Sick Bees Part 18A: Colony Collaspse Revisited
Gone, or Just Taking a Breather?
The Anatomy of Colony Collapse
Yet Another Sign of Impending Collapse
Sick Bees Part 18a: Colony Collapse Revisited
Randy Oliver
ScientificBeekeeping.com
First published in ABJ May 2012
In my article on almond pollination last month I pointed out that beekeepers in the U.S. started experiencing increased colony mortality in the mid 2000’s. What made the headlines was an unusual form of sudden colony mortality eventually given the name “Colony Collapse Disorder” (CCD). But this season CCD has sort of fallen off the radar. So perhaps it’s time to look back at what we’ve learned.
Gone, or Just Taking a Breather?
The question is, has CCD now gone the way of previous cases of “Disappearing Disease”—episodes in which some disease caused bouts of sudden mortality, and then disappeared before anyone could figure out what caused it? A number of researchers suspected that CCD would do the same, following the typical progression of a pathogen-induced plague. The surprise was that it stuck around as long as it did.
If CCD is indeed caused by one or more novel virulent pathogens, we’d expect that pathogen’s virulence to be burning out by now. On the other hand, if CCD is caused by an extraneous environmental factor, such as cell phones, GMO’s, or some pesticide, we would not expect to see a change until that factor was removed from the environment.
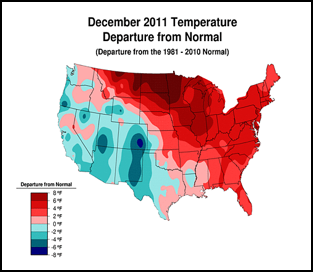
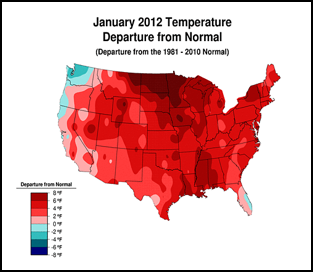
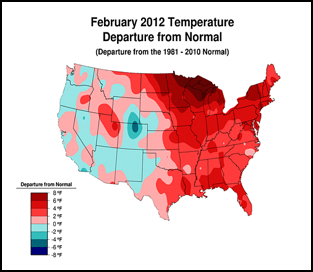
Or perhaps, CCD simply requires enough chilling of colonies to kick it into gear (figure 1):
Figure 1. . The unusually warm winter may have contributed to better colony survival this year. These maps show winter temperature “departures from normal” for December, January, and February. Red areas experienced higher than normal temperatures; blue areas colder. Each color step indicates an additional 2°F deviation. Colony mortality is often related to weather patterns. Source http://www.ncdc.noaa.gov/img/climate/research/cag3/asos-feb2012-nocities.gif.
Practical application: to minimize mortality, winter your colonies in the warmest possible locations—sunny southward slopes, out of cold pockets, or move them to warmer areas.
CCD in Retrospect?
I wish that I could have entitled this article “CCD in Retrospect,” but I’m still seeing some colonies suffering sudden collapse! This winter when I moved my strong hives to almond pollination, I left the dinks behind in my bee yards in the foothills. The apparent reason for many of these colonies being dinks is that they are fighting a virus or nosema infection, which they may eventually get the better of. I’ve learned that if you simply leave them to their ways, they will often dwindle down to a couple of frames of bees, and then either ramp up their antiviral response or gain the upper hand over nosema, and turn around, vibrantly recovering with the first pollen flows as though nothing had happened!
I feel that in many cases in which the queen is blamed, the actual culprit is a virus or nosema infection of the workers, as these queens often show every indication of being able to lay vigorously, given a healthy cohort of foragers to bring home the bacon. In my neck of the woods, if the colony has “turned around” by late February, it will generally be fine. As it happened, late this February I enjoyed a visit by Bee Culture editor Kim Flottum, accompanied by photographer Kodua Galieti. I took them out to look at some of the recovering dinks (Fig. 2). Kodua snapped some photos, which she has generously granted me permission to use—see more of her photos at www.koduaphotography.com.
