The Varroa Problem: Part 15 – Modeling the Effect of Mite Treatments
Contents
Early-season mite management. 2
Late-summer mite management. 4
The basics of oxalic vaporization. 6
The optimal interval for OA vaporization treatments. 9
Fall-winter mite management. 13
The Varroa Problem: Part 15
Modeling the Effect of Mite Treatments
Randy Oliver
ScientificBeekeeping.com
First published in ABJ February 2018
We beekeepers are nearly blind as to varroa. Yes, we see the occasional mite on a bee, or in torn drone brood. And the more diligent beekeeper may even perform occasional alcohol washes or stickyboard counts. But few beekeepers have anything but a remote grasp of how varroa is actually living and dying in the hive.
All that changed for me when I started mathematically modeling varroa population dynamics. This required me to account for every aspect of varroa life and death. In this article, I’m going to focus upon the death part of the equation—that which the beekeeper causes when he/she applies a mite control measure or miticide treatment.
My modeling of mite population dynamics suggests that if you keep bees in an area in which your colonies rear brood for at least 6 months of the year, that you’ll need to reduce mite levels by 90% at least once each season; if there’s 8 months of brood rearing, this will require two reductions per season, and if more than 10 months with brood, likely at least three reductions over the course of the season. In this article, I’ll quickly hit a few points on early- and mid-season management, and then focus upon late-season treatments, especially oxalic acid vaporization.
In my previous article, I presented a graphic of a varroa management strategy that my sons and I successfully use in the California foothills. But as Maine beekeeper Bill Truesdell is fond of pointing out, “All beekeeping is local.” Thus, I studiously avoid making any specific recommendations regarding hive management. That said, I can share some of the pros and cons of the various choices of treatments used for mite management.
Early-season mite management
In order to help your colonies avoid being forced to deal with a varroa/virus epidemic during midsummer, it’s important to reduce their mite levels early in the season. This is also the time of year that the greatest proportion of the mites are in the brood—which creates both problems and opportunities for management.
BIOTECHNICAL METHODS
USE RESISTANT STOCK
For beekeepers who run truly mite-resistant stock, varroa may not be a serious problem, so long as the bees themselves are able to keep the mite from multiplying to levels that allow viruses to run rampant.
Practical application for those using mite-resistant bee stock: it’s sooo easy to delude oneself—just because you “believe” that your stock is mite resistant, you can’t really be sure unless you confirm it by checking. In order of reliability, I’d suggest alcohol wash, sugar shake, accelerated mite drop [[1]], or stickyboard natural drop, in that order. If you don’t deal with mite-infested colonies early, when they inevitably then collapse later in the season they may overwhelm any truly varroa-resistant hives with an influx of mites. A quote from the Cookeville Beekeepers perhaps says it best: Treatment Free beekeeping and just hoping for the best while doing nothing are NOT the same thing.
BROOD REMOVAL
As colonies approach swarming season, my modeling suggests that some 80% of the mites may be hidden—and actively reproducing—in the worker and drone brood. Although this makes miticide applications less efficacious, it does allow for the physical removal of mites from the hive. Removal of a single drone brood trap frame can potentially remove 15-20% of the mites.
Practical application: removing 15% of the mites certainly helps, but a more realistic target would be to reduce the mite population at this critical time by 90%.
SPLITTING
When a swarm issues from a colony, that may reduce the subsequent buildup of the parent hive’s mite population by up to 50% over the rest of the season [[2]]. On the other hand, a natural or shook swarm will start with a much lower mite infestation rate than the remaining parent hive, since the phoretic mites that it carries would constitute only about 15% of the parent hive’s total mite population. The removal of shook bees or brood for nucs can also set mite buildup in the parent hive back substantially [[3]].
Practical application: The low starting varroa burden gives a swarm a leg up on the mites, compared with the parent hive, which got stuck holding most of the bag. But keep in mind that if that swarm is successful at establishing itself, it will still likely eventually succumb to varroa. The last thing you want is for your escaped swarms to be later sending their mites back to your hives as those swarm colonies inevitably collapse.
Dividing a hive equally into two, results in each split starting with only half of the original mite population (I’ll let you figure out the math by yourself). Even better, if you start the splits with queen cells (or make walkaway splits), you would be able to take advantage of the brief window of opportunity when there would be no sealed brood in the split in which varroa could hide. This occurs at around 18-24 days after the split is made [[4]]. By applying an oxalic dribble or vaporization during this window, one can achieve a 95% reduction in a hive’s mite population.
Practical application: my sons and I have now used the above method for six years, on thousands of nucs each spring, with great success.
Unfortunately, such an oxalic treatment would be of little help to the queenright split, since that hive never gets a brood break.
