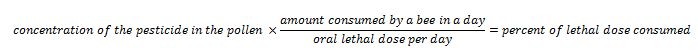First published in: American Bee Journal, October and November 2013
Pesticide Exposure
Oh No, Not Pesticides Again!
Reality Checks
The Two Worlds of Beekeeping
Pesticides and Bee-pocalypse
A Comparison To Some “Control Groups”
The Four Horsemen And The Tip Point
Could Pesticides Cause Colony Mortality And CCD?
Short Memories
The Heart Of The Hive – The Nursery
Industry’s Arguments
But Don’t We Already Know That It’s The Neonicotinoids?
An “Acid Test” Of Neonic Seed Treatment
So Which Pesticides Are Actually To Blame?
The Evidence
Oh Boy, Let’s Do Some Math!
And How About The “Inerts”?
Choosing To Ignore The Obvious
Blinded By Bias
No More Safe Home To Return To
A Historical Artifact
The Beekeeper Contribution To Shifting The Tip Point
Stop Right There!
Undetectable Levels And Hormesis
Wrap Up
Acknowledgements
References
Sick Bees Part 18f7: Colony Collapse Revisited
Pesticide Exposure
Randy Oliver
ScientificBeekeeping.com
Originally published in ABJ Oct and Nov 2013
Oh No, Not Pesticides Again!
Some readers may wonder why I am spending so much time on the issue of pesticides, since to many (if not most) beekeepers, pesticides are a non issue. In answer, the main reason is that the public (and our lawmakers) are being hammered by the twin messages that the honey bee is on the verge of extinction, and that the reason is pesticides. In my writings, I’m attempting to address the validity of both of those claims. Let’s start with the first.
Reality Checks
Honey bees have clearly (and deservedly) become one of today’s most charismatic environmental poster children, and as such are a useful bioindicator that our human activities are having a negative impact upon pollinators, and wildlife in general.
But I also feel that we take care to not overstate or exaggerate our case. One of my greatest concerns is that beekeepers are allowing the media to scare the public with all the hue and cry of an impending bee-pocalypse (and that it is due to a certain type of pesticide). Our complicity in this message (as we enjoy the luxury of basking in the warmth of all the public support) may backfire on us one of these days—putting us into the position of the little boy who cried wolf. Some in the media are starting to notice that the facts don’t support the claim that bees are disappearing (Fig. 1).
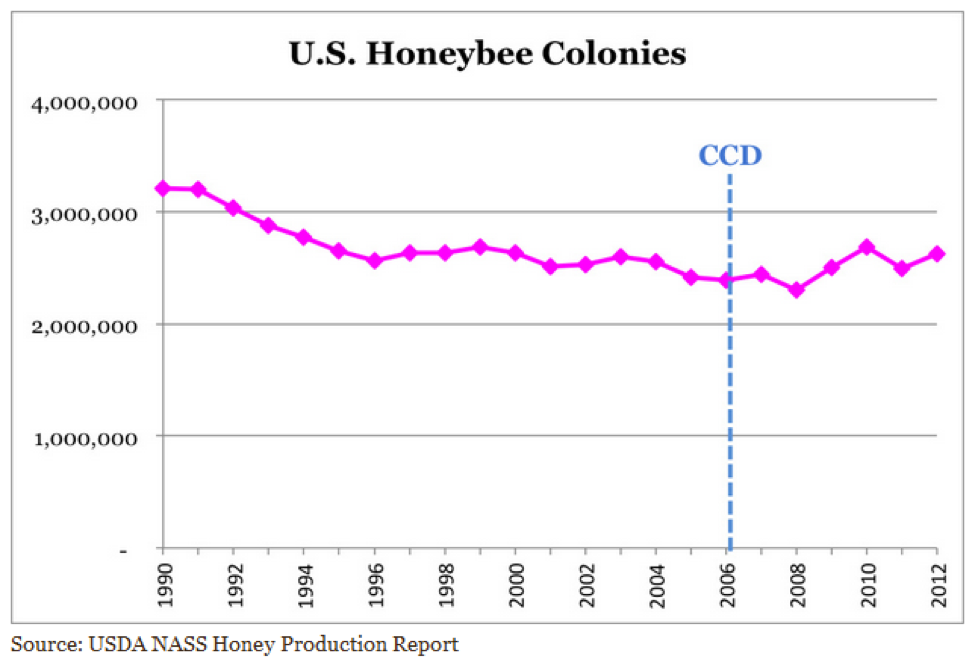
Figure 1. It’s true that it’s more difficult to keep bees healthy these days, but it doesn’t look like bee-pocalypse is imminent (as evidenced by this recently-published chart). Whenever honey and pollination prices are high enough to make beekeeping profitable, resourceful beekeepers somehow manage to recover their colony losses [[i]]. Chart courtesy Shawn Regan [[ii]].
[i] Rucker, RR and WN Thurman (2012) Colony collapse disorder: the market response to bee disease. http://perc.org/sites/default/files/ps50.pdf
[ii] (Broken Link) http://perc.org/articles/everyone-calm-down-there-no-bee-pocalypse
In the rest of the world, the number of managed hives has actually been increasing [3]. And as far as claims that pesticides are driving bees to extinction, Hannah Nordhaus, the author of the excellent book The Beekeeper’s Lament writes:
Reflexively blaming pesticides for all of the honey bee’s problems may in fact slow the search for solutions. Honey bees have enough to do without having to serve as our exoskeletal canaries in a coalmine. Dying bees have become symbols of environmental sin, of faceless corporations out to ransack nature. Such is the story environmental journalism tells all too often. But it’s not always the story that best helps us understand how we live in this world of nearly seven billion hungry people, or how we might square our ecological concerns and commitments with that reality. By engaging in simplistic and sometimes misleading environmental narratives — by exaggerating the stakes and brushing over the inconvenient facts that stand in the way of foregone conclusions — we do our field, and our subjects, a disservice [4].
