Guessing the Future of Varroa: Part 2 – Ways that Bees Can Manage the Mite
Guessing the Future with Varroa: Part 2
Ways that Bees Can Manage the Mite
Randy Oliver
ScientificBeekeeping.com
First published in ABJ in January, 2019
I suspect that our problems with varroa—at least for commercial beekeepers—may get worse before they get better. But I’d be willing to bet that eventually, we’ll all be keeping bees that are naturally resistant to the mite. It’s quite clear, that left to its own means, Apis mellifera is able to force varroa into the position of being a relatively benign parasite. It is only our current beekeeping practices that are causing this natural evolutionary transition to take so long.
In order to work with our bees to turn varroa into a “benign” parasite (as it is in its natural host Apis cerana), we need to understand what we’ve been doing wrong, and what we can do better. As I’ve tried to explain in previous articles, we’re now dealing not just with varroa, but with the new synergistic coupling of the mite and Deformed Wing Virus (DWV), which I’m calling The Monster. Of interest, it’s only in recent years that parasitologists have started paying much attention to multiparasitism and co-infection [[1]].
When I think of how varroa has turned DWV into the problem that it is today, I love the descriptive terms that Steven Frank [[2]] used to describe competition between genotypes; as you read the snip below, think of how it applies to the varroa/DWV Monster, and the evolutionary forces upon benign or virulent forms of the parasite combination:
There is, however, a problem when two or more genotypes occupy the same host. If one genotype extracts host resources rapidly and reproduces quickly, then the host may die in a short time. A prudent genotype would have relatively low fitness when paired in a host with a rapacious genotype because, for both genotypes, the host is short-lived, and the rapacious genotype reproduces more rapidly than the prudent one [emphasis mine].
Our current beekeeping practices favor the most rapacious genotypes of The Monster by:
- Maintaining multi-hive apiaries favorable to the easy dispersal of virus-vectoring mites from collapsing colonies to many other hives, and
- Then replacing those fallen colonies each year with more of the same mite-susceptible bee stock.
So long as we continue this hopeless and unsustainable farce, which is completely dependent upon continually-increasing applications of miticides, our dismal rate of 33% annual colony losses is unlikely to improve [[3]] (Fig. 1).
Figure 1. The unfortunately all-to-familiar first signs of a colony entering the death spiral due to the varroa/DWV “Monster.” Note the bee with deformed wings, and the slumping prepupae. Even a strong late-season miticide treatment will not be enough to save this unfortunate colony.
The long-term solution
Most of us are not going to keep smaller apiaries, nor stop replacing deadouts, so the only way that we’re going to solve The Varroa Problem is by focusing upon the genetics of our bees–that is, we need to start demanding that our queen producers get serious about selecting for mite resistance–which would favor the more prudent forms of varroa and DWV.
Practical application: so long as our queen and package producers breed from stock that is dependent upon multiple miticide treatments in order to survive, the situation is unlikely to improve. Only when they start breeding only from colonies that have survived for a year without treatment, will we then stop rewarding the most rapacious forms of the varroa/DWV Monster, and start favoring a more prudent parasite.
The good news is that there are a whole lot of ways that bees can fight The Monster.
Understanding varroa and Apis cerana
The first thing that I’d suggest is for every beekeeper gain an understanding of how varroa’s natural host, the Eastern honey bee (Apis cerana) evolved to keep this parasite in check. It’s only recently, however, that we may have learned one of its critical tactics. It’s always puzzled me exactly why varroa doesn’t attempt reproduce in cerana worker brood to any extent. Sure, it could be due to the extreme varroa-sensitive hygiene (VSH) of cerana, but that doesn’t fully explain why the mites don’t at least try, or how the nurse bees tell that a pupa is infested.
Three recent studies have greatly helped to clear up the mystery. Lin [[4]] demonstrated that varroa indeed has the ability to successfully reproduce on cerana worker pupae. Page [[5]], showed that the pupae fight back by “social apoptosis”—being entirely intolerant of having mites feed upon them. But the most recent paper was the clincher–Zhang [[6]] discovered that cerana worker larvae appear to cue on a particular protein in the saliva of the mite, which then triggers the larva to self-sacrifice for the good of the colony. Amazingly, this cue is specific only to late-instar cerana worker larvae—it has no, or only slight, effect upon any other life stage or sex of either A. cerana or mellifera.
