The Nosema Problem: Part 6 – Treatment
Contents
What else is on the market?. 6
What if you don’t want to treat?. 9
The Nosema Problem: Part 6
Treatment
First published in ABJ November 2019
Randy Oliver
ScientificBeekeeping.com
OK, so you’ve checked under a ‘scope to confirm that some of your hives actually exhibit nosema prevalence levels of concern ― so what can you do about it?
2023 Update: see https://scientificbeekeeping.com/field-trial-of-several-nosema-treatments/
But First, let’s revisit queen failure
Root in 1940 [[1]], observed:
During the past few years, or since the shipping of package bees has come to be such an enormous industry, there has been not a little complaint that the queens in these packages, even from reputable breeders, are superseded either before they get to laying at all, or sometimes after they have a nice lot of good brood in all stages of growth.
I still hear this complaint today, despite lots of work by Pettis, Tarpy, and the queen producers to figure out why. Yet the Tarpy lab’s webpage suggests that nearly 30% of newly-installed queens die in the first 6 months.
I was curious as to how that figure compared to back in the old days. Farrar, from 1938-1946, kept track of the survivorship of 1540 package-bee queens over the first two months after installation (Table 1).
Table 1. Queen losses in a total of 1540 packages over a period of nine years, data reworked from Farrar [[2]] (packages presumably shipped to Wisconsin).
| Percentage of queen losses due to abnormal supersedure | ||||
| Time of loss | First 2 weeks | Second 2 weeks | Second month | Total losses |
| 5% | 8% | 6% | 18% | |
| Percent of losses suspected to be due to nosema | 17% | |||
Farrar’s data suggest that early queen failure rates may have been substantially less in the old days than those of today. Of even more interest is that Farrar blamed most of the failures that he observed upon Nosema apis (although he didn’t associate nosema with colony mortality).
But now that N. ceranae has nearly completely replaced N. apis, is that still the case? Tarpy [[3]] purchased 10 queens each from seven California queen producers in 2011 and analyzed them for the presence of nosema 3-5 weeks after being introduced into nucs (which may have already been infected). His technicians didn’t observe any spores in the queens, and only three of the 59 queens tested had detectable levels of nosema as determined by PCR analysis [[4]].
An early failure rate of some queens is to be expected ― we observe it often if poor weather had contributed to poor mating, but such nucs typically quickly requeen themselves; Root suspected that early supersedure may be due to the aging package bees not getting the right signal that enough young bees were emerging, thus inducing them to replace the queen. Anyway, something other than nosema appears to be at play.
But the real question is the very high rate of colonies dying due to queen issues nowadays ― surveyed beekeepers put the blame for 25-40% of their colony losses on “queen failure” [[5]]. I’ve had trouble finding historical records of the rates that colonies used to go queenless, but it didn’t appear to be a common phenomenon. Langstroth [[6]] wrote in 1878:
Queens sometimes die of disease or old age, when there is no brood to supply their loss. Few, however, perish under such circumstances; for either the bees build royal cells, aware of their approaching end, or they die so suddenly as to leave young brood behind them. Queens … are usually the last to perish in any fatal casualty. As many die of old age, if their death did not ordinarily occur under favorable circumstances, it would cause, yearly, the loss of a very large number of colonies.
And then in 1940 Root wrote:
As a rule, the average colony will supersede its own queen in two years without any attention on the part of its owner. The process is a perfectly natural one and goes on in the best regulated yards year after year [Fig. 1).
Figure 1. This colony is rearing a fat supersedure cell, during the “normal” process of replacing their failing queen. Some beekeepers cut such cells out; I figure that the bees likely know what they’re doing. The question is, why do so many colonies these days fail to successfully supersede their queens, thus allowing the hive to go queenless?
Practical application: Back in the day, at least in my operation, I don’t remember colonies going queenless that often ― they’d just replace a failing queen themselves, as described by Dadant and Root. But nowadays, colonies appear to commonly go queenless. Since few of today’s queens appear to be infected with ― much less die from ― N. ceranae infection, it appears that today’s reported high failure rates can’t be blamed on nosema.