Further reading: for a detailed and sober analysis of the factors that affect managed bee populations, I highly recommend the review by Drs. vanEngelsdorp and Meixner [5].
The reality is that it is not the honey bee that is being driven to extinction—it is instead the commercial beekeeper who is finding that his traditional business model is becoming less profitable due to today’s greater degree of colony losses and the decreasing availability of good summer forage.
The question is, to what extent are pesticides involved in those problems?
The Two Worlds Of Beekeeping
There are two very different worlds of beekeeping—small scale (hobbyists, who constitute the vast majority of beekeepers by number) and large scale (commercialized professionals, who manage the vast majority of hives), with a small continuum of sideliners bridging the gap.
Hobby beekeeping is currently enjoying a bubble of resurgence, but in the Big Picture in the U.S., hobbyists manage an insignificant number of hives. And those small-scale beekeepers tend to keep their hives close to home, largely avoiding serious exposure to pesticides. But that’s not to say that small-scale beekeepers are immune to pesticide kills; I’ve heard of several this season, and what with all the spraying for West Nile virus and the citrus psyllid, we can expect more of the same. And since there are far more small-scale beekeepers to put pressure on regulators and legislators, I feel that it is a good idea for them to be informed about pesticide issues.
Large-scale beekeepers, on the other hand, typically run migratory operations—moving their hives to almond pollination, and then to other agricultural areas (it’s problematic to keep apiaries of hundreds of hives in the suburbs). The fate of those bees (and their keepers) is largely determined by agricultural land use practices and their degree of exposure to agricultural pesticides.
It is some of those large-scale beekeepers for whom extinction is a valid concern. The reason (as with any other enterprise) is financial—they can only survive so long unless their businesses continue to be profitable [6]. In recent years, they’ve had two things going for them—sky-high honey prices and elevated pollination fees. But all is not rosy—there are reasons for those prices going up; these days it’s simply more costly to produce honey or to provide bees for pollination.
Today’s breathtakingly-high high almond rental rates typically don’t even cover operating costs—even if most of one’s colonies make it through the winter! Today’s 30% average winter loss rate is bleeding profitability from many operations. Not only does the beekeeper need to rebuild his numbers after almonds, but to stay in the black he must also make additional income from paid pollinations or a decent honey crop. And that may no longer be as easy as it used to be for various reasons:
- Formerly bee-friendly farmland has been turned into agri-deserts devoid of any bee forage.
- Honey producers on field crops (such as alfalfa, sunflowers, or cotton) get hammered time and again by pesticide spraying, sometimes watching whole yards of colonies dwindle or go queenless weeks afterwards.
- Paid summer pollination contracts (such as for vine crops) may leave colonies in poor shape for the winter, due to the heavy stocking, the lack of nutritious pollen, and the exposure to multiple pesticides.
These days, the sad fact is that many good beekeepers are barely keeping their heads above water. So the beekeeper’s lament continues—varroa, high winter mortality, and lack of good forage are driving a number of operations into the red.
Practical application: although the “extinction of the honey bee” makes for a good rallying cry, the real concern is the possible extinction of the migratory beekeeper who supplies necessary pollination services to agriculture. So far, the almond industry has been economically propping up the bee industry, but I’m not sure how long that arrangement will be sustainable.
Pestcides And Bee-pocalypse
For some beekeepers, “bee-pocalypse” has already occurred. New York beekeeper Jim Doan, whose case I detailed in a previous article [7], sadly gave it up this year. Here is a beekeeper whose apiaries had been in the same locations for many years without noticeable pesticide problems, but who apparently suffered from devastating spray or dust kills this season and last, as evidenced by piles of fresh dead bees in front of his hives in spring.
Residue analysis of those dead bees clearly showed that they had been exposed to several pesticides, but none of the detects were at levels that would be expected to cause such carnage—so we don’t even know which pesticides or practices to point the finger at! To my scientific mind, this is very frustrating—that our “system” was not able to identify the cause of Jim’s bee kills, to change anything to keep them from recurring, nor compensate an innocent beekeeper for the loss of his livestock and livelihood.
As unlucky as Jim has been, his case is not necessarily the norm. Overall, the issue of environmental toxins is improving. In my own lifetime I’ve seen us clean up our pollution of the air and water, cease atmospheric testing of nuclear weapons, ban DDT, fluorocarbons, and PCB’s, phase out the worst pesticides, and raise the general environmental consciousness. Humans still inflict far too damaging an environmental footprint on Earth, but we are moving in the right direction, and should give ourselves some credit for that!
There is no doubt that pesticides are often involved in bee health issues, but can we blame them for all our problems? That question is best answered by considering the health of those colonies that are not exposed to pesticides:
A Comparison To Some “Control Groups”
There are plenty of beekeepers in non agricultural areas whose apiaries are not exposed to pesticides to any extent. Those hives serve as a “control group,” whose health we can compare to those colonies that do have to deal with pesticides.
For instance, in my own operation of about a thousand hives, their only exposure to pesticides is to the fungicides in the almond orchards (from which they don’t appear to suffer to any serious extent). I haven’t used synthetic miticides in over a decade, rotate my combs, and rarely feed syrup.
