Selective Breeding for Mite Resistance: Walking the Walk
Contents
Selective breeding for mite resistance. 4
Selection vs. Bottlenecking. 16
The big question ― heritability. 18
The importance of the drone pool 19
Selective Breeding for Mite Resistance:
Walking the Walk
Progress Report 2019
First published in ABJ June 2019
Randy Oliver
ScientificBeekeeping.com
I’m writing these words in mid-April. It’s been a miserable wet spring in Northern California (Fig. 1), and we’ve been furiously trying to catch up with delayed queen rearing while trying to stay ahead of swarming. I struggled to find time to type up my next installments on nosema and pesticides, then decided to instead write about what I was currently immersed in — my selective breeding program for mite resistance.
Figure 1. If you had trouble getting queens or packages this spring, you can blame the weather in California. I snapped this photo of an almond orchard on a cold day shortly after a hard rain had knocked some of the flower petals to the ground. These surreal black-and-white manmade forests never cease to amaze me with their unusual beauty.
Selective breeding for mite resistance
The varroa/virus complex is the number one problem for most beekeepers worldwide. As a beekeeper and biologist, I feel that our industry, after 30 years with varroa, has been derelict in not yet fully adopting honey bee stocks that are naturally resistant to the mite. To help get the ball rolling, in the spring of 2017 I wrote “Bee Breeding for Dummies” [[1] ] to suggest how most any medium-to-large-scale queen producer could engage in a serious, yet simplified, low-cost selective breeding program for varroa-resistant bees. I then promised to “walk the walk” in order to determine how much it would cost, and what sort of progress I could make.
In spring of 2018 I shared my experiences in “Selective Breeding for Mite Resistance: 1000 hives, 100 hours,” [[2]] being surprised at the minimal costs involved. And now in spring of 2019, I’m sharing another progress report after two years of experience.
| Full transparency: These articles are not a sales pitch — I’m not looking for new customers. Instead, what I’m trying to do is to see whether selective breeding for mite resistance is something that Joe Queenproducer can do without any scientific training or equipment, using his own stock of bees as the starting point. If I’m not successful, I’ll tell you. If on the other hand, I am successful, then there will be no excuse for every queen producer not to be breeding and selling mite-resistant queens. |
The 2018 season
After heavy culling of our remaining potential breeders in the fall of 2017, the following spring we bred from the queens of only the 20-some colonies that exhibited minimal mite levels after a year without treatment (roughly 2% of our 2017 starting count of 1000 hives in the program). I was somewhat disappointed that by April, the mite counts in most of those breeders had started to climb a bit. But I had to keep in mind that those queens had been mated to a drone pool coming from almost entirely non mite-resistant stock, so I really couldn’t expect to see much improvement yet.
Anyway, for 2018 we started over 1500 new colonies with daughters from those twenty queens. Come late June, when I asked my sons for help with mite washing, they enthusiastically jumped on board, saying that the last-season’s across-the-board mite washes had actually paid for themselves in the savings from unneeded treatments, and resulted in lower varroa levels across the operation — perhaps due to our identifying and culling any hives with extremely high mite levels.
Mite assessment went even quicker this season, since following my article “Smokin’ Hot Mite Washin’,” [[3]] I had built several prototypes to come up with a portable, rechargeable motorized mite washer, which sped up our assessments immensely (Fig. 2).
Figure 2. My assistant Brooke Molina, with the portable mite washer. The washer plugs into a cigarette lighter socket to recharge between yards, and can be easily carried right into the apiary. We’ve performed nearly a thousand washes with this prototype, and absolutely love it. I plan to make it available to all as soon as I can, as it revolutionizes the concept of monitoring for varroa — taking less than a minute to get an accurate mite count. You can see a short video of it in use at [[4]].
Brooke has by now counted enough mites to last a lifetime — often needing to count 50 or more in a cup. But with last year’s potential breeders, their low mite counts allowed us to move very quickly. Zeroes get big smiles!
Practical application: What we found was that with a crew of five, we could obtain alcohol wash counts from a yard in less than a minute per hive — from truck doors opening to doors closing. And instead of being tedious work, it was actually fun to do as a crew — at each stop we all jump out and hit the ground running — each of us with specific jobs, racing against each other, joking and laughing (Figs. 3- 5). Then off to the next yard.