A CLEVER OPTION FOR SMALL-SCALE BEEKEEPERS
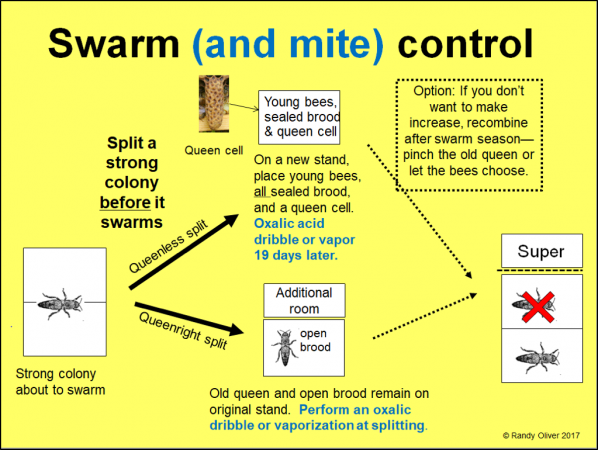
For those willing to locate the queen and swap around the brood frames, there is an option that not only reduces the varroa population in both the parent and split by 95%, but also suppresses swarming, requeens at least one of the pair, and allows you to make an increase only if desired (Fig. 1).
Figure 1. By placing all the sealed brood into one (or more) queenless splits, you can obtain high-efficiency mite reduction with carefully-timed oxalic acid treatments even in springtime. The timing is critical in order to hit the mites when they aren’t hidden under the cappings. If the splits are forced to rear their own queens, then you’d get even better mite control by waiting 24 days ‘til you treat.
SUGAR DUSTING, HEAT TREATMENT, ETC.
I couldn’t in good conscience recommend sugar dusting in the spring, due to its low efficacy at that time. And I have no experience with heat treatment or the new sonic mite killers.
MITICIDES
Again, due to the majority of the mites being in the brood in the spring, for good efficacy you need to apply either a time-release miticide, or formic acid—the only miticide that kills mites under the cappings
Time release treatments: Apivar® (amitraz) strips, or perhaps back-to-back treatments with Hopguard® can work. Extended-release oxalic acid treatments show considerable promise, and we’re working hard to get the oxalic/glycerin formulation registered for use. Thymol treatments (Apiguard® or ApiLife Var®) could be used, but have the disadvantage of suppressing brood rearing during the course of the treatment. Note that of all the registered treatments above, only Hopguard can be used during a honey flow.
Formic acid: Due to the typically lower temperatures at this time of year, formic acid (MAQS or other application methods) can be very effective, and MAQS has no restriction against its application during a honey flow.
Practical application: due to its lack of residues, proven efficacy, and relatively brief brood rearing interruption, formic acid has a lot going for it as a spring treatment. Small-scale beekeepers can protect the queen by setting her aside during treatment, either in a tiny nuc, or in a queen cage in the house. Tip: use a pair of 7.25” stainless steel medical bandage scissors [[5]] to open MAQS wrappers–the angle of the handles and the blunt ends makes opening the packs a breeze.
Mid-season mite management
While you’ve got honey supers on the hive, you’re really limited as to mite treatments. Formic acid is likely the best choice, although repeated sugar dusting is an option. During hot weather, in order to avoid queen loss, some apply only a single MAQS strip for a mite “knock back” until they can pull the honey. What I’ve observed is that even with a strong formic treatment in hot weather, it is usually only already-failing queens that get lost, and the colonies typically successfully replace them.
Late-summer mite management
This is the most critical time for mite management—you want to get the mite count down close to zero at least six weeks before the colony rears the last rounds of brood destined to become its winter bees. As a general rule, figure mid-August treatment at the latest. If you need to wait, buy time with MAQS or a formic flash.
If your mite levels are high, slow-release formulations such as Apivar or Hopguard may not do the trick quickly enough. Although I’m aware that some beekeepers are still getting good results with off-label amitraz, I’m not going to discuss illegal treatments here.
Practical application: a glaring problem faced by many is the amount of mite immigration coming into managed hives in late summer and fall. I’ll be writing at length about this in an upcoming article, but suffice to say, that even if you completely control mites in your own operation, some of your hives may still be invaded by hundreds to thousands of mites from neighbors and collapsing feral colonies. This makes late-season mite management very difficult in some areas with large numbers of beekeepers. This mite influx may call for an additional “clean up” treatment.
THE “FORMIC BLAST”
It’s difficult to turn a “varroa bomb” around this late in the season. The virus epidemic is entrenched, and without immediate kill of all the mites in the hive, the colony is more of a threat to the rest of your hives than it’s worth. So in our operation, we simply break our mite bombs down into singles, and apply 300 mL of 65% formic in a time-release plastic bag (or 3 MAQS) in a rim on top.
Practical application: this harsh treatment kills all the mites in the brood, but not the sealed brood itself, nor the adult bees (but does kill some queens, whose genetics we don’t really want anyway). After treatment, we then place the now mite-free box of bees and brood on top of a strong nuc with a fresh queen with very good success. We love the hard formic blast—quick, cheap, no residues, no mite drift; a fresh start.