More to the point, since a healthy colony would be expected to replace a failing queen by normal supersedure (or emergency cells), perhaps we should instead be asking the question, Why are we seeing so much “failure to successfully supersede” these days?
An interesting study by Butler in 1957 [[7]] indicated that a failing queen, should she continue to produce adequate pheromones to suppress her workers from starting supersedure cells, could put the colony in the position of not being able to replace her once she’s unable to lay any more eggs. Could today’s queens be sending the wrong signals for some reason? Before you point fingers, I see this happening even in my pesticide-free apiaries. I’ve discussed this apparent phenomenon with other researchers; it is currently an open and nagging question towards which we should devote more study.
OK, I’ve perhaps diverged a bit; let’s get back to treatment for nosema.
Treatments for nosema
We are still on the learning curve as far as figuring out how microsporidians such as nosema infect host cells [[8]], and thus largely shooting in the dark as far as how to figure out which directions to take in our search for potential treatments. In today’s backdrop, “antibiotics” are being more closely regulated, and we beekeepers need to keep in mind that the public is concerned about what chemicals get into our honey. So how about fumagillin, which has recently come back on the market?
fumagillin
Back in 1969 Moffett [[9]] tested 24 different compounds against N. apis ― only fumagillin gave evidence of any control. But what we’re now realizing is that all antibiotics come with potential “side effects.” One of the issues with fumagillin is that, as pointed out by van den Heever [[10]], the commercial formulation consists of the dicyclohexylamine salt (DCH) of the product, which is coming onto the radar of those who are concerned about consuming DCH-contaminated honey:
The contribution of DCH present in the commercial fumagillin formulation to the overall toxicity and resulting food safety implications in products destined for human consumption is definitely not negligible.
Not only that, but fumagillin does not appear to be as effective against N. ceranae as it was against N. apis, and may even exacerbate the problem [[11]]:
Fumagillin suppresses reproduction of microsporidia but disease prevalence and hive performance in treated apiaries were similar to untreated apiaries 6 months after treatment. We compared responses of N. apis and N. ceranae to the antibiotic at concentrations that simulated fumagillin degradation in hives during the honey bee foraging season when use of the drug is discontinued. N. ceranae spore production recovered at a higher fumagillin concentration than N. apis. At lower fumagillin concentrations, significantly more infective N. ceranae spores were produced in treated bees than in untreated infected bees.
Practical application: After the application of fumagillin was banned in Europe and illegal applications were also reduced, some expected an increase in colony losses caused by nosemosis. However, this did not happen.
I also find of great interest that when a group of Canadian researchers [[12]] followed up after fall treatments with fumagillin, they concluded that:
Fall treatment with fumagillin of hives in Canada did not appear to decrease either winter mortality nor spring spore counts.
A colleague in Spain, Antonio Pajuelo, tells me that:
Although treatment with fumagillin will generally reduce spore counts of Nosema ceranae, that does not necessarily correlate with improved colony strength or productivity.
Practical applications: Fumagillin used to work well against N. apis; it may not be as effective against N. ceranae. Treatment with fumagillin for Nosema ceranae may not improve winter survivorship, and may actually have negative health effects upon the colony. And ― if buyers start testing for residues ― it may not only be a waste of money, but also affect the salability of your honey.
Alternative treatments
Hey, we’d all love to have a food-grade treatment for nosema. Some years ago, two Canadian beekeepers hired me to determine what doses of Generally Recognized as Safe (GRAS) antifungal food preservatives could be safely fed to bees in syrup, and then to see whether such doses would control nosema. I never published the results, so here’s the short version.
I found that caged broodnest bees fed sugar syrup could tolerate up to 4000 ppm of any of three common food preservatives, although all the preservatives at high doses caused serious defecation of previously-consumed pollen (perhaps due to disturbing the gut endosymbionts) (Fig. 2).
Figure 2. A preliminary run, demonstrating the dysentery caused by feeding a preservative, compared to the sugar syrup controls (the two cages in the upper left). I then chose “safe” doses of each preservative to test for their effects upon nosema.