Figure 2. A recovering dink in late February, building up on early alder pollen. This colony was a bit stronger than three frames of bees, but clearly is on the road to recovery! It does not appear that this colony being a dink was the fault of the queen. But note the barely adequate bee to brood ratio—this amount of bees will have a hard time keeping the brood warm in the event of a cold snap!
Practical application: I find that a low bee to brood ratio is a typical sign of a colony struggling with a virus or nosema infection—sick bees abandon the hive. Should the colony in the photo above manage to hatch out its brood before a cold snap, then it will then have a whole new crop of healthy young bees, and a good crack at complete recovery.
The Anatomy of Colony Collapse
Unfortunately, one of the dinks in the same yard told a different story (Fig. 3). It had also built up similarly to the colony pictured above, but then had a relapse during a two-day cold snap—cold snaps being a common precursor to sudden colony collapse. If the bees and brood get chilled, the colony may go into a rapid downhill spiral. I’ve previously described in detail the progression of colony collapse–see https://scientificbeekeeping.com/sick-bees-part-2-a-model-of-colony-collapse.
Figure 3. This colony is in the middle of a sudden collapse. You can easily see the outline of the brood area, delineated by the crescents of freshly-packed pollen, which must have been covered by bees a few days prior. This colony continued to collapse quickly, and finally died in a cold snap a week later—with only silver-dollar sized patch of dead bees remaining.
Note also the lack of stored nectar around the brood area—this is despite the fact that a nectar flow was on, and adjacent colonies were whitening comb. This is exactly what I observed when I inoculated colonies with IAPV—they would collapse hungry due to lack of foragers, even in the midst of plenty. Plus the bees in sick colonies seem to be unable to utilize honey stored in close proximity to the broodnest. Collapse can then come suddenly (Fig. 4) in the event of even a minor chill, although the sick bees leave the nest, rather than dying in the typical pathetic cluster indicative of starvation (Fig. 8).
Practical application: sudden collapse can happen seemingly overnight. But it is, in my experience, preceded by a low bee to brood ratio and lack of nectar foraging, which are signs to be aware of. Sick colonies may or may not take syrup.
Figure 4. Collapse came quickly, as evidenced by this fresh, white pupa outside the cluster. Abandoned larvae and pupae soon die and turn gray, and can be used to estimate the amount of time since bees were covering the brood.
The queen, however, had not given up (Fig. 5). This is typical in colony collapse—the disease does not appear to be directly attributable to the queen. It’s just that the bees get sick and fly off to die!
Figure 5. The queen appeared healthy and robust, as evidenced by fresh eggs in nearly every single cell in the abandoned brood area (two eggs in some cells). Note the lack of signs of varroa (no guanine deposits, deformed wings, nor partially emerged adults). Also note the presence of “snotty brood.”
Practical application: In deadouts, check to see whether varroa/DWV were the culprits. Inspect the remnants of the broodnest for white guanine deposits on the cell “ceilings,” bees with deformed wings, and partially uncapped mature worker brood, often with deformed wings. However, DWV can also take down a colony with few of those signs present.
I’d like to draw your attention to the snotty brood. The diseased brood generally matched the signs of EFB, but was not quite typical. Dr. Jeff Pettis now uses the catchy term “Idiopathic Brood Disease Syndrome” for this atypical sick brood, which means “we haven’t completely figured out what causes it yet”.
So this poor colony looked like it was going to make it, but then lost its battle with the pathogens. Note that this yard was in a pesticide-free area, and there was no sign of mites.
Relative Risk
Relative Risk: the ratio of the chance of a disease developing among members of a population exposed to a factor compared with a similar population not exposed to the factor.
Dr. Dennis vanEngelsdorp and co researchers determined the “relative risk” of several factors often associated with colony collapse. A factor with a high relative risk doesn’t necessarily mean that the factor will cause the collapse, but rather that it is associated with a higher incidence of collapse (for example cold weather has a high relative risk for colony mortality, even though it isn’t necessarily directly responsible for their death). Determining relative risk is like setting the odds for eventual collapse—a relative risk of 2 doubles the odds that the colony will die.
Practical application: If snotty brood is present, the relative risk of that colony dying is nearly [redacted] times that of a colony without it.