A day-by-day model
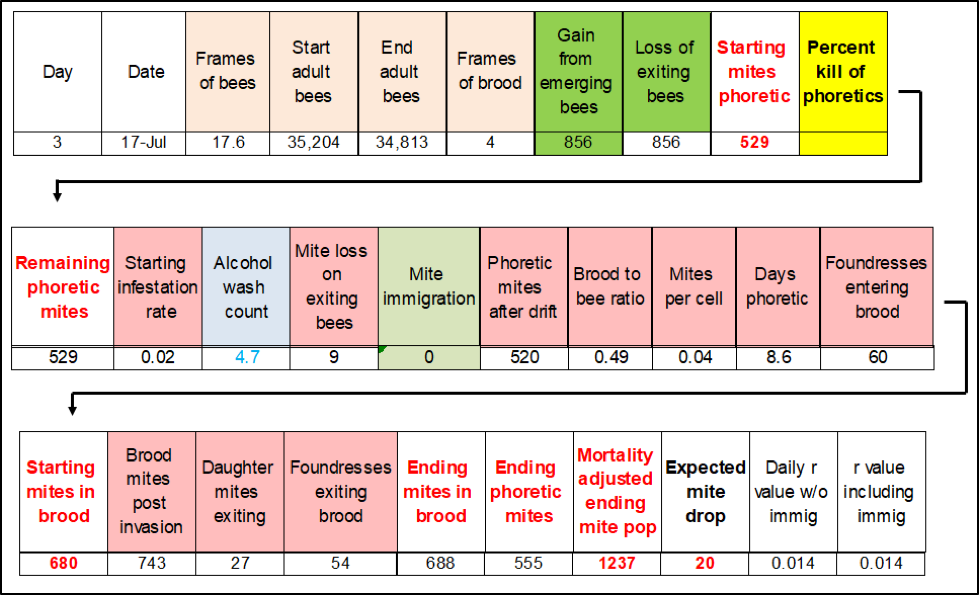
I created Randy’s Varroa Model [[6]] to be user friendly by limiting it to calculate changes in the mite population only twice a month. This works fine when one can input the overall mite reduction from an occasional treatment. But when I started getting questions about how to input two oxalic acid vaporizations within one 15-day time period, I needed a model that calculated on a daily basis. Such a model is unwieldly, since one must input daily values for the bee and brood areas. Anyway, I’m developing a simplified model that takes into account daily immigration and emigration of mites, as well as the number of foundresses entering a cell each day, and the number of daughters emerging (Fig. 2).
Figure 2. The flow chart for the simplified basic model. The outputs can then be checked against stickyboard counts and alcohol washes for validation of accuracy.
With a model such as this, I may be able to answer questions about the optimum spacing of short-term oxalic acid treatments.
The safest and simplest way to apply oxalic acid is as a dribble, but it is truly effective only when applied during a natural or induced brood break. And as evidenced by Buddy May’s recent field trial [[7]], repeated dribbles may be tough on the colony. Oxalic vaporization (sublimation) may get around this problem, as it appears to be a bit gentler on the bees. It’s also attractive to beekeepers who want to avoid opening their hives to apply a treatment. Anyway, oxalic vaporization is a hot topic among beekeepers, many of whom may not quite understand the details.
The basics of oxalic vaporization
In the first place, you can’t see oxalic vapor—it’s invisible, and being produced at around 350°F, it would cook any bee that it came into contact with. It’s only the cooled fog of condensing oxalic micro crystals that you see. Those crystals float in the air, eventually settling on, or sticking to, surfaces with which they come into contact—such as comb surfaces or the bodies of the bees (Fig. 3).
Figure 3. A bee covered with microcrystals of oxalic acid. Surprisingly, bees tolerate such exposure with minimal adverse effects—perhaps because there is no sugar involved to encourage them to ingest it. On the other hand, the mites are apparently killed by the absorption of oxalic acid from crystals with which their sticky tarsal pads come in contact [[8]]. Photo courtesy Chamblis, Wikimedia Commons.
Keep in mind that unless that fog of crystals actually penetrates the cluster to make contact with the bees inside, it likely won’t have much effect upon varroa. When using a slow vaporizer such as the Varrox®, the cluster may open up enough to allow the warm fog to be carried inside by the convection current, resulting in better penetration of the acid into the cluster. In cold weather, however, when the bees are in a “hard,” relatively airtight cluster, it may require a forced air stream to get the fog into the cluster [[9]]. I question how well the 20-second blasts from some of the “fast” vaporizers touted on the internet actually penetrate the cluster—if you have checked bees from inside the cluster under a ‘scope after such a vaporization, please let me know.
Practical application: later in the season, as the colony is reducing its broodnest, 40-50% of the mites may be phoretic at any time, making oxalic vaporization a reasonable candidate for mite reduction. That said, confirm that your application method is actually getting to all the phoretic mites—perform an alcohol wash a few days after your vaporization.