Yet, I’ve experienced CCD firsthand, see more queenlessness, unsuccessful supersedure, and experience somewhat higher winter losses than in the old days (meaning before varroa). I hear the same from many others in the pesticide-free control group. The simple fact is that these days it requires better husbandry to maintain productive colonies. Yet we in the “control group” can hardly blame pesticides to be the cause.
And then there are the stationary “treatment free” beekeepers in the middle of intense agriculture who suffer no higher colony loss rates than the norm, despite their apiaries being surrounded by corn and soy [8]. How the heck do we reconcile their success to the problems that the commercial guys experience in the same areas?
Do they owe their success to keeping fewer hives in a yard? To keeping locally-adapted survivor stock? To their placement within flight range of patches of undisturbed forage? To the fact that they don’t move to multiple crops? Or is it because they aren’t contaminating their combs with miticides? Believe me, if I knew the answer, I’d tell you!
As (the very successful) beekeeper Dave Mendes observes, colonies just seem to be more “fragile” these days. It’s no surprise then that the addition of toxins of any sort can help to tip a colony over. The Ericksons [9] put it this way:
Pesticides and their residues in the hive stress bees as do other factors such as weather extremes, food shortages, pests, predators, and disease. Conversely, stress induced by other factors undoubtedly has a significant impact on the level of damage that a pesticide inflicts on a colony.
Note that the above words were written prior to our colonies having to deal with varroa, the varroa-vectored viruses, Nosema ceranae, our evolving brood diseases, GMO’s, neonicotinoids, or Roundup Ready corn.
The Four Horsemen And The Tip Point
Colony growth is a function of the recruitment rate via successful broodrearing vs. the attrition rate of workers due to age, disease, the altruistic departure of sick bees, or the loss of foragers in the field. When recruitment exceeds attrition, colonies grow; when attrition exceeds recruitment, the colony population shrinks. Environmental factors, including toxins, can shift the tip point for colony growth (Fig. 2).
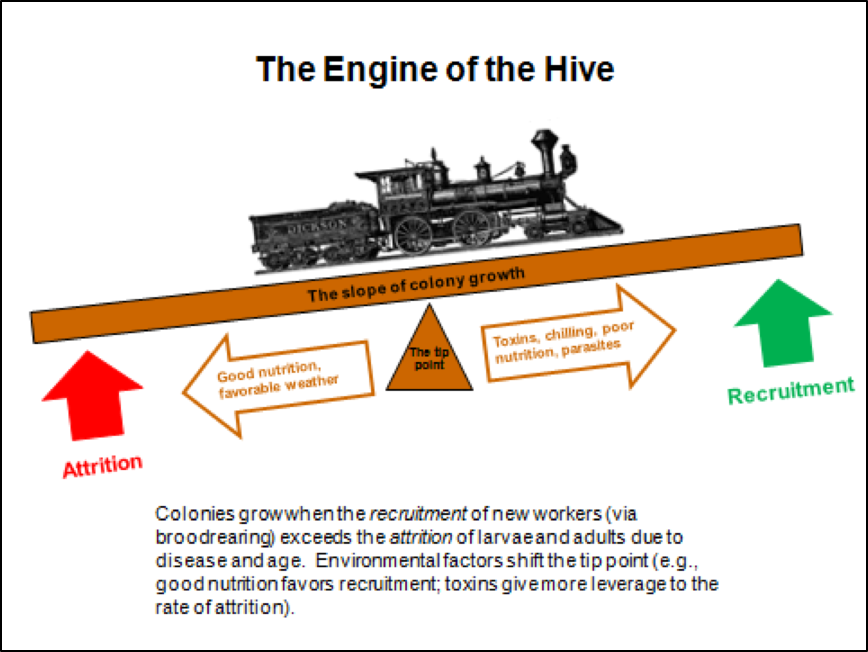
Figure 2. Any colony with a good laying queen has the potential to grow rapidly—the greater the rate of recruitment (successful broodrearing), the steeper the slope of the growth curve. In the real world, such potential growth is often held back by the lack of nutritious pollen, or by the stresses of toxins, chilling, or pathogens (especially the mite-associated viruses, nosema, or EFB). Any of those can strongly shift the tip point, slowing, or even reversing, the rate of colony growth.
In the last decade, something appears to have shifted that tip point—colonies today seem to more readily go into a downhill spiral and queens no longer hold up as well. Could it be due to pesticides?
Could Pesticides Cause Colony Mortality And CCD?
Of course they could! In 2010, after closely observing the progression of experimentally-induced CCD with my collaborator Dr. Eric Mussen, I published the flow chart below (Fig. 3) to detail the interactions and feedback loops involved in the step-by-step collapse of a colony [10]. At the time, I fully intended to further elaborate upon the contribution of toxins, but didn’t get around to it until now.