Practical application: as far as I’m concerned, the above is a revelation, and gives us a prime target for selection in our breeding programs [[7]].
With this understanding, I can take a stab at listing the set of rules that Apis cerana sets for the mite:
Apis cerana’s Rules for Coexistence with Varroa
- Don’t waste your time trying to reproduce in our worker brood—our larvae will “just say no,” and we adults will try to kill you as we remove our self-sacrificed sisters.
- But we’ll offer you a carrot, along with a stick. We’ll allow you to reproduce in our drone brood, under the condition that we’ll maintain a sniffing hole in the capping, and monitor for any signs of stress to that pupa. Hurt it, or transmit harmful viruses, and we will seal you and your bloodline into a waxen grave.
- And we’ll further restrict your reproduction by rearing drones only from time to time.
- At all times we are going to make your life miserable. We will self-groom and allo-groom (groom our nestmates) with fervor. That means that every time you are forced to shift to a younger worker in order to avoid being carried out by an aging ride that might not return, you’ll have to run the gamut again.
- If you play by our rules by being a “prudent” minor parasite, we’ll allow you to vertically transmit to the next generation when we swarm.
By forcing the mites to reproduce only upon the haploid drones (which possess only one allele for each gene), and since only strong drones can catch up with a virgin queen, this confers rather intense selective pressure upon A. cerana genetics, since those colonies that produce a lot of healthy drones have the best chance at getting the genetics of their queen (as opposed to those of the drones that she mated with) into the next generation [[8]] (Fig. 2).
Figure 2. I shot this photo of a drone comet chasing a queen in a commercial mating yard. The queen (apparently no longer a virgin) is at the top left. What appears to be a lucky drone is tumbling upside down slightly below her and to the right.
Practical application: in order to produce and maintain mite-resistant queen lines, our queen breeders will need to shift the genetics of all the drones within flight distance of their mating yards—something that I’m attempting to do over a three-year period [[9]].
The natural evolution of bees towards mite resistance
Apis cerana has figured out a way to live with varroa. Apparently natural selection did not find that it was worth it to eliminate the mite entirely–instead, it relegated the mite to the status of a minor parasite, similar to how we humans allow follicle mites to live on our bodies.
Direction of evolutionary pressure: There is strong natural selective pressure for any wild-living population of Apis mellifera to also work out a deal with varroa—we’ve already seen this happen in South Africa, South America, and in other regional bee populations.
I’d be remiss not to mention varroa tolerance. Honey bees have always been quite tolerant of high varroa levels, so long as DWV or other varroa-vectored viruses are not involved. But some colonies appear to exhibit a high degree of immunity to DWV, even at elevated mite levels. This is an intriguing way for bees to deal with varroa.
Direction of evolutionary pressure: Dr. Eyal Maori has been on the cutting edge of how bees evolve resistance to viruses, and has shown that they can confer resistance via the endogenization of part of the virus genome, as well as via the jelly produced by the nurses [[10]]. We may see some bee strains develop better resistance to DWV.
The future of managed bee populations
But such natural evolution has been inhibited in our commercial stocks–due to our reliance upon miticides, and general lack of hard selection for resistance by most queen producers. On the other hand, a number of breeders who have allowed Bond Selection to take place now observe substantial resistance in their stocks—so long as their bees are kept relatively isolated and away from apiaries of commercial stock. If not kept isolated, the immigration of mites from surrounding apiaries and swarms of the non-resistant bees may overwhelm what would otherwise be an adequate degree of resistance.
This drift problem is exacerbated by the coevolution of varroa and DWV to create the colony-killing Monster about which I’ve written in recent articles. The presence of virulent DWV does not allow for “mite-tolerant” bee stocks, since as soon as their mite level starts to climb, DWV takes the reins and causes the colony to collapse, thus effecting dispersal of the most virulent symbiotic strains of varroa and the virus. I can’t get excited about breeding for “tolerance”—we want to breed for bees that by one means or another, reduce the reproductive success of varroa, at least in the worker brood.