Figure 3. In this snapshot, Brooke caught five of us blasting through a yard for the mid-June first assessment. These colonies had been started as late nucs in April, and had nearly finished drawing the foundation in their second brood chambers. At this time point, any colony with an alcohol wash count of over 2 mites per half cup of bees, or that hasn’t grown to above yard average, didn’t make the grade. This eliminates around 90% of the colonies in each yard from further consideration as breeders. We also use this inspection to take care of any hives that need attention.
Figure 4. In order to keep track of which mite count goes with each colony, we use color-coded cups and matching hive markers (not shown). The person counting mites simply looks for the matching color marker on a sampled hive, writes down the mite count, and then puts the now-refilled cup and marker on the next hive to be tested. The system works beautifully, with minimal confusion.
 Figure 5. Move fast, but don’t rush! My sons found an exceptionally productive hive last season, and suggested that I check it for mites. It was late on a cold day, so I hurriedly took a sample. To my horror, as soon as I dumped the bees into the alcohol, my eye spotted the queen’s abdomen. Unfortunately, there’s no way to save a queen once she gets immersed. The mite wash turned out to be zero, so I immediately grafted 100 daughter larvae in order to save her genetics.
Figure 5. Move fast, but don’t rush! My sons found an exceptionally productive hive last season, and suggested that I check it for mites. It was late on a cold day, so I hurriedly took a sample. To my horror, as soon as I dumped the bees into the alcohol, my eye spotted the queen’s abdomen. Unfortunately, there’s no way to save a queen once she gets immersed. The mite wash turned out to be zero, so I immediately grafted 100 daughter larvae in order to save her genetics.
Our mid-June assessment of 1500 hives in 50+ yards took us several days. Only 171 hives made the grade of having mite infestation rates of less than 1% (Fig. 6).
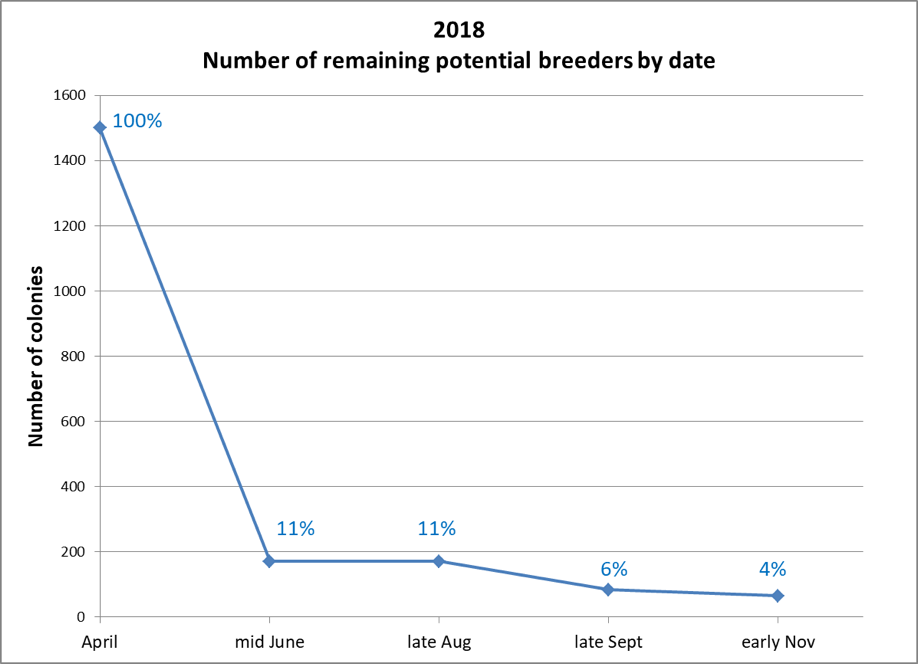
Figure 6. Of our starting 1500 hives, only 171 (11%) made it past the first assessment. By November, there were only 80 (4%) still in the running.
We minimize recordkeeping by simply stapling an index card on the lid of any potential breeder hive (Fig. 7).
Figure 7. I keep it as simple as possible. At the first assessment, any colony making “potential breeder” status gets a card stapled to the hive cover (it doesn’t rain during the summer where we live). We write the dates and alcohol wash count of the hive at each assessment, or immediately eliminate the colony as a potential breeder if the mite count is above what we consider to be acceptable — at which point we remove the card and treat the hive. In case you’re curious, the above hive scored zero mites at the following late March assessment (not shown).