Sorry, when I asked Dr. vanEngelsdorp how to cite the preliminary relative risk figures that he and Dr. Jeff Pettis presented at the major conventions this season, he asked me to hold off until they are more firm on the numbers—so I am respectfully deferring to his request. I find this line of research to be of great potential value to beekeepers, and I want to only publish accurate information!
I’ve currently got a number of colonies with spotty brood and signs of “EFB-like” snotty brood. I’m familiar with the classic symptoms of EFB—curled, twisted young larvae with white tracheal tubes standing out against a yellowish background, slight sour odor, minor ropiness, and drying to a removable rubbery scale. I’d occasionally see EFB in springtime; especially when strong colonies were deprived of pollen due to rain, and then the disease would spontaneously disappear with the advent of a good pollen flow.
But nowadays I’m seeing a great deal of “atypical” EFB. It may be associated with mites and viruses, since the signs are similar to those seen in sick brood in colonies suffering from Parasitic Mite Syndrome (PMS), but I now see it in colonies with low mite levels!
One odd thing is the smell, since this dead brood gets really putrid stinky! The scientist who first identified the bacterium responsible for EFB was G.F. White (yes, the same guy who identified Nosema apis). Dr. White stated that EFB “is characterized by the death of the brood during its uncapped stage…In advanced cases the disease may be accompanied by an odor, but in the writer’s experience this never has been marked and never offensive” [1]. But other bacteria are commonly associated with EFB; perhaps these are causing the differences in smell and appearance. An excellent page on the diagnosis of EFB (with color photographs) can be found at Extension.org [2].
So I sent samples off to the USDA Bee Diagnostic Lab, and wish to thank Sam and Bart for helpful discussion and a speedy turnaround so that I could make deadline for this article. The samples came back positive for EFB.
In the past several years, I’m seeing what appear to be two different forms of atypical EFB-like brood disease:
Syndrome 1:
- Larvae prior to propupal stage turning bright corn yellow, usually remaining in the “C” shape without much twisting. I’ve heard of this same symptom from many beekeepers across the country.
Syndrome 2:
- A greater proportion of older, rather than younger, sick larvae in the combs, many of them capped over, and having sunken, perforated cappings. These older larvae often turn flaccid without twisting, and eventually melt down into strongly putrid-smelling goo (but with a very different odor than AFB).
- The dead capped brood may melt down into a dark watery pool, rather than the translucent-opaque, slightly ropy goop typical of EFB. The liquid also really stinks with a putrid, rather than sour, odor!
- Capped pupae dying in the same colonies. EFB should not kill pupae [3], since the larva sheds its infected gut lining when it pupates. However, these pupae may be infected by something else, such as a virus—they look very much like pupae dying from DWV.
- The disease does not necessarily go away with a pollen flow.
Practical application: Some of these symptoms can be confused with those of AFB—I was surprised when I sent a very similar-looking sample to the lab last season and it came back positive for AFB! My quandary is that I wish to burn any AFB combs, whereas EFB responds well to the antibiotics oxytetracycline or tylosin (I hear anecdotally that OTC gives better control, plus has less chance of getting into the honey). A brood break, such as by making splits, may help as well.
Update 9/5/2012
Bee Parasitic Mite Syndrome (BPMS)
First reported when bee colonies were stressed by varroa mites, the name BPMS or PMS was given (189) to explain why colonies infested with both HBTM and varroa were not thriving. BPMS may be related to both mites vectoring a virus, such as acute paralysis virus (106, 108). The symptoms, which can be present any time of the year, include the presence of mites, the presence of various brood diseases with symptoms similar to that of the foulbroods and sacbrood but without any predominant pathogen, American foulbrood-like symptoms, spotty brood pat- tern, increased supersedure of queens, bees crawling on the ground, and a lowered adult bee population. Although BPMS remains an enigma, feeding colonies Terramycin (antibiotic) syrup or patties and pollen supplements and using resistant bee stock have shown promise in keeping bees alive.
