An Experiment to Improve Pollen Sub: Part 1
Beekeeper-Funded Research
An Experiment to Improve Pollen Sub: Part 1
Randy Oliver
ScientificBeekeeping.com
First published in ABJ April 2020
In my location, we may not see a drop of rain all summer long, so our colonies become pollen-starved. In order to prepare strong colonies for almond pollination, I used to move them to better forage out of state, but now instead feed pollen sub to build them up before winter. Today’s pollen subs are really good, but I’m always looking for ways to improve them.
Introduction
With the demand for strong colonies of bees for almond pollination, coupled with landscapes that are offering less and less bee forage, we beekeepers are feeding more and more artificial diet in the form of pollen subs. Not only is forage being affected by changes in agriculture, but temperature and rainfall are changing with the climate. Of serious concern is a finding brought to our attention by USDA researcher Lewis Ziska [[1]], who found that during my lifetime, in response to rising levels of CO2 in the atmosphere, that the amount of protein in goldenrod pollen has apparently decreased by a third, thus forcing our bees to work even harder to obtain critical nutrients. Thus there is huge interest by beekeepers as to what are the “best” pollen subs.
Practical application: There is a crying need for someone to perform the service for beekeepers that Consumer Reports does for other consumers — to test products on the market one against the other.
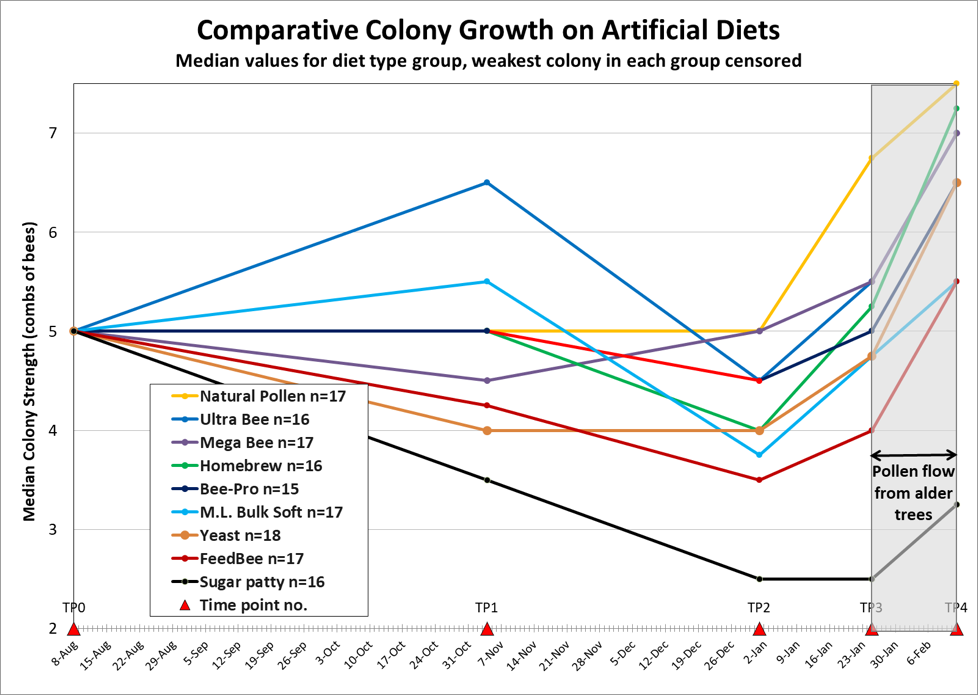
With that intent, in 2013 I ran a field trial to compare the performance of various pollen sub formulations on the market [[2]] — testing to see how well they supported colony growth over the six months prior to almond pollination. The results clearly demonstrated that the feeding of protein patties resulted in far greater colony strength. I also found that although some of the pollen subs initially outperformed natural pollen (on a pound-per-pound basis), that over time, they appeared to lose the lead (Fig. 1).