Once we’re down to fewer than 100 potential breeders remaining, I pin a numbered stainless steel tag on the landing board and record all the previous mite counts from the index cards, so that I don’t lose track of the information over the winter (Fig. 8).
Practical application: We’re learning as we go. I want to keep the system as simple as possible, and keep paperwork to a minimum. To date I haven’t done formal tracking of bloodlines, but am planning to make an attempt to do so this season.
Figure 8. I find these off-the-shelf stamped stainless steel tags to be handy to identify potential breeder colonies, since our hives are otherwise unmarked. We record the mite counts from the index card when we apply the metal tags in fall.
Last season (2018) we didn’t seem to experience the same degree of September mite immigration that we had in 2017 — which in that year had knocked around half of our August potential breeders out of the running. In correspondence with Dr. John Kefuss, he suggested that I should have allowed some of those hives to try to recover. So in November of 2018 I retained potential breeders with infestation rates up to 3 mites/hundred bees (Fig. 9), and was glad I did.
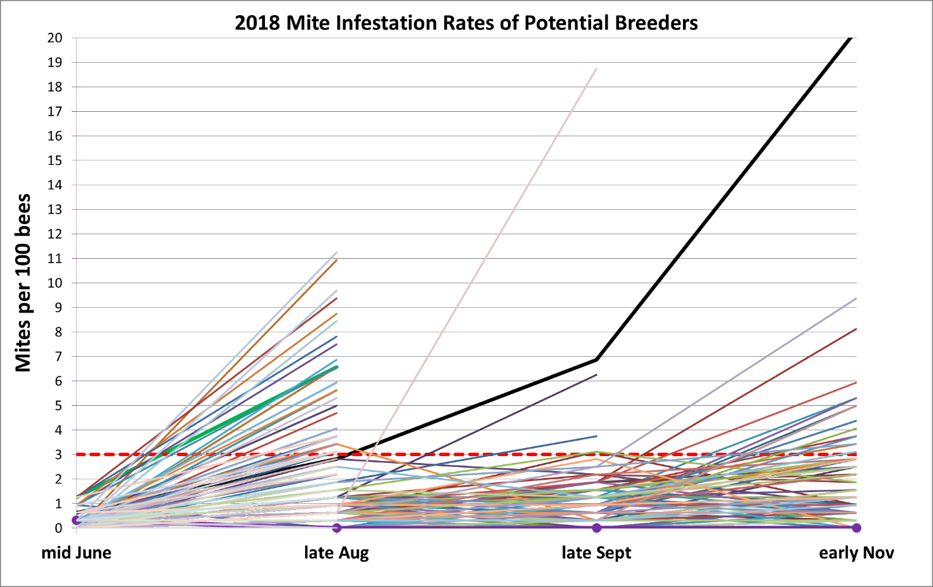
Figure 9. Note the typical trajectory of mite increases, between the June and August assessments, even in a number of colonies tentatively labeled as potential breeders in June. Since we assess these potential breeders roughly once a month, no colonies die from mites in this program — those not making grade are immediately treated with an appropriate organic acid or thymol. The heavy black line represents the average expected progression of mite increase in a typical untreated hive in our area — generally reaching a 20% infestation rate (60+ mites in an alcohol wash) by October. The light pinkish line going up from mid-August is a colony in which the varroa count for some reason exploded. Note how few potential breeders needed to be culled after the late August assessment, despite late-season mite drift.
A visit to France
I’d long corresponded with Dr. John Kefuss — the man who coined the term “The Bond Method” for allowing nature to take its course in selecting for varroa-resistant bees. My own proposed breeding program is modeled on his “Modified Bond” method, applies the same selective pressure, but without the loss of any colonies, so I wound up being invited to present the method to the French National Association of Bee Breeders (ANERCEA). Afterwards, John took me to visit his main apiary (Fig. 10).
Figure 10. Dr. John Kefuss in his main apiary in Toulouse, France, where he invited me to participate in his “World Varroa Challenge” and earn a shiny new French penny for each mite that I could find. This was in November, so it required me to dissect brood in six hives before I could add my name to the list of notables who had earned their penny. John has clearly demonstrated how the application of strong selective pressure can result in the production of stock that requires no varroa treatments.