Diana Sammataro, et al
Annu. Rev. Entomol. 2000. 45:519–548
* * *
The ectoparasitic mite V. destructor impairs both brood and
adult bees causing a non-uniform disease pattern called
varroosis or parasitic mite syndrome and including a
specific form of brood damage termed “snotty brood”
(Shimanuki et al. 1994). The symptoms of varroosis are
dependent on the rate of mite infestation of a given colony
and on viral infections vectored to individual bees by the
parasitizing mites
Honey bee pathology: current threats to honey bees and beekeeping
Elke Genersch
Appl Microbiol Biotechnol (2010) 87:87–97
The symptoms that I see these days are similar to the PMS that I saw in the mid 1990’s, but occur without the associated high mite levels.
The Holst Milk Test
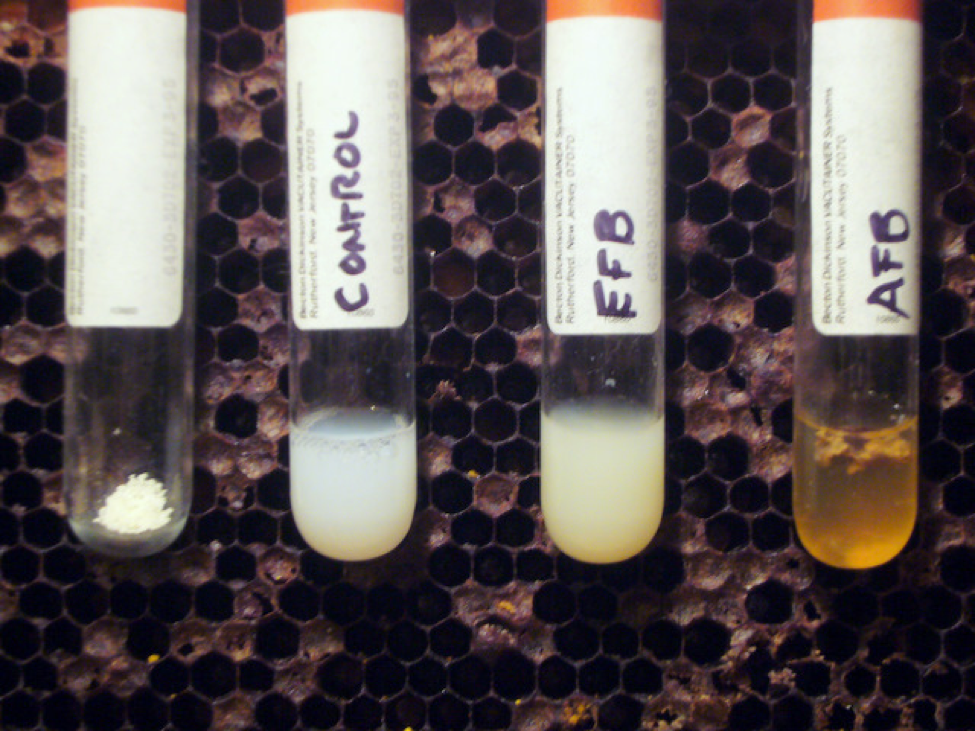
While I was waiting for the lab results, I tried a diagnostic technique that I’d never done before—the Holst Milk Test for AFB [8]. This test detects the presence of the strong proteolytic enzyme that the sporulating AFB bacteria release in order to melt down the larval bodies in the last stage of infection. The enzyme will also break down milk protein—and “clarify” a weak solution of powdered milk within minutes. Luckily, I had a comb of obvious AFB on hand to show to my beginners classes, so I tested fully decomposed suspect EFB-like larvae against known AFB scale. The results were impressive (Fig. 6)! I highly recommend this test for beginners who suspect AFB in deadouts.
Figure 6. The Holst Milk Test—a simple field technique for diagnosing AFB from dried scales. Put a tiny pinch of nonfat milk powder into a clear tube, add ½ tsp water, and then add one or two well-decomposed suspect larval remains (scale). Keep the tube at body temperature for 20 minutes, shaking occasionally. In the background is a typical comb from an AFB deadout. See [8] for additional details.
I experimented with the test a bit. The more concentrated the milk solution, the longer it takes AFB to clear it—sometimes overnight (use less milk powder than in the photo above for a quicker field test). EFB will cause the milk to clump, but you don’t get the clear “apple juice” look as with AFB.
