Being Part of The Solution
Click on the link below to view a ppt presentation.
Beekeeping Through the Eyes of a Biologist
I’ve updated at the link below:
@Citizen Science Mite Drift Instructions
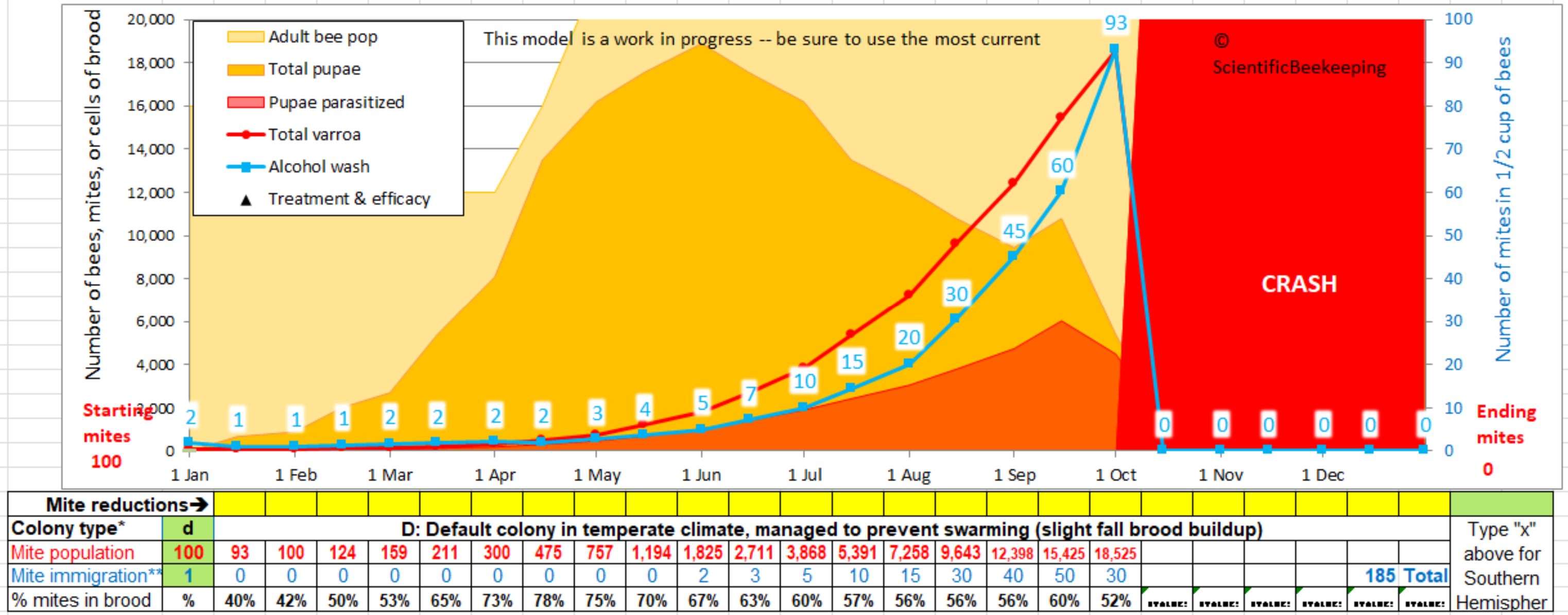
I feel that our industry and research community has long needed a useful, accurate, and user-friendly varroa population model–so I spent a year of early mornings and weekends creating this one. You can use this model to predict what sort of mite management strategy will work in your area.
Be sure to view the tutorials listed below before you attempt us use the model!
The model runs best in Excel. If you don’t have Excel on your computer, Trish Harness has a simplified online model that you can use on your cell phone at https://www.chickabuzz.com/model.html
For Joe Beekeeper, the model is easy to use (see the snip below)–you simply type inputs into the three green cells to the left to see whether your colony is likely to crash. In order to avoid such a crash, you can then apply one or more “treatments” (percent mite reductions) on any date in the yellow cells below the graph–each time you click on another cell or hit “enter,” the model instantly runs a new simulation. By this method, you can test various mite management strategies at your computer.
To use the model, you will need to have Microsoft Excel installed on your computer (it doesn’t work well in Google Sheets or Open Office, but appears to work with Linux Libre Office and with Numbers in Mac, but the output may not be the same). I hope someday to have an online version (I’d appreciate help). See *** below if you are using an older version of Excel.
The model is quite simple to use, but I’ve found that a visual demonstration really helps those trying it for the first time. To that end, I created a few tutorials on YouTube. I strongly suggest that you view them step by step, by clicking on the blue links below:
Tutorial #1 (10:02): Basic introduction–how to download and open the Excel workbook (including the best viewing settings), and how to use an included simplified model to explain basic varroa population dynamics and necessary mite reductions (I’ve also included printable written instructions at the bottom of this webpage).
Tutorial #2 (8:56): How to work with the model to understand varroa buildup and the effects of treatments. I show the example of how I use the model for mite management in my own operation.
Tutorial #3 (8:29): For moderately-advanced users who wish to customize the model by inputting the regionally-specific “Colony” parameters for their ecoregion and management style.
Tutorial #4 (5:35): Intended for bee researchers, bee breeders, and advanced users who want to gain a deeper understanding of varroa population dynamics, mite management, and the effects of various traits for mite resistance. It also shows how to better understand how the model produces its simulations.
Attention Excel geeks, modelers, and varroa researchers
*** Beekeeper Matthew Waddington points out that pre-2008 Excel uses a different Poisson function. The fix is to change the function name in all the cells in column BO to
=1-(POISSON(0,BN4,FALSE))
That said, I am not a mathemetician, a modeler, nor good with Excel. This model is a work in progress, and I’ve revised it nearly every day over the past several months. Please help me to further fine tune and improve it for use by all.
I’ve put explanatory comments in any cell that says “comment’–just click on it to view. I’ll be happy to unlock the formulas for you in return for an agreement to respect my intellectual property.
Once you open the workbook in Excel, click “enable editing.”
In order to prevent inadvertent changes to the wrong cells, I’ve locked all the cells other than those ready for user inputs (the yellow cells).
Then go to the bottom right corner of the window and set to 90% view, then to the “View” tab at the top, and hit “Full Screen” (hit “Escape” on your keyboard if at any time you wish to exit Full Screen view).
I suggest that you first open the “Necessary mite reduction” tab at the bottom to get an idea of the basic concept. Type in various numbers of months with brood, and different treatment percentages in order to understand how much varroa control you will need for your region.
After you’ve got the concept, then switch over to the “Current Version” tab to use the model itself.
All the instructions that most beekeepers will need are under the “Instructions” on rows 26-34. There aren’t very many instructions, so please read these first!
I suggest starting with the Default “Colony” (type “d” into cell B29, “100” mites in B31, and “1” for immigration in B33. If you keep bees in the Southern Hemisphere, type an “x” into P27.
After you’ve played with the Default colony by entering mite treatments, then you’ll want to go to the “Colony” tab, and either choose a colony type for your area, or create your own colony profile (please send me any accurate regional colony profiles, so that I can post them). View Tutorial #3 before attempting to create your colony!
Those with greater interest can go down to the green cells in the “Advanced” section of the Current Version tab, and type in the word “custom” to investigate the effects of changing key parameters involved in mite population buildup.
Have fun!
Randy
Open the link below to view the annotated pictorial presentation.
and if you want to see us doing smokin’ hot mite washin’ in real time, Rachel surprised me by figuring out how to prop up her cell phone to take a video of us washing a yard–to see the 36-second video, click here :
The varroa mite does not care that you’re keeping your bees in a top bar hive–colonies in top bar hives are just as susceptible to the mite. Unless you are running a truly mite-proof bee stock, you can expect the same sort of buildup of mite populations as in Langstroth hives in late summer, and I have seen plenty of top bar deadouts with clear signs of mite overload.
You will still need to knock the mites back by 90% at least once, and generally twice a season, depending upon your circumstances. Perform alcohol washes or sugar rolls to monitor your mite levels.
Unfortunately, the lack of space between the top bars makes mite management a bit more difficult than with Langstroth hives, since there would be little bee or vapor movement downward from treatments applied above the broodnest, and oxalic acid solution can’t simply be dribbled into the Langstroth frame interspaces.
However, that doesn’t mean that you can’t apply mite treatments, although depending upon the exact configuration of your top bar hive, you might need to use some ingenuity. I have zero personal experience with mite control of TBHs, so the following information has been largely gleaned from others who know what they are talking about.
Dr. Wyatt Mangum, a pioneer of TBHs, no longer finds mite treatment necessary, since he runs resistant bee stock, but he suggests that strip treatments can be applied between the top bars, at the same rate, according to colony strength, as in Langstroth hives (typically 1 strip per 5-10 frames of bees).
Synthetic Miticides
The strips currently available would be:
Essential Oils
Don’t waste your time. Bees generally find essential oil treatments to be stressful, and other than thymol, have little effect upon varroa.
Thymol
Apiguard gel can be used effectively, and is organically approved. For efficacy, the bees must have access to the gel particles, and carry them through the hive (there is very little vaporization). Thus, the gel must be placed such that the cleaner bees carry it throughout the hive. You can place 50g below the combs, but might get better results if you can figure out how to place it someplace else that bees will remove it. Please let me know if you find a method that works well.
Formic Acid
Formic acid is excellent for mite treatment—it’s organically approved, leaves zero residues, works fast, and is the only treatment that penetrates the cappings of brood cells. Formic acid vapors are denser than air, so it is best applied above the combs. I suspect that it would work well if you were to spread the top bars slightly, and apply a flash treatment overnight. You can remove the queen for protection, and keep her in a cage elsewhere during treatment, although young, vigorous queens are not generally harmed by formic treatment.
Unfortunately, formic flash treatment requires mixing formic acid with water and special application. MiteAway Quick Strips (MAQS) on the other hand are available off the shelf. They do a decent flash on the first day, but take 5 days for full treatment. Some report success by applying them below the frames. You’d likely get best results if you closed off screened bottoms during treatment.
Oxalic Acid
I really like oxalic acid, and Christy Hemenway covers the use of OA in TBHs fairly well in her book Advanced Top Bar Beekeeping.
.Although you can’t dribble as with Langstroth frames, you could temporarily spread your frames tiny bit and dribble between them. Or better yet, invest in an oxalic vaporizer, close off any bottom screens, and apply vapor. Vaporization might be easier, but is more potentially hazardous to the applicator.
The caveat with oxalic acid is that either treatment is only effective for about 3 days, and it doesn’t affect the mites in the brood. Thus, unless you’re applying during a broodless period, either dribble or vapor must be repeated at 4-day intervals at least 4 times (dribble is stressful to the colony if applied that often).
If you are dribbling, adjust the dosage per comb relative to the size of a deep Langstroth comb (134 sq. in. per side), which takes 5 mL.