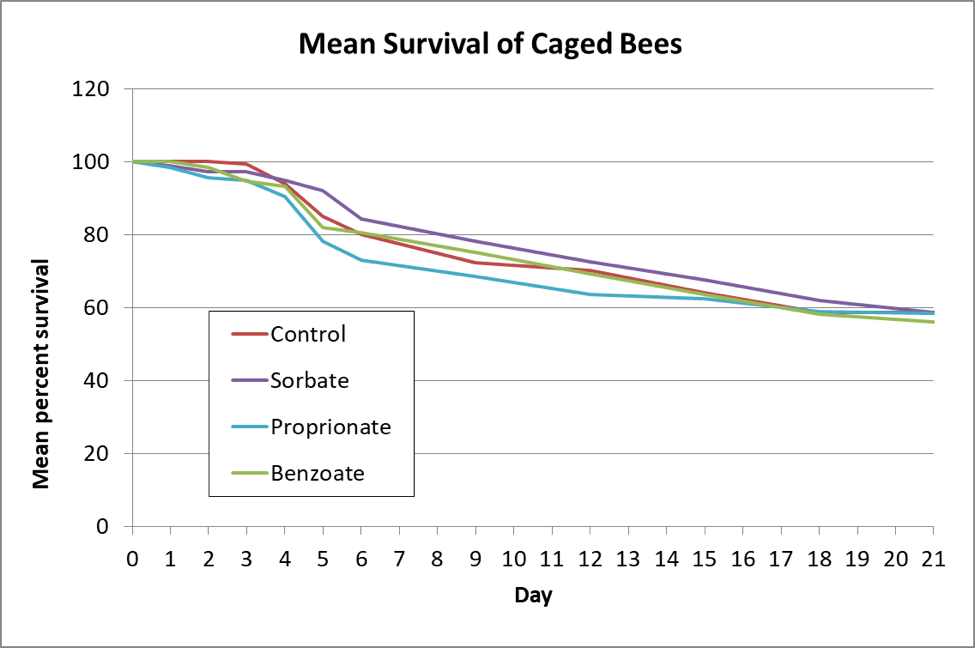
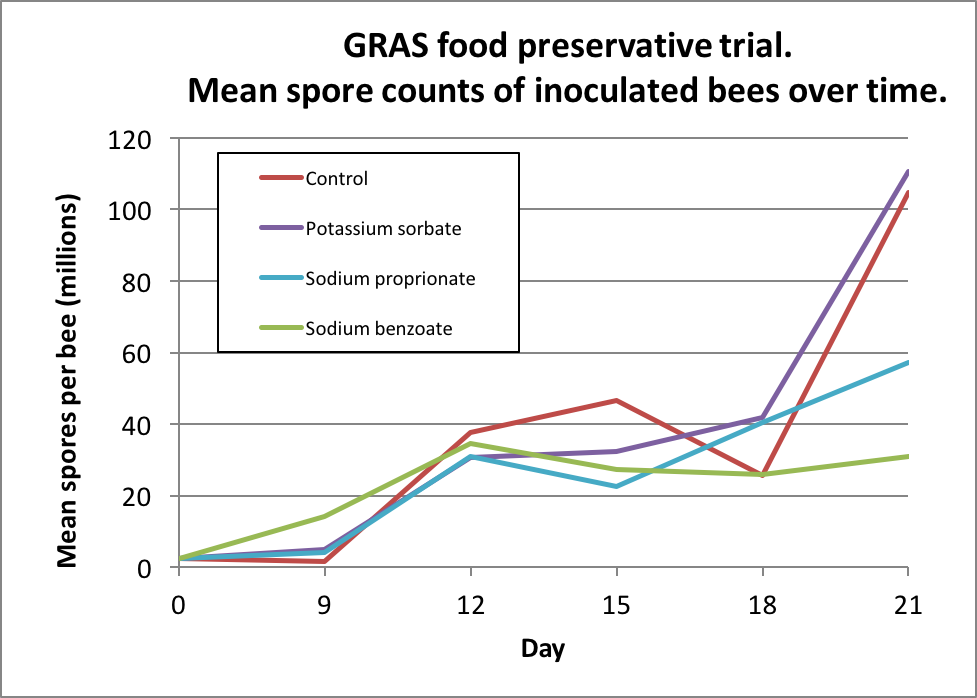
I then inoculated workers shaken from brood combs of a lightly-infected colony into 6 replicate cup cages per treatment, and fed them a freshly-prepared inoculum of ~20,000 spores each. I maintained the caged bees in an incubator and fed them (based upon my previous findings) sugar syrup laced with either 3000 ppm potassium sorbate, 1000 ppm sodium proprionate, or 1000 ppm sodium benzoate, and then tracked both bee mortality and nosema spore counts of some surviving bees each day (Figs. 3 & 4).