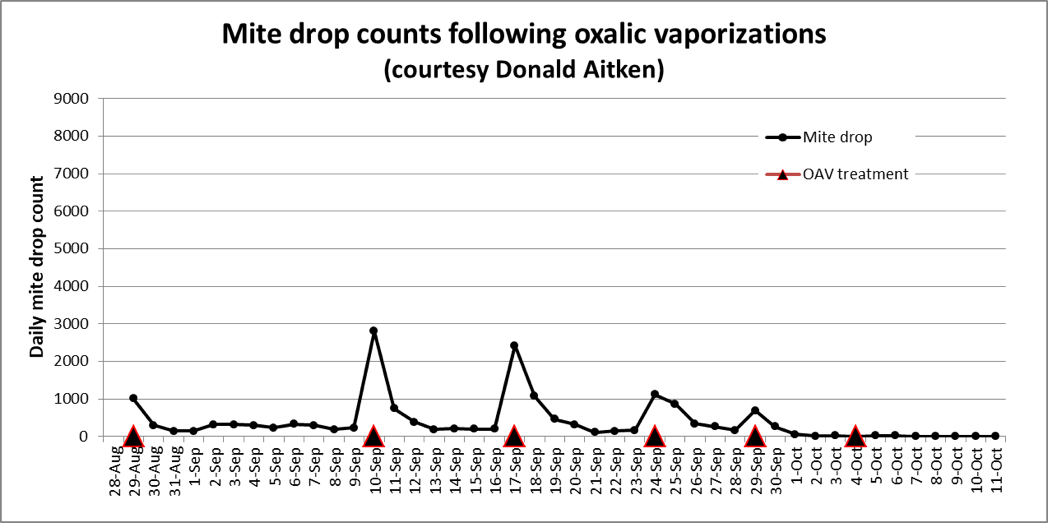
The second thing to keep in mind is that oxalic acid, whether applied by either sugar dribble or vaporization, appears to be quickly groomed from their bodies by the bees, and thus exhibits a relatively short-term effect upon the mites—typically for only a few days [[10]]. There is surprisingly little data available on daily mite drops following either of these treatments—if you have some, please send it to me! Below (Fig. 4) is one data set shared with me by beekeeper Donald Aitken of Edmonton, Alberta [[11]], who took the time to count his dropped mites when he did a series of vaporization treatments starting at the end of August, following the end of his main honey flow.
Figure 4. Donald treated this hive by oxalic vaporization six times (red triangles). Each treatment consisted of roughly 2 g of oxalic acid vaporized under a screened bottom board. The hive was fairly well sealed for 15 minutes after each application. Note that mite drops were only elevated for about three days after each treatment. I asked Donald why the first peak was lower than expected; his notes suggest that he may not have allowed enough time for a full vaporization of the OA on that initial treatment.
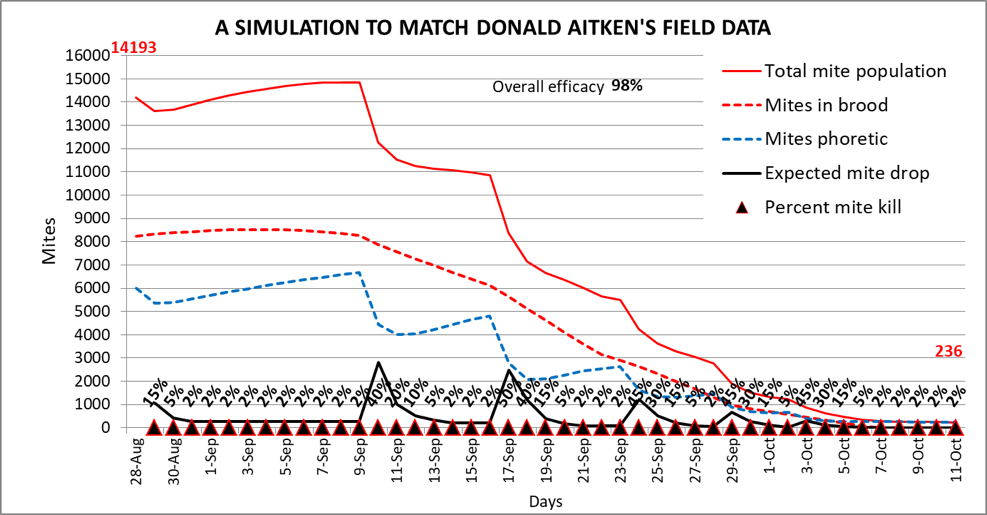
I used the daily mite model described above to attempt to duplicate Donald’s mite drop counts, hoping to back-calculate the daily kill rates resulting from his OA vaporization treatments. I started by inputting values for his colony [[12]]. And then by trial and error I estimated a starting mite count that would result (from treatment effect plus natural varroa mortality) in a total stickyboard mite count that matched Donald’s actual total count of ~17,000 fallen mites (yes, there can be a lot of mites in a hive)—the model also suggests that Donald’s hive’s starting alcohol wash count would have been in the ballpark of 60 mites in a half cup of bees. The output of my best [[13]] simulation is shown in Fig. 5.
Figure 5. A simulation to match Donald’s mite-drop data (the black line). His mite drop counts suggest that with his six oxalic vaporization treatments, he obtained 98% efficacy overall [[14]]. I then hand inserted daily varroa kill rates to match the model’s mite drop curves to those of his recorded counts (those daily kill rates are in boldface above the mite drop curve). At only one time point did the daily kill rate of phoretic mites reach as high as 50%, but of interest is that there appeared to be an extended slightly elevated kill rate for some time after each treatment.
In the above and following graphs I included dotted plots to indicate the proportions of mites on the bees or in the brood. Note how the treatments reduced the number of phoretic mites stepwise with each treatment (blue dashed line)—after which they’d slowly rebound as more mites exited the brood. Compare that to the red dashed line indicating the number of mites in the brood, which was instead reduced slowly and steadily.
Practical application: note also how any sticky board count (solid black line) taken a few days after treatment could be misleading, since it can drop to near zero despite there still being plenty of mites left in the brood. The same would apply to an alcohol wash count.
It appeared that each of Donald’s treatments typically resulted in around 70% overall kills of the phoretic mites present at the time [[15]]. This is close to the kill rate found by Radetski [[16]] for colonies with “some” brood, but less than that determined by Enzo or Al Toufailia [[17]]—both in hives free of brood.