Figure 1. The results of the 2013 trial (product formulations may have changed since then). I shaded the plots of colony growth after 25 January, since that growth was sustained by worker emergence resulting from a natural pollen flow from alder, which began three weeks earlier. Note that two of the artificial diets initially outperformed the natural pollen patties. This may have been due to the higher protein content of the artificial diets. My question was whether those diets were deficient in a limiting nutrient that eventually prevented full utilization of the protein.
Practical application: At this point, allow me to make it clear that for short-term colony buildup, some artificial diets performed as well or better than natural pollen. It is only under conditions of sustained artificial feeding that any nutritional deficiency may become apparent.
Full disclosure: This article is about a follow-up pollen sub trial that I ran in 2018. I had to decide whether to name the commercial product that I used for the test, since I knew that beekeepers would ask me. I left it up to the manufacturer, who granted me permission to do so. That said, I pride myself on my objectivity, and have no wish to endorse any product. In our commercial beekeeping operation, we do use commercial products, but our use does not constitute endorsement or favoritism. In the case of pollen subs, a number of us California beekeepers prefer to chop pollen sub into chunks for feeding, rather than purchasing it in preformed flat patties (since we can place more of a soft chunked patty within the cluster). One manufacturer offers such a patty for sale, thus eliminating our need to mix our own. That product is Mann Lake’s Ultra Bee Bulk Soft Patty (hereafter called the Control sub). Since I was familiar with the field performance of that product, for this trial I approached the manufacturer to see if they were willing to prepare a custom batch to my specifications. Since they would benefit from knowing the results, I asked them to donate the feed for this trial, which they did. I received no other compensation from the company, and all other costs were funded by donations from beekeepers. The results of this trial do not constitute endorsement of any product, but rather information of use to anyone producing pollen subs. FWIW, since new pollen subs have come on the market since my 2013 trial (notably Dadant’s AP23 and HealthyBees spirulina patty), I’m planning to run another comparative trial this summer.
My experimental objective
I wondered whether there was something missing or in short supply in the best-performing subs — That could be considered as “limiting nutrients.” So I ran this experiment to see whether I could improve the performance of the Control sub by supplementing it with two nutrients that I hypothesized in which it may have been deficient. Allow me to walk you through my rationale for the experimental design.
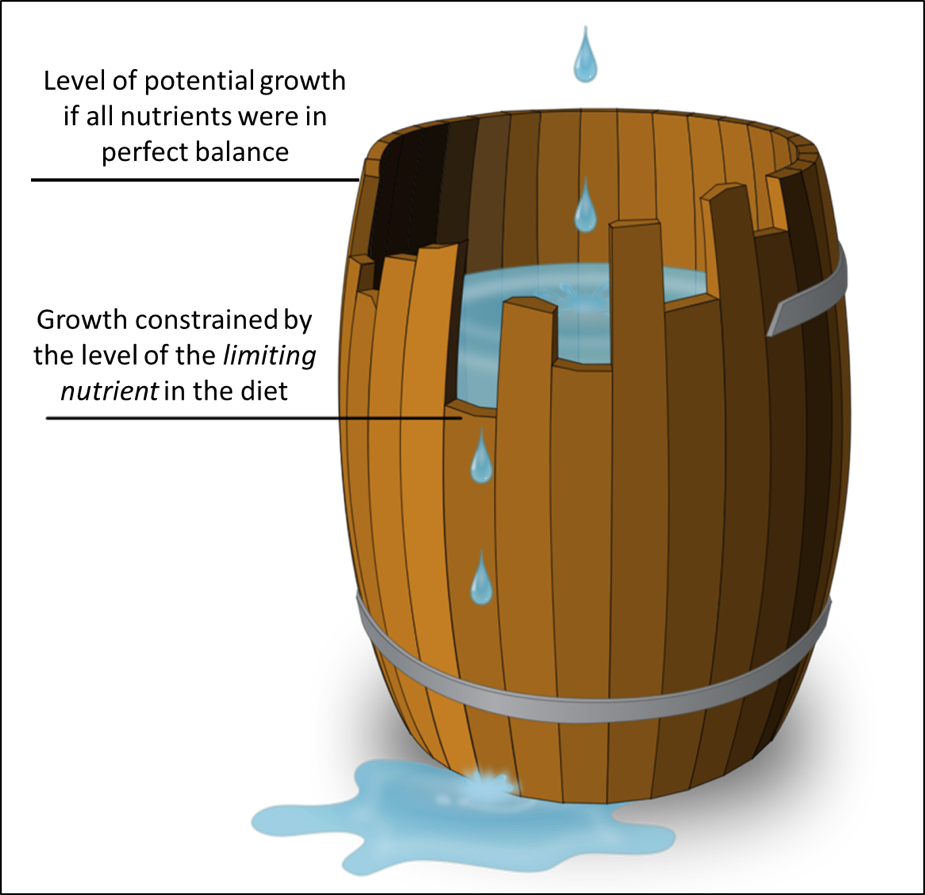
The concept of a limiting nutrient is commonly illustrated by the barrel analogy of “Liebig’s Law,” shown in Fig. 2.