Update March 2015: The amount of milk powder shown in the photo above is more than needed–use only enough to make the liquid translucent white. A couple of drops of liquid milk works even better than dry milk powder.
Treating for nosema
Speaking of treatments, since I recently found a correlation between weak colonies and the prevalence of nosema (percentage of bees infected), I tried treating all my dinks once a week from late January through early February with a drench of fumagillin (Fig. 7), 3-4 times total [4].
Figure 7. I find that using a garden watering can to apply medication by drench is very quick. Since I only apply ¼ cup to small colonies, I stand little chance of contaminating my spring honey. Multiple weekly drenches spread the medication out over an entire brood cycle. Take caution if you add essential oil “feeding stimulants”—they can cause wetting and drowning of bees! Note the uneaten pollen supplement patty.
Because I had treated these dinks recently against nosema, I assumed that I could rule it out as the cause for the collapse. But beekeeping certainly has a way of making an ass of you when you assume anything! As I’m writing this article, it occurs to me that I should go back to the yard and grab a sample of the remaining dead bees to test for nosema…
O.K., I’m back now– Result: 8 out of 10 of them had their guts loaded with spores! And one contained amoeba cysts to boot. That was an eye opener!
Dr. vanEngelsdorp found that even a relatively low level of nosema (1M spores in the house bees) increases the odds of a colony collapsing by a factor of [redacted]. More to the point, having 8 bees out of 10 infected is a death sentence for a colony! Oh, and by the way, there was no sign of dysentery.
Disclaimer: Now please don’t get me wrong—I’m not saying that Nosema ceranae is the cause of CCD! It is not necessarily present when colonies suffer sudden collapse—the viruses are quite capable of doing that on their own!
Practical application: The “spring turnover” of colony population, during which the aged winter bees must rear replacements, is a dicey time for weak or sick colonies. The old bees must not only fight parasite buildup and winter cold, but must also generate heat for the broodnest, and produce jelly in order to rear healthy young bees to take their places. If they don’t pull this feat off, the colony can quickly crash! This colony collapsed during a mild California foothill winter, with a good nectar and pollen flow on, interspersed with occasional bouts of snowfall. The chill events appear to be the fatal factor.
Practical application: My guess as to why fumagillin did not clear the infection is that I was simply too late in applying it—the infection had already seriously taken hold, and this colony was simply unable to replace the infected bees with healthy ones in time. But remember, most of the dinks recovered quite handily!
As we looked through the dinks, we came upon some with yet another indicator of impending collapse—the presence of supersedure cells (Figures 8 and 9).
Figure 8. This is a two and a half-frame dink in late February with numerous supersedure cells. And yes, I realize that they are on the bottom bar, but this colony was in no shape to swarm, and had no drones yet. A healthy virgin emerged from a ripe cell that broke open. The presence of supersedure cells suggests that this colony has a [redacted; but substantial] relative risk of dying.
I commonly see unexpected supersedure cells in heavily infected colonies—it doesn’t really seem to matter what the pathogen or parasite is. I’m not sure whether the bees are blaming the queen for their misery, or whether the queen gets infected and puts out a pheromonal signal to replace her.
Figure 9. Here is yet another dink that died with supersedure cells (I’m pointing to two), despite the presence of an apparently healthy queen (see Figure 10). You can see that this colony had recently covered a fairly large brood area. Note also the remains of a pollen patty fed at an earlier date.
Of even more import is that some risk factors may synergize. If you multiply the relative risk due to a nosema infection times the relative risk due to the presence of supersedure cells, you then get a combined relative risk of [redacted; but much higher].
Practical application: the combination of a nosema infection plus supersedure cells does not bode well for a colony’s survival!
Figure 10. A close up of the dead cluster—you can see the queen near the top center. In none of these deadouts were there any appreciable numbers of dead bees on the bottom boards. Note the absence of “heads in the cells” typical for starvation.
As I’m looking at this photo I realize that my readers would be curious as to whether this colony also tested positive for nosema, so off I go again back to the same yard (through the rain—what I do for my readers)…
O.K. I eventually found this exact same frame of dead bees, and squashed the abdomens of ten of them. Wanna guess? Nine of them were chock full of spores! So the infection had clearly moved into the last few young bees. Unfortunately, I had given the dead queen to Kodua to photograph, so couldn’t test her (the queen) for spores.