Figure 3. The bees in all the treated groups survived as well as the untreated controls.
Figure 4. Bottom line: unfortunately, although it was a great idea, none of the preservatives appeared to adequately suppress nosema reproduction in the bees.
Since then, researchers (including myself) have tested many other possible treatments, generally with disappointing results. I’ve reviewed some of those treatments previously [[13]]. Thymol and resveratrol have shown some promise [[14]].
What else is on the market?
Nozevit®
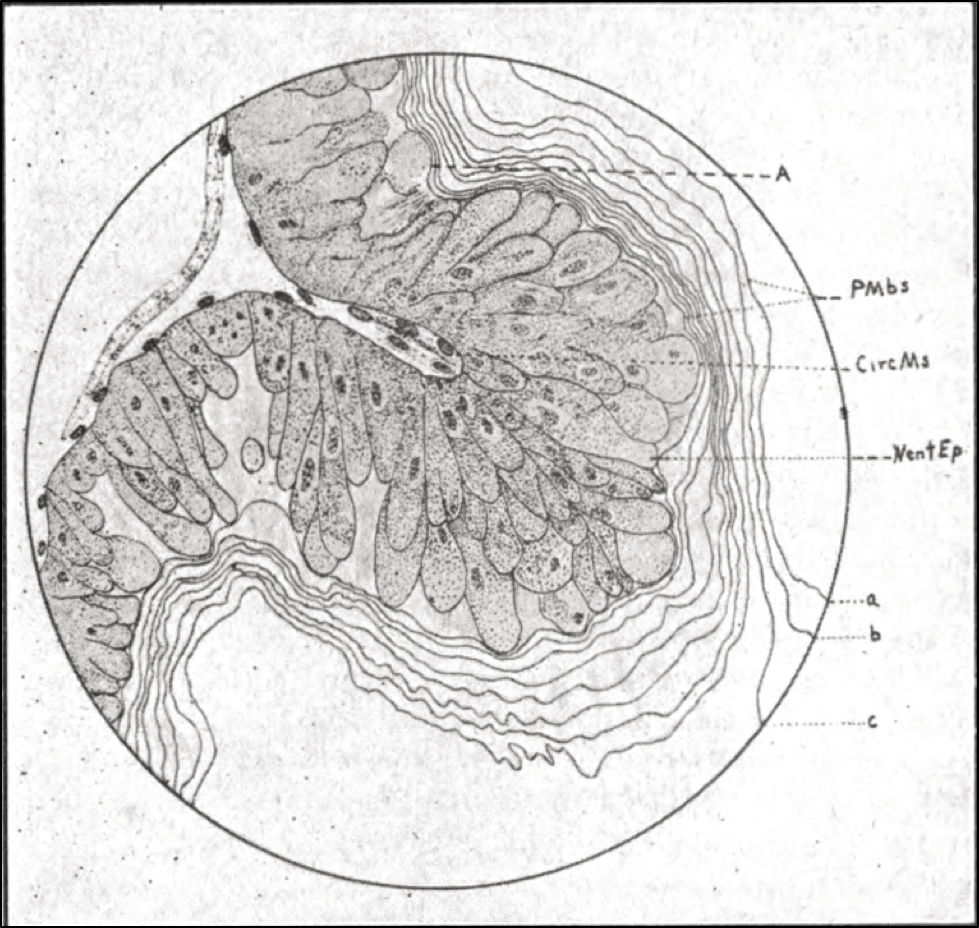
For anyone with a deeper interest in the bee gut and pollen digestion, I suggest perusing a great paper by Whitcomb and Wilson from 1929 [[15]], which contains some great drawings of the honey bee peritrophic membrane, such as the one below (Fig. 5).