My spring surprise
We couldn’t resist the offered fee for almond pollination, so took most of our remaining 70 potential breeders to the orchards, where they easily made grade. Most continued to build up even during the miserably-rainy bloom period — we only consider colonies as potential breeders if they perform well. As they returned from almonds, I took mite washes, ready to dismiss those in which mites had built up since the November assessment. The colonies had begun broodrearing in earnest 10 weeks earlier — that’s over 4 varroa reproductive cycles — after a very brief winter brood break. Thus that time period would have allowed the mites to build up. But one must correctly interpret alcohol wash counts:
Practical application: This entire selection method is based upon using alcohol wash assessments to identify colonies that somehow restrict varroa reproductive increase. So it’s important to understand at every time point how the alcohol wash counts relate to the total mite population in the hive. For this, it’s helpful to use my open-access varroa model. [[5]] The model suggests that during the period from January 1 through April 1, that the mite population in a California hive would have built up 11-fold. But due to the large increase in the amount of worker and drone brood present, an alcohol wash count would only double. Take home message: Alcohol wash counts underestimate varroa buildup early in the season, but then overestimate mite population increase once the colonies begin to reduce broodrearing later in the season.
To my great surprise and delight, as of mid-April, 25 of the returned potential breeders exhibited mite wash counts of zero or only a single mite (well less than a 1% infestation rate). Incredulous, I made a point of inspecting the drone brood of these hives to see whether there were mites in hiding. As you can see in Figure 11 (from a representative breeder hive), no mites were to be seen.
Figure 11. Note the absence of mites in the drone brood of this breeder colony after return from almonds in early April. I checked each breeder colony’s drone brood to confirm the results of the alcohol wash.
Practical application: A mite count of zero does not mean that a colony at this time of year is devoid of mites, but it does indicate that the mite infestation rate has certainly not increased to any extent over the course of a full year without treatment.
Selection vs. Bottlenecking
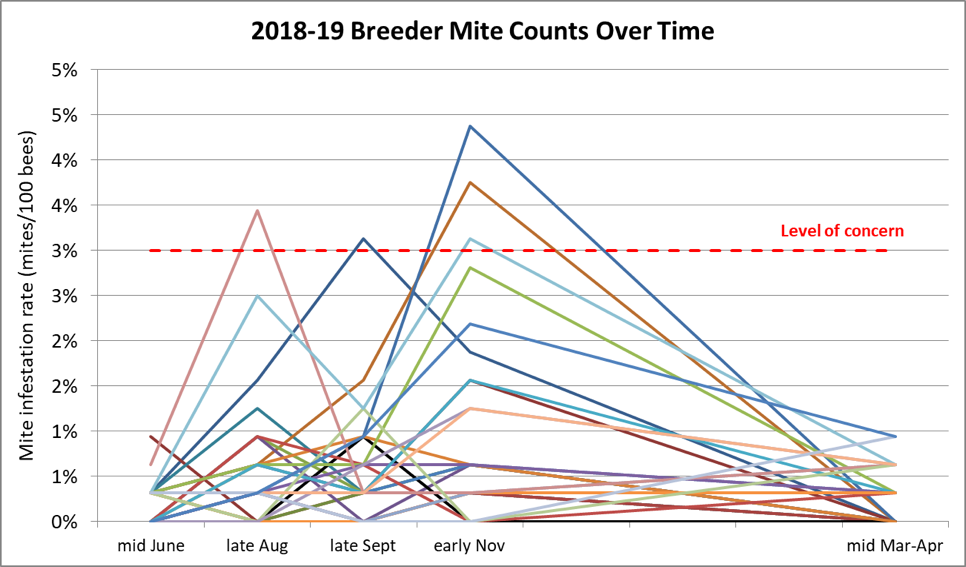
Any selective breeding program consists of bottlenecking the genetic diversity of the breeding population — in this case by applying strong selective pressure for the trait of mite resistance. But in the case of honey bees, we must always keep in mind that one needs to maintain enough diversity in the sex alleles in order to ensure viable worker brood. Thus, I try to strike a balance by breeding off of at least 25 different queen mothers each season. Below are the track records for my 28 breeders chosen for this spring (Fig. 12).