Figure 2. Liebig’s Law states that the growth of an organism is limited by the nutrient in shortest supply. Using the analogy of a barrel being filled with water, the maximum fill (total growth) is limited by that “limiting nutrient.” That nutrient could be an amino acid, a vitamin, a mineral, or perhaps (in the case of bees) a sterol. Image credit [[3]].
Keep in mind that honey bees grow at an incredible rate — faster than any other livestock species [[4]]. In order to do so, they require a well-balanced, nutrient-intense diet of nectar and pollen. Any deficiency in an artificial diet (pollen sub) will limit its overall utilization by the bees for colony growth.
Practical application: Beekeepers, researchers, and manufacturers tend to focus upon the protein and amino acid contents of pollen subs, but I wonder whether those are actually the true limiting nutrients in artificial diets. There is an economic reason to see whether this is so, since protein is the most costly component of a pollen sub. But that costly protein may be wasted if some other limiting nutrient prevents its full utilization by the bees.
Based upon my reading of the literature, two potential limiting nutrients in pollen subs came to mind: the first a plant sterol.
24-methylenecholesterol
Let me start by quoting Dr. Allen Cohen, the author of the textbook Insect Diets [[5]]:
Because insects, unlike vertebrates, cannot make sterol to support their needs, they must get it from their diet, thus making sterols, by definition, essential nutrients.
Sterols are essential components of cellular membranes, the molting hormone ecdysone, and other fundamental biological processes. Back in the ‘60s through ‘80s, researchers at the USDA ARS labs delved deeply into the sterols of honey bees [[6]], concluding that one specific sterol — 24-methylenecholesterol (24mCh) — was a major component of the jelly produced by nurse bees. More recently, Tian [[7]] found 24mCh to be a component of Major royal jelly protein 1 (MRJP1).
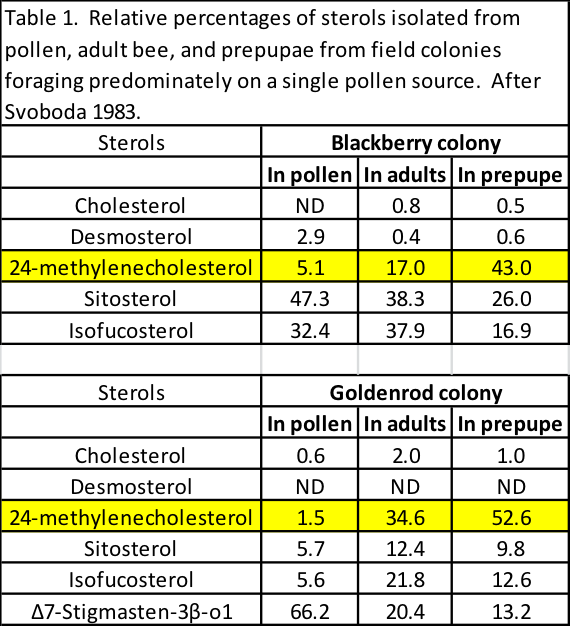
Each plant species produces pollen with different relative proportions of sterols, of which only a few appear to be utilized by bees. Stone and pome fruit tree pollen is notable for having high levels of 24mCh [[8],[9]], but what is especially of interest is that in colonies foraging upon low-24mCh pollens, such as blackberry or goldenrod, that the nurse bees concentrate 24mCh to levels much higher than it is in the pollen that they’re feeding upon, as evidenced by analyses by Svoboda [[10]] (Table 1).