Figure 5. This drawing from Whitcomb is a side view of a bee’s midgut wall, outer surface to the left, gut lumen to the right, with an infolding of the nutrient-absorbing intestinal villi cells (the darkened structures) in which nosema is able to reproduce. The layers of the protective peritrophic membrane lie to the right.
Whitcomb pointed out that honey bees, compared to other insects, create a more multilayered peritrophic membrane. More recently, other authors have found that the membrane serves several important functions. Within the midgut, the membrane not only compresses any ingested pollen into a bolus for passage, but also traps some toxic chemicals. As the food bolus makes its way down the midgut, the peritrophic membrane allows for a countercurrent flow of fluids inside or outside of the membrane, thus allowing the bee to also better utilize its digestive enzymes.
But none of the above valuable functions explain why honey bees would create so many layers in the membrane. Some researchers suggest that the membrane may help to protect the insect from infection by ingested parasites, such as viruses, bacteria, or (I’m adding this) nosema. And this is where it gets interesting.
Practical application: as far as I can tell, the only place that nosema can infect a bee is in the epithelial (surface) cells of the intestinal villi. In order for a nosema spore to gain entry, it needs to eject its polar body and first penetrate the peritrophic membrane, and then harpoon an epithelial cell. So one possible way to thwart nosema would be to toughen the peritrophic membrane.
Nozevit® is a natural extract of oak bark, following a traditional beekeeper recipe used in Croatia. Oak bark is well-known to contain plant polyphenols commonly called “tannins.” Tannins give plants an astringent taste due to their property of binding with proteins, thus making those proteins unavailable for digestion. Humans use tannins to toughen up the collagen proteins in animal skins during the process of “tanning.” We’re also learning that some tannins may have beneficial effects when consumed in small quantities.
As found by Tlak Gajger [[16]]
Nozevit induces the production and secretion of mucous from the epithelial layer of treated bees, and additionally coats the peritrophic membrane to form a firm and resilient envelope. Thus, the preparation may ensure protection from new invasion with Nosema sp. spores and also from normal physiological processes.
Thus the mechanism of action of Nozevit may be due to containing infective nosema spores within the peritrophic membrane, and/or preventing them from harpooning an epithelial cell.
Nosevit can be applied by any of three methods, or some combination thereof. Since I found application methods hard to find, I asked the manufacturer directly:
- Drenching: Mix 1.5 ml in 8 oz. thin syrup solution. Apply this mixture by drenching 4 times 4 days apart. Apply drench when bees have at least 4 hours of flying time left in the day and above 60 F.
- In syrup: Mix Nozevit PLUS in feeding syrup at the rate of 1.5 gallons Nozevit Plus in 1000 gallons normal feed syrup.
- In patty: Mix Nozevit or Nozevit PLUS in protein patty mix at the rate of 1.5 ml per 1# finished weight patty. 200# final weight batch of patties needs 300 ml or 1 1/4 cup Nozevit or Nozevit PLUS.
Practical application: I’ve reviewed a number of lab studies and field trials of Nosevit [[17]]; in general, researchers have found some reduction in spore counts.
Update: A small SARE study on caged bees found Nosevit to reduce spore loads, but not to improve the survivorship of the caged bees.