For best results with OA, apply only during natural or induced brood breaks (when there is no sealed brood in the hive). An induced break can be created by splitting the hive or caging the queen for a minimum of 12 days. The queen can be released after 12 days, and the OA needs to be applied before 8 days have passed since her release. This method can result in over 90% mite kill.
Although not yet approved, the oxalic shop towels covered at https://scientificbeekeeping.com/oxalic-shop-towel-updates/ hold great promise for TBHs, if applied as some sort of strip (such as Strait-Flex Drywall Shims).
Kristina Williams (Boulder, Colorado)
I’ve successfully used MAQS or Apiguard on the on the bottom of the
hive. I sometimes have to trim the bottom edge of 2-4 comb to get the
treatments to fit. If using Apiguard in the single serve trays, I dump it
upside down onto the foil cover, more like the bulk form. Those trays are
just too thick. I’ve used Hopguard II in a TBH. It’s as effective as it
is in a US standard hive. That is to say, not very, but it will keep the
mites down to a dull roar until the weather cools off somewhat. I’ve used
OA vapor in January, but there has to be a slot type entrance for the
cooker. Some TBHs only have bung holes.
One problem with treating a TBH is that there are no honey supers to
pull off. I tell people to mark ALL the bars in the hive when Apiguard or
OA is applied as not-for-humans. I put Hopguard only in the brood area. I
put Apiguard far from the entrance so the bees have to carry it through the
hive.
There’s no standard size or entrance configuration. For dosage of the
organic acids and thymol I estimate volume (they’re all different), and
sort of take into account the shape and how much air flow there is. Some
are pretty congested with cross comb and attachment to the walls. I
haven’t used an OA dribble, but it could be done IF the combs are free
enough to be moveable a half inch apart.
For monitoring, it’s a little tricky getting a sample off those combs,
especially the new ones. Maybe brushing bees into a tub and then scooping
up a half cup would be safest. And of course the sugar shake is what most
prefer, at least to start. Three to four years of dead bees will sometimes
change minds, though.
I have alcohol wash data before and after on my hive. On other
people’s hives I have pretreatment data, but only whether the hive survived
or not for post treatment. Generally they do survive if they’re treated
adequately. My steady TBH customers are splitting hives, cancelling
package orders, and saying they don’t want more hives. So, it can be done.
I have a talk with people about ‘natural’ treatments. Plants and
insects are mostly enemies and those secondary compounds we like in our tea
are mostly made by plants to repel or kill insects. So hold off on those
plant products unless they’re necessary, effective and safe for the bees
and you. Add that lemongrass and wintergreen to your own tea. It’s lovely.
I also try to point people at evidence based resources no matter what
type of hive they have – University, USDA, extension, your site – and away
from blogs, opinions, Facebook unless backed by the former. Most people
really don’t know where to find good information.
Bill Hesbach (Connecticut)
The treatments that are most effective are HopGuard during bloodless periods with small populations. You can open the top bars and slip the strips in then move the bars back together – no problem. The same is true for an OA dribble- separate the bars, dribble the seam and close them again. I don’t use Amitraz but I’d assume those strips could also be placed between bars. The easiest broodless treatment is sublimating OA. My TBHs allow rear access (see photo) so I can slide my varrox right under the cluster or place in the center of the colony.
During periods with brood I use MAQS. I just slide the pads along the bottom board and position them under the brood. Even though the formic acid vapors are heavier than air they seem to work just fine. If you monitor the colony and keep the numbers low, same as you would in a lang box, you get the same results- colonies that survive – I just split one two days ago after its third year.
This page is for the sharing information on the extended-release method for oxalic acid application–by dissolving it in glycerin, and then applying to the hive on a cellulose matrix. You can view my article on the subject here: Beyond Taktic. A big thanks to EPA, ARS, and CDPR for working with me toward registering this application method for the benefit of the bee industry!
Warning: this method of treatment is only approved in some countries, but not yet registered in others, including the U.S. It is illegal to apply oxalic acid to a hive in this manner unless it is registered for use in your jurisdiction. However, you may be able to obtain an “Experimental Use Permit” or a “Pesticide Research Authorization” from your State Lead Agency for pesticide regulation.
I do not encourage nor condone the illegal application of any pesticide, including mite treatments to bee hives. The information on this page is solely to report on my progress (working in conjunction with ARS) towards getting this application method approved by EPA , and is not intended to promote illegal use in any way. I get sent anecdotal reports from all over the world, and I assume that anyone reporting such information to me has obtained the proper permit. I neither approve of, nor encourage, applications not sanctioned by local authorities.
Additionally, this application method is a work in progress, with only scant supporting data on efficacy at reducing mite populations. I will be performing formal trials this season.
If you have previously viewed this page, please reload it to ensure that your computer is not showing you an outdated cached copy. Only then, if after reading all the updates, you have questions or you’ve figured out ways to improve the method, please write me and I’ll post here.
Update 29 June 2017
Please folks, read all updates before you write me!
I no longer press the towels; instead, I’m mixing in water in order to get the right amount of glycerin into the towels. The formulation that I’m currently testing is:
We just finished our midpoint grading of the Calif early-season trial. A heat wave quashed our honey flow, so the colonies did not build up and remove the towels very well in some of the test hives. That said, at 3 weeks into the trial, mite suppression was erratic. I haven’t yet worked the data, but it appears that the towel application method may not be adequate for mite control early in the season. I’ll update soon.
The July issue of ABJ has an extensive update of what I’ve learned to date, with details on preparation methods, and will soon be posted to this website–I’ll update this page when it’s up.
Please realize that this application method is a work in progress–it is not yet approved, and may not result in adequate mite control. I will keep you updated…
Update 8 June 2017
The OA/gly shop towel is currently work in progress, with no claims of efficacy. This update page is informational only; the towels should only be used by those who have received authorization for experimentation.
I’ve sent an extended progress report to ABJ for publication, and will post it here shortly after it’s published. The report covers what I’ve learned to date, and details how I am currently preparing towels (using the 5 May formula) at home.
We’ also now started the first formal trial for EPA registration of the method (in conjunction with ARS) here in Grass Valley, California, with a replicate trial in Georgia (under conditions of higher humidity) to soon begin.
Day Zero of Calif trial. We graded 64 hives for strength, and are here weighing each hive.
We also took honey samples from hives in the Test and Control groups to determine whether treatment substantially increases the natural oxalic acid content of honey.
As a result of testing this spring, I questioned whether a single-towel treatment provides adequate mite control when colonies are building up in spring and early summer–so I was planning on testing three different doses (1,2, or 3 towels per hive). Unfortunately, in two test hives, I found that I can’t increase the dosage by stacking towels one above the other, since this prevents the bees from chewing them adequately. Thus, I changed the protocol shortly before starting the trial, and we are applying only a single dose of three half towels (a 1.5-towel equivalent) per hive (I will also test back-to-back applications).
Applying three half towels to a hive. I’m hoping that the additional exposed edges will increase the rate of distribution of OA in the hive by promoting more chewing at the edges. This application also allows for better vertical movement of bees through the cluster.
Another thing that we’ve learned is that the bees must have access to both the top and bottom of the towels to stimulate them to chew up the towels (and thus distribute the OA)–they do not work well if simply placed under the hive cover. The best position appears to be between the two brood chambers.
Update 5 May 2017
I placed the towels from the previous update into strong colonies, in order to see how quickly the bees would start chewing them–they quickly started chewing holes. So here’s my current best recipe, per Scott Shop Towel:
12 g oxalic acid dihydrate, stirred into
10 mL boiling water until fully dissolved (reheat if necessary), then stir in
13 mL food-grade glycerin.
The above towels, if allowed to air dry, with be a little stiff. Once in the hive, they will quickly rehydrate, and the bees will get to work at removing them (which allows for the mechanical transfer of the solution to the bees’ bodies). The question now is what is the proper dose per hive. Although a dose of one towel per hive in September gave good results last season, the nucs into which I had applied a half towel this spring exhibited unacceptable mite counts when I checked a few days ago. I plan to soon start some formal testing, using different rates of application (including 2 towels laid one on top of the other). Please keep in mind that every one of these updates are merely reports of my preliminary experimentation–NOT recommendations. I still need to confirm efficacy under different conditions, as well as lack of adverse effects upon the colony. There is still much for us to learn!
Addition of an irritant: as I’ve stated before, bees avoid cellulose that is saturated with glycerin. Thus, in order to encourage the bees to chew out the shop towels, one might add an irritant to the glycerin that initiates a hygienic removal response. I’ve thought of using cadaverine or putrescine (and have purchased the raw materials to concoct them), but first was curious about using thymol, since in playing with thymol dissolved in glycerin two years ago, I found that bees readily removed cotton saturated in a thymol/glycerin solution.
Beekeeper Chuck Cook volunteered to run some tests (legal, since he wasn’t using for mite control). He mixed thymol into glycerin at the rates of 0, 0.25, 0.5, 1.0, 1.5 and 2.0 g of thymol per mL of glycerin, and then soaked strips of shop towels in the mixture and presented them to 5 hives. After 14 days, the bees exhibited differences in hygienic removal of the strips:
The above photo is from the hive showing the clearest results–pure glycerin at the bottom, 2 g/mL at the top. It appears that the optimal concentration for eliciting removal would be in the 1 g/mL range. However, Chuck found that two queens were lost during this test–there was simply too much thymol applied in the tests per hive! If one were to add 12 g of thymol to a shop towel, it would also likely be too much. Again, we have much to learn!
Update 2 May 2017
I just came across a very exciting recent finding, by Argentine researcher Matías Maggi, who wondered whether repeated exposure to OA would lead to OA-resistant mites (for some reason the full paper has been taken down, but you can read the abstract here: The Paper). Maggi found that even after being exposed to 64 consecutive treatments by OA dribble over a period of 8 years, the mite population in that apiary was still highly susceptible to OA (surprisingly, apparently even more susceptible than the control group of mites that had never been exposed to OA). This finding suggests that OA, if used judiciously, and in rotation with other treatments with different modes of action, may remain effective for the long term.
Update 30 April 2017
We’re finally getting caught up with beekeeping, so I’m back to kitchen chemistry.
I tried mixing up different proportions of OA, glycerin, and water, for one towel at a time, in order to check the wicking action, crystallization of OA, and texture of the towels. I mixed up individual solutions, each containing 12 g of OA, and differing amounts of glycerin and water. I also ran one wicking test for the highest water concentration (foreground, photo below). I laid out the individual towels over Saran wrap and allowed them to air dry outdoors.
The towels had essentially the same texture once the the water had evaporated off, and whether I used 13 or 14 mL of glycerin. I did notice a slight amount of crystallization of OA on the surfaces after drying. So I placed them in my queen incubator overnight. At broodnest temperature and humidity (95F and 60-70% RH), 12 g of OA in 13 mL of glycerin remains in solution (covered to avoid absorption of water). With the towels, their texture completely changes.