Note in the above table how the proportion of 24mCh increases dramatically from the amount in the pollen, to that in the adult bees, and then especially in the prepupae that had grown on a diet of jelly produced by the nurses.
Experimental question: The big question for those of us trying to understand bee nutrition is just how important it is for bees to obtain 24mCh directly from their diet, or how readily they are able to convert other pollen sterols into it. Svoboda [[11]] concluded that they were indeed capable of sterol conversion to some extent:
… previous studies with chemically-defined diets have demonstrated the capacity of the worker bees to provide 24-methylenecholesterol, as the major sterol in the brood food when no pollen is fed to the brood and even when no sterol is added to an artificial diet. These results indicated that the nurse bees could readily cycle certain sterols from their endogenous pools to maintain high levels of 24-methylenecholesterol, sitosterol and isofucosterol in the brood food for the developing larvae. In addition, bees from free flying colonies were found to produce brood in which there were high levels of these three sterols even when their pollen source contained low levels of these sterols.
Practical application: Although bees appear to possess the ability to convert some other plant sterols to 24mCh, I was curious as to how much 24mCh there was in the Control sub.
I had long looked for an inexpensive commercially-available source of 24mCh. Ten years ago, I couldn’t find one, since few vegetable oils contain it to any extent [[12]]. But nowadays, off-the-shelf borage oil — presumably high in 20mCh — is readily available, but I didn’t have any way to confirm that. As luck would have it, in 2017 I heard Drs. Ramesh Sagili and Priya Chakrabarti from Oregon State University present that they had paid thousands of dollars to have a batch of pure 24mCh synthesized, and that the OSU lab could use it as a reference standard to quantify 24mCh in pollen samples. This opened an opportunity for me to see whether I could use borage oil as a source of 24mCh in pollen sub. So I contacted Dr. Chakrabarti to see whether the lab could run analyses for me, to which they graciously agreed (Disclosure: I was also involved in helping the researchers to obtain funding for their research).
I sent a sample of the off-the-shelf Control sub, which tested at containing only a trace [[13]] of 24mCh (compared to a detection of over 4122 mg/100g in a sample of almond pollen). A sample of borage oil tested at 170 mg/100 g 24mCh — far less than almond pollen, but perhaps enough to make a difference in an artificial diet.
Experimental design: I would ask the manufacturer to replace the canola oil used in their pollen sub with borage oil, in order to provide a source of 24-methylenecholesterol in the diet.
The second potential limiting nutrient on my mind was a trace element:
Zinc
Zinc is well known to be a necessary nutrient in animal diets, critical for metabolic function, growth, immunity, and appetite. It is typically supplemented in animal diets to the 50-75 ppm level [[14]]. But natural bee-collected pollens typically contain only from 24-50 ppm zinc [[15]], from which nurses produce royal jelly with zinc in a narrow range of 20-25 ppm [[16]]. Zhang [[17]] fed colonies during a nectar and pollen dearth sugar syrup supplemented with zinc, and found that it took at least 60 ppm zinc for the nurses to produce jelly with a normal zinc content.
Practical application: The zinc content of natural pollens [[18]] is often below that considered optimal for animal nutrition, so it’s possible that zinc may be more of a limiting nutrient than is the protein content of incoming pollen. Of concern is that even that potential deficiency may be being exacerbated by plant response to the elevating level of carbon dioxide in the atmosphere.