Hive alive®
Another product on the market is Hive Alive, which contains seaweed extracts. The product makes a number of claims, the ability to suppress nosema among them. But careful inspection of the supportive studies suggest that the claims may be a little bit wishful. The most supportive study was run in Greece by Charistos [[18]]; however, the results are somewhat “polished” in the sales brochures. What beekeepers are really interested in is the effect of treatment upon colony strength and survival ― despite application of the treatment, Charistos’ study found no appreciable differences in colony strengths between the test and control groups during the first eight months (November through June). By the next November the colonies had dwindled to only 3-4 frame strength ― too small for me to consider any subsequent results to be meaningful. I’d need to see stronger supportive data before making any further assessments of the product. So until I can review further supportive data on Hive Alive, I am unable to provide an assessment.
Acids in syrup
Beekeepers just love to dump this, that, and the other thing into sugar syrup, often without any supportive evidence that it is of any benefit to the bees. Many add acid, in the hope that it will help with nosema. Swedish researchers Eva Forsgren and Ingemar Fries found otherwise [[19]]:
No effect from altering the pH by addition of acetic acid, or from benzoic acid could be found, neither on the quantitative disease development of the parasite, nor on the infection rate of individual bees. The results from the field experiments support the laboratory results: addition of acetic or benzoic acid to the feed of honey bees has no influence on nosema prevalence or development.
Possibilities on the radar
There is now renewed interest in the development of products to take the place of fumagillin. Dr. Boris Baer has devoted a number of years studying how queen bees manage to keep sperm viable in their spermatheca. His lab recently found that the seminal fluid contains chitinases and proteases (enzymes that degrade the chitin and protein in the nosema spore’s protective “shell”) that “kill” the spores [[20]]. It appears that drones surround their semen in protective agents. Further study may come up with something that we can use as bee medicine.
Another group of compounds of interest are porphyrins, pigments commonly found in nature. Ptaszyńska [[21]] found that feeding some porphyrins in syrup increased the longevity of nosema-infected bees, and reduced spore counts.
Practical application: Researchers continue to test substances against nosema. Perhaps some will get lucky.
Note: I’m happy to update this page if anyone comes across additional studies, or new products come on the market. Let me know!
There is a nice review of the state of the art of nosema control prodcts in: The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits. https://www.mdpi.com/2076-0817/10/2/234/htm
I’ve also personally tested zeolite, but didn’t find any benefit.
What if you don’t want to treat?
Then just don’t worry about nosema (and focus upon varroa instead). Most colonies survive nosema infection, and one can argue that those that don’t should be removed from the gene pool anyway. I’ve previously mentioned studies that indicate that having young queens, a large winter cluster, a south-facing sunny winter location (to allow cleansing flights, Fig. 6), and abundant pollen allow colonies to hold their own against nosema.
Figure 6. This colony is clearly suffering from dysentery. Nosema-infected bees tend to chill during cold-weather cleansing flights, thus eliminating themselves from the hive, which is a good thing.
Allow me to end this section with a quote from USDA researcher Floyd Moeller [[22]]:
The results … suggest that two natural factors can help to reduce the level of Nosema infection: emergence of brood allows replacement of infected bees with healthy young bees; winter flights reduce the amount of defecation in the hive, and help to eliminate diseased bees by chilling them in flight.
The resistance of colonies to this infection should, therefore, be increased by encouraging the production of brood (e.g. by having vigorous productive queens, and abundant pollen or pollen supplements as appropriate), and by allowing winter flight whenever weather permits.
Aknowledgements
Thanks to Peter Borst, Dave Tarpy, Jeff Pettis, the late Ingemar Fries, Mariano Higes, Antonio Pajuelo, and the other researchers and beekeepers who’ve helped me.
citations and notes
[1] Root, AI & ER (1940) The ABC and XYZ of Bee Culture
[2] Farrar, CL (1947) Nosema losses in package bees as related to queen supersedure and honey yields. J. Econ. Ent. 40(3): 333-338.
[3] Tarpy, D, et al (2012). Assessing the mating ‘health’ of commercial honey bee queens. Journal of Economic Entomology 105: 20-25.
[4] I’ve cited other studies indicating that N. ceranae is not normally a problem for queens in Part 4 of this series.