Practical application: a single carefully-applied OA vaporization may be all that’s needed for a broodless colony. On the other hand, if a colony’s got a fair amount of brood, you can’t realistically expect an efficacy (as far as reduction of the expected mite buildup) of greater than 25-50% from a single vaporization (as indicated by the steps in the solid red line)—you need to keep hitting the hive until you’ve purged all the mites in the brood and killed them before they could reenter. This is exactly the reason that I’m enthusiastic about the extended-release OA/glycerin treatment [[18]].
The question then is what is the best interval for repeated vaporizations?
The Optimal Interval for OA Vaporization Treatments
Beekeepers have been asking me how to use my mite model in order to determine how frequently to apply oxalic vaporization to best effect. So I ran quite a bunch of simulations in order to see.
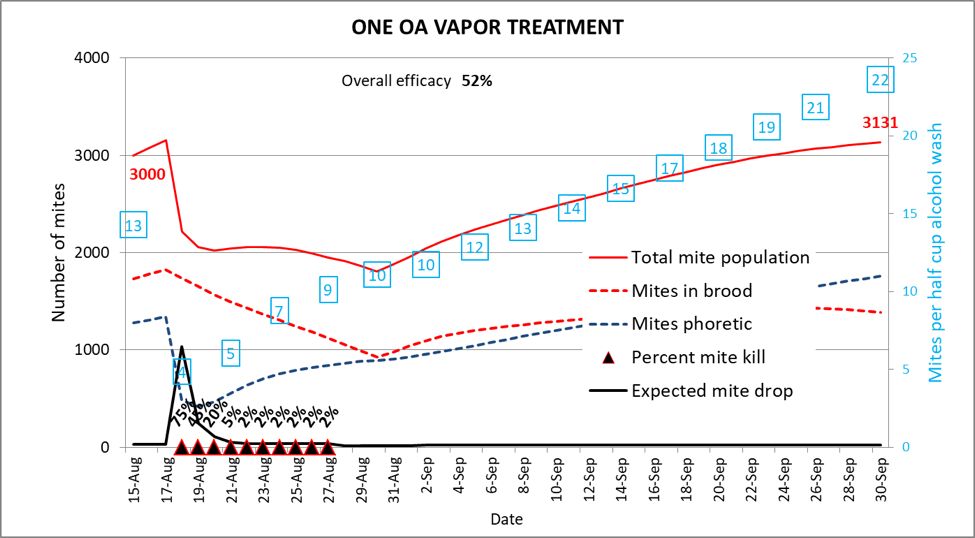
For these calculations I ran all the simulations for a mid-latitude colony, starting mid-August, and running through the end of September. The modeled hive starts with 15 frames of bees, dropping to 12, and 4 frames of brood, dropping to one half frame. I started with an arbitrary 3000 mites (a 13-count alcohol wash), with a 300-mite overall immigration, peaking on 5 September. The expected ending mite population (absent any treatments) would be 6,523 mites (a 46-count alcohol wash). I set the total kill rate for each oxalic vaporization to result in an overall 90% mortality of the phoretic mites present during the time of each treatment [[19]]. Here’s a simulation for a single oxalic vapor treatment (Fig. 6).
Figure 6. The effect of a single oxalic vaporization was not impressive—over the course of the 47-day period, the colony’s mite population went from 3,000 up to 3,131 (compare this ending mite population to that in the following simulations). And remember that I’m using (in this and subsequent simulations) an optimistic kill rate better than that achieved by Donald Aitken.
Each simulation also calculates the overall efficacy of the treatment(s), comparing the ending varroa population (in red) to the 6,523 mites which would have been expected without any treatments. In the above case, despite the mite population increasing slightly, it was less than half what would have been expected—thus the calculation of 52% efficacy.
I’ve also included the expected alcohol wash counts (mites per ½ cup of bees) in the blue squares. Note how this count, after the treatment, went up even more rapidly than did the total mite population. This was because of the reduction in the amount of brood present—forcing more of the mites out onto the adult workers. As I type this words, I just checked back to find that this iteration of the model is suggesting that nearly half the mites would still be in the brood on the last date. I suspect that that is an overestimate, so I will continue to fine-tune this daily model. Until then, please take the results of any of these simulations with a grain of salt.
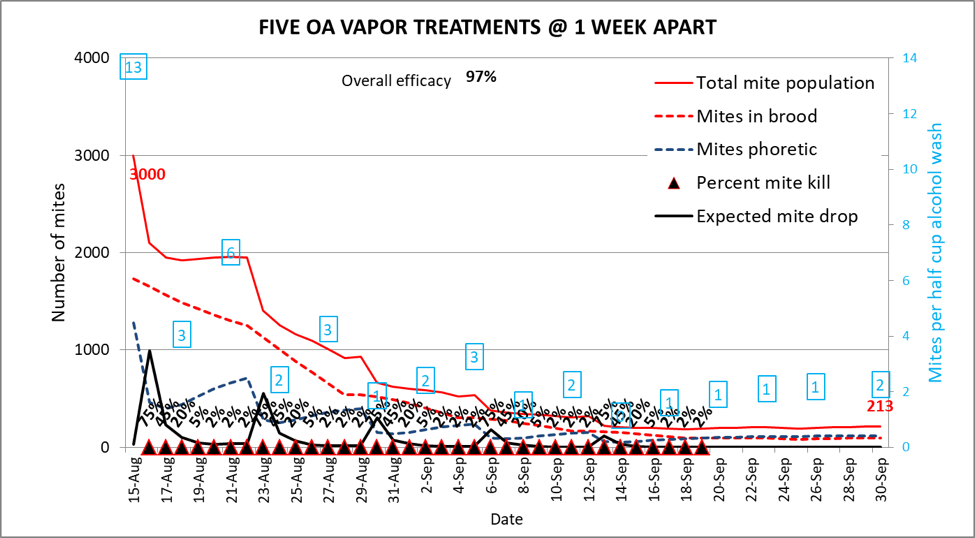
In any case, it appears that a single vaporization isn’t likely to do the trick. So let’s try five treatments, each a week apart (Fig. 7).