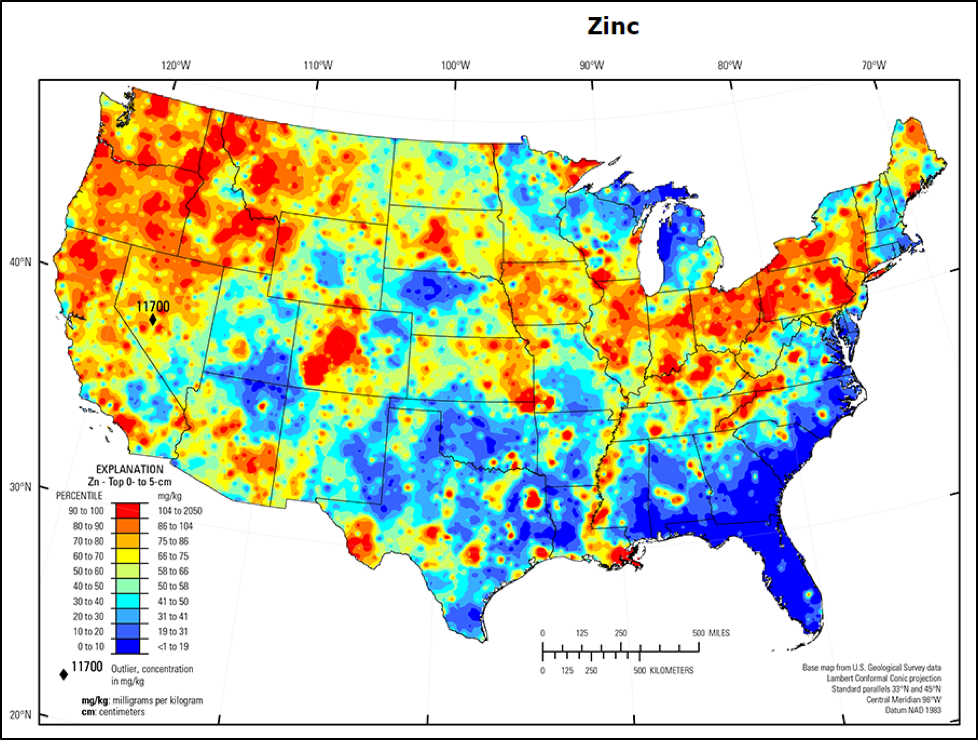
Loladze [[19]], in reviewing multiple studies, found that the levels of zinc greatly decreased in plants grown under conditions of elevated CO2. And that’s not to mention that in some regions (notably the Southeast) that zinc would be expected to perhaps be a limiting nutrient in pollen, due to the deficiency of the element in the soil (Fig. 3).
Figure 3. The availability of trace elements in the soil varies greatly across the country. The map above is for the concentrations of zinc in the upper soil level, with red indicating the highest concentration, blue a deficiency. Map credit [[20]].
Practical application: Although my experiment was run where zinc is relatively abundant in the soil, beekeepers in the blue areas of the map above may consider zinc supplementation of their colonies. To view maps of trace element contents of the soil in your area, go to [[21]].
So zinc was clearly on my radar as possibly being the limiting nutrient in the pollen sub that I’ve been using. I asked the manufacturer for analysis of the Control sub, and it came back at only 20 ppm — perhaps less than optimal.
Experimental design: I would supplement the Test batch of pollen sub with zinc to 75 ppm, by adding 25% as zinc sulfate, and 75% as zinc proteinate, based upon a recommendation from an animal nutritionist.
With the object of efficiency in mind, I decided to kill two birds with one stone, and simultaneously test another potential limiting nutrient on my radar in the same batch.
Practical application: Running a controlled trial of pollen subs is costly in labor and materials. My thought was that if the double-supplemented batch exhibited increased performance, then I could later tease out which of the nutrients was responsible.