[5] https://bip2.beeinformed.org/survey/
[6] Langstroth, L (1878) Langstroth’s Hive and the Honey-Bee. Dover.
[7] Butler, CG (1957) The process of queen supersedure in colonies of honeybees (Apis mellifera Linn.). Insectes Sociaux 4(3): 211―223.
[8] Franzen C (2004) Microsporidia: how can they invade other cells? Trends in Parasitology 20(6): 275-279.
[9] Moffett, J, et al (1969) Compounds tested for control of nosema in honey bees. J. Econ. Ent. 62(4): 886-888.
[10] van den Heever, J, et al (2014) Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 62(13): 2728-2737.
[11] Huang W-F, et al (2013) Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog 9(3): e1003185.
[12] Williams, G, et al (2010) The microsporidian Nosema ceranae, the antibiotic Fumagilin-B®, and western honey bee (Apis mellifera) colony strength. Apidologie 42: 15-22.
[13] https://scientificbeekeeping.com/the-nosema-twins-part-5-alternative-treatments/
[14][14] Costa, C, et al (2010) Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 41: 41―150.
[15] Whitcomb, W & H Wilson (1929) Mechanics of digestion of pollen by the adult honey bee and the relation of undigested parts to dysentery of bees. Vol. 92 Agricultural Experiment Station of the University of Wisconsin. Available at https://babel.hathitrust.org/cgi/pt?id=wu.89098844962&view=1up&seq=4
[16] Tlak Gajger, I, et al (2011) Effect of the herbal preparation Nozevit on the mid-gut structure of honeybees (Apis mellifera) infected with Nosema sp. spores. Veterinarni Medicina, 56(7): 344―351.
I’ve discussed Dr. Gajger’s detailed and methodical research regarding Nozevit with her over the years, and find the results compelling. She’s published twice in this journal:
Tlak Gajger I, Petrinec Z, Pinter Lj, Kozaric Z (2009a): Experimental treatment of nosema disease with “Nozevit” phyto-pharmacological preparation. American Bee Journal 149, 485―490.
Tlak Gajger I, Vugrek O, Pinter Lj, Petrinec Z (2009b): “Nozevit patties” treatment of honeybees (Apis mellifera) for the control of Nosema ceranae disease. American Bee Journal 149, 1053―1056.
[17] Rhoades, P (2009?) Testing the efficacy of three new alternative treatments for Nosema disease of honey bees in Tennessee. https://projects.sare.org/project-reports/gs09-086/
Higes, M, et al (2014) Preliminary effect of an experimental treatment with “Nozevit®”, (a phyto-pharmacological preparation) for Nosema ceranae control. Journal of Apicultural Research 53(4): 472-474.
van den Heever, J, et al (2016) Evaluation of Fumagilin-B® and other potential alternative chemotherapies against Nosema ceranae-infected honeybees (Apis mellifera) in cage trial assays. Apidologie 47(5): 617―630.
Michalczyk, Maria, et al (2016) Evaluation of the effectiveness of selected treatments of Nosema spp. infection by the hemocytometric method and duplex PCR. Acta Veterinaria-Beograd 66 (1): 115-124. Open access review of other studies to date.
[18] Charistos, L, et al (2015) Long term effects of a food supplement HiveAlive™ on honey bee colony strength and Nosema ceranae spore counts. Journal of Apicultural Research 54(5): 420-426.
[19] Forsgren, E & I Fries (2005) Acidic-Benzoic Feed And Nosema Disease. Journal of Apicultural Science 49(2): 81-86.
[20] Peng Y, et al (2016) Seminal fluid of honeybees contains multiple mechanisms to combat infections of the sexually transmitted pathogen Nosema apis. Proc. R. Soc. B 283: 20151785. It’s a bit more complicated than just “killing” the spores.
[21] Ptaszyńska, A, et al (2018) Porphyrins inactivate Nosema spp. microsporidia. Scientific Reports 8: 5523.
[22] Moeller, F (1972) Effects of emerging bees and of winter flights on nosema disease in honeybee colonies. J. Apic. Res 11(2): 117-120.