At the elevated temperature and humidity, the glycerin absorbs water, and the towels go from feeling crisp and dry with a slight “oiliness” to feeling very wet and oily. This wetness is what I’m aiming for, since I want the acidic liquid to cling to the bees’ cuticle, but not so oily that the bees avoid chewing the towels.
Update 27 April, 2017
Q: If you use enough ingredients to properly saturate a half roll of shop towels, then apply one shop sheet per hive, if you have, say 5 hives and can’t store the balance, isn’t that a waste of resources?
A: Of course it would be. In the first place, I’m not recommending that anyone use this method–I am merely posting my progress on getting this application method legally registered. I have not yet run formal trials to collect data on efficacy, adverse effects, or contamination of honey stores.
However, for those who can legally experiment with it in their jurisdictions, it is an easy matter to use simple math to make smaller batches. My current working recipe, per full towel, is:
12 g OA dihydrate
13 mL food-grade vegetable glycerin
5 mL distilled water 0r 10 mL
However, I’m finding too much crystallization over time with the above formulation, and need to either increase the glycerin or decrease the amount of OA slightly. And I’m going to experiment with increasing the amount of water.
Update 26 April 2017
Q: Have you been able to successfully store the towels and or solution? I can foresee smaller operations wanting to get in on this and not necessarily need 55 towels at one go. Would you recommend just preparing less solution or can it be stored after it’s made.
A: I’ve been storing a few rolls of prepared towels at cool room temperature for a couple of months. As prepared, the towels get a bit more “delicate.” Coupled with some crystallization of the OA, it becomes more difficult to unroll the towels without tearing them (I may need to either increase the glycerin or decrease the OA). In addition, over time, some of the OA may react with the glycerin to form esters (which may or may not have effects on the bees or degree of efficacy for mite control). So I suggest that towels be made fresh for use, and not stored for prolonged periods of time.
Update 21 April 2017
It’s been too crazy with bee work to run experiments, but I’m getting feedback from other beekeepers. One compared February treatment with Apivar vs. OA/gly shop towels (2 half towels/hive), prior to blueberry pollination in North Carolina, and recorded frame strength, frames of brood, and starting and ending alcohol wash counts. After 46 days, there were no differences in frame strength, frames of brood, or queen loss between the groups. Mite counts started close to zero in both groups, and declined in both groups. Unfortunately, he didn’t run an untreated control group, so we can’t calculate efficacy.
After the 46 days, the colonies had removed most of the towels, which is my goal:
Update 6 April 2017
In response to the two reports below that OA/gly treatments had killed bees, I ran a quick and dirty experiment. I made up 10 medium-strength 5-frame nucs with 2nd-yr queens, and laid a half an OA/gly shop towel across the top bars of each one. Weather conditions were initially cool and rainy.
I checked the nucs each day for any signs of dead bees at the entrance, and never saw any. After a week, the bees had not yet chewed the towels to any extent, so I added another 5-frame box of drawn comb above, in order to give the bees access to the tops of the towels. At that time, due to the cool, wet weather, I fed each nuc a half gallon of 1:1 sugar syrup via a top feeder.
I still saw no dead bees at the entrances, and then the weather warmed, and the bees took the syrup, and then began foraging on a nice nectar and pollen flow. Yesterday I went into 6 of the nucs to check for signs of adverse effects. Every nuc that I checked was thriving and expanding as well as other untreated nucs in my operation. The brood patterns were lovely:
A typical brood pattern of a treated nuc. Note the partially-chewed towel that I lifted with my hive tool in order to remove the frame. I do not understand why some others are reporting adverse effects.
Of interest, in one of the nucs that I checked, the syrup had dripped onto the towel, giving the bees an opportunity to consume oxalic/glycerin/sugar syrup solution. Again, even in this nuc I observed no signs of adverse effects. In this particular experiment, I was only interested in seeing whether there would be adverse effects from treatment of small nucs under poor weather conditions in early spring. Due to the small clusters and poor weather, I did not take mite washes, so cannot comment on efficacy of the treatment. I plan to begin a larger, controlled trial on nucs next week.
Update 12 March 2017–updated measurements into grams. Until we better understand the chemistry, I suggest heating the mixture quickly in order to dissolve the OA, and then pouring it immediately onto the towels and allow it to cool. The point is to avoid holding the OA/gly solution at high temp any longer than necessary.
Update 11 March 2017
A beekeeper just reported to me that he placed 2-3 cardboard strips following the Argentine formula into 15 nucs and killed them all. I’ve not yet tested on nucs, so be careful!
Another email today: “Two weeks ago, I treated one strong hive in a remote yard with glycerin and oxalic acid saturated in a Brawny Dine-a-Max towel – I like the untreated Dine-a-Max towel as a trap for small hive beetles, and thought it might do well as a carrier for the gly/ox. When I checked the hive last week, there was a large pile of dead bees on the ground at the entrance of the hive – probably the nurse bees, since the dying field bees probably flew away before dying.
Folks, experimentation carries risk. I’ll be testing this application method thoroughly this season, and will report my results.
Update 10 March 2017
I’m currently working with EPA and Dr. Jay Evans at USDA ARS to add this method as an approved application method (so that it can be used legally). The current sticking point is to get it approved for use while honey supers are on, which will apparently require us to do further testing, and to have EPA set an acceptable maximum residue level for OA in honey. Luckily, natural honeys and especially some honeydews contain a fair amount of OA, as do many veggies. So this likely won’t be an insurmountable problem, but will need to work through the bureaucratic requirements. A big thanks to EPA, ARS, and CDPR for working with me for the benefit of the bee industry!
Good news, my experimentation is now legal! California DPR has issued me a Pesticide Research Authorization to run field experiments with the OA/gly towels (snip below).
There are quite a few beekeepers worldwide currently experimenting with this method of application–keep in mind that OA/gly extended release is not yet registered for application in the U.S. I encourage others to apply for experimental permits in their jurisdictions.

Photo sent to me by a commercial beekeeper running tests (name withheld for his protection).
I had a brainstorm–instead of using rolled towels, to instead use the folded ones (below) that come in an 85-towel pack, and are dispensed one at a time like Kleenex tissues.
I purchased a stainless steel steam table insert that fit the stack of towels perfectly, and poured in the solution (below).
Result: it worked beautifully! I was sure that this would be the answer for easy towel dispensing. But alas, the proof of the pudding is in the eating. When I attempted to pull the towels out, the OA/gly prevented them from separating easily (below).. It looks as though I’m back to the rolls.
OK, you may have noticed that I’m not wearing protective nitrile gloves. I’ve gotten so casual with these towels, that when testing in the kitchen, I often handle them with bare fingers, and then immediately wash the solution off afterwards. I’ve not noticed any skin irritation, but you sure wouldn’t want to rub your eyes or pick your nose! Best to ALWAYS wear gloves.
Current best formulation from my testing to get the maximum amount of OA into a usable shop towel that is not too “oily” to discourage removal by the bees:
Per towel Per full roll of 55 towels
OA crystals 12 g 672 g
Glycerin 13 ml 728 ml (917 g at room temp)
Water 5 ml 280 ml (280 g at room temp)
Be sure to measure each ingredient before adding it to the mix, in order to avoid the overshooting that can easily occur if you try to weigh the ingredients as you add them to the mixing pot.
For a roll of 55 towels, I multiplied the amounts by 56 (in order to account for the absorption by the cardboard roll in the center). Tip, if you measure the glycerin in a measuring cup, after you pour it out, place the cup in a microwave for a few seconds to heat the remaining glycerin, and the remainder will pour out easily).
We may not need this much OA in a towel. My preliminary testing with cardboard strips to replicate Maggi’s trials in Argentina indicated that it required 4 strips per box for adequate varroa reduction. That worked out to 40 g of OA per box, or 80 g per double-deep hive. A shop towel using the above formula holds only 12 g per hive. Its increased efficacy over the cardboard strips is apparently due to the chewing-removal action by the bees.
Tip: I noticed yesterday that it appeared to help to heat the water first, then add the OA. This appeared to allow me to break up the chunks better than when I add the OA to the hot glycerin first (I need to confirm this).
I’d had a number of people suggest rather complex manufacturing methods for spraying, vacuum suction, etc. methods for mass producing OA shop towels. I appreciate your suggestions, but I will leave mass production methods to manufacturers. What I’m focusing on are simple preparation methods for beekeepers. My most recent formulation is at the 12 Feb update.
Some other recent questions:
Q: I don’t remember reading how long you leave this treatment in place.
A: In the first place, this application method is not yet approved for use in the U.S. You may be able to obtain an experimental use permit from your State department of pesticide regulation. That said, the application method is designed for an extended release over a period of roughly one month, by which point the bees should have removed most of the shop towel. Mite reduction is not immediate, and from my limited testing, appears to take about a month. Therefore, if legal in your state or country, apply it as soon as the mite infestation rate (as determined by alcohol wash) exceeds 2 mites/100 bees.
Q: Can it be used with supers in place?
A: According to Maggi’s testing, this treatment does not appear to increase OA residues in honey. I’m asking EPA to approve it for use with honey supers on.
Q: Does the ambient temperature matter?
A: I’ve tested at up to 100°F (at low humidity), and did not observe any adverse effects. I’ve also tested during our California winter (for adverse effects only–not efficacy), during sustained cold rain and some freezing temperatures. Again, I observed no adverse effects upon the bees or brood.
Q: When sourcing your Oxalic acid/Glycerin etc have you looked at different grades of OA? I see you have food grade gly. I have been sifting through OA msds sheets from what we have locally available and the chemical composition varies wildly from agricultural bore cleaner or wood bleach to technical grade OA with prices that vary even more. The lower end “bore cleaner” OA Dihydrate has Sulphate at 1000ppm and some metal elements included, Iron at 100ppm and Lead at 50ppm.
A: The EPA requires registered “Distributors” of OA for varroa treatment to certify the purity of the OA. Currently, Brushy Mountain is the only registered distributor. I’m working with another registrant to bring prepared towels to market at an inexpensive cost.
Update 12 Feb 2017
I checked several hives yesterday that have had a OA/gly towel in them for the past two months during rainy, cold spring buildup. There does not appear to be any adverse effect upon the colony.
Please remember that this formulation is not yet approved for use in the U.S. (I am in the process of obtaining an Experimental Use Permit for the State of California). It is against the law to apply OA to hives in an unapproved manner. I’m working with EPA and ARS to get this application method added to the label, but it’s a slow process.
Meanmwhile, I’ve been doing lots of kitchen chemistry to figure out how to avoid the need to press the excess OA/glycerin out of the shop towels in order to obtain a texture that the bees will remove. It appears the total glycerin should not exceed 13 mL per towel. This amount of glycerin will hold 12 g of OA, without the OA coming out of solution at hive temp.
I think that I’ve finally hit upon a formula that works. It is difficult to get the solution to wick by capillary action into the full length of a roll of shop towels. (For non U.S. readers, the Scott shop towels are a cellulose heavy-duty, highly absorbent towel http://www.kcprofessional.com/products/wipers/general/scott-shop-towels).