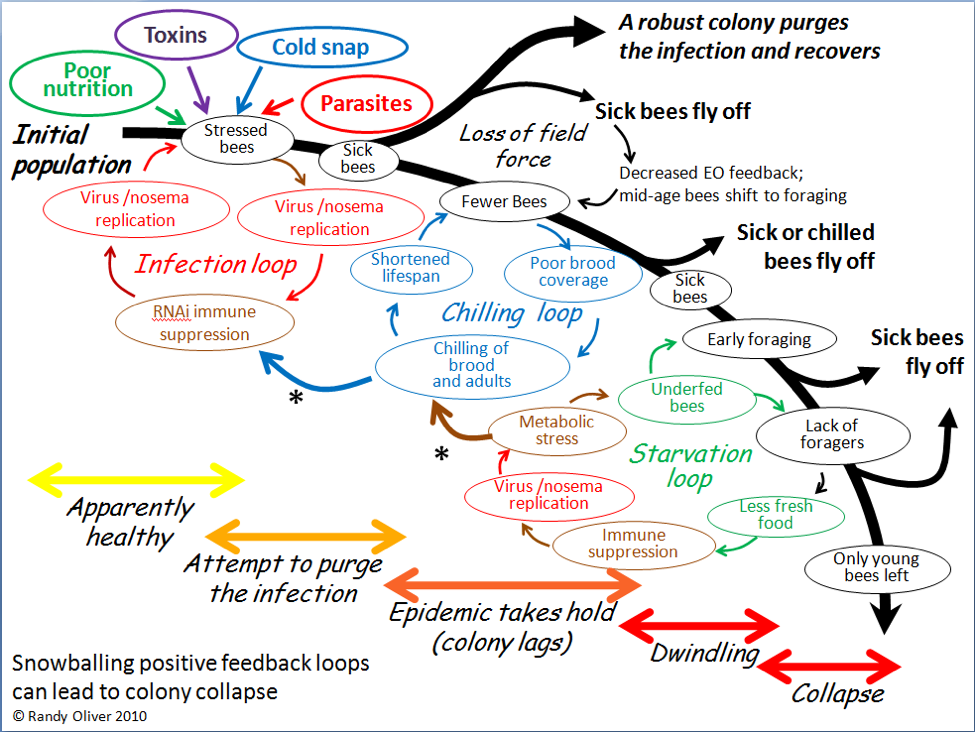
Figure 3. The positive feedback loops that can lead to colony dwindling and/or sudden depopulation. I’ve since observed this process take place in sick colonies time and again.
In the above chart, I called out toxins (which would include pesticides) as one of the “Four Horsemen of Bee Apocolypse” (the four factors at top left). Below I’ve indicated in red those points at which toxins may exacerbate the downhill process (Fig. 4).
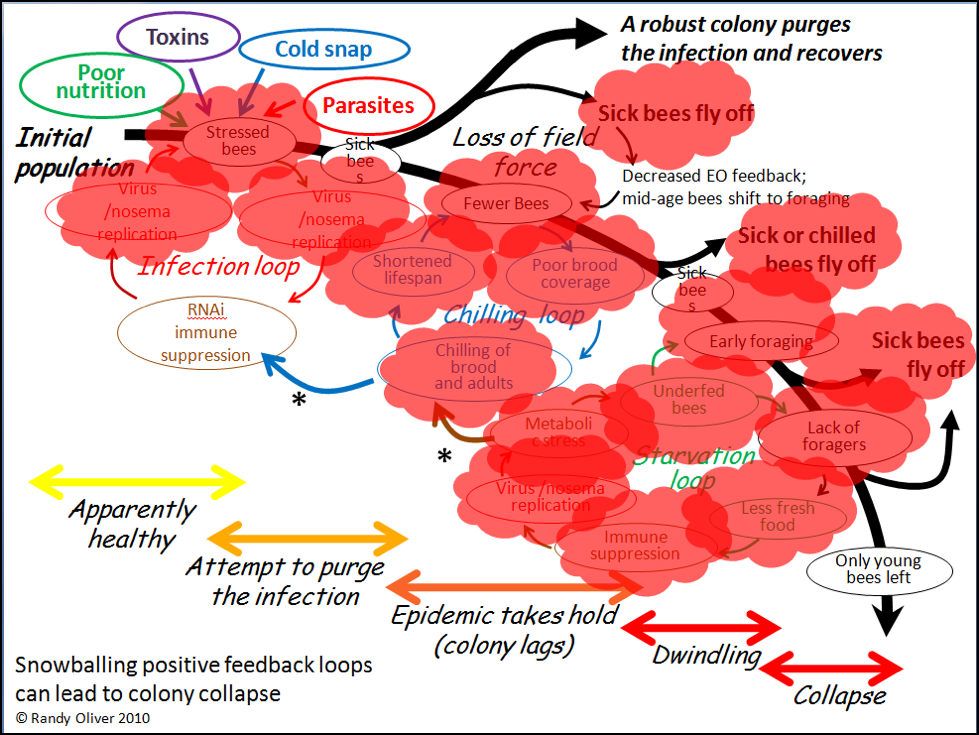
Figure 4. Note that toxins can exert lethal or sublethal effects (red bubbles) at every step in the process of colony dwindling or collapse. Pesticides may in some cases be the prime cause of colony mortality; more frequently they might be “contributory factors,” especially due the prolonged sublethal effects of residues in the beebread or wax.
Please note that in these charts I’m referring to toxins generically, not specifically to manmade pesticides. Such toxins would include natural plant allelochemicals, industrial pollutants, metals such as arsenic or selenium in soil and dust, fungal and bacterial toxins (which may be altered in beebread by the presence of pesticide residues), beekeeper-applied varroacides, HMF in overheated corn syrup, all in addition to any agricultural pesticides. In the words of ecotoxicologist Dr. Helen Thompson, we must pay attention to the total toxin load of the hive, plus any interactions between those chemicals, as well as other contributory factors [11]—a sentiment also echoed by the Fraziers at Penn State [12]. So, back to our original question: Can toxins, including synthetic pesticides, cause colony morbidity or mortality?
Verdict #1: clearly, synthetic pesticides and varroacides may constitute the most serious toxin load for managed bees in agricultural areas, and have the potential kill a colony outright, or to exacerbate positive feedback loops that can result in dwindling, poor overwintering, or collapse.
But does any pesticide specifically cause CCD—“the disappearance of most, if not all, of the adult honey bees in a colony, leaving behind honey and brood but no dead bee bodies” [13] (and no sign of brood diseases or varroa-induced DWV collapse).