Figure 7. Five weekly vaporizations, on the other hand, resulted in an overall efficacy of 97%, dropping the mite population (solid red line) pretty quickly to only 213 mites.
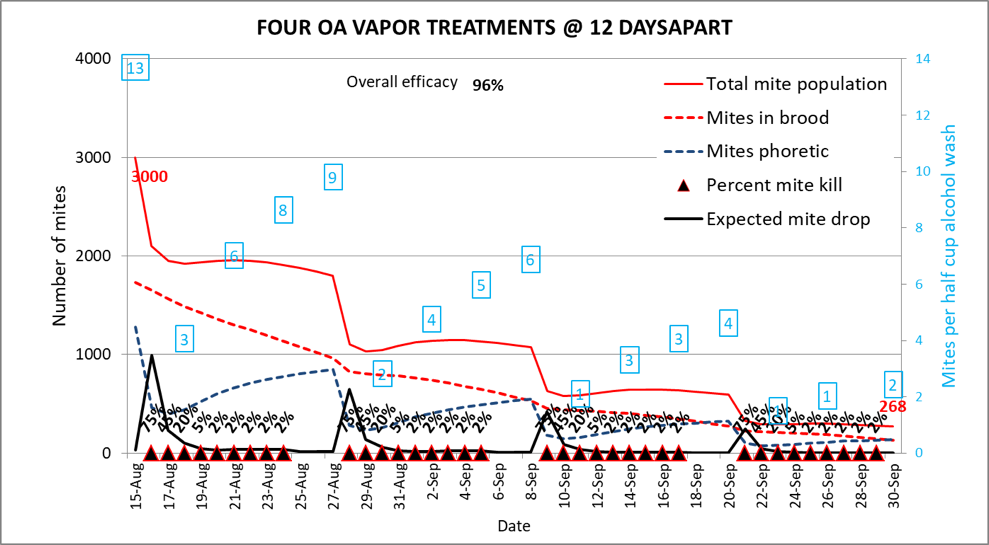
But do you really need to do five treatments? How about only four? (Fig. 8).
Fig. 8. Skipping one of those treatments, and spacing them further apart, still resulted in 96% overall efficacy, partly due to killing late-immigrating mites. Ending mite population of 268 mites.
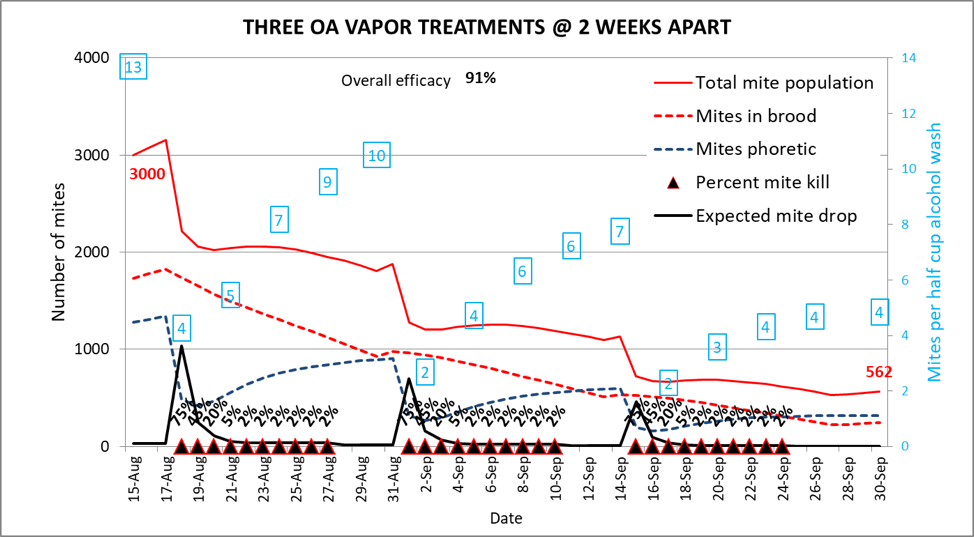
Not bad, you say—so how about only three treatments, one every other week? (Fig. 9)
Fig. 9. We’ve saved quite a bit of field time here, yet still obtained a decent efficacy of 91%. However, mite levels remained a bit high for the first two weeks, allowing for a degree of deleterious virus transmission to continue. The ending mite pop of 562 mites is higher than that of the two previous simulations.