So here’s a step-by-step process that appears to make the ideal towel, although I need to confirm for efficacy in the hive.

Step 1. Use a sharp kitchen knife to cut the roll of towels exactly in half crosswise.
Step 2. Find a stainless steel saucepan that just fits the roll (stood on end). The optimal formula appears to be (per towel) 12 g of OA dihydrate, 13 mL of glycerine, and 5 mL of water. Multiplied by 55 towels + the cardboard roll = 56/2, for final amounts per half roll of towels of:
| OA | 336 g |
| glycerin | 364 mL |
| water | 140 mL |
Yes, adding water turned out to be OK, so long as I use slightly more glycerin in mL than OA in grams. The water will mostly evaporate from the towels, depending upon temp and humidity. I show a graduated cylinder above on the right (most accurate), and a measuring cup on the left.
Weigh out the 336 g of OA (I actually use a more accurate beam balance, but it’s not critical).

You can preheat the water and glycerin in the microwave, or on the stove. It doesn’t appear to be critical when you add the water, but if you add it first to the glycerin before adding the OA, it may help to prevent the formation of oxalic esters during the heating.

Heat to 140-160°F. Higher temps will cause degradation of the OA and accelerate ester formation (both of which we wish to avoid).

Add the OA crystals, and use a stainless steel spoon (it may discolor) to stir while you dissolve the OA. Wear nitrile gloves and safety glasses! Keep the burner on the mixture while stirring, but do not allow the temp to exceed 160F. With a modicum of caution, this is safe to do.

As soon as the mixture is completely clarified, remove it from the heat and place the pan on a plastic tray.

Preheat the roll of dry towels for 1 minute in the microwave–this preheating helps the solution to wick into the roll. Although you can insert a piece of 1-1/4″ PVC pipe into the roll, I find no benefit from doing so, since the cardboard cylinder will come loose anyway. Also no benefit to leaving the wrapper around the roll, since I get better absorption if the roll is allowed to expand.

Carefully place the roll into the hot solution. The pan should first be placed on a plastic tray (not as shown). The solution will quickly wick up part way. When it gets to about 1/3rd of the way up, then flip the roll.

Use kitchen tongs to flip the roll. The mixture will not immediately irritate your skin, and can be easily washed off with warm water. But it is sticky, and will get on all surfaces if you’re not careful. It is really important not to allow traces of the solution to get on surfaces, as it is slowly corrosive, and a hazard. You don’t want it ending up in your eyes! Keep warm water and a wet sponge on hand for wiping surfaces. A little bit of baking soda dissolved in water will immediately neutralize any acid.

The solution will wick up from the other end.
You may find it to be of benefit to flip the roll again. The roll will absorb every last drop of solution. Allow the roll to cool, and for the water to evaporate off (I’m not sure that this is necessary, as it would also occur later in the hive). You can then place the roll into a secure plastic container or ziplock bag for storage.
I found that when I held towels in the solution at 150F for a few days (with or without water), that the acid dissolved the towel. I don’t observe this happening at room temp, but have not had time to test to determine how long the treated towels can be stored before losing strength. The Aluen CAP strips claim to be good for an extended storage period. I will be performing tests to see whether the OA or towels degrade with extended storage. For now, probably best to make them up fresh.
One would then apply two half sheets of towel per hive for a treatment, which would apply 12 g of OA to the hive.
I’m not completely sold on the rolled towels, and have the folded version on order, which I will test when they arrive.
I’m buried in email from those wanting more info. Please only email me if you have something important to offer, or a serious question. If you are not comfortable with accurate weighing, measuring, and safe handling of acids, YOU SHOULD NOT ATTEMPT TO DO THIS!
I am encouraging one cooperating bee supply company to bring an inexpensive premade version to market.
Update 24 Jan 2017
There has been lots of response to my article on OA/gly in ABJ. I’m in communication with EPA to get this application method approved.
I’m in the middle of a trial of OA/gly shop towels on 6 hives during our wet, cold winter. As of today, after ~3 weeks, the colonies are not showing any adverse effects upon the bees.
I’m currently working on is the best ratio of OA to glycerin, and the best amount per towel. The problem is that the towels don’t want to evenly absorb the optimum amount of glycerin to leave them less “oily” so that the bees will chew at the towels. I’m trying to figure out how to get a full roll of towels to absorb the right amount of solution without the need for squeezing out the excess.
I tried 700g OA in 700ml of glycerin, heated to 160F, and the roll of towels preheated 1.5 minutes in the microwave. I poured the hot solution into a tall, narrow asparagus pot, and set one end of the towel roll into the pot. When the solution had soaked half way up, I flipped the roll over, and placed the roll into a warm oven. After 15 minutes, the solution has still not absorbed to the center of the roll. So I left it for another hour in the warm oven. This proved to be a mistake, since the acid degraded the lower part of the roll into a soft mess.
The 700ml of glycerin also appeared to be too much–leaving the towels too “oily” feeling.
I’m now going to experiment with adding isopropyl alcohol (boiling point 180F) to the solution, and decreasing the amount of glycerin.
Dear U.S. beekeeper,
Recent studies by Dr. Stephen Martin and associates have found that there is apparently a benign form of DWV that can out compete the virulent form, thus allowing colonies to survive despite varroa infestation.
If this is true, it raises the possibility that we may be able to minimize the effect of varroa by inoculating our colonies with the benign form of DWV.
We obtained funding from Project Apism to survey bee colonies across the U.S. to determine the distribution of the strains of DWV. We’re especially interested in adult bee samples from feral and survivor stock that have survived for some time without treatment. We also need reference samples from “normal” managed apiaries.
If you are interested in contributing samples, please write to Randy at randy@randyoliver.com, with the word “kit” in the subject line, the sort(s) of hive(s) that you’re able to sample, and the state in which the hives are located. Please also include your mailing address.
I will reply, and send a postpaid sampling kit. It should take less than an hour of your time to contribute to this research.
Please print out these sampling instructions
Here is a slide show for general public presentation. Contains some old 35mm photos, which I hope to soon replace.
Public general presentationPublic general presentation
There are a number of other resources for reliable information on varroa management, some of which I’ll include links for:
The Honey Bee Health Coalition released this summary of mite management tools in 2015. Free download at Tools for Varroa Management
Updated 15 January 2016 Updated oxalic acid ppt presentation
Since the EPA registered oxalic acid for the use in beehives (as far as I know, Brushy Mountain has the only registered product to date), I’ve been flooded with questions about using it (since I’ve used it steadily in my operation for over a decade).
Therefore, I’m making a Powerpoint presentation on using oxalic acid available. You may use this presentation at your local bee club meetings. Most of the slides are self explanatory, plus I’ve added notes to those needing more explanation. The presentation consists of 84 slides:
If you use this presentation, I’d appreciate a donation to ScientificBeekeeping.com to help offset my time in preparation.
The Powerpoint presentation can be downloaded at: 2016 Oxalic acid
For those without Powerpoint, a pdf version is at: Oxalic pdf with comments Thanks to beekeeper Dan Lindamood III for taking the time to add my comments as sticky notes (they do not show fully in Chrome–you may need to open in another pdf viewer).
Update 29 Dec 2015: An email from a recreational beekeeper:
“Hey Randy, first let me thank you for all your good work and your ability to transfer information and making it palatable to newbies such it myself. Just want to drop you a quick line about Oxalic acid. This is not scientific–just a practical application advice. I’ve been using it for about a year and a half. Started out with the dribble method and had excellent results. My mite count is practically nil. I recently purchased the vaporizer and would like to make a couple of quick non-scientific observations. First being a backyard beekeeper I have to pull the battery out of my car and drag it out to the backyard hive locations. Treat my five hives and then return the battery to my vehicle and hope that it still has a charge to start my car up. No little task for an auto neophyte. Next if you’re using the vaporizer you can kiss your screen bottom board goodbye. It will most likely eat right through the screen and the heavy Styrofoam type removable bottom used in most commercial screen bottom boards. Next if you’re using plastic frames there an excellent chance that the intense heat will melt them. At least any in the immediate location of the heating element. Last but not least as you mentioned in your articles the fumes of the acid is intense and dangerous but with the added odor of any Bees that may have been cooked in the heating element the smell is pretty bad. Just my two cents of a couple of problems which I don’t ever recall being mentioned or spoken about.”
There are pros and cons to every treatment method, and the decision to dribble or sublimate OA is no exception. I’ve listed the pros and cons in the ppt presentation.
6 Jan update:
A team at the University of Sussex published an excellent paper (not yet published) comparing three methods of oxalic application: spraying each frame of bees individually, dribbling the “seams” of bees, or sublimation with a Varrox M3080 vaporizer. They tested the three methods at different doses of oxalic acid.
A brief take-home on their findings:
Spraying was effective, but tough on the bees, invasive, and time consuming, so I will discuss it no further.
Dribbling and sublimation were fairly comparable, with some notable differences.
Updated Nov 2: The science of the effects of neonics upon pollinators is so contentious, that I am often asked to comment on new published studies. This study, which claimed an effect of neonics on queen mating, immediately garnered such requests. Unfortunately, the lead author was at conference at the time, and was unable to fully discuss the paper and answer all my questions until recently. I now wish to revise my comments accordingly.
I’ve now had the chance to discuss the paper at length with the author, of whom I think highly (and with whom I previously coauthored a paper). We’ve discussed the shortcomings in the original design of the experiment, which come back to haunt us when we try to interpret the results.
In this study, queens were reared in either Test or Control cell builder colonies–the Test group being fed neonic-spiked pollen; the Control group received unspiked pollen.
The main finding of the study was that after four weeks of being in mini mating nucs stocked with roughly 1000 workers, fewer queens were successfully laying in the Test group that had been fed neonics during the cell building period. The question then, is why?
Since nurse bees typically produce jelly free of pesticide residues, and since the researchers did not test the jelly in the queen cells, we have no way of knowing whether the developing queens were directly exposed to neonics. Without such confirmation of exposure, one cannot say that the queens were actually exposed to the treatment. The bees that were clearly exposed were the nurses that reared the queen larvae and later used to stock the nucs.
For the experiment, the newly-emerged queens were placed into mini nucs stocked with roughly 1000 workers.
The bees used to stock the Test nucs came from colonies that had been fed neonics for 36 days, ensuring that those workers had been reared under continuous neonic exposure, and then when they emerged had likely consumed neonic-spiked pollen for their entire nursing period up ’til when they were transferred to the mating nucs.
The bees used to stock the Control nucs had received no exposure to the insecticides.
Interpretation of the results is thus confounded by our not knowing whether the observed effects were due to changes in the queens or changes in the workers in the nucs.
The result was that there was greater mortality and/or failure to lay eggs of the queens in the neonic-treated nucs.
But it was difficult to tease out exactly why. The Treated queens flew and mated exactly the same as the Control queens and had significantly more ovarioles than the Control queens, but may have had somewhat fewer stored spermatozoa(it was difficult to tease out statistical significance due to the small numbers and high variability).