Practical application: don’t count on dysentery to be a reliable indicator of serious Nosema ceranae infection—it isn’t, although I see it occasionally over the snow in front of infected colonies. If you’re not testing your dinks and deadouts for nosema, you have no idea as to whether Nosema ceranae is causing winter/spring mortality!
Practical application caveat: over the past few years when I’ve tested the few remaining bees in collapsed deadouts, nosema was generally not evident (leading me to conclude that nosema was not a problem). In those cases, collapse was likely due to a virus infection. I’m not clear as to why this year is different, although my nosema levels have been ramping up each year (I hadn’t been treating against nosema). Overall, my bees this year have not been at their best, although a strong majority went to almonds and look fine. I’ll spot test them when they return (I’m typing these words in early March).
Pollen Supplement Patties
I haven’t heard the researchers mention it, but I observe that yet another indicator of elevated risk for later collapse is that the colony does not consume pollen supplement in the fall. Beekeepers have long noticed that untouched protein patties are generally a sign of queenlessness, but I’m finding that they are also often a sign of a sick colony. Most all of my dinks had uneaten patties on the top bars in February (Figs. 7, 9, and 11).
Figure 11. It seems that I could largely predict which colonies would eventually dwindle or die by whether they consumed protein patties in fall (the size of this patty suggests that the cluster was much larger when we put it on—we only put patty over seams filled with bees). Healthy colonies gobble supplement up, leaving no scraps. We checked every colony that didn’t consume their patty in the fall to confirm that they indeed had a laying queen; yet few that had patty remaining in February were strong enough to go to almonds.
Practical application: a good indicator that a colony is going downhill is that it doesn’t consume protein patties. It might be a very good idea to quarantine those colonies to an isolated “sick yard” (like far away from my hives in the almonds!).
Yet Another Sign of Impending Collapse
I thought that I was perhaps the only one who noticed this one, but in conversation with other beekeepers who suffered from CCD, I found that others also observed that the cappings over the brood sometimes turned a very dark reddish tint, and instead of being slightly rounded outwards, would be flat. The brood appeared to be perfectly healthy under the cappings. I found this to be a sign that a colony was in trouble. However, I didn’t notice it when we later experimentally caused colony collapse with IAPV, nor do I notice it with colonies collapsing from Nosema ceranae.
Ag Exposure as a Risk Factor
Oh man, is this a touchy subject, with some very strong and adamant opinions out there! Beekeepers have long noticed that colonies set in certain agricultural areas go downhill, or may not make it through the following winter. Sometimes a certain pesticide is clearly to blame; other times it may simply be due to the lack, or poor nutritional quality, of the forage in the agricultural area, especially in this age of “clean farming” and wall to wall corn or soy.
When I first published my model for colony collapse, mentioning that some pesticides become more toxic to bees at cooler temperatures, Dr. Eric Mussen sent me this note:
“When Dr. Eric Erickson was employed at the USDA laboratory in Madison, WI, he conducted a small demonstration study based on observations reported by a number of nearby beekeepers. The beekeepers told him that when their hives were located near commercial corn fields and permethrin was applied to the crop during “bloom” (tassels producing pollen), the colonies did not survive over winter.
“Dr. Erickson had half the lab colonies moved into an area near a corn field and had an application of permethrin made during bloom. No noticeable bee loss was noted in the apiary at the time. The colonies were then returned to the lab apiary and all colonies were prepared for winter. During the winter all the permethrin-exposed colonies died, whereas all the stay-at-home colonies survived. It wasn’t clear as to whether the mortality was due to the corn pollen, the pesticide, or something else, but the point is that colonies foraging upon sprayed corn fields in tassel exhibited a delayed effect of high winter mortality–greater than that of the colonies that were not exposed to the treated corn.”
Practical application: in the registration process of pesticides, they are normally only tested on bees at about 80°F. We really have little idea as to the effect of pesticides upon bees at 45°F—the temperature of the bees in the outer shell of the winter cluster!