Analysis: The most direct way to answer that question is to see whether we can fulfill Koch’s third postulate [14]: can we experimentally create the symptoms of CCD by treating a healthy hive with the pesticide in question?
Verdict #2: to the best of my knowledge, no one has yet duplicated the symptoms of CCD by treating a colony with any pesticide (the most obvious difference being that there are generally plenty of dead bees present in the case of pesticide toxicity). This is notably true for the neonicotinoids, for which any number of researchers have attempted to duplicate CCD symptoms by continually feeding colonies neonic-tainted syrup or pollen.
Hold on—drop those stones! I am not saying that pesticides cannot contributeto CCD or colony morbidity or mortality in general—my chart above clearly illustrates that they have the potential to do so. Yet even those beekeepers who manage to completely avoid pesticides may still experience sudden colony depopulations, dwindling, or excessive winter losses due to some combination the Four Horseman (as in the perfect storm detailed at [15]).
I feel that it is a serious error for us to try to link CCD to pesticides. Pesticides have always been an issue to beekeepers, but CCD-like events have historically come and gone (as in Disappearing Disease—read the description at [16]). Pesticides will remain an issue long after the term “CCD” is forgotten.
Bottom line: Despite the fact that the evidence at hand does not support the case that CCD is directly caused by any pesticide, that fact certainly does not mean that we should ignore pesticide issues. If anything, we beekeepers ourselves have helped to make pesticides even more of an issue these days.
Short Memories
There is a popular myth going around that pesticides only started to become an issue to honey bee colony survival in 2007. In fact, the sublethal effects of pesticides were well known to beekeepers and researchers long before then. If we review the older literature [17], we find that it was already well known that contaminated pollen was a more serious issue to colony health than the in-field kill of foragers. We knew that colonies might collect such tainted pollen from miles away, that dusts were worse than sprays, that young bees may be more susceptible than older bees, and that temperature and humidity had a great deal to do with pesticide toxicity. Pesticide issues were actually far worse in the 1960’s and ‘70’s than they are today, and have generally improved since then (not to say that some new issues haven’t arisen).
On the other hand, the overall contamination of combs with pesticides has increased in recent years due to the direct contribution by we beekeepers ourselves. In virtually any residue analysis of beebread or beeswax these days in any country with varroa, the most prevalent toxins are the beekeeper-applied varroacides [18]–you may wish to refer back to my chart of the “toxicological eras of honey bee evolution [19].
So one question is, To what degree we have shifted the tip point of colony health by contaminating our brood combs with miticides? Let’s explore the broodnest…
The Heart Of The Hive – The Nursery
The insidious, long-term effects of total toxin load (including pesticide and varroacide residues) would be from those that made it into the heart of the hive—the critical stored beebread and the wax of the brood combs (Fig. 5). Note: Dr. David Fischer of Bayer brings to my attention that in the case of imidacloprid, the results of his testing indicates that bees in the hive are more affected by residues in the nectar than by those in the pollen.

Figure 5. Long after a pesticide-sprayed field force has been replaced by newly-recruited foragers, the colony may still need to deal with the lingering effects of pesticide residues in the combs, and especially in the all-important stores of beebread. It is here that such persistent residues can affect colony health and buildup for many months after the initial exposure, and exactly where we should focus our attention.
Here’s some food for thought: a toxin need not actually kill a single bee to mess up a colony. There are many ways in which sublethal levels of toxins can negatively affect the colony population curve. A few examples would be:
- By decreasing the survival rate of larvae (as from residues of varroacides [20, 21], or fungicides [22]) , or by increasing their development time (as effected by various pollutants, plant alleleochemicals, pesticides, or miticides).
- By affecting the proper fermentation of beebread (fungicides).
- By affecting the sensitive nurse bees that must digest that beebread and produce the critical jelly used to feed the brood, queen, and other workers (natural plant toxins, pollutants, or pesticides).
- By affecting the normal behavioral progression of the workers. E.g., if workers initiate foraging prematurely, this greatly reduces their overall longevity, and results in severe depression of colony growth [23] (much more research is crying to be done, but many chemicals would be suspect).
- By requiring bees to allocate precious resources toward the detoxification of the poisons (as per my leaky boat analogy [24]).
- By increasing the virulence of varroa, nosema, or viruses (any number of pesticides and miticides have been implicated [25, 26])
- By affecting normal colony homeostasis, such as thermoregulation of the brood, which is dependent upon the proper assessment of temperature, and the ability to effectively generate heat by the vibration of the wing muscles (neurotoxins would be expected to affect this ability).
- By affecting the longevity of the queen, the viability of spermatozoa, or the ability of a colony to successfully supersede (coumaphos notably had this effect)
- By affecting the production of, or normal communication via pheromones (which include the recognition of brood and the queen) [27] (essential oils, formic acid, other pesticides?)
- By affecting foragers’ ability to communicate by dance, to navigate, to learn (a wide range of pesticides [28]), or to react properly to normal stimuli (neonics can clearly do this [29]; but similar effects could be due to any number of other pesticides).
Bottom line: the toxin load in the broodnest can greatly affect a colony in many ways, generally (but not necessarily always) negatively. The greater the total toxin load with which the colony is forced to deal, the more likely that it will suffer from the combined ill effects.
Industry’s Arguments
In order to present an objective review of pesticide issues, we should also hear Industry’s side of the argument. The industry-funded think tank OPERA [30] takes the position that:
Although, based on the facts outlined above, there does not appear to be any strong evidence that sublethal effects of pesticides play a key role as causative factors behind bee colony mortality (which is likewise supported by the fact that in several monitoring projects no correlation has been found between colony losses and pesticide exposure), sublethal effects are certainly a point where more fundamental research is needed to obtain a clearer picture of the nature of the issue.