I’d heard some experienced European beekeepers recommend repeating the vaporizations at shorter intervals– every 4 days–in order to prevent any mites from exiting and reentering the brood between treatments. This sounds compellingly sensible, but lacking hard data, I wondered whether this would be worthwhile. So let’s run the numbers (Fig. 10).
Figure 10. Four back-to-back treatments certainly dropped the mite level quickly, but there was then enough mite increase after treatment from reproduction and immigration to drop the overall efficacy to only 91%, with an ending mite population of 576 mites.
Practical application: I’ve run the numbers—you decide what to do. Let me be clear that the above simulations were run on a simplified and preliminary daily model, which still needs to be validated and fine tunes by comparison to hard mite count data from the field. If you’re willing to take such daily mite drop counts, please let me know.
Update May 2019: compare the above simulations to my data on repeated OA vaporizations in Fig. 12 at https://scientificbeekeeping.com/extended-release-oxalic-acid-progress-report-4/
Thymol
Now I was curious about running a simulation for the mid-August treatment with Apiguard thymol gel that we’ve been using in our own operation. When I’ve opened brood cells during strong thymol treatments, I don’t observe any mite kill beneath the cappings, so I assumed for the simulation that the treatment would kill only a percentage of the phoretic mites, and not affect the reproductive success of the remaining mites (although I strongly suspect that it actually does). From this I hoped to be able to again back calculate the daily additional mortality of the phoretic mites due to treatment.
At this time of season, due to lack of forage, my colonies are already decreasing in strength, and the thymol treatment only accelerates that process as the bees reduce brood rearing and move the broodnest away from the fumes. So I ran a simulation to match (we feed pollen sub immediately after treatment, so that the now low-mite colonies can build up for winter).
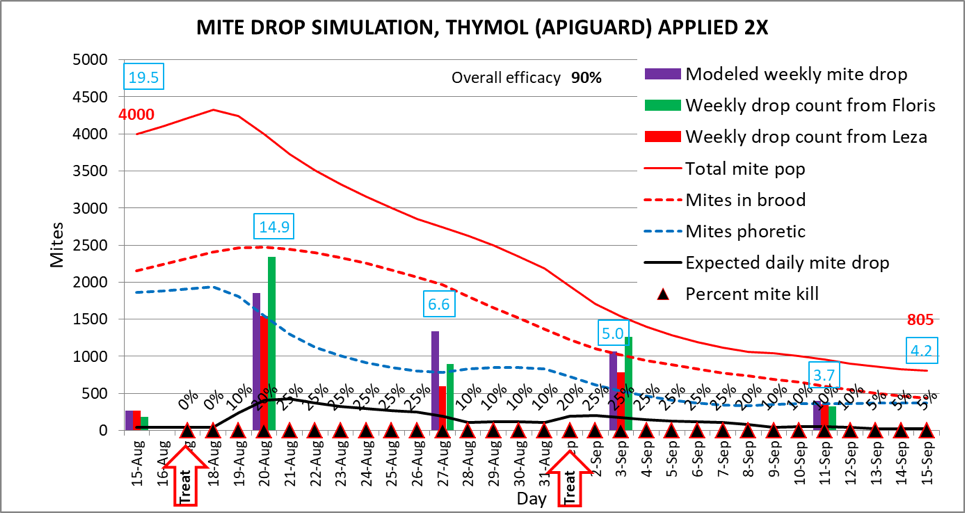
In order to validate the output of my model, it needed to match field data for both overall efficacy of the treatment, as well as for mite counts from stickyboards. I knew from experience that we obtain roughly 90% efficacy with the Apiguard treatment, which matches that of other published trials. I then reviewed published studies in which researchers had recorded mite drop data during late-summer treatment. I converted the mite drop data from summer and autumn trials by Floris and Leza [[20]] in Italy and Spain, respectively, to proportionally match the modeled results of a starting mite population of 4,000 mites—an expected number for a hive that had not been treated in the springtime (if you’re surprised, compare to Donald Aitken’s 17,000 counted mites). I then again back calculated the daily kill rates of the phoretic mites required to match the field data (Fig. 11).
Figure 11. A simulation for two applications of Apiguard thymol gel, applied 14 days apart (red arrows). Note how the model’s output (purple bars) is close to that of Floris’ and Leza’s proportional hard data. This simulation is based upon a colony starting at 15 frames of bees–dropping to 13, with 4 frames of brood–dropping to 2 due to the suppression of brood rearing due to the thymol fumes; it also assumes a daily immigration of 10 mites.
In order to match the field data, the model suggests that treatment with Apiguard kills roughly 25% of the phoretic mites every day for about a week (the small percentages above the triangles) [[21]]. This rate of slow attrition resulted in an overall modeled efficacy of 90%. But note that the mite population reduction is not as steep as with repeated oxalic vaporizations.
Practical application: note that in the above simulation an efficacy of 90% was obtained. That would be fine if you started with an alcohol wash count of say 8, which would have ended with a count of less than 2. But if you started with a count of 20, you’d still be going into the winter with an unacceptable number of mites in the hive (likely over 1,000). Take home message: your required treatment efficacy must be figured relative to your starting mite infestation rate—high-mite hives need stronger treatment.