Figure 12. Mite infestation rates over time of the colonies whose queens who made the grade for 2019. Note that of the 28 selected breeders for this season, none exhibited infestation rates of over 1% after a year without treatment. The red dashed line indicates the 3-mites-per-100-bees infestation rate that might have been cause for concern in the fall. The few that I chose to keep despite their showing higher counts at that time were kept in the running because they were exceptionally gentle, productive, and came from high-mite yards. But perhaps most impressive, they then brought those counts back down without help.
I’m also going to graft from a breeder now in her second year without treatment. The incredibly exciting thing for me is that 13 of those breeders never reached a mite infestation rate above 1% over the entire course of a year — again without any treatment. And every breeder had mothered a gentle and productive colony.
Practical application: When I see such lovely colonies handling varroa on their own, it gives me goosebumps!
Choosing the breeders
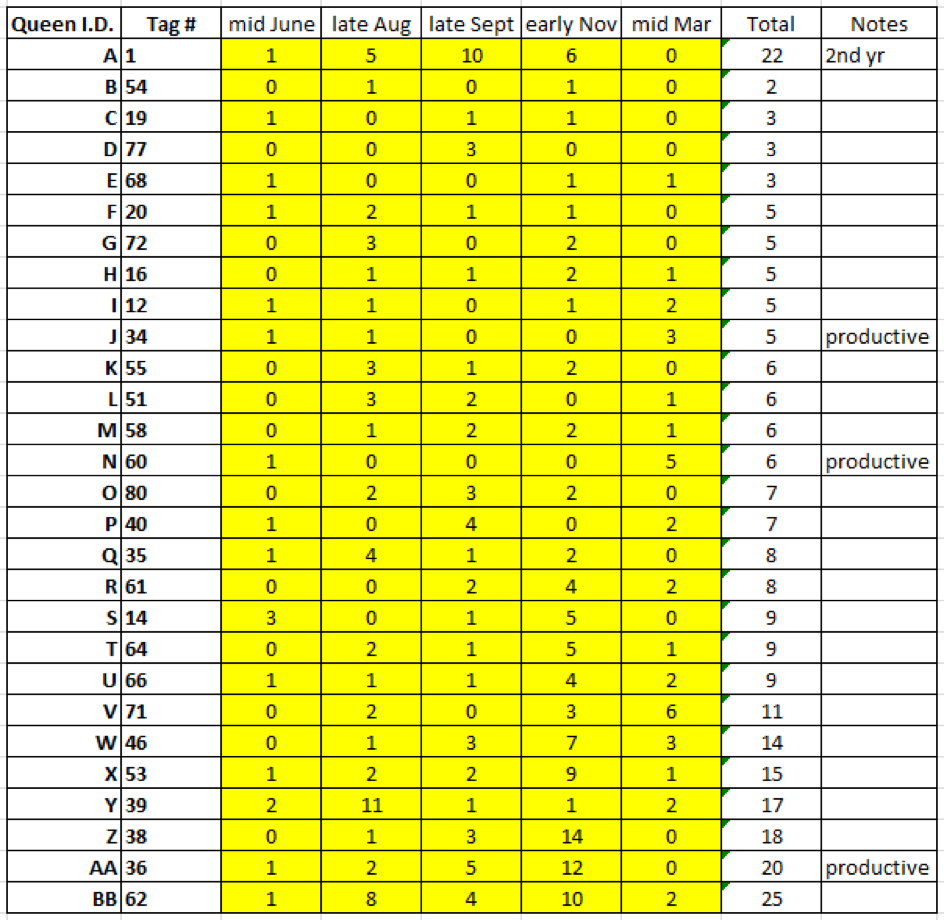
Despite exhibiting low mite counts, no queens were chosen as breeders unless they went to, and returned from, almonds strong and healthy. I entered their returning mite wash counts into my spreadsheet, along with notes from last summer, and then used the sort function to rank them by the combined total number of mites from those five assessments. I then assigned each colony an additional letter name (since we use letter names to keep track of which mothers we’ve grafted from); this also now ranks the breeders alphabetically by preference (Fig. 13).
Figure 13. I prioritized Queen A, whose colony in its second year continued to control the mite. Those that made an exceptional amount of honey last summer were labelled “productive.” Note that colony #54 had a combined total of only 2 mites in the 5 alcohol washes taken over the course of a year. Since I always worry about a breeder queen swarming prior to grafting, I graft from the most desirable first (hence my ranking of them alphabetically).