It’s easy to see that the way we’re headed is a dead end street—our dependence upon miticides, and our continual restocking of our apiaries with mite-susceptible stock, favors the evolution of the varroa/DWV Monster. Our current practices are pushing evolution in the wrong direction. Luckily, a number of forward-looking researchers and breeders are working towards solutions, and I feel that the market for queens is about to reach the tip point, where the buyers will start to demand proven resistant stock. At that point, the genetics of our managed bee populations may shift quickly.
Direction of evolutionary pressure: the consumer will likely place strong pressure upon our queen producers in the near future. Once proven mite-resistant stock hits the commercial market, evolution in the bee industry could occur rapidly.
That said, let’s look at which traits we might expect to see evolve…
It’s not necessary to kill a single mite in order to control varroa
Although we beekeepers generally consider the killing of mites to be the most important aspect of varroa management, I suggest that we step back and look at the Big Picture. As evidenced by Apis cerana, it’s not necessary to actually kill a single mite in order to control varroa—the mite’s Achilles Heel is its success at reproduction.
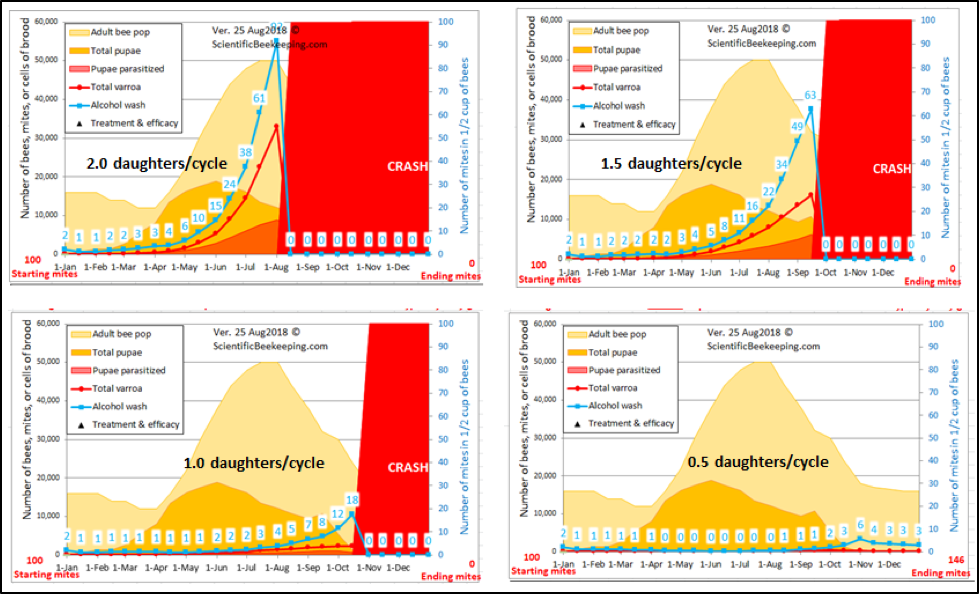
Feel free to play with my varroa model [[11]] and use the “custom” setting to reduce the average number of daughters per foundress per reproductive cycle in half (from 1.45 to 0.72)—at that point, the reproductive success of the mite becomes so low that the varroa population in the hive can’t keep up with its natural rate of attrition (Fig. 3).
Figure 3. I ran four simulations for typical colonies, with the average number of daughters (mated or not) per foundress ranging from 2 to 0.5 (“normal” is around 1.5). Note the differences in the rate of mite buildup. No mites were killed in these simulations—the only holdback upon the buildup of mites was reducing the average fecundity of the foundresses, which can be accomplished by any number of means—several detailed below.
Fortunately for us, it’s becoming clear that the bees have any number of ways for reducing a foundress’s degree of fecundity or reproductive success—I’ll elaborate further on.