The above statements are factually correct in that there is to date no compelling evidence that pesticides are at the root of the elevated rates of colony mortality seen in recent years, and that more fundamental research is clearly needed. But a long history of practical experience by beekeepers with the sublethal (as well as lethal) effects of pesticides leaves no doubt that pesticides certainly have the potential to cause colony health issues.
But Don’t We Already Know That It’s The Neonicotinoids?
The media have already tried and convicted the neonicotinoids as the cause of all bee problems, and it’s currently fashionable to celebrate the restrictions recently imposed on them by the European Union. But it is rational? No one has ever shown convincing evidence that neonics are linked to colony collapse; conversely, there is abundant experimental and on-the-ground evidence that the residues from seed-treated plants do not appear to cause observable harm to colonies [31].
Planting dust, soil drenches, or foliar applications are a different story, but these are generally drift or misapplication issues, hitting individual apiaries, not the bee population as a whole. Our regulators are well aware of these issues, and working to fix the problems.
Regarding the completely unacceptable bee kills due to the dust from corn seeding, of interest is a recent paper by Drs. Chris Cutler and Cynthia Scott-Dupree [32]—environmental toxicologists from Canada’s Dalhousie University—who analyzed the 110 pesticide incident reports received by Canada’s PMRA since 2007. Ranking the reports by the degree of severity of the bee kill, they found that there were over five times as many “major incidents” due to non-neonicotinoid products (including carbofuran, chlorpyrifos, coumaphos, diazinon, dimethoate, fluvalinate, formic acid, permethrin, and phosmet) as there were due to neonics, yet that these incidents are largely ignored by the press and beekeepers, who for some reason single-mindedly focus upon the neonics.
Hey, I’m as concerned about pollinators and pesticides as anyone. A recent review by Goulson [33] points out the excessive use of neonics (actually all pesticides are greatly overused), and details the many environmental questions about this class of chemicals. But here’s the thing—I can read studies all day long, but what I prefer to seek out are actual on-the-ground, real-life observations. Let me share one with you:
An “Acid Test” Of Neonic Seed Treatment
Activists are calling for a ban on clothianidin—the most common neonicotinoid seed treatment. Although honey bees appear to do just fine on seed-treated canola, their species has an advantage over solitary bees and other pollinators, due to their foraging on multiple plant species over a wide area, their social structure, and their processing of the pollen by nurse bees. So honey bees may not be the best indicator of neonic toxicity.
On the other hand, solitary bee species may be a better indicator as to whether neonic residues cause subtle adverse effects. Many solitary bees are “monovoltine,” meaning that they only raise a single generation per year. Because of this, a negative effect on any single female bee could prevent the production of the next generation. It occurred to me that the Alfalfa Leafcutter Bee (Megachile rotundata), which is used to pollinate clothianidin-treated canola (Fig. 6), would provide an excellent “acid test” of clothianidin for several reasons:
- Clothianidin has been shown to be highly toxic to leafcutter bees by topical application [34]. Since neonics are typically an order of magnitude more toxic by oral exposure [35], it is reasonable to expect that the leafcutter bee would be even more susceptible to residues consumed in food.
- Leafcutter bees do all their foraging within a few hundred feet of the nest [36], so those placed in the middle of a canola field would forage solely upon treated canola.
- Each individual female alone forages and provisions her nest, feeding upon the contaminated pollen and nectar as her sole protein and energy sources. If the insecticide negatively affected her behavior, navigational ability, health, or longevity she would be unable to reproduce effectively.
- The male bees use canola nectar as their sole energy source, and if the insecticide residues interfered with their behavior or longevity, the female bees might not get properly mated.
- The larvae consume a diet consisting solely of unprocessed contaminated pollen and nectar (rather than royal jelly), and thus every item in their diet would contain verified concentrations of clothianidin (approximately 1.7 ppb in the pollen; 0.8 ppb in the nectar [37]). Note: as with honey bees, neonicotinoids are virtually nontoxic to the larvae of the leafcutter bee [38].
- The female constructs her nest by cutting (with her mouthparts) leaves from the treated canola plants, which contain even higher residues of clothianidin than the pollen, thus exposing her to even more of the chemical. The larva then develops surrounded by these contaminated leaves, and the pupa overwinters in them.

Figure 6. Tents covering Alfalfa Leafcutter bee nest boxes in a canola field.
In short, the leafcutter bees would constitute the most severe test case for clothianidin exposure from a seed-treated crop. So I phoned a commercial supplier of leafcutter bees in Ontario (who declined to be named) and asked him whether he had any problems with his bees reproducing or overwintering after being set in clothianidin seed-treated canola. He said that he had been rearing them on such fields for many years and did not observe any problem. I put a good deal of faith into such unbiased field experience by a commercial bee man. You can draw your own conclusions.
So Which Pesticides Are Actually To Blame?
It’s pretty easy to diagnose an acute bee kill, what with piles of twitching bees in front of the hives (see “Signs and symptoms of bee poisoning” at [39]), and in many cases the responsible pesticide can be identified. To sidetrack briefly, remember when I mentioned a few articles back that the residues in Jim Doan’s bee kills did not indicate that the bees contained lethal doses of the chemicals? This made me strongly suspect that we can’t apply the LD50 data (in nanograms per bee) to the values obtained from actual field samples of dead bees. The recent report from Canada [40] confirms this. The highest residue level of clothianidin (from corn planting dust) found in any sample of dead bees from the entrances of a hive was 24 ppb, which works out to about a tenth of the theoretical amount necessary to kill a bee . This finding could be due to the metabolic degradation of the insecticide, but it certainly suggest that the LD50 value should be adjusted lower for samples of dead bees! I am greatly heartened that Canada is moving forward in addressing this issue of bee kills from corn planting dust [41].