The big question ― heritability
Hey, colony #54 is a gem, but is that apparent trait of mite resistance actually heritable via grafting from their queen? I have no idea how these colonies managed to keep their mites in check, but if the trait isn’t heritable via her daughters, a selective breeding program would be a waste of time.
Here’s the problem: The trait of “mite resistance” is something that we observe, not a definition of how the bees do it. As an analogy, we may observe that a basketball team appears to express the trait of consistently winning games. It could be pure luck, or something inherent in the team. Different teams may be winners for different reasons — it may be due to a couple of star players, or the team’s offense, their defense, their ability to rack up three-point shots, or the coach’s skill at choosing which players work well together on the court.
Similarly, there are any number of ways that a colony could gain resistance to varroa, [[6]] and as with basketball, it may be something in common for every member of the team, or due to different members having specific abilities that all come together in the hive.
So when I identify a colony exhibiting consistently low mite levels, that may be due to all the workers in the hive expressing the same genes, or it may be due to some lucky combination of sister patrilines (each fathered by different drones that the queen had mated with) all working together.
In order to develop a bloodline of bees that can consistently control the mite, the entire suite of necessary alleles (forms of each gene involved) must be carried by each breeder queen, and perhaps most of the drones that she mates with (this is likely why so many beekeepers wind up being disappointed when they’ve open mated the daughters of instrumentally-inseminated resistant queens).
Practical application: It’s not the queen who fights the mite — it’s a team effort of the various patrilines of workers in the hive. Thus, although a colony may exhibit mite resistance, that doesn’t necessarily mean that new colonies founded by daughters of the resistant colony’s queen will carry all the critical alleles.
In my own program, going into its second season of mating daughters solely from the queens of colonies exhibiting apparent mite resistance, it’s difficult for me to resist a glimmer of hope:
- During breeder selection in 2017, none of them exhibited mite counts of zero the next spring.
- This year, at least 12 did.
So it would be easy for me to think that I’m making progress. But I must temper my excitement, since the other 98% of the hives in our operation still required mite treatments. The big question is, is the apparent [[7]] mite resistance of my selected breeders heritable? Other breeding programs have demonstrated that it can be, so I have hope, but I don’t expect a miracle.
Some things to keep in mind are that not all those selected colonies necessarily used the same mechanisms to control the mite, meaning that when their sisters (yes, next season’s queens are still daughters of their mothers, and hence sisters of the workers) cross mate, those mechanisms may conflict. And even assuming that their apparent resistance is genetically determined, it’s likely that there would be a number of protein-coding or regulatory genes involved, and if so, it then makes a huge difference whether those genes exist on the same chromosomes, and furthermore, whether they are close enough on the DNA strand to be “linked.” Not only that, are those alleles dominant or recessive (in which case the drones would need to carry the same alleles)? And I’m also fighting the high recombination rate of the bee genome during reproduction, which keeps mixing the genes up. [[8]]
Practical application: Apologies for the scientific jargon, but I just want to assure you that I’m not approaching this blindly.
The importance of the drone pool
Over the years, many of us breeders have brought in instrumentally-inseminated mite-resistant queens, only to watch that desirable trait disappear in subsequent generations. So you have every reason to ask, Randy, (1) why aren’t you using instrumental insemination, and (2) what makes you think that you can exercise control of the open matings of the daughters of your selected queens? My answer to the first question is: because I want to keep it as simple and inexpensive as possible. As far as the control of matings, luckily, some USDA researchers answered that question back in the 1990s by cleverly allowing daughters of double-recessive cordovan queens to open mate in or around commercial queen breeding apiaries. [[9]] Their conclusion was:
We consider 90-95% to be a realistic level of mating control that most queen producers will be able to attain without substantially modifying existing practices.
Practical application: I have the greatest respect for our commercial queen producers — they serve our industry well. But if you’re going to seriously breed for mite resistance, you need to go all in.
The point is, that in order to control matings, you must restrict the production of drones in all nearby colonies solely to daughters of selected breeders (similar to how the producers of the hybrid Starline and Midnight queens did back in the day). In the case of breeding for mite resistance, that means that every queen in the operation must come from the mother of a colony exhibiting resistance.