Breeding for resistance
Although many beekeepers fancy that they are helping to breed for resistance, simply withholding treatments is a far cry from “selective breeding.” Any meaningful breeding program would require:
- A valid assay for actual resistance. This may be difficult in many areas, since colonies exhibiting some degree of resistance may get overwhelmed by mite drift from other hives [[12]].
- The breeder needs to control the drone pool, which can only be accomplished by isolation, the flooding of the area with chosen drones, or instrumental insemination.
- And then that breeder would need to propagate and disperse thousands of daughters from the resistant queen line.
Realistically, the best that Joe or Jane hobby beekeeper can do is to vote with their dollars—by supporting those breeders who are indeed making a serious effort at selecting for resistance.
Practical application: it’s hard for me to get excited about breeding for mite tolerance—simply because colonies with a heavy mite load will always be more stressed. So I feel that we should focus upon selecting for resistance to mite buildup. We don’t initially need completely mite-proof bees; partially-resistant colonies that required only a single treatment a year would be a big step in the right direction.
That said, I’ve bred bees for certain traits for many years, and generally found it pretty easy to breed for color, temperament, productivity, and resistance to AFB and tracheal mite. I was completely surprised by how difficult it’s been to breed for bees that can handle varroa. I figured that if Apis mellifera came from an Asian heritage, as has long been assumed, that it would still possess deep in its genome some tools for fighting mites—but if that were the case, it shouldn’t be so hard to tease those resistance traits back to the forefront. I mean, what gives—it took only about five years for bees to develop widespread resistance to the tracheal mite.
A possible explanation for this conundrum has been recently suggested by Dr. Keith Delaplane, based upon a revision of the course of migration and evolution of the genus Apis by Kotthoff [[13]]. It may be that our Western honey bee actually split off as a sideline of the genus before the other honey bee species evolved in Asia—where they were only then exposed to parasitic mites. Thus, Apis mellifera may have never needed to assemble a toolbox for fighting varroa, and was blindsided by the mite when it later jumped host from the bees’ mite-resistant cousin.
But that certainly doesn’t mean that our bees can’t repurpose their existing tools to do so. There are any number of possible ways that the honey bee can fight back against varroa—the ones that we’ve already identified are well reviewed at [[14]]. Here’s a quick rundown on some promising traits to select for:
Grooming and biting