At this point I was ready to write up a protocol for a field trial. To be continued…
Acknolwedgements
I thank Mann Lake and Drs. Priya Chakrabarti and Ramesh Sagili for providing chemical analyses of products involved in this experiment.
References & Notes
[1] Ziska LH, et al (2016) Rising atmospheric CO2 is reducing the protein concentration of a floral pollen source essential for North American bees. Proc. R. Soc. B 283: 20160414
[2] https://scientificbeekeeping.com/a-comparative-test-of-the-pollen-sub/
[3] Image from https://en.wikipedia.org/wiki/File:Minimum-Tonne.svg#filelinks, modified by the author.
[4] See Figs. 1 & 2 at https://scientificbeekeeping.com/sick-bees-17-nosema-the-smoldering-epidemic/
[5] Cohen, A (2015) Insect Diets, second edition. CRC Press.
[6] Herbert, E Jr., et al (1980) Sterol utilization in honey bees fed a synthetic diet: effects on brood rearing. J. Insect Physiol.. Vol. 26, pp. 287 to 289.
Standifer, L et al (1968) Pollen sterols — a mass spectrographic survey. Phytochemistry 7: 1361-1365.
Svoboda, J, et al (1986) Selective sterol transfer in the honey bee: its significance and relationship to other hymenoptera. Lipids 21: 97-101.
[7] Tian, W, et al (2018) Architecture of the native major royal jelly protein 1 oligomer. Nature Communications 9:3373 DOI: 10.1038/s41467-018-05619-1.
[8] Loper, G, et al (1980) Biochemistry and microbiology of bee-collected almond (Prunus dulcis) pollen and bee bread, I. – Fatty Acids, Sterols, Vitamins and Minerals. Apidology 11(1): 63-73.
[9] Chakrabarti, P, et al. (2019) The omics approach to bee nutritional landscape. Metabolomics 15: 127 https://doi.org/10.1007/s11306-019-1590-6
[10] Svoboda, J, et al (1983) Comparison of sterols of pollens, honeybee workers, and prepupae from field sites. Archives of Insect Biochemistry and Physiology 1(1): 25-31.
[11] Svoboda, J, et al (1986) Sterols of organs involved in brood food production and of royal jelly in honey bees. Insect Biochem 16(3): 479-482.
[12] Reina, R, et al (1999) Sterol and triterpene diol contents of vegetable oils by high-resolution capillary gas chromatography. J of AOAC International 82(4): 929-935.
[13] I was a bit surprised by this, since I would have expected there to be a measureable amount, from the canola oil in the patty.
[14] Sloup, V (2017) Zinc in the animal organism: a review. Scientia Agriculturae Bohemica 48(1): 13—21. Sloup states that: “The amount of Zn found in compound feed for livestock is around 100 mg/kg [ppm].” Older texts suggest the 40-70 ppm range. My consultation with an animal nutritionist suggests the 50-75 ppm range.
[15] Zhang G, et al (2015) Zinc nutrition increases the antioxidant defenses of honey bees. Entomol Exp Appl 156:201—210. https://doi.org/10.1111/eea.12342
[16] Stocker, A, et al (2005) Trace and mineral elements in royal jelly and homeostatic effects. Journal of Trace Elements in Medicine and Biology 19: 183—189.
[17] Zhang (2015) op. cit.
[18] Bonvehí, J & R Jordà (1997) Nutrient composition and microbiological quality of honeybee-collected pollen in Spain. J. Agric. Food Chem. 45: 725−732.
Szczêsna, T (2007) Concentration of selected elements in honeybee-collected pollen. Journal of Apicultural Science 51(1): 5-13.
[19] Loladze, I (2014) Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife DOI: 10.7554/eLife.02245. See Figure 1.
[20] Smith, DB, et al (2019) Geochemical and mineralogical maps, with interpretation, for soils of the conterminous United States: U.S. Geological Survey Scientific Investigations Report 2017-5118, https://doi.org/10.3133/sir20175118
[21] https://pubs.usgs.gov/sir/2017/5118/sir20175118_geo.php