Practical application: 90% of control of matings is good enough for me. We replace every one of the queens in our operation with daughters from selected breeders each season, and run a drone frame in our hives when we go to almonds, resulting in colonies bursting with drones. We thus flood our mating yards year after year with our own “selected” drones. My hope is that this will result in balancing our inbreeding for mite resistance vs. the maintenance of sufficient genetic diversity in my breeding population.
The above said, I was curious as to just how much progress I might expect from open mating.
A Primer on Bee Genetics
The production of eggs and sperm during meiosis involves a mixing of alleles from the father with those of the mother, in order to create novel genetic combinations. In honey bees, the mixing up of genetics each generation is amplified, due to the multiple matings by the queen, and the bees’ unusually high rate of genetic recombination. Such genetic blending helps to allow honey bees to rapidly evolve, but may make it tough to breed for specific traits.
Half of any queen or worker’s alleles (on average) come from her mother, and half from only one of the many drones that her mother had mated with. And I have no idea which drones carried the alleles critical for mite resistance. Thus, without incorporating single-drone instrumental insemination into a breeding program, it may be more difficult to select for the critical alleles involved. I’m fully aware of this, but choose to stick with open mating since that’s what Joe Queenproducer can easily do.
That said, his/her only control of the genetics of the drone pool is by making sure that all drones in that pool come from daughter queens produced in the previous year solely from larvae of queen mothers of colonies exhibiting strong mite resistance — knowing full well that not all of those drones will necessarily carry alleles for mite resistance.
Practical application: Each season’s virgin queens will supply all the genetics for the next season’s drones — I’m counting on this, rather than using instrumental insemination. But since queens come from fertilized eggs, half the genes of the daughters of a breeder queen come from their mother, and half from one of the drones that their mother had mated with (the new drone’s grandfathers) — but no telling whether that particular drone carried genes for mite resistance. Thus, even with complete drone flooding, the best that one can hope for is to shift the genes responsible for mite resistance in the drone population by less than 50% each season.
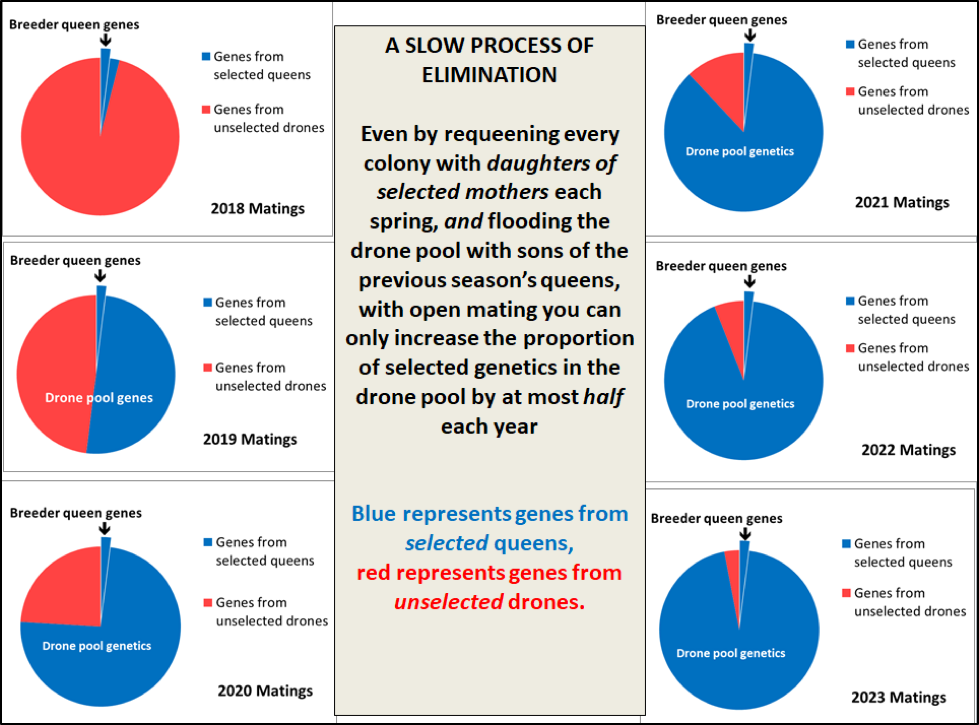
In order to illustrate this visually, I created the diagram below (Fig. 14) in which, I’m selecting a small percentage of my stock to be breeders each season (surprisingly, that percentage doesn’t affect the proportional progression illustrated). The red area of each pie represents alleles from unselected colonies; the blue area represents alleles carried by the selected queens (although not necessarily alleles for resistance). The chart assumes a best-case scenario of a full 50% shift of the genetics of the drone population each season — but that shift does not necessarily represent the degree of shift of the genes responsible for mite resistance.
Figure 14. Even though I selected the queens from our most mite-resistant colonies as breeders last season, their daughters mated with a drone pool coming from 98% unselected stock. The virgins that we produce this season will mate with a drone pool in which half the genes will now trace back to those from selected queens.
Practical application: The above illustration represents the absolute best case scenario for genetic progress, since I have no idea as to how many genes are involved in the mite resistance of my breeders, nor whether all the breeder colonies depended upon the same genes. Thus I certainly don’t expect to see much improvement in mite resistance for at least another couple of years, since it will take a while to fully shift the genetics of the drone pool. But seeing how many apparently-resistant breeders I have this spring, I can’t help but being “guardedly optimistic” about what I hope to observe in 2020.
Bottom line
Can breeding for mite resistance be a simple and straightforward process that does not require scientific training or loss of colonies, and only minimal expense? In theory, yes — indeed, this sort of “directed evolution” was how all breeds of vegetables, fruits, and livestock were created prior to Gregor Mendel’s explanation of inheritance. What I’m doing is to see the extent of progress that a thousand-hive operation can expect to make in a reasonable amount of years.
Practical application: In order to prevent loss of sex alleles while applying strong selective pressure, it will likely take having at least 1000 hives in a breeding program. This is why I’m targeting commercial queen producers.
Note to hobby beekeepers: I say this to make clear that beekeeper Jane or Joe Treatment-Free is dreaming if she or he think that allowing their colonies to die from lack of mite management is going to improve honey bee genetics overall. If they want to improve honey bee genetics, they’d do best to treat their hives to keep them healthy, but put pressure on whoever they purchase queens from to seriously select for mite resistance.
Note to commercial queen producers: I’m making no promises, but am merely sharing what I learn as I determine whether my proposed simplified selective breeding program is worth pursuing. If it proves out, it’s so simple and cost effective that it will eliminate all excuses for you not to be seriously breeding for resistance. If it doesn’t work, rest assured I’ll let you know as well.
I gotta tell you though, seeing these strong, gentle, productive hives that are able to maintain minimal mite levels over the course of an entire year of near-continuous broodrearing in California has got my crew danged excited about the possibility of a future in which we would no longer need to worry about varroa and its associated viruses.
Acknowledgements
Thanks to my hosts in France ― John Kefuss, Yves Le Conte, Benjamin Poirot, and Gabrielle Soland, with whom I discussed this proposed breeding program. Also Albert Robertson (Saskatraz queens), and all the beekeepers and queen producers supporting me in performing this project. And as always thanks to Peter Borst for his library assistance.
References
[1] https://scientificbeekeeping.com/the-varroa-problem-part-6a/
[2] https://scientificbeekeeping.com/selective-breeding-for-mite-resistance-1000-hives-100-hours/
[3] https://scientificbeekeeping.com/the-varroa-problem-part-10/
[4] https://www.youtube.com/watch?v=NVcV7ofrwlc
[5] https://scientificbeekeeping.com/randys-varroa-model/
[6] https://scientificbeekeeping.com/guessing-the-future-of-varroa-part-2/
[7] I’m being careful to qualify what “appears” to be resistance. My tracking of mite levels over the course of a full year tends to support the assumption that the low mite levels in the breeders are not simply flukes.
[8] “High recombination rates appear to have evolved independently in several eusocial insects, leading to the hypothesis of its association with the evolution of eusociality, particularly caste specialization. For example, high recombination can allow simultaneous positive selection of beneficial mutations and negative selection of deleterious mutations by decoupling the effects of linked alleles.” From:
Dogantzis KA, & A Zayed (2018) Recent advances in population and quantitative genomics of honey bees, Current Opinion in Insect Science https://doi.org/10.1016/j.cois.2018.11.010
[9] Hellmich, R & G Weller (1990) Preparing for Africanized honey bees: Evaluating control in mating apiaries. ABJ 130(8): 537-542.
Hellmich, R, et al (1993) Evaluating mating control of honey bee queens in an Africanized area of Guatemala. ABJ March 207-211.