The first mite-resistance trait that often comes to mind is grooming/biting behavior. And although it makes us feel good to imagine our bees crushing mites in their jaws, I find little compelling evidence that they actually kill healthy adult mites very often, and suspect that most mites that get crushed were already too feeble to get away [[15]]. So although we clearly want bees that groom mites vigorously, I doubt that grooming alone will be enough to solve The Varroa Problem [[16]]. So selection for grooming, yes–but along with selection for other more effective traits.
Entombment under the cocoon
Update: Dr. Jeff Harris, working at the Baton Rouge lab, found that in one mite-resistant bee stock, that the 5th-instar larvae were able to kill foundress mites by trapping them under the silk as the larva spun its cocoon. Note: this would be a very easy trait to assay for, by simply inspecting the cocoons in the cells of any colonies exhibiting low mite counts. See Jeff’s video, in which he mentions this trait, at https://www.youtube.com/watch?v=a2vg59Snt6c
Postcapping duration
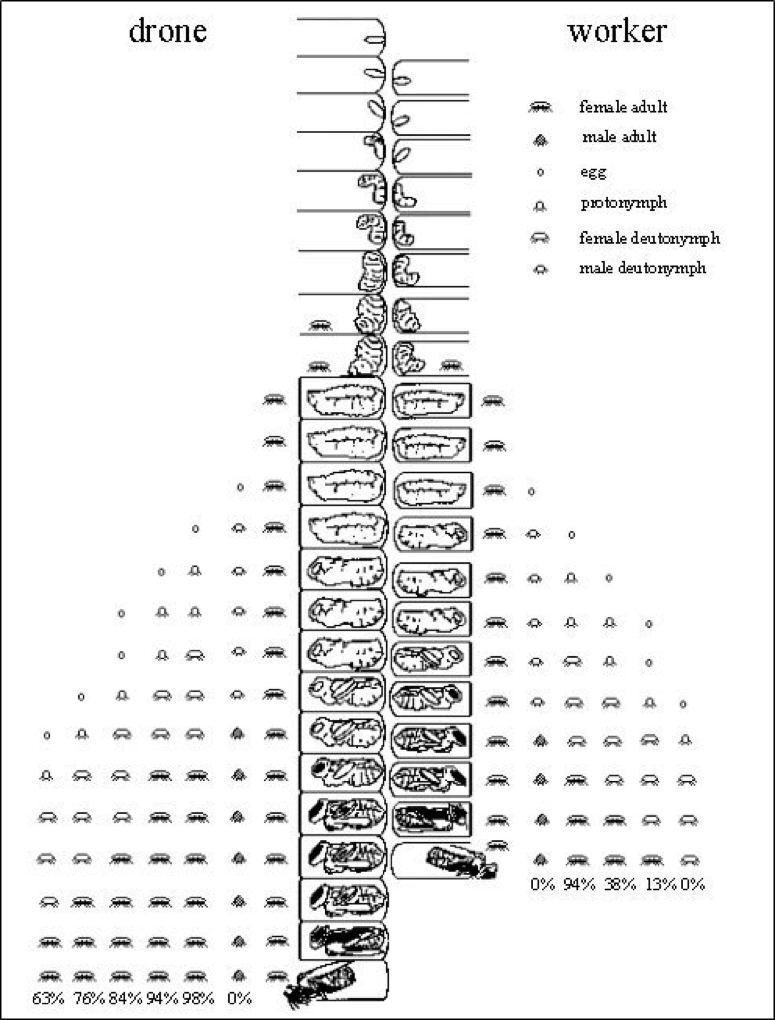
Varroa enjoys far greater fecundity (mature daughters per foundress) in drone brood than in worker brood, due to the longer postcapping duration for the drone pupae (15 rather than 12 days) (Fig. 4).
Figure 4. The longer postcapping duration of immature drones allows for more daughter mites to successfully complete their development. This allows for a theoretical average emergence of roughly 4 daughters emerging from a drone cell vs. 1.45 daughters per worker cell. The actual number of mated daughters per foundress, however, may be considerably less, especially when more than one foundress invades a cell. Image courtesy Dr. Stephen Martin [[17]].
Some early research by Büchler [[18]] suggested that selecting for shorter postcapping duration might be constructive, and a number of teams have since tried this approach, but generally not found it to be of substantial benefit. However, a recent study by Oddie [[19]] found what appeared to be a slight benefit, which in conjunction with others resistance traits, might help to do the trick.
Biological observation: bee immatures develop more quickly if broodnest temperature is raised a bit. It’s not clear whether varroa responds similarly, since the mite appears to reproduce better at lower temperatures. I see no reason that Apis mellifera couldn’t use broodnest temperature regulation to its advantage.
Varroa-sensitive hygiene
Colonies may differ greatly in the degree of diseased brood hygiene that they exhibit, which is surprising, since rapid hygienic removal of sick brood is a very effective way for colonies to control diseases [[20]]. It’s very easy to select for strong hygienic behavior by using the freeze- or pin-killed brood test. This trait will also confer some degree of mite resistance, since mite- or virus-sickened, or abnormally-developing brood may emit odors that initiate generic hygienic removal (Fig. 5).
Figure 5. These bees have uncapped, and are chewing out apparently-abnormal pupae. This lack of tolerance for abnormal development or olfactory signals is a well-proven means for bees to prevent the mite from reproducing.
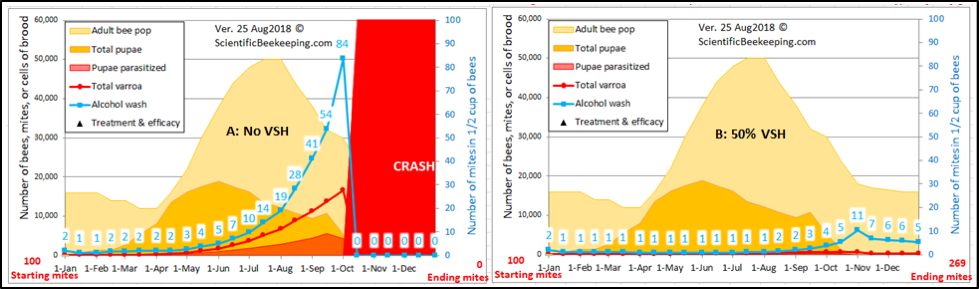
Even more specific is some sort of varroa-sensitive hygiene (VSH). Apis cerana exhibits VSH to the extreme, due to the self-sacrifice of its worker larvae. VSH is a generic term for any sort of hygienic behavior that by some means specifically targets mite-infested brood cells or cells in which mites are reproducing (generally specific to the worker cells), and is a well-proven trait–a colony that exhibits even 50% VSH can pretty much keep varroa in check (Fig. 6). The USDA VSH and Pol lines, as well as the Russian bees all exhibit strong VSH.
Figure 6. The strong effect of VSH upon varroa reproductive success. (A) A simulation for mite buildup in a colony not exhibiting VSH; (B) The result of a 50% rate of VSH. I suspect that my model currently underestimates the full effect of VSH, since it does not yet account for an increase in mortality of the disturbed foundress.
Practical application: VSH is a well-proven and effective trait for mite resistance.
I hesitate to mention specific queen producer names, but open-mated Minnesota Hygienic queens are commercially available, and a number of California breeders have also been selecting for freeze-kill hygiene. Certain breeders offer purebred Primorsky Russian stock (outbred stock may not perform the same). Dr. Albert Robertson from Canada is collaborating with U.S. producers to make his Saskatraz line available in the US. Other breeders may also offer bee stock selected for resistance, but as yet there is no testing organization to verify their degree of resistance.
Practical application: our industry needs to start an “All-America” type of organization of volunteer testers to evaluate stocks with claimed mite resistance.
An exciting development is that the USDA-ARS, Project Apis m, and Arista Bee Research are collaborating with a large breeder to make VSH production queens available on large scale—perhaps by next year. This stock will indeed have hard mite-resistance data to back it up.
Along with VSH there is also the uncapping/recapping trait, which may include the creation of “bald brood” (Fig. 7)—we are still working on how to better understand this trait [[21]].
Figure 7. Many of us have observed “bald brood” in our colonies—something that we didn’t notice often prior to varroa. Perhaps such uncapping (apparently sometimes only temporary) may disrupt mite reproduction, or desiccate the unsclerotized male or immature female mites.
Other traits that may confer resistance
VSH is effective, but it’s also pretty ugly and wasteful, since it involves the death and physical removal of the brood involved. Something that I find fascinating is what the ARS used to call SMR—Suppression of Mite Reproduction. SMR appears to be the main resistance mechanism of the African savannah bee (A. m. scutellata), for which beekeepers find no need to control varroa. As straightforwardly noted by Nganso [[22]]:
…hygienic and grooming behaviors did not significantly differ between subspecies [African and European] with respect to Varroa mite-infestation levels recorded, suggesting that other resistant mechanisms such as suppression of mite reproductive success and/or lower viral prevalence within honeybees and mites might play an important role in honeybee responses to mite infestation.
This is also something that appears to have been selected for by some populations of resistant bees in Europe (well reviewed by Panziera [[23]]. We’re watching evolution in action—and our bees, given the chance, may surprise us by coming up with different ways to deal with the mite. Unfortunately, intentional selection for SMR requires tedious examination of the brood (Fig. 8).
Figure 8. I’m removing mature brood one at a time, under a dissecting ‘scope (the spotlight is turned off for the photo). Such dissection can be used to determine whether there was a mite in the cell, and whether it reproduced. Yes, tedious.
Kairomones and proteins—Achille’s heels of varroa
Other potential resistance mechanisms have to do with olfactory cues used as kairomones by varroa. A foundress mite must start (and then end) two critical, yet separate reproductive processes, carefully cued and timed to coordinate with the timing of the development of its immature bee host. The first step is oogenesis (the creation of the egg cell), followed by vitellogenesis (yolk formation, which is also dependent upon the mite’s feeding on the larva’s fat bodies). Oogenesis is triggered by one or more olfactory cues from the late-instar bee larva [[24] [25]]. And somehow, the foundress is cued to stop producing eggs five days after the larva pupates, despite the fact that the pupa still has four days before it will emerge.
These cues are absolute requisites for successful mite reproduction, plus, the cues must cause the mite’s first egg to be a haploid male (which in varroa, surprisingly comes from a fertilized egg) [[26]]. The tweaking of any of these cues by the bees has the potential to mess up successful reproduction by the mite.
Now here’s where it gets interesting. Although varroa has a larger genome than does the bee, it appears to have given up the ability to produce some critical proteins—instead assimilating them directly from its bee host without digestion. Although discovered by Tewarson over 25 years ago [[27]], only recently have molecular biologists started looking into this fascinating aspect of varroa biology, publishing a protein atlas for varroa by McAffee [[28]] and an informative breakdown by Mondet [[29]], by which we may figure out some of the mite’s weak spots.
One recently-found possibility is suggested by Conlon [[30]], who notes that mite vitellogenesis is triggered by the molting hormone ecdysterone (ecdysis is the scientific term for molting). He notes that:
[Varroa’s reproductive] pathway is incomplete with only three of the seven genes from the ecdysone biosynthetic pathway present in the V. destructor genome… Functional forms of ecdysone are capable of ingestion by Varroa; suggesting the reduced number of genes may be an adaptation of the mite to its parasitic lifestyle and missing compounds are acquired through its haemolymph diet. This raises the possibility that the pulse of prepupal ecdysteroids is not a signal but a necessary physiological component for the successful initiation of reproduction in V. destructor.
The reason that Conlon’s suggestion is of great interest is that his research suggests that two independent populations of mite-resistant bees may both be downregulating their ecdysone-linked genes, thus possibly conferring resistance at the molecular level of the mite’s reproductive pathway [[31]].
Evolution is not limited by our imagination. And this is the reason that I’m curious to see what happens if I simply apply strong selective pressure upon the breeding population of my own operation, based upon alcohol wash count monitoring alone (plus them mothering productive, gentle colonies). Who knows what traits they might come up with?
Acknowledgements
Thanks to Peter Borst for his assistance in literature search, to all the bee researchers I’ve spoken with on this subject, and to my wife Stephanie for her suggestions on my manuscript.
References
[1] Furthermore, the reality, more or less ignored until recently, is that most parasites co-occur with other parasites. Vaumourin, E, et al (2015) The importance of multiparasitism: examining the consequences of co-infections for human and animal health. Parasites & Vectors 8:545 Open access.
[2] Steven A. Frank, SA (1996) Models of parasite virulence.The Quarterly Review of Biology 71(1): 37-78.
[3] https://beeinformed.org/results/colony-loss-2016-2017-preliminary-results/ Early indications are that losses may be higher this year (pers. comm. from pollination brokers).
[4] Lin Z, et al. (2018) Reproduction of parasitic mites Varroa destructor in original and new honeybee hosts. Ecol Evol. 2018:1–11.
[5] Page, P, et al (2016) Social apoptosis in honey bee superorganisms. Nature Scientific Reports 6:27210
[6] Zhang, Y & R Han (2018) A saliva protein of varroa mites contributes to the toxicity toward Apis cerana and the DWV elevation in A. mellifera. Scientific Reports 8:3387.
[7] It would be a relatively simple bioassay or gene for Marker-Assisted Selection.
[8] Since, other than some recombination, the haploid drones carry only the genetics of their mother.
[9] It’s easy to talk the talk; I’m walking the walk, and openly sharing my methods, successes, and failures.
https://scientificbeekeeping.com/the-varroa-problem-part-7/
https://scientificbeekeeping.com/the-varroa-problem-part-10/
A spoiler—we’re pretty excited about what we’re seeing so far in our November mite washes of our “potential breeders” this season.
[10] Maori, E., et al (2007) Reciprocal sequence exchange between nonretro viruses and hosts leading to the appearance of new host phenotypes. Virology 362 (2): 342-349.
Garbian Y, et al (2012) Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces varroa population. PLoS Pathog 8(12): e1003035.
Maori, E, et al (2018) A transmissible RNA pathway in honey bees. https://www.biorxiv.org/content/early/2018/04/12/299800
[11] https://scientificbeekeeping.com/randys-varroa-model/
[12] This was a problem during ARS’s development of the Primorsky Russian stock.
[13] Kotthoff U, et al (2013) Greater past disparity and diversity hints at ancient migrations of European honey bee lineages into Africa and Asia. Journal of Biogeography. 40(10):1832-1838.
[14] https://aristabeeresearch.org/varroa-resistance/
[15] My varroa model indicates that if the bees were able to increase the baseline varroa mortality rate from 0.5% a day to only 2% a day, that varroa would barely be able to survive in the hive. Since mites can be easily found in colonies selected for “biting,” I must question whether this trait has substantial effect.
[16] Nganso BT, op cit.
[17] Published in Munn & Jones, ed (1997) Varroa! Fight the mite. Conference proceedings, International Bee Research Association.
[18] Büchler, R & W. Drescher (1990) Variance and heritability of the capped developmental stage in European Apis mellifera L. and Its correlation with increased Varroa jacobsoni Oud. infestation, Journal of Apicultural Research, 29(3):172-176.
[19] Oddie, MAY, et al (2018) Reduced postcapping period in honey bees surviving Varroa destructor by means of natural selection. Insects doi:10.3390/insects9040149
[20] The question is, if rapid hygienic behavior is of general benefit to the colony, we would expect evolutionary pressure to have selected for all bees to be “hygienic.” Why that’s not so, I don’t know. See:
Al Toufailia, H, et al (2018) Both hygienic and non-hygienic honeybee, Apis mellifera, colonies remove dead and diseased larvae from open brood cells. 373: 1751.
[21] Oddie, M, et al (2018) Rapid parallel evolution overcomes global honey bee parasite. Scientific Reports 8: 7704.
[22] Nganso BT, et al (2017) Hygienic and grooming behaviors in African and European honeybees—New damage categories in Varroa destructor. PLoS ONE 12(6): e0179329.
[23] Panziera, D, et al (2017) Varroa sensitive hygiene contributes to naturally selected varroa resistance in honey bees. Journal of Apicultural Research 56( 5): 635-642. Open access.
[24] Garrido, C & P Rosenkranz (2004) Volatiles of the honey bee larva initiate oogenesis in the parasitic mite Varroa destructor. Chemoecology 14: 193–197.
[25] Frey, E, et al (2013) Activation and interruption of the reproduction of Varroa destructor is triggered by host signals (Apis mellifera). Journal of Invertebrate Pathology 113: 56–62.
[26] Sabelis, MW & CJ Nagelkerke (1988) Evolution of pseudo-arrhenotoky. Experimental & Applied Acarology 4: 301-318.
[27] Tewarson, NC & W Engels (1982) Undigested uptake of nonhost proteins by Varroa jacobsoni, Journal of Apicultural Research 21(4): 222-225. Since unaltered bee proteins are directly utilized by varroa, this is a potential way for bees–or beekeepers–to sneak something disruptive into the mite, such as RNAi or some other attached chemical.
[28] McAfee, A, et al (2017) A Varroa destructor protein atlas reveals molecular underpinnings of developmental transitions and sexual differentiation. Mol Cell Proteomics 16(12): 2125–2137.
[29] Mondet, F, et al (2018) Transcriptome profiling of the honeybee parasite Varroa destructor provides new biological insights into the mite adult life cycle. BMC Genomics 19:328.
[30] Conlon, BH, et al (2018) A modified honey bee Ecdysone pathway inhibits reproduction in Varroa. In Of Mites and Men: The independent evolution of host-induced Varroa infertility in the drone brood of Apis mellifera. PhD Dissertation, Martin-Luther-Universität.
[31] Some astute research groups will likely apply to patent varroa-control products based upon suppressing the production of mite-required proteins by the bees, or by using those undigested proteins as transporters of a bound chemical.