberg for his help with the chemistry, and those beekeepers who have shared their experimental results with me. And of course to Jay Evans and David Epstein of the USDA ARS, and to the EPA personnel working with us to register this application method for the benefit of U.S. beekeepers.
Notes and Citations
[1] Oliver, R (2017) Beyond Taktic®. ABJ 157(1): 43-50. https://scientificbeekeeping.com/beyond-taktic/
[2] I’ve successfully used it in my commercial operation for over ten years.
[3] I haven’t found need to any synthetic miticides for going on 17 years now.
[4] Aliano, NP, MD Ellis, BD Siegfried (2006) Acute contact toxicity of oxalic acid to Varroa destructor (Acari: Varroidae) and their Apis mellifera (Hymenoptera: Apidae) hosts in laboratory bioassays. J. Econ. Entomol. 99(5): 1579-1582
Aliano, Nicholas P. and Ellis, Marion D. (2009) Oxalic acid: A prospective tool for reducing Varroa mite populations in package bees. Experimental and Applied Acarology 48: 303–309.
[5] https://www.regulations.gov/document?D=EPA-HQ-OPP-2015-0043-0119
[6] Something that I find interesting: EPA calls for data, but leaves it to the registrant to design the experiments. Only after the data is submitted, does EPA decide whether the experiments were properly designed.
A frustrating thing is that our current Administration, apparently in a politically-motivated effort to suppress the public’s access to taxpayer-funded scientific information, has across the board blocked online access to previously accessible supportive documents, including those for EPA’s registration of oxalic acid.
[7] I’m not completely clear as to the legality of individuals using oxalic acid dissolved in glycerin for their own use (not for sale to others). I’m also not clear to what degree the label restricts the allowed timing of treatments, since the label states both, “Use only in late fall or early spring when little or no brood is present, as well as “This product can also be used as a “clean up” Varroa treatment following the application of a different acaricide where Varroa infestations continue to be problematic.”
Not only that, but FIFRA Section 2(ee) (3) allows an exemption for “employing any method of application not prohibited by the labeling unless the labeling specifically states that the product may be applied only by the methods specified on the labeling.” After speaking with an EPA representative, I found that this would likely apply if the OA were purchased from Brushy mountain, with the caveat that application would still be restricted to use only when honey for intended for harvest was not on the hive.
The EPA is concerned that off-the-shelf oxalic might contain contaminants that might get into honey. Oddly, the label for OA as a mite treatment lists it as being only 97% pure—but on Amazon, you can purchase virtually pure (99.6+%) oxalic acid cheaply (I’ve been happy with Wintersun’s 55-lb bags).
Oddly, it is perfectly legal to use oxalic wood bleach to whiten the top bars of your frames, so long as your intent isn’t to use it as a miticide. Although disingenuous, I’ve heard that some beekeepers claim to use it for that very purpose, saying that whitened top bars look so pretty.
The reality is that each state’s Lead Agency has limited funds for enforcement, and may be reasonable. You may wish to speak with your local pesticide enforcement office.
[8] Please do not bother your EPA district office—the EPA Registration department would prefer to handle the process without needless confusion.
[9] U.S. beekeepers may not be aware that there are a lot of very smart beekeepers elsewhere in the world, and some great beekeeping magazines—if you can figure out how to translate them.
[10] Maggi, M, et al (2015) A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 47(4): 596–605.
[11] I’ve read every bit of chemistry that I can find on the subject, and have discussed this at length with retired chemist Dick Cryberg. It is easy to observe that some sort of chemical reaction taking place between OA dihydrate and glycerin once the temperature reaches 160°F (since bubbles are produced). I’d appreciate the help of any chemist who’d like to do lab analysis on this.
[12] There is likely the added benefit that the water shifts the chemical equilibrium against the formation of oxalate esters that occurs if the acid is first dissolved in hot glycerin.
[13] Other experimenters—please let me know if you try this.
[14] We don’t yet have a special hat for the designated Towel Applicator, but we’re working on it.
[15] Maggi, MD, et al (2017) The susceptibility of Varroa destructor against oxalic acid: a study case. Bulletin of Insectology 70 (1): (preprint).
[16] https://www.google.com/patents/US20070059333. Disclosure: I tested some strip formulations for Stuart.




