Fall-Winter Mite Management
Once a colony’s gone broodless, mites are much easier to kill (even sugar dusting may produce adequate results). But you want to make sure that any treatment given at this time is not too stressful on the bees. Although there’s no temperature restriction on Apivar, I’ve seen no reports on winter treatment, nor for Hopguard. A gentle formic treatment can be effective. Thymol may be both stressful and ineffective due to temperature.
Beekeepers worldwide have good success with a single oxalic acid dribble or vaporization. In some countries, beekeepers are substituting glycerin for the sugar in the dribble. This is for two reasons: (1) the bees have an aversion to glycerin, so aren’t tempted to lick up the acid, as they do when sugar is used, and (2) the glycerin maintains acid residues on the bees’ bodies for much longer than with either sugar dribble or vaporization [[22]]. My sons and I are currently testing this method; I’ll let you know the results.
Acknowledgements
Thanks as always to Peter Borst for research assistance, and to all the dedicated and hard-working bee researchers from whose publications I draw useful information.
Notes and Citations
[1] Accelerated by the application of a quick-acting miticide or a sugar dusting.
[2] Since only 20%-25% of the mites would be phoretic at this time of season, assuming that 70% of the adult bees left with the swarm, the swarm would carry only about 15% of the total mite population. The rest of the reduction (from the potential buildup) would be due to the lack of mite reproduction during the period before the new queen begins laying.
[3] Maucourt, S, et al (2017) Comparison of three methods to multiply honey bee (Apis mellifera) colonies. Apidologie DOI: 10.1007/s13592-017-0556-9 See their Fig. 4a.
[4] At 20 days, all the original worker brood will have emerged; by 24 days, any drone brood. The earliest that brood from the new queen coming from an inserted cell will be receptive to mites is typically around Day 20. See https://scientificbeekeeping.com/simple-early-treatment-of-nucs-against-varroa/
[5] Amazon: “Prestige Medical Bandage Scissor with One Large Ring”
[6] Updated regularly at https://scientificbeekeeping.com/randys-varroa-model/
[7] May, RC (2017) The effects of repeated summer application of oxalic dribble. ABJ 157(10): 1123-1125.
[8] Papežíková, I, et al (2017): Effect of oxalic acid on the mite Varroa destructor and its host the honey bee Apis mellifera, Journal of Apicultural Research DOI: 10.1080/00218839.2017.1327937
[9] Dr. Medhat Nasr has investigated this pretty thoroughly for Canadian application.
[10] Al Toufailia, H, et al (2015) Towards integrated control of varroa: 2) comparing application methods and doses of oxalic acid on the mortality of phoretic Varroa destructor mites and their honey bee hosts, Journal of Apicultural Research 54(2):108-120.
Schneider, S (2015) Subletale Wirkungen von Oxalsäure in Kombination mit Zuckerwasser oder Glycerin auf Apis mellifera: Untersuchung der Toxizität, der Pharmakodynamik, des Verhaltens und der Lebensdauer, sowie der Rückstände auf Bienen und Beutenmaterial. Doctoral Dissertation, Freien Universität Berlin.
[11] Shared here by permission.
[12] I guessed that it started at the end of August with 15 frames of bees, dropping linearly to only 10 frames by 11 October (remember, that it was highly infested with mites). I also assumed that it started with 4 frames of brood, an amount that dropped steadily until the hive went completely broodless by 26 September. Because his bees enjoyed good flight weather through 11 September, I included a mite immigration count from other hives of 20 per day during that period.
[13] I ran dozens of simulations figuring out how to do this. During this process I’d recognize some error or another in the model, and then start all over again. But I’m finally getting more comfortable with the utility of the daily model. However, at this time, it is not a done thing!
[14] Calculating efficacy as 1-(final mite pop/expected final mite pop of 13,186).
[15] For example, a kill of 45% the first day, 30% the second, then 15%, 5%, and then 2% daily over the next week results in an overall kill of 72%. The 17 September treatment suggested an 80% kill.
[16] Radetski, T (2001) Vaporisation of oxalic acid in a field trial with 1,509 colonies. http://www.honeybeeworld.com/diary/files/__www.mellifera.de_engl2.pdf Back calculating from Radetski’s tables, and adusting for natural mite mortality in the control group, his mite reduction appeared to be roughly 77%.
[17] Enzo, N, et al (2004) Oxalic acid by Varrox® to varroa control control in central Italy. Apiacta 39: 39-43.
Al Toufailia (2015) op. cit.
Enzo’s kill rate was around 80%; Al Toufailia’s about 95%.
[18] Refer to my “Extended-release oxalic acid progress reports” in this journal, and at my website.
[19] A series of daily kill rates of 75%, 45% , 20%, 5%, 2%, 2%, 2%, 2%, 2%, 2%. This 90% total phoretic figure may be overly optimistic.
[20] Floris, I, et al (2004) Comparison between two thymol formulations in the control of Varroa destructor: effectiveness, persistence, and residues. J. Econ. Entomol. 97(2):187-191.
Leza,M, et al (2015) Comparison of the efficacy of Apiguard (thymol) and Apivar (amitraz) in the control of Varroa destructor (Acari: Varroidae. Spanish Journal of Agricultural Research 13(3): 1-5.
[21] Assuming that it does not affect mite reproductive success. If it indeed does so, then the daily kill rate would be lower.
[22] Schneider, S (2015) op cit.