I recently attended a presentation in Oregon in which commercial beekeepers described the number of pollination contracts that they moved their bees to during the summer, and more to my interest, the sheer number of pesticide applications that the bees had to deal with. To my mind, it’s a wonder that their bees survive at all!
Practical application: some ag crops in certain areas are the kiss of death to bees, but the effect may not be noticed until winter. This is a complex subject, having to do not only with pesticides, but also with colony population dynamics, parasite dynamics, nutrition, symbiotic fungi and bacteria, pesticide and miticide synergies, pesticide/pathogen synergies, and who knows what else! I doubt that the problem has a simple answer.
Dr. Mark Carroll of the ARS is currently engaged in a project called “The Costs of Following the Bloom–Nutrient Processing, Microbial Dynamics, and Colony Health in a Migratory Beekeeping Operation” [5]. I hope that his research can help to answer some of the above questions. In a recent presentation, he spoke of “overextended bees” in migratory operations, suffering from poor nutrition and parasite buildup. Perhaps he should also have spoken of “overextended beekeepers,” who are simply trying to run more colonies than they can properly manage!
There is a raging debate as to whether the systemic neonicotinoid insecticides are causing CCD, fueled by a great deal of conjecture and hyperbole that is often confused with fact. I’ll discuss this in my next article.
Reliving History
There is nothing new about the phenomenon of sudden colony collapse. In 1897 R.C. Aikin, from Colorado, described a similar phenomenon [6]:
“In [May] of 1891…I had been watching carefully the progress of brood rearing, and had the colonies quite strong in both bees and brood. … Many colonies were so strong that they were clustering out, although we had not unpacked yet…While they were so, I had looked all over and equalized stores and brood, then was absent about ten days… [when] I went to the out-apiaries to again inspect as to stores… I was astonished at the very few bees in them. I went to some hives that I knew had been clustered out about ten days previously, and I found not enough bees to cover the brood. The weather was warm, the bees packed in chaff, and the few bees left were spread all through the hives caring for the brood.
“Some of the colonies were so depopulated that, when I lifted combs, I could lay my open palm on the face of a comb of brood and scarcely touch a bee. It was not what I understand as spring dwindling. The bees were mainly young, for bees had been hatching for weeks…
“The bees just vanished, and were nowhere to be seen. If they had died in or about the hive, possibly we might have found out what was the matter; but they seemed to evaporate, hence I have called it “evaporation.” The loss of bees was so complete that many colonies had not half a teacupful of bees left, where, less than a week before, they covered brood In three combs and upward. The queens, it seems, were always left; but the workers so completely evaporated that the brood perished.”
Beekeepers have always had to deal with episodes of colony collapse, sudden or not, and depending upon their fortitude and perseverance, use the bees’ biological capacity for rapid increase to recover. This “get over it and get going” attitude is exemplified in a scene from the movie “The Last Beekeeper.” While sitting at a diner, a woman commercial beekeeper is despairing about how CCD has financially ruined her operation; meanwhile, her companions, an older beekeeper couple, between bites of food, just keep saying “You gotta restock, you gotta restock.” That scene to me was the archetype of the attitude necessary for successful beekeeping.
CCD was never actually a serious bee issue—there was little doubt (other than in the minds of fanatics) that the honey bee would survive. CCD was really about whether commercial beekeepers could financially survive such serious losses. And as I pointed out last month, were it not for the “generosity” of the almond growers, many of us would not have!
In my own beekeeping career, my operation has been devastated by collapse events four times: first by tracheal mite in the late 1980’s (to which many beekeepers lost 70% of their colonies); then by varroa (I lost 97% around 1996); then again when Apistan strips failed in the late 1990’s. And this dismal record doesn’t even take into account those El Niño years in which I decimated my operation myself in order to fill my nuc orders! By the time I got hit by CCD in 2005, collapse events were old hat!
However, each of the above events was followed by seasons in which I could recover. But as we picked up each new parasite, recovery got a bit more difficult, due to increased levels of colony morbidity and mortality. Preceding, and concurrent with CCD, our colonies were already experiencing an elevated rate of winter loss over the norm. What the heck is the “norm”? Back in the “good old days,” beekeepers expected to lose maybe 5% of colonies over the winter; perhaps 10% where winters were severe. Losses were mainly blamed upon starvation, queen failure, or Nosema apis.