The question then is, were the apparent differences between queen performance due to direct effects of exposure to the queens during their larval development, or were they due to the performance of the workers in the nucs?
Could the apparent differences in the number and viability of the spermatozoa be due to poor queen care by those 1000 treated workers, or due to poor thermoregulation by the neonic-exposed workers, or simply due to their premature death leading to rapid dwindling of those 1000 bees in the nucs? The experiment was not designed to answer those questions.
In answer to my questions, Dr. Williams tells me that none of the queens were observed to be drone layers. Those which had not laid worker eggs had simply not laid any eggs at all (although there is the possibility that the workers cannibalized them; this often occurs in hungry nucs).
I also asked whether the worker population had dwindled in any of the nucs. Answer: “If the queen was there, regardless of egg-laying, there were always ample numbers of workers.” Unfortunately, this does not exclude the possibility that the workers had abandoned some nucs.
Although the findings of this study are strongly suggestive, the small number of subjects, plus the innate variability of mating success (I rear thousands of queens each spring), makes me hesitant to draw firm conclusions until we see the experiment replicated, which Dr. Williams intends to do. I have also volunteered to collaboratively replicate it in California next spring.
This paper is open access, and can be downloaded at Neonicotinoid pesticides severely affect honey bee queens
A Review of Dr. Lu’s paper on neonics in Massachusetts
By Randy Oliver ScientificBeekeeping.com
August 18, 2015
I was recently asked by a couple of extension horticulturalists to comment on Dr. Alex Lu’s most recent publication on the neonicotinoid insecticides. I hesitated to do so, since Dr. Lu feels that I’ve picked on him. But since his papers carry the prestige of the Harvard name (and will thus grant him unwarranted attention from the media), I felt compelled to give it an honest review. But first let me explain why I go to the effort.
I’ve been an environmental activist since my teens, and am happy to see the progress that we’ve made since then. Our air and water are cleaner, and society’s environmental consciousness is at an all time high. That said, there is still much work to be done in reducing humanity’s negative impact upon the Earth’s environment. We still need watchdogs and activists to monitor our business and personal activities that may be contributing to environmental and health problems, and I applaud such watchdogs and activists for doing so.
But doing so also confers a responsibility not to exaggerate the facts, nor to unduly alarm the public, mislead the media, or to foment fear for fundraising purposes. Unfortunately, when I became involved in the politics of beekeeping I quickly learned how the media, in their quest for sensational stories, pander to activists and advocacy groups that are guilty of all the above. The public, which is unfortunately largely scientifically illiterate, then swallows these compelling false narratives hook, line, and sinker, leading well-intentioned folk to make poor judgments (such as going on low-fat diets, not vaccinating their kids, or worrying about the wrong things). Even worse, their misdirected activism subsequently leads to protests and public pressure on our representatives to “do something” about a problem that may be greatly exaggerated, and directs our energies away from more important environmental concerns.
Practical application: each of us can only devote a limited amount of our time towards constructive environmental activism. Such activism should thus be based upon sound science and rational discussion. To that end, I’ve attempted to objectively review the neonics in such a manner [[1]].
Prior to World War II, farmers commonly used truly dangerous insecticides such as lead arsenate and nicotine to control pests. Then when the first synthetic insecticides were introduced, they were heralded as miracles—DDT was nearly nontoxic to humans, but wiped out mosquitoes, lice, and many other insect pests. But then Rachel Carson brought to our attention that we had not foreseen that the organochlorines not only did not degrade in the environment, but actually bioaccumulated, culminating in the devastation of some raptor and pelican populations.
Important fact: the neonics are not DDT. They don’t bioaccumulate, nor do they appear to build up in the soil to any appreciable extent despite repeated use [[2]].
This, and the Big Tobacco debacle, left the public with the impression that our regulators are not to be trusted. In reality, the newly-founded EPA did indeed take those lessons to heart, and now does a great job of following its mandate to use the best science to carefully regulate pesticides to avoid “unreasonable risk to man or the environment.” What we need to keep in mind is that the EPA must attempt to find that difficult balance between our society’s demand for cheap, cosmetically-perfect food, versus the obvious negative effects of pesticides upon the environment (while worrying that a Republican-controlled Congress may cut its budget).
The problem is that for the foreseeable future, agriculture (conventional or organic) is going to require some use of pesticides in order to produce enough food for our burgeoning human population. The question then, is to figure out which pesticides should be allowed, and which restricted. These decisions are best based upon sound science, not misinformed activism.
In recent years, the bee research community was surprised to see publications by a Dr. Alex Lu, an Associate Professor of Environmental Exposure Biology at Harvard Medical School. Why in the world, we wondered, was a medical researcher without any background with bees attempting to perform bee research? His biography may give us a clue: “Alex’s research focuses on understanding how ecological and human health are being affected by the pervasive presence of chemicals in the environment [[3]].”
I applaud Dr. Lu on his bringing his concern to our attention, since human health is his field of expertise. Unfortunately, bee biology is not. Yet Dr. Lu seems determined to convince us that the neonicotinoid insecticides are both the cause of elevated honey bee colony mortality, as well as being a threat to human health, which due to the sensationalism of the subject, has made him a darling of the media. But for anyone with a good understanding of bee biology and the running of field trials, his papers tend to be sadly amusing; unfortunately, the press loves to trumpet his widely discredited “findings” as fact.
To his credit, Lu’s papers have improved over the years (perhaps in response to my critical reviews). But his latest paper, Distributions of neonicotinoid insecticides in the Commonwealth of Massachusetts: a temporal and spatial variation analysis for pollen and honey samples [[4]], still follows his pattern. Dr. Lu is a man on a mission—to get us to fear the neonicotinoid insecticides—which comes as a surprise to the EPA, whose risk assessors consider them to be “reduced risk” products to both man and the environment, replacing the more harmful organochlorines and carbamates, and the failing pyrethroids.
Before I go any further, allow me to clearly state that I have no beef with Dr. Lu, I’m sure that he is a fine scientist and human being, and thank him for investigating any adverse effects of neonics upon humans. My problem is not with him personally, but rather with any scientific publications based upon sloppy science, or that play loose with facts and interpretation; and especially those that are announced at press conferences prior to publication in order to promote the authors’ agenda in the media.
Lu makes the claims that neonic residues are causing honey bee colony collapse, killing birds and other wildlife, and possibly causing neurotoxicity in developing mammalian brains [[5]]. The above claims are cause for serious concern, and call for deeper investigation, which I’ve done. What I found was that each of the claims was speculative and only weakly substantiated.
At this point allow me to make clear that I believe that our agricultural system is far too dependent upon pesticides, and that no insecticide is without fault. Neonic planting dust from corn seeding is sometimes a serious problem for pollinators. Neonics also appear to be more of an issue for some pollinators (such as bumblebees and mason bees) than to honey bees. And there is suggestive evidence that neonics may be involved in premature queen failure and immune suppression. I’d be the last person to argue that neonics are harmless. Insecticides by definition kill insects. Ideally, any registered use of an insecticide will provide the most targeted impact on the pest(s), with the least collateral damage to other species.
That said, let’s look into Lu’s claim that that neonics might cause neurotoxicity in the developing brains of humans. Again, I’d sincerely like to thank Dr. Lu for being a watchdog for our health. In another paper [[9]], he states:
In light of new reports of toxicological effects in mammals, the results strengthen the importance of assessing dietary neonicotinoid intakes and the potential human health effects.
The mention of these “new reports” got my attention, so I read further. He actually cites only a single in vitro (test tube) study [[10]] on the action of neonics on human neurons (which did not even mention toxicity); this is a good example of how Dr. Lu creatively extrapolates others’ findings.
Lu, as a toxicologist, fully understands that risk is the product of hazard (toxicity) times exposure. In order for a neonic to be a risk to the developing brain, the person must first consume in excess of a defined amount of the chemical via residues in food, the chemical must then pass through the blood-brain barrier, and then it must actually cause a negative effect in the brain itself.
So let’s then look into each of these conditions in turn, in order to see whether there is data to back up Lu’s dogma.
EXPOSURE THROUGH FOOD
Our first question should be, is it realistic for a person to consume enough residues of neonics to be of concern? In order to answer that question, we first need to know the NOAEL (No Observable Adverse Effects Level) of the chemical, as determined from long-term feeding studies of surrogate animals [[11],[12],[13]]. For imidacloprid in rats, mice, rabbits, and dogs, this was found to be around 10 mg/kg/day. The EPA, heeding the precautionary principle, then goes much further, and sets the chronic dietary reference dose for humans nearly 200x lower–at 0.057 mg/kg/day.
I understand that the above figures are gobbledygook to most readers, so let me put them into understandable (and personal) terms. I love apples, and eat at least two a day. Could I be poisoning myself with neonics? As luck would have it, Lu himself conveniently supplies us with analyses of seven different apple cultivars [[14]], for which residues of imidacloprid averaged less than 1 ppb.
So how many neonic-tainted apples would I need to consume in a day in order to approach the extremely conservative “safe” level of exposure as determined by the EPA? I of course did the math [[15]]. Result? I’d need to eat nearly 10,000 pounds of apples a day to approach any level of concern.
GETTING THROUGH THE BLOOD-BRAIN BARRIER
OK, so let’s say that I was really craving apples, and managed to consume 10,000 pounds today. Some of the imidacloprid residues would be absorbed through my gut; but could they then make it through my blood-brain barrier (which our body uses to protect ourselves from dietary neurotoxins)? Lu sounds the alarm that:
Neonicotinoids and some of their metabolites are also shown to be able to pass through the blood-brain barrier in mouse, and some metabolites having enhanced potency to nAChR are even more toxic than their parent compounds [[16]].
This surprised me, so I checked his reference. As Lu has a habit of doing, he distorted the findings of others. For example, he ignored the authors’ finding that:
Brain levels of 3–16 ppm can be achieved without obvious poisoning signs.
Now keep in mind that 3-16 ppm is equivalent to 3000-16,000 ppb directly in the brain, yet still no obvious toxicity! Lu also neglected to say that these levels were only obtained by the researchers intentionally adding a second solvent (DMSO) in order to help the insecticide to pass through the blood-brain barrier. In fact, one of the attractions of the neonics is that they only penetrate the mammalian blood brain barrier to a very limited extent.
Practical application: it is actually difficult to intentionally poison oneself by drinking imidacloprid straight up [[17]]. This simple fact alone speaks volumes about how little we should be concerned about neonic toxicity to humans, relative to the alternatives [[18]].
The other thing that Lu does is to raise our concern about the metabolites that are of increased toxicity, but as a toxicologist, he should also explain that overall toxicity of the metabolites would not exceed that of the parent compound as a whole. I find this sort of intentional omission to be both misleading and indefensible.
CITED EFFECT UPON THE BRAIN
Despite the evidence so far that I really didn’t need to worry about unintentionally poisoning myself with neonics, I was still concerned about Lu’s suggestion [[19]] of the “possible neurotoxicity in developing mammalian brain.”
I certainly don’t want my grandkids to develop brain abnormalities from consuming neonic-tainted produce. So I dug a bit deeper into the cited study. What the researchers had actually demonstrated was that when neuronal cells in a petri dish were directly flooded with a 1μM solution of imidacloprid, that they fired similarly to those exposed to the same concentration of nicotine, but took a bit longer to recover. The authors didn’t even mention neurotoxicity.
And just how strong is a 1μM concentration? It works out to 256 ppb actual exposure to the neurons. Compare this to the typical concentration of imidacloprid residues in apples of less than 1 ppb [[20]], and the tiny amount that would ever make it through the blood-brain barrier. Why the researchers used such an unrealistically high concentration of imidacloprid is beyond me. It would have been scientifically prudent for them to continue to reduce the dose to determine the NOAEL at which the neurons no longer exhibited any excitation.
Practical application: scientists perform in vitro (“test tube”) experiments to screen for potential health effects; real life application can only be determined by in vivo testing on live animals (generally lab rats as surrogates for humans), testing on human volunteers, or from the effects of accidental (or intentional) poisonings.
So I continued my literature search for such in vivo studies (which are also what the EPA focuses upon). What I found distressing is that the titles of scientific papers again tend to sensationalize their findings. For example, Duzguner [[21]] showed “acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system.” But he did so by injecting ¾ of the lethal dose (26,000 ppb by my calcs, along with DMSO penetrant) into rats. I question the relevance of such studies to the sort of actual exposure to humans that humans would get from eating produce with residues of insecticides.
Practical application: if you are a young scientist wanting to get your paper widely cited in order to improve your advancement and funding, all you need to do is to include the word “neonicotinoid” in the title, and then speculate in the abstract that there could conceivably be some effect upon human health, and the media will trumpet your name across the globe.
Due to Lu’s association with the name “Harvard,” his efforts at scientific research gain far more attention than they deserve. Indeed, he comes off more as a showman than a scientist (as evidenced by his latest publicity stunt of analyzing the food served to Congress in order to claim that the traces of neonics found in their produce were cause for concern). His “research” so far may be politically provocative, but unfortunately, for all practical purposes, irrelevant to meaningful discussion of the neonics.
Perhaps we can better put things into perspective by returning to our neonic-poisoned congresspersons, many of whom smoke cigarettes (if not something else). The nicotine in cigarettes is roughly 100 times as toxic to mammals as is imidacloprid. When a congressperson smokes a cigarette, their blood concentration of nicotine goes to about 12 ppb [[22]]. To match that toxicity by the consumption of apples, a congressperson would need to consume at least 1200 times his/her body weight in apples in one sitting (and repeat that performance for the next cigarette). It seems to me that the members of Congress should be more concerned about secondhand smoke than with the produce on their table.
At this point in my research, and being a nontoxicologist, I was getting frustrated by trying to get at the truth of the matter. So I searched for the most conservative opinion by actual toxicologists that I could think of. The most precautionary assessment would likely be that from the European Union’s EFSA (analogous to our EPA), the members of which go out of their way to cover their butts to appease the European anti-neonic activists. These learned toxicologists concluded that [[23]]:
As the current ARfD and AOEL [[24]] for imidacloprid may not be protective enough for potential developmental neurotoxicity of this active substance, the Panel also recommends to conservatively lower these reference values to the same level as the ADI (0.06 mg/kg bw per day) [emphasis mine].
In case your brain is already fogging over with numbers, that’s the same figure used by the EPA (and that which I used to calculate the 10,000-pound dose of apples that you’d need to consume a day).
CONCLUSION
I’m as concerned as anyone about being exposed to pesticides, and especially about unforeseen effects upon my health, or the developing brains of my grandkids (or our congressmen). However, contrary to Lu’s alarmism about the neonics, any objective review of research to date would suggest little cause for fear. All the evidence that I’ve seen to date concur with the EPA’s assessment that neonics are indeed reduced risk insecticides, so far as humans are concerned, and it appears highly unlikely that the amount in produce is likely to harm you in any way.
So let’s now return to Lu’s current paper, in which he sticks to neonic residues in pollen and honey, which are likely more relevant to the diet of bees than to humans. Unlike humans, some insects exhibit behavioral effects from neonics at concentrations as low as a few ppb. So this study had the potential to determine whether neonic residues in pollen affected colony health. Alas, Lu did not bother to do so; instead he used his state of the art equipment essentially to show off that he could detect residues of neonics in the vanishingly small parts per trillion—levels likely of biological irrelevance.
In a typical scientific paper, the author proposes a testable hypothesis (in this case, that neonics are present in the environmental at a level that is causing health problems to bees and humans), then designs an experiment to test that hypothesis (in this case by measuring levels of neonic residues in honey and pollen), and then seeing whether the results of the experiment either support or refute his hypothesis (by seeing whether there was a correlation between neonic exposure and colony performance).
But Lu’s study was not a test of a hypothesis, but rather an extremely informal survey for neonic residues in bee-collected pollen (due to his anti-neonic tunnel vision, he didn’t bother to analyze for any other pesticides). The paper starts off on shaky ground by referring to an excellent paper by Mullin, and points out that neonics were previously found in beebread samples. But what he doesn’t mention was that no neonic was found in more than 5% of Mullin’s samples, refuting his next claim that:
Neonicotinoid insecticides, in particular imidacloprid and clothianidin, have long been implicated and recently shown complicit in honeybee colony collapse disorder (CCD).
The above claim completely flies in the face of the fact that the bee research community has largely come to the conclusion that this is not the case [[25]], as well summarized by Dively [[26]]:
Given the weight of evidence, chronic exposure to imidacloprid at the higher range of field doses (20 to 100 μg/kg) in pollen of certain treated crops could cause negative impacts on honey bee colony health and reduced overwintering success, but the most likely encountered high range of field doses relevant for seed-treated crops (5 μg/kg) had negligible effects on colony health and are unlikely a sole cause of colony declines.
Lu further goes on to claim that:
No study has yet been published that demonstrates the prevalence of neonicotinoids in the environment where bees are foraging to elucidate the temporal and spatial variations of neonicotinoids in pollen.
While perhaps technically true, numerous researchers have been tracking pesticide residues in pollen since the early 2000’s [[27]].
We all want scientific papers to be technically accurate. One expects any scientific paper to be thoroughly reviewed by other peer experts for accuracy in technical details, and reasonality in its conclusions. Don’t count on this for Lu’s work. For example, he includes flonicamid as a neonicotinoid, although its mode of action is completely different—it does not target the same nAChR neuronal receptors [[28]]. As explained by another researcher [[29]],
Flonicamid has no effect on the nAChR and according to both the IRAC and the EPA, flonicamid is not a neonicotinoid by classification or mode of action.
Lu goes on to calculate the “relative potency factor” for combinations of neonic residues, but the information that he used to calculate those factors frustratingly cannot be found at the cited source, and he didn’t bother to state whether the toxicity values were calculated for rats or for bees (critical, since neonics are vastly more toxic to insects). Without such information, his RPFvalues are nearly meaningless. It appears, based upon the values, that he used mammalian toxicities, which would be misleading if applied to honey bees.
One may wonder why Lu’s team manages to detect a greater prevalence of neonic residues than other researchers, such as the huge data sets for produce by the USDA [[30]], or by bee researchers [[31]]. The reason appears to be that the Chen lab is comfortable with the accuracy of their “sensitive and modified LC-MS/MS method along with the QuEChERS procedure to simultaneously measure 8 neonicotinoid residues” [[32]].
Unlike other labs, which detect neonics at the ppb level, Chen’s lab claims to set their level of quantification in the parts per trillion.
Lu’s methodology is explained in great detail in some areas, but to my untrained eye seemed to be lacking in other critical aspects; so I asked two experts in the field for their opinions. They pointed out that pesticide residue analysis is complicated and difficult–that’s why it costs so much. Lu described the easy part in the paper. What he didn’t detail were the difficult parts–his quantification and quality control of the mass spectrometry. This is critical, since there are many things that must be carefully controlled in order to maintain precision and accuracy in residue analysis. As one reviewer explained:
[Lu] describes the LC program well that was used for the analyte separations but doesn’t say what parent and product ions he screened for each compound or any information at all about standard reference materials that were used for identification and quantification. Did he use matrix matched standards? How many levels in his calibration curves? Is the method validated/verified? What were the analyte recoveries? Is his lab accredited? Does he participate in a third party proficiency sample program that would lend credibility to his residue analysis? Does his lab have a Quality Management System? There are a lot of ways for a lab to demonstrate competency and proficiency, but none were referenced in this paper.
But let’s give Dr. Lu the benefit of the doubt and accept his residue results at face value. What I’m more concerned about are his interpretations of the scientific literature as he attempts to put his results into perspective.
For example, one of Lu’s claims caught my attention:
A comparable study has demonstrated that bees living and foraging near cornfields in Indiana are being exposed to neonicotinoids…When maize plants reached anthesis [tasseling], maize pollen that was collected directly from bees using a pollen trap from treated seed was found to contain clothianidin and thiamethoxam, ranging from non-detectable to 88 [ppb] and non-detectable to 7.4 [ppb]respectively.
The 88 ppb level in the pollen of seed-treated corn was nearly 50x higher that any measurement that I’d previously seen. How could I have missed this? So I reread the study that he cited [[33]]. It seems that Dr. Lu has trouble in understanding scientific papers, since the 88 ppb figure was from bee-collected pollen from flowers on July 10, not from the pollen of seed-treated corn, which measured at only 3.9 ppb clothianidin. Dr. Lu’s misrepresentations of the scientific literature makes his work an embarrassment to other researchers.
Lu further stretches credibility by then stating:
In brief, honeybees exposed to imidacloprid or clothianidin at levels ranging from 0.5 to 30 ppb…could lead to CCD, a systematic disease still lingering in many countries after its first occurrence in the winter of 2005–2006.
In the first place, signs of CCD were already common in the winter of 2004-2005. In the second place, there is no such thing as a “systematic disease,” and CCD is a disorder, not a disease. You’d think that a researcher from the Harvard Medical School (or his reviewers) would understand disease terminology. And then Lu’s very own previous research [[34]] contradicts his statement, since in his 2012 paper, it was obvious that there was no apparent effect from feeding colonies syrup spiked with up to 20 ppb of imidacloprid. So he has zero supporting evidence for his claim that a dose 40x lower could cause CCD. On a roll, Lu continues:
Sub-lethal exposure to acetamiprid, imidacloprid, clothianidin, dinotefuran, fipronil, thiacloprid or thiamethoxam could lead to developmental impairments, including poor colony growth at levels of 2–5 ppb.
I again have no idea as to how Lu could make this claim. The two citations that he uses to support the claim did not involve any neonic other than imidacloprid, so he can’t legitimately mention any others. And as far as claiming poor colony growth at 2-5 ppb, in the two cited studies, colony growth between the treated and control colonies was identical at up to 20 ppb in the first study, and identical at 135 ppb in the second. These sorts of contradictory and unsupported claims are simply unacceptable in a scientific paper.
To Lu’s credit, he was able to come to some coherent conclusions, for example:
Lastly, the use of the LOAEL as the basis of calculating imidaclopridRPF may be a reasonable approach for estimating the aggregate neonicotinoid exposure by pollen ingestion, but one should be cautious when interpreting the cumulative risks in bees.
Yes, a neonic RPF would be a reasonable approach, if one leaves chemicals with dissimilar modes of action (such as flonicamid) out of the equation (and uses values for bees, not mammals). And yes, one should be extremely cautious about interpreting the cumulative risk (especially if you screw up the basic assumptions in the first place). It would have been of far greater interest had Lu had taken the time to identify the pollens associated with the few unusually high detects of neonics in his samples. But then Lu returns to his deductive logic of putting the cart before the horse:
Considering various sublethal effects of neonicotinoids on bees, it is prudent to identify ways to reduce the uses of neonicotinoids in order to reverse the deteriorating trends in honeybee health and the declining numbers of honeybee colonies.
Yes, it would indeed be prudent to reduce the uses of all pesticides! But Lu ignores the plain fact that the trend for honey bee health has improved greatly in recent years (provided that beekeepers manage varroa), and that the numbers of colonies are hardly declining, but rather increasing [[35]].
If we give Lu the benefit of the doubt that his methodology was valid, his results indicate that traces of neonics can be commonly detected in pollen and honey samples from Massachusetts. This comes as no surprise to anyone who has checked the pest control product shelves in any hardware or garden store, and Lu rightly concludes that:
The ubiquity of neonicotinoids in states such as Massachusetts where the planting of neonicotinoid-treated crops only represents a very small fraction of overall agricultural activities suggests that non-agricultural uses of neonicotinoids is likely the main contribution to the levels found in pollen and honey that bees brought back to their hives.
Of interest is that in some honey samples, levels of dinetefuran or imidacloprid reached nearly 15 ppb (of no concern to humans, since few people consume honey by the gallon in a day). Such levels are not consistent with agricultural applications, suggesting that it is homeowner or landscape applications that are putting that much insecticide into the nectar—I agree that this is cause for concern regarding pollinators. Unfortunately, Lu didn’t take the obvious step of then performing pollen analysis of those honey samples, which may have indicated from which floral sources that tainted nectar came—data which would have been of interest to the EPA.
As far honey bees are concerned, I must take issue with Lu’s exaggerated claim that:
The levels of neonicotinoids that we measured in pollen samples have significant implications for honeybee health.
One should look at his Figure 4, which clearly shows just how negligible most of those residues were. In only 6 of 45 monthly samples did residues exceed 2 ppb, with the highest being roughly 5 ppb. Based upon numerous studies, and summarized by Dively, levels of neonics in the range of 5 ppb have negligible effect upon honey bee colonies (although other pollinators may be affected at that level).
What troubles me is that Lu’s agenda appears to be to circumvent the EPA, and instead use the media to put pressure on Congress to ban the neonics, despite the fact that EPA’s registration of neonic products has allowed us to greatly reduce our exposure to the more harmful organophosphate and carbamate insecticides. I’d much rather see Dr. Lu use his training and talents to help collect data that might be usable by the EPA.
I appreciate that Dr. Lu is acting on his convictions, but I’d rather that he do so honestly and based upon sound science. He is exhibiting a classic case of deductive reasoning—prior to any research, he came to the conclusion that neonics are bad, so he deduces that there must be an effect on bees and human health. Such erroneous logic is anathema to science, which instead uses inductive reasoning—coming to conclusions only after careful observation, extensive testing of hypotheses, and the collection of supportive data. Lu’s data do not support his hypotheses, yet he continues to stick with his preconceived agenda.
Although I fault Dr. Lu for the above, the real responsibility lies with others. We trust the editors and referees of scientific papers to recognize errors and reject papers not suitable for publication. Every one of the egregious errors that I’ve pointed out in Lu’s papers should have been caught by his reviewers and been corrected, or been cause for the paper to be rejected for publication—this is their duty to the scientific community and the public. I’m appalled that a trusted institution such as Harvard allows its name to be associated with examples of science at its worst. And how about our media, who stoop to tabloid sensationalism by uncritically parroting this sort of unscientific nonsense upon an unsuspecting public?
Data vs. Dogma
We have a regulatory mechanism in place to assess the risks of pesticides. The EPA is tasked with a job that is guaranteed to never please everyone. However, I speak with its risk assessors regularly, and feel that they are indeed basing their decisions on the best available data.
The public debate about pesticides is a good thing. We need to keep pressure on industry and the regulators. But we need to do so with solid supporting evidence—good scientific data that tells us where there are truly issues that need to be addressed. Researchers such as Dr. Lu are in a position to test for adverse effects, and to supply such needed data to the EPA. Wild unsubstantiated claims only confuse the issue. Speaking of such, Dr. Lu showed his true colors by saving one for the press conference held for the release of his paper.
Although Lu’s findings confirmed that neonics were sometimes present in pollen at extremely low levels, in his press release [[36]] he used the Harvard name to promote the preposterous claim that those minuscule residues “may pose health risks for people inhaling neonicotinoid-contaminated pollen.” Such knowing contortion of science is a deplorable use of the Harvard press office.
In the first place, Lu’s study had absolutely nothing to do with the airborne pollens that we inhale—he collected only the typically sticky pollens gathered by bees. But let’s do the math anyway. Worst-case exposure to pollen grains is in the order of less than 10,000 grains per day [[37]]. For neonic-treated corn, there are around 3000 pollen grains per milligram [[38]], and median neonic residues of hand-collected pollen is about 2.8 ppb [[39]]. This works out a 170-lb person inhaling at worst 0.0000000001 mg/kg body weight/day of neonic residues. Compare that figure to the conservative safe intake of 0.06 mg/kg, which works out to you’d need to inhale tainted corn pollen every day for million years straight in order to meet even one day’s level of concern. As a medical researcher and “expert” on pesticides and toxicology, Dr. Lu knows full well how to do calculations such as the one above. I find it inexcusable for a Harvard scientist to promulgate clearly unwarranted fear in a trusting public.
Practical application: I’m as concerned about the impacts of pesticides upon the environment and human health as is anyone, and I submit formal comments (backed by good science) to the EPA detailing specific issues. On the other hand, I find the gratuitous fomenting of fear in a concerned public by a medical researcher of Dr. Lu’s stature to be reprehensible, and an embarrassment to the Harvard institution. Lu could benefit by the example of the Harvard students who recently published an excellent review on GMOs and our food [[40]].
You can decide for yourself, but if I were a resident of Massachusetts, I’d continue to eat commercial apples without undue fear (and inhale deeply as I do so). And as far as the bees, despite Lu’s unshakeable belief that the major bee problem is neonics, for some reason in this study he did not to attempt to link cause and effect. I cannot fathom why he did not ask his cooperating beekeepers to additionally record colony health, productivity, or survivorship, so that he could have told us whether there was any correlation between neonic exposure and colony performance [[41]]—data which would have been of far more interest than the colorful graphics in the paper.
Nor did he make the effort to determine the sources of neonic contamination in nectar or pollen, which he could have easily done by simple microscopic analysis, and which would have had great practical application. I implore Dr. Lu, should he continue his studies on neonics, to consult with experts who can help him to better design his experiments or surveys, so that his results will be meaningful to beekeepers, the public, and our regulators.
In conclusion, I’m no salesman for any pesticide, and am concerned about the general overuse, and some specific uses of the neonics. But I realize that growers are going to protect their crops. I’d like the products used to be regulated based upon good science, rather than showmanship. I urge the reader to review three recent well designed and executed studies on actual field exposure of bees to seed-treated canola or corn [[42]], none of which found any effect from the neonic residues in the pollen and nectar upon foraging, orientation, colony performance, or survival.
In the spirit of my review, if any reader finds even a single error in fact or interpretation in this article, I will be glad to correct it here.
The full citation for the paper is: “Distributions of neonicotinoid insecticides in the Commonwealth of Massachusetts: a temporal and spatial variation analysis for pollen and honey samples,” Chensheng (Alex) Lu, Chi-Hsuan Chang, Lin Tao and Mei Chen, Journal of Environmental Chemistry, online July 23, 2015, doi: 10.1071/EN15064
One researcher points out that “CCD is, by definition, a new name for an old disorder,” and suggested that I cite the source for the term “CCD”: http://www.beekeeping.com/articles/us/ccd.pdf
Correction: Dr. Lu is an associate professor of environmental exposure biology in the Department of Environmental Health at the Harvard T.H. Chan School of Public Health.
Aug 28–I’ve now received a large amount of positive feedback for this review from other bee researchers; most of whom, out of professional courtesy, are hesitant to publicly criticize other scientists. I wrote the review as a call to action for scientists and editors to start cleaning up their act before the public loses all faith in the integrity of science. I hope that it makes a difference.
[1] https://scientificbeekeeping.com/what-happened-to-the-bees-this-spring/#contribution-from-pesticides
https://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science/
https://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science-part-2/
[2] Whiting, SA, et al (2014) A multi-year field study to evaluate the environmental fate and agronomic effects of insecticide mixtures. Science of the Total Environment 497–498: 534–542. (See Fig. 2).
Jones, A, et al (2014) Neonicotinoid concentrations in arable soils after seed treatment applications in preceding years. Pest Management Science 70(12): 1780–1784.
[3] http://www.chgeharvard.org/about/people/chensheng-alex-lu
[4] Lu, C, et al (2015) in Environ. Chem. http://dx.doi.org/10.1071/EN15064
[5] citing Kimura-Kuroda J, et al (2012) Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS ONE 7(2): e32432.
[6] https://scientificbeekeeping.com/the-extinction-of-the-honey-bee/
https://scientificbeekeeping.com/the-harvard-study-on-neonicotinoids-and-ccd/
https://scientificbeekeeping.com/news-and-blogs-page/
[7] https://scientificbeekeeping.com/the-birds-and-the-bees/
[8] https://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science/
https://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science-part-2/
[9] Chen, M, L Tao, J McLean, C Lu (2014) Quantitative analysis of neonicotinoid insecticide residues in foods: implication for dietary exposure. J. Agric. Food Chem. 62: 6082. doi:10.1021/JF501397M
[10] Kimura-Kuroda (2012) op cit.