Overt bee kills aside, more insidious are the residual effects due to contaminated dust, pollen, or nectar that foragers bring back into the broodnest. I’m told by beekeepers with far more experience with pesticides than I, that after exposure to certain pesticides, colony growth and production come to a standstill, sometimes for months, until the colony clears itself of residues and perhaps eventually recovers (or not). The problem is that few beekeepers (if any) can look inside a hive and diagnose which pesticide (or combination thereof) is causing the problem. He may notice spotty brood, poor buildup, winter dwindling, or queenlessness, but it is very hard to isolate the effect any particular pesticide residue, especially in today’s stew of residues in combs. But that doesn’t mean that we are completely blind…
The Evidence
Due to the rapid turnover of bees in a hive (other than the queen or “winter bees”), if a pesticide were indeed exerting a long-term effect upon colony health, then there would by necessity need to be residues of that pesticide or its degradation products persisting in the combs. With today’s testing equipment, we can detect residues to the parts per billion level, and have quite a large database of residue analyses of beebread samples, which we can perhaps use to either finger or exonerate certain pesticides suspected of being involved in colony health issues.
In a court of law, all evidence would be laid out before the court to determine whether it was substantial enough to make a case against a particular suspect. We can do something similar by reviewing two large publicly-available datasets of actual pesticide analyses of beebread from across the country—one by the Penn State team , the other by the USDA (Tables 1 and 2). I’ve condensed their data to only those pesticide detects that were found in at least 10% (Penn State) or 5% (USDA) of the samples, following this reasoning:
If a pesticide isn’t present in at least 10% of samples, then it isn’t likely to be the cause of widespread problems.
I’ve also color-coded the results as to the type of pesticide, and included the median detection level (to help us to determine whether that dose would be expected to cause colony health problems, or whether it would be insignificant).
| Pesticide |
Present in percent of samples*
|
Median detection if positive for target (ppb)
|
Type of pesticide
|
| Fluvalinate |
88.3
|
40.2
|
Beekeeper-applied miticide
|
| Coumaphos |
75.1
|
13.1
|
Beekeeper-applied miticide
|
| Chlorpyrifos |
43.7
|
4.4
|
Insecticide
|
| Chlorothalonil |
52.9
|
35
|
Fungicide
|
| Pendimethalin |
45.7
|
13.4
|
Herbicide
|
| Endosulfan I |
28
|
4.2
|
Insecticide
|
| Endosulfan sulfate |
26.3
|
2.2
|
Insecticide
|
| DMPF (amitraz) |
31.2
|
75
|
Beekeeper-applied miticide
|
| Atrazine |
20.3
|
8.9
|
Herbicide
|
| Endosulfan II |
20
|
3.8
|
Insecticide
|
| Fenpropathrin |
18
|
7
|
Insecticide
|
| Azoxystrobin |
15.1
|
10.2
|
Fungicide
|
| Metolachlor |
14.9
|
8.1
|
Herbicide
|
| THPI (Captan) |
14.2
|
227
|
Fungicide
|
| Captan |
12.9
|
103
|
Fungicide
|
| Esfenvalerate |
11.7
|
3.3
|
Insecticide
|
| Carbaryl |
10.9
|
36.7
|
Insecticide
|
| Cyhalothrin |
10.9
|
1.7
|
Insecticide
|
Table 1. The 2010 survey by the Penn State team [42], based upon (depending upon the pesticide) either 350 or 247 samples. This study (plus numerous others worldwide) clearly point out that the predominant pesticide residues in brood combs are typically those from the beekeeper-applied miticides (yellow).
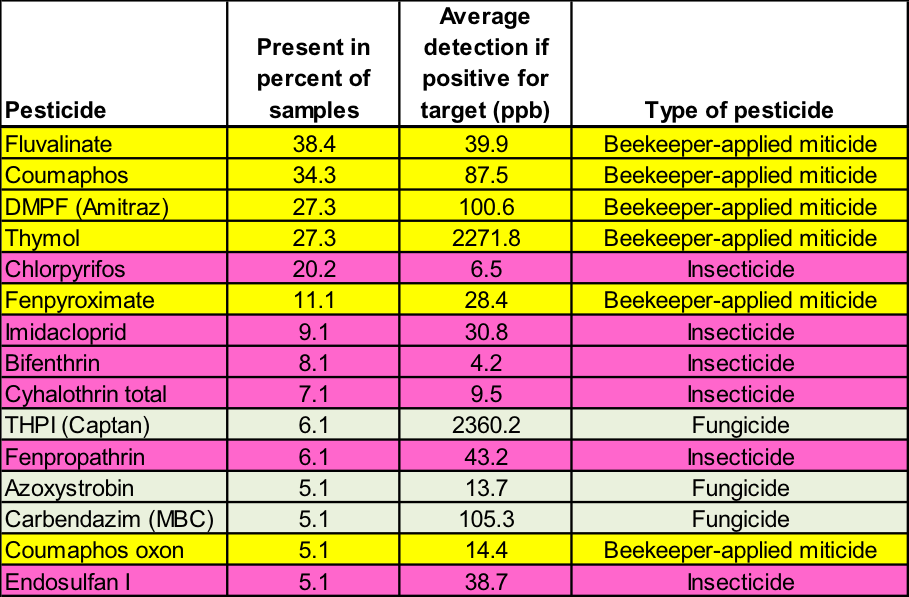 Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.
Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.
Keep in mind that the above surveys screen only for 174 chosen pesticides—compare this number to the roughly 1000 pesticide active ingredients and adjuvants registered for use in California. I’ve discussed the composition of this list with USDA’s Roger Simonds, who runs the tests. It is prohibitively costly to test for every possible pesticide, so one must arbitrarily draw up a limited list of the chemicals of most concern. All are aware that this is a difficult task, since we don’t even know which toxins with which we should be most concerned!
Note that in both surveys, the most common insecticide present was chlorpyrifos– an “old school” (introduced in 1965) organophosphate neurotoxin classified as being “highly toxic” to bees, and marketed as Dursban and Lorsban. Chlorpyrifos was previously widely used by homeowners and residential pest control companies. EPA has since restricted its use due to its toxicity to wildlife and aquatic organisms, and possible links to human health issues [44]—some of the reasons that EPA favors the neonicotinoids as “reduced risk” products.
Oh Boy, Let’s Do Some Math!
But just because a pesticide is present, doesn’t necessarily mean that it is causing measureable harm. A nurse bee may consume about 10 mg of beebread per day [45], so if she consumed that amount of pollen contaminated with chlorpyrifos at 6.5 ppb, then she would have been dosed with 0.065 ng (1 nanogram = 1 billionth of a gram) of the chemical. The question then is, how much chlorpyrifos does it take to actually harm a bee?
One commonly cited figure is that the LD50 for chlorpyrifos given orally is 360 ng/bee. Compare those figures (360 ng for toxicity vs. 0.065 in the daily diet)! Even though chlorpyrifos is a disturbingly common comb contaminant, it is unlikely that the median detected concentration (alone) would be causing colony health problems (not to say that higher doses don’t hurt colonies).
But, you say, some of the neonics are even more toxic than chlorpyrifos. How about the mean 31 ppb found by the USDA in the few samples positive for imidacloprid? The typical nurse bee would consume 0.31 ng, compared to the oral LD50 of about 4-40 ng, so she’d be eating a tenth to a hundredth of the lethal dose. This would be cause for concern, tempered by the fact that a bee can easily metabolize that amount of imidacloprid a day [46]. Such consumption could legitimately be suspected of causing sublethal effects. However, keep in mind that that 31 ppb was an average, which was strongly skewed by a few samples with very high concentrations (which I’d fully expect to cause colony health problems). Plus this is not simply a matter of the average amount of contamination; one must also look at the percentage of positive detects. The Penn State team [47] puts it well:
Our residue results based on 1120 samples which include Mullin et al. (2010) and subsequently more than 230 additional samples do not support sufficient amounts and frequency of imidacloprid in pollen to broadly impact bees.
OK, so how about the varoacides fluvalinate at 40 ppb or the amitraz degradate DMPF at 100 ppb? Surprisingly, I can’t find an oral LD50 for fluvalinate, so the contact toxicity figure (200 ng/bee) will need to suffice. Those residues work out to about 1/500th expected toxicity.
Amitraz scored a bit better, with the nurse bees consuming about 0.01 ng—far below the lethal dose. But a recent study found that an oral dose of 0.2 ng of amitraz causes more than a doubling of the heart rate of a bee [48]—that’s at 1/20th of the average detect! The authors dryly state:
The above responses clearly show that the heart of the honeybee is extremely vulnerable to amitraz, which is nevertheless still used inside beehives, ostensibly to “protect” the honeybees against their main parasite, Varroa destructor.
How vulnerable? Frazier [49] observed that “Dead and dying bees collected around colonies in association with corn had only residues of 2,4-DMPF at 5,160 ppb.” Looks like perhaps the beekeeper inadvertently killed his own bees with an off-label mite treatment that may have overworked their little hearts!
And if those miticide and insecticide residue weren’t enough alone, some of the toxicity of these chemicals is additive or synergistic. The Penn State team again says it well:
[The] pyrethroids… were found in 79.4% of samples at 36-times higher amounts than the neonicotinoids, on average… The mean neonicotinoid residue was 37 ppb (scoring non-detects as 0 ppb), of which only 6.7 ppb was imidacloprid. Pyrethroids, by comparison, were present at a mean residue of 106 ppb and a frequency of 80.3% in pollen samples… Indeed, if a relative hazard to honey bees is calculated as the product of mean residue times frequency detected divided by the LD50, the hazard due to pyrethroid residues is three-times greater than that of neonicotinoids detected in pollen samples [emphasis mine].
The pyrethroids are popular because they are relatively nontoxic to humans. But they can sure kill honey bees. More so, they can cause sublethal effects, such as irreversible inhibition of olfactory learning ability [50].
Hey, we’re only getting rolling! Mussen [51] pointed out a decade ago that fungicides could kill larvae; recent research from the Tucson lab [52] and elsewhere confirm that fungicide residues can mess up the colony (we sometimes observe this in almonds). Of note is that colonies treated with some fungicides were unsuccessful at requeening themselves! And recent research by Zhu [53] found that the relative toxicity of larvae to the commonly-detected fungicide chlorothalanil was almost 40 times higher than that of chlorpyrifos. Fungicides are frequently found at high concentrations in beebread.
I cannot help from returning to the refrain that instead of limiting our concern to any single pesticide, that we should be looking at the total toxin load that the colony is forced to deal with.
And How About The “Inerts”?
The pesticide detection analyses above do not look at the “inert” adjuvants in the pesticide “formulation.” These chemicals not only help to disperse the pesticide over the waxy leaf surface, but also aid in its penetration through the insect cuticle, thus making the pesticide relatively