After the arrival of varroa, the “normal” winter loss rate ramped up to the 15-20% range. CCD (and perhaps Nosema ceranae) notched that average rate up to above 30%–a level at which winter losses really started to hurt! The interesting thing is that about a quarter of commercial operations get hit disproportionately hard, and about a quarter have few problems. Beekeepers and researchers are pulling their hair out trying to find the causes for the difference!
What made CCD stand out was our inability to blame it on the “Usual Suspects.” However, it also became an oh so handy scapegoat for absolving oneself from blame for losses due to mismanagement, poor timing, lack of varroa control, or anything else. I’m not depreciating the devastation and distress caused by CCD in some operations (the film “The Last Beekeeper” is heart wrenching), but most beekeepers found that given enough financial incentive, they could recover and figure out how to avoid major losses.
Practical application: those beekeepers who diligently keep mite and nosema levels down, ensure that their bees get good nutrition, and are not continuously exposed to commercial agriculture tend to have fewer problems with colony collapse (not that I expect you to take that as any sort of revelation!).
As you can see by the blow-by-blow account that I’m giving of my own learning curve, I personally sure haven’t figured everything out—beekeeping presents new challenges every year! I like the recent quote from California beekeeper Henry Harlan [7]:
“If you meet a beekeeper who says he knows it all, his bees will probably be dead next year.”
In any case, Colony Collapse Disorder has presented us with an opportunity to learn a great deal about bee health. I will follow this article with a critical analysis of the suspect causes of both CCD specifically, and increased colony mortality and morbidity in general, followed by a summary of experiments that actually tested various hypotheses.
Acknowledgements
As always, I am deeply indebted to Peter Borst for his help with research, and to my wife Stephanie for her critical reading of my manuscripts. And thanks to Dianne Behnke of Dadant for digging out and scanning old articles from ABJ for me! I am especially appreciative of the worldwide community of bee researchers, who take the time to discuss my questions at length.
References
[1] White, GF (1920) European foulbrood. USDA Bulletin No. 810.
[2] http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.02.03_EUROPEAN_FOULB.pdf
[3] http://www.extension.org/pages/23693/european-foulbrood:-a-bacterial-disease-affecting-honey-bee-brood
[4] The drench consisted of 2 Tbl Fumagilin-B (200mg fumagillin) per ½ gal of 1:1 syrup, which allowed me to mix up fresh batches as needed. At the rate of ¼ cup of drench per dose, each dink received a dose of 12.5mg of active ingredient, which would be the recommended rate for a 3-frame dink to be given multiple treatments.
[5] http://www.ars.usda.gov/research/projects/projects.htm?ACCN_NO=420102
[6] Aikin, RC (1897) Bees evaporated: a new malady. Glean. Bee Cult. 25(13):479-480.
[7] http://www.latimes.com/business/la-fi-california-bees-20120304,0,2026018,full.story
[8] The Holst Milk Test
Holst, EC (1946). A simple field test for American foulbrood. Am. Bee J., 86: 14–34.
The enzyme is produced by the bacteria only when the larvae reach the “ropy” stage or later, and persists in the dried “scale”—it won’t work with larvae at an earlier stage of disease. It is often easiest to simply rip out the entire bottom of the cell with forceps and drop it all into the tube. You can also drop in the twig that you use to test for ropiness. The more diseased larval remains you add, the quicker the reaction; however, if you use only a partial scale, or a twig, then either use less solution, or dilute the milk even further.
The reaction can also be speeded up by warming the water to up to 165°F (as hot as you can hold your fingers in). I could get tubes of ½ tsp of weak milk solution inoculated with a single scale to clear in less than 5 minutes by incubating them in a cup of hot water.
It is easy to make up a field AFB test kit consisting of a pair of tweezers, a vial of milk powder, and some clear glass vials for running the tests. At home, you can use liquid milk (skim preferred) diluted 1:4, again at the ratio of 1 scale per ½ tsp of diluted milk. When first trying this test, I suggest that you run an uninoculated vial of milk solution side by side for comparison.
Note that the test is retarded if the combs have been stored with paradichlorobenzene for wax moth control.