[11] http://www.inchem.org/documents/jmpr/jmpmono/2001pr07.htm#2.1.2
[12] A 2-year feeding study in rats fed up to 1,800 ppm resulted in a No Observable Effect Level (NOEL) of 100 ppm. A 1-year feeding study in dogs fed up to 2,500 ppm resulted in a NOEL of 1,250 ppm http://extoxnet.orst.edu/pips/imidaclo.htm
[13] Researchers estimated the NOAEL at 14 mg/kg/day for rats. The chronic dietary reference dose (RfD) has been set at 0.057 mg/kg/day based on chronic toxicity and carcinogenicity studies using rats. http://npic.orst.edu/factsheets/imidacloprid.pdf
[14] Chen (2014) op cit.
| 175 | lbs my weight | |
| 79.54545 | kg my weight | |
| 0.057 | mg/kg/day EPA safe exposure level | |
| 4.534091 | mg/day number of mg of imidacloprid that I can safely consume | |
| 0.004534 | mg/day converted to grams/day | |
| 1 | ppb = | 4.54E-07 |
| 9987 | lbs apples at 1ppb/day | |
[16] Chen (2014), citing: Ford Kevin A. Ford and John E. Casida (2006) Chloropyridinyl neonicotinoid insecticides: diverse molecular substituents contribute to facile metabolism in mice. Chem. Res. Toxicol 19 (7): 944–951.
[17] Mohamed, F, et al. (2009) Acute human self-poisoning with imidacloprid compound: a neonicotinoid insecticide. PLoS ONE 4(4): e5127.
[18] For those interested in comparing the toxicity of other insecticides to that of the neonics, I suggest reading this great online lecture by Dr. Allan Felsot (see Tables 3 and 4):
http://feql.wsu.edu/esrp531/Fall05/101005DOC.pdf
[19] In Chen (2014) op cit.
[20] Chen (2014) op cit.
[21] Duzguner, V & S Erdogan (2010) Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pesticide Biochemistry and Physiology 97(1):13–18.
[22] Digard, H, et al (2012) Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine Tob Res doi: 10.1093/ntr/nts123.
[23] http://www.efsa.europa.eu/en/efsajournal/pub/3471
[24] The ARfD of a chemical is the estimate of a substance in food or drinking water, expressed on body weight basis, that can be ingested over a short period of time, usually during one meal or one day, without appreciable health risk to the consumer on the basis of all known facts at the time of evaluation. The AOEL is the Acceptable Operator Exposure Level.
[25] Carreck, NL,et al (2014) The dose makes the poison: have “field realistic” rates of exposure of bees to neonicotinoid insecticides been overestimated in laboratory studies? Journal of Apicultural Research Vol. 53(5): 607-614.
Cutler et al. (2014), A large-scale field study examining effects of exposure to clothianidin seed-treated canola on honey bee colony health, development, and overwintering success. PeerJ 2:e652; DOI 10.7717/peerj.652[25]
Dively GP, Embrey MS, Kamel A, Hawthorne DJ, Pettis JS (2015) assessment of chronic sublethal effects of imidacloprid on honey bee colony health. PLoS ONE 10(3): e0118748. doi:10.1371/journal.pone.0118748
[26] Dively (2015) op cit.
[27] For example, the heroic efforts of Marie-Pierre Chauzat in France, Helen Thompson in Great Britain, Jim and Maryann Frazier and Chris Mullin in Pennsylvania, and Jeff Pettis and Dennis vanEngelsdorp in the U.S. in general (not to mention several others).
Chauzat, MP, et al (2006) A survey on pesticide residues in pollen loads collected by honey-bees (Apis mellifera) in France. Journal of Economic Entomology 99: 253-262.
Pettis JS, et al (2013) crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 8(7): e70182. doi:10.1371/journal.pone.0070182
[28] Nicotinic acetylcholine receptors. Both nicotine and neonicotinoids bind to these receptors and allow sodium ions to flood through, initiating an “action potential.” In layman’s terms, they are stimulants, and kill insects by overstimulating them.
[29] Whipple, SD (2015) Letter to the Editor regarding “Simultaneous determination of residues in pollen and high-fructose corn syrup from eight neonicotinoid insecticides by liquid chromatography–tandem mass spectrometry”. Anal Bioanal Chem 407:1273.
[30] http://www.ams.usda.gov/sites/default/files/media/2013%20PDP%20Anuual%20Summary.pdf
[31] For example:
Mullin, CA, M Frazier, JL Frazier, S Ashcraft, R Simonds, D Vanengelsdorp, JSS Pettis (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One 5, e9754. doi:10.1371/JOURNAL.PONE.0009754.
Rennich, K, et al (2015) 2013-2014 National Honey Bee Pests and Diseases Survey Report. http://beeinformed.org/wp-content/uploads/2015/07/2013-2014-NHBS-Report.pdf
[32] Chen (2014) op cit.
[33] Krupke, CH, et al (2012) Multiple routes of pesticide exposure for honey bees living near agricultural
fields. PLoS One 2012, 7, e29268.
[34] Lu, C, et al (2012) In situ replication of honey bee colony collapse disorder. Bulletin of Insectology 65 (1): 99-106. Refer to his Fig. 1.
[35] http://www.usda.gov/nass/PUBS/TODAYRPT/hony0315.txt
[36] http://www.hsph.harvard.edu/news/press-releases/pesticides-found-in-most-pollen-collected-from-foraging-bees-in-massachusetts/
[37] Rosenberg, GL, et al (1983) Inhalation challenge with ragweed pollen in ragweed-sensitive asthmatics. Journal of Allergy and Clinical Immunology Volume 71 (3): 302–310.
[38] http://www.bio.net/bionet/mm/maize/1999-November/000599.html
[39] Dr. Jerry Bromenshenk (in press)
[40] http://sitn.hms.harvard.edu/signal-to-noise-special-edition-gmos-and-our-food/
[41] Typically, other researchers who have done so, conclude that, as did Chauzat, “No statistical relationship was found between colony mortality and pesticide residues.”
Chauzat, MP, et al (2009) The influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environmental Entomology 38: 514-523.
Chauzat, MP, et al (2010) A case control study and a survey on mortalities of honey bee colonies (Apis mellifera) in France during the winter of 2005-6. Journal of Apicultural Research 49(1): 40-51.
[42] Cutler, GC, et al. (2014) A large-scale field study examining effects of exposure to clothianidin seed-treated canola on honey bee colony health, development, and overwintering success. PeerJ 2:e652; DOI 10.7717/peerj.652
Pilling E, et al (2013) A four-year field program investigating long-term effects of repeated exposure of honey bee colonies to flowering crops treated with thiamethoxam. PLoS ONE 8(10): e77193.
Thompson, H, et al (2015) Thiamethoxam: assessing flight activity of honeybees foraging on treated oilseed rape using rfid technology. Environ Toxicol Chem., Accepted Article • DOI: 10.1002/etc.3183
Knowing the intrinsic rate of reproduction of the honey bee colony and of varroa, the proportion of mites that are phoretic at the time, and the efficacy of a powdered sugar dusting at removing those phoretic mites, one can create a spreadsheet that will calculate the estimated effect of repeated sugar dustings.
I’ve done so, and found that unless you dust regularly, and at frequent intervals (weekly during broodrearing season) that sugar dusting is not a very effective method of mite management.
I was recently asked by beekeeper Amanda Millar from Sussex to estimate the effect of weekly sugar dusting. To do so, I created an interactive spreadsheet, which you can download to play with: Dusting simulation spreadsheet
Here are a couple of sample simulations:
Beekeeper Justin Kay asked the following questions:
>Two questions:
1) Where did you get the assumption that 40% of the phoretic mites drop as
a result of the sugar dusting?
>2) More of a comment – the spreadsheet doesn’t account for the theoretical
increase or decrease in the population of the colony itself, i.e. the “%
infestation” rather than the total number of mites per colony.
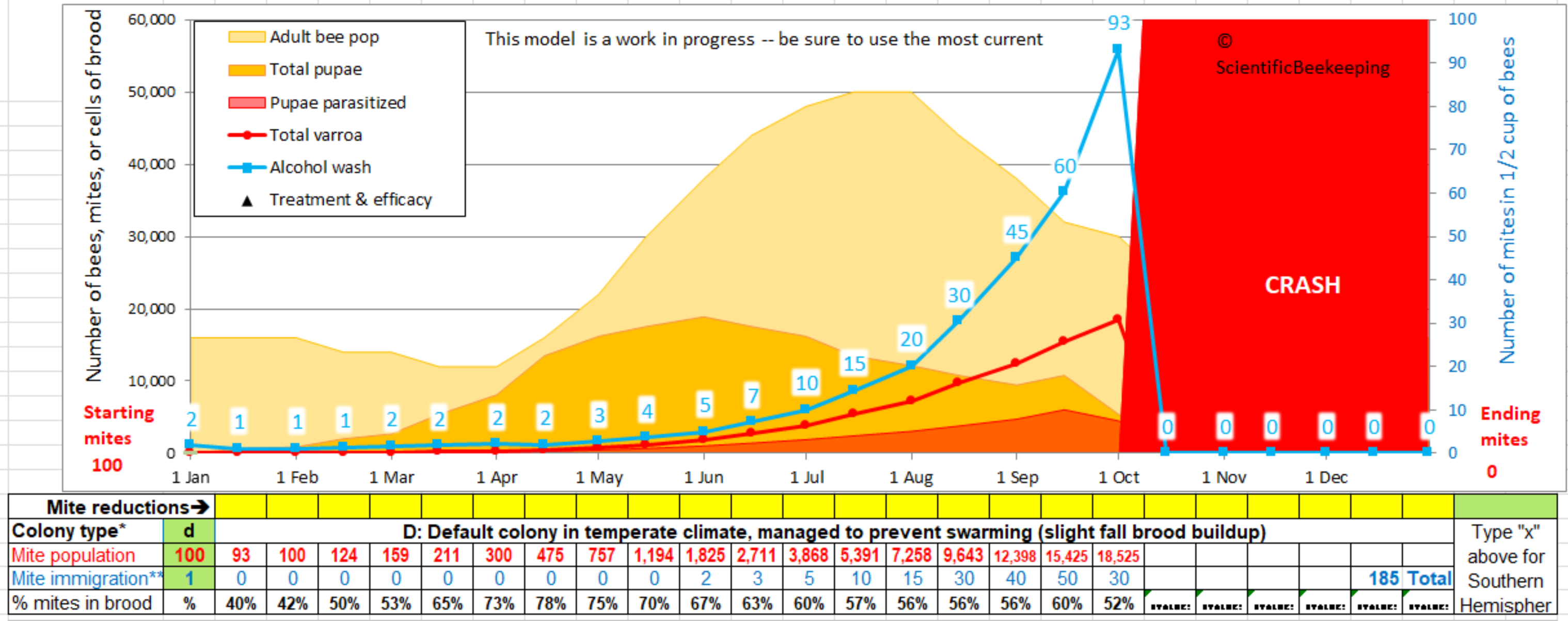
Note how the mite infestation rate “explodes” in late summer/ fall, despite the fact that the total mite population of the hive appears to have only increased relatively slightly. This illusion is due to the scale of the y axis. Allow me to plot out the exact same data for the mite population on a more illustrative scale below: