Re-Evaluating Varroa Monitoring: Part 3 – How Does Mite Distribution Vary Frame-to-Frame in a Hive?
Re-Evaluating Varroa Monitoring
Part 3
HOW DOES MITE DISTRIBUTION VARY FRAME-TO-FRAME IN A HIVE?
Randy Oliver
ScientificBeekeeping.com
First published in ABJ May 2020
In the previous articles in this series I evaluated the different methods for varroa monitoring, and then discussed the state of our knowledge as to where mites would be most prevalent in the hive. The remaining question then is, from what type of comb would you obtain the most representative and consistent sample of bees to assess the mite infestation rate of the colony as a whole?
Based upon a review of the literature, I’d convinced myself that I could take a bee sample from nearly any frame in a brood box [[1]]*. But any time that I convince myself of something, I then try to see whether it will hold up to scrutiny. Last autumn, as my colonies were tapering down broodrearing — at the time when many beekeepers are finally getting around to monitoring their hives for mites — I happened to have seven high-mite double-deep hives that hadn’t made the grade in my breeding program. Since I also had on hand mechanical agitators calibrated to give accurate alcohol washes, I decided to collect some hard data to see just how those mites were distributed on the bees on each comb.
*Update: See also my analysis of Dr. Frank Eischen’s data in Fig. 3 at https://scientificbeekeeping.com/tag/mite-drop/
Materials and methods
Helped by beekeeper Sandy Honigsberg, we smoked and then gently moved each hive backwards from its location, and placed another bottom board and empty box in its place (Fig. 1). I then gently separated the two brood chambers, and pulled frames one at a time, starting with the bottom box, shook the bees into a tub, allowed any to fly off (there was not much return flight for most hives), and then scooped up a level half cup of bees to alcohol wash. In this way, there was no agitation or movement of bees from comb to comb before I shook them, and any flown bees returned to the original stand, rather than to the yet unshaken combs.
Fig. 1 The observational study equipment and setup. I shook a separate bee sample from each of all 20 combs in each hive. If a comb did not supply enough bees for a half-cup sample, I shook the next comb into the tub and indicated a combined comb sample on the data sheet. We used color-coded cups to avoid confusion.
In order to see whether the infestation rate of a bee sample tends to correlate with how the colony is using that comb, I took a quick look at each frame after I shook the bees into the tub, and recorded an abbreviation for whether it was drawn comb only, or contained honey, beebread, mixed brood, mostly open brood, or the queen. I then replaced the comb back on the original stand. Sandy then double-washed each bee sample to ensure 99%+ mite recovery.
Results
After collecting the data, I had to think about how best to display it. I realized that it would be easier to visualize the results if I color-coded the cells representing each frame, above the mite count for the bee sample from that frame (Fig. 2).
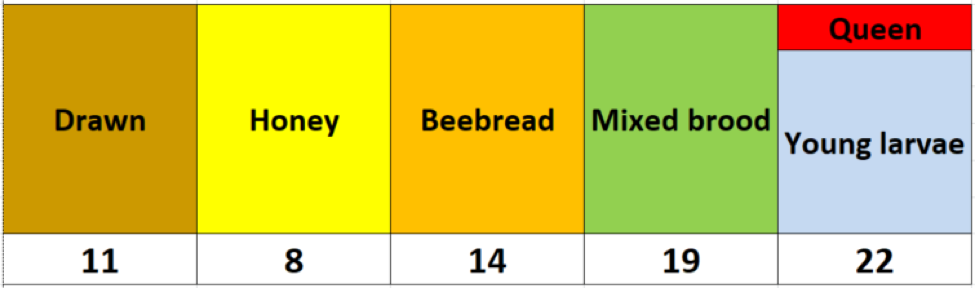
Fig. 2 I used the above colors to indicate comb type, with the mite wash counts for a half-cup bee sample from that frame recorded underneath.
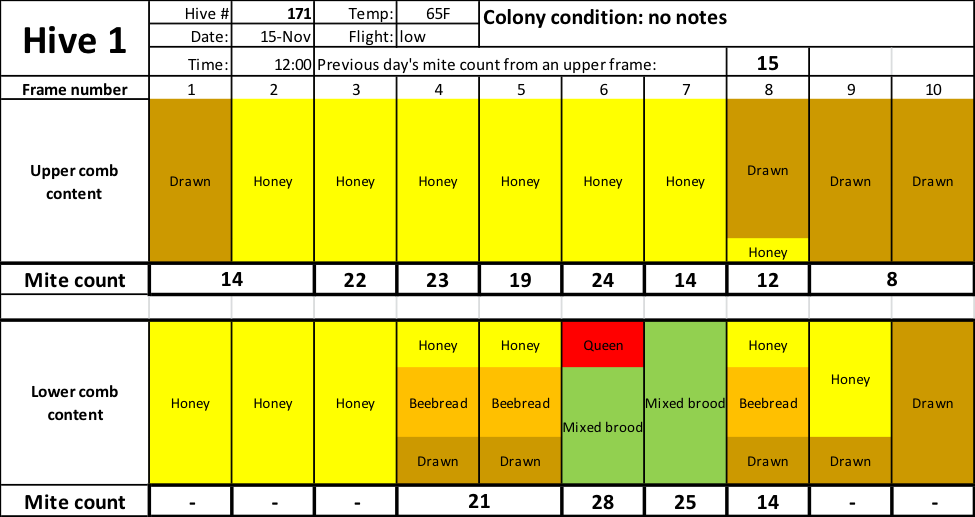
Below are the mite counts of the bee samples from each comb of one hive (Fig.3). I used considerable liberty in guessing the proportions and placement of color for each comb based upon my minimal notes — they are not necessarily representative of actual proportions on that comb, but do indicate how a beekeeper (me) would describe the comb type.
Fig. 3 Note that mite wash counts ranged from a low of 8 to a high of 28 (the average is 19) — all from the same hive. So which one best represents the impact of varroa upon the colony?
Of interest is a recent report by Sankey & Dolezal [[2]], in which they observed greatly differing infestation rates in newly-emerging bees from day to day, and large differences in the infestation rates of newly-emerged and “nurse” bees. I’ve also observed wild day-to-day swings in mite counts taken from brood frames. These observations suggest the mite counts from frames with emerging brood could be pretty dynamic.
Practical application: In my selective breeding program, what I’m looking for is a representative and consistent count, rather than either extreme. It’s up to the individual beekeeper (or researcher) to determine the sort of bee infestation rate that they’re specifically interested in, and all studies and recommendations should specify from what sort of frame bee samples were (or should be) taken.
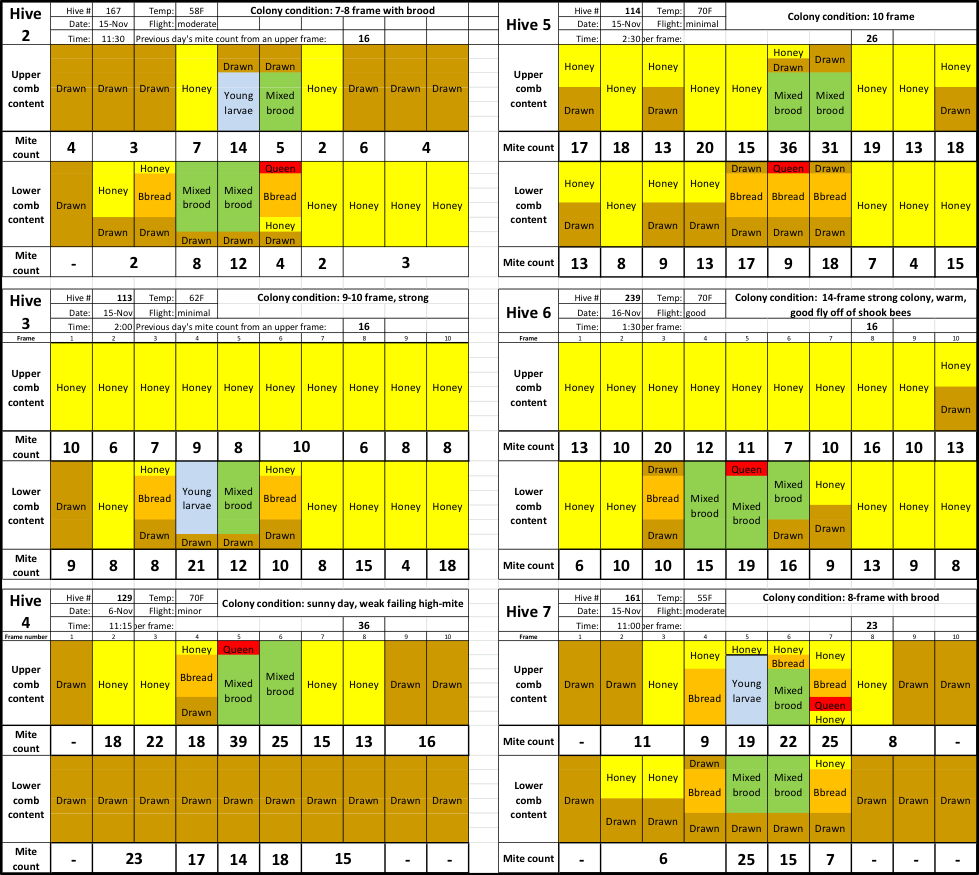
I assume that my readers would be interested in the data from the six other hives. Due to space limitations, I shrunk the size of the charts, but in order to free you from the need to find a magnifying glass, I enlarged the font for the mite counts (Fig. 4).
Fig. 4 The color codes for comb types are the same. As you can see, mite counts from the same hive can be all over the place. The question then is, can we detect any pattern?
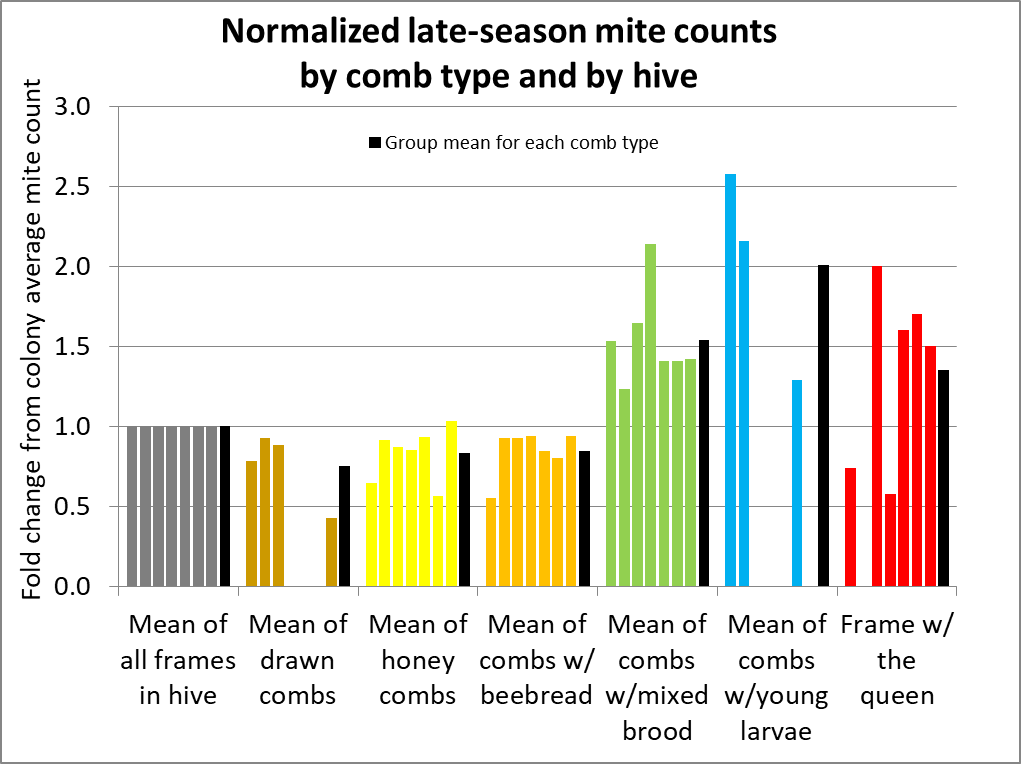
The figure above has a lot of information to digest, but it shows you the fundamental variability of mite counts between bee samples taken from the same hive. To better make sense of the data, I normalized the counts from all hives for comparison (Fig. 5).
Fig. 5 I’ve normalized the counts to set the average count for all frames in a hive to 1 (gray columns). Each group of 6 columns represents the counts, by comb type, from each hive. They are all in the same order, by hive, with the average for all the hives in black. Missing columns indicate that a hive did not have bees on that comb type.
Interpretation: As in other studies, a bee sample taken from a frame containing brood is clearly likely to exhibit a higher mite infestation rate than the across-the-hive average. But if you refer back to the original hive data in Fig. 2, you can see that a sample even from the same comb type can vary greatly in a hive. In order to better illustrate that, I calculated the standard deviations for samples from each comb type within each hive below (Fig. 6).
Fig. 6 The chart above illustrates the variation in mite counts by comb type by hive – the taller the column, the greater the variability. In the last group I calculated the average. On average, mite counts in bee samples shaken from combs other than brood combs are more consistent.
Bottom line: It’s a crapshoot. If you want to find the highest infestation rate, take a bee sample from a brood comb with young larvae, but be prepared for wide variabilty. Otherwise, if your goal is to obtain a consistent and representative sample of the adult bee infestation rate, shake it from a drawn or honey comb free of brood, but adjacent to the broodnest. Doing so has the huge added advantage of greatly reducing your chance of inadvertently harming the queen.
Update: after the fact, I realized that I neglected to include the most important chart!
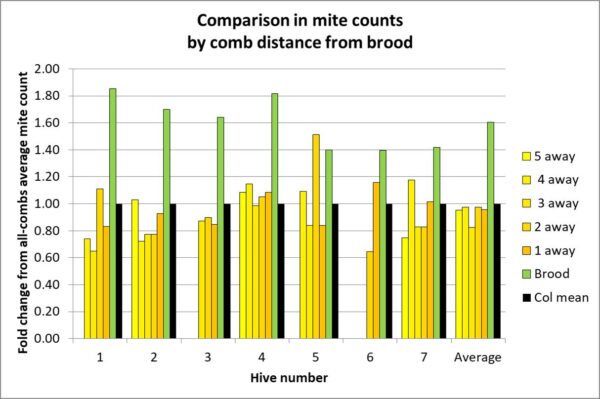
The above chart is for frames in the upper brood chamber. Notice how closely the mite count of a frame adjacent to the brood matches the average.
Coming next: Should you allow the shook bees to fly out of the tub before scooping your sample?
References
[1] Lee, KV, RD Moon, EC Burkness, WD Hutchison, M Spivak (2010) Practical Sampling Plans for Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) Colonies and Apiaries. J. Econ. Entomol. 103(4): 1039-1050; DOI: 10.1603/EC10037
[2] Sankey, A & A Dolezal (2019) We “mite” not know we’re losing until it’s too late. Illinois State Beekeepers Bulletin 102(6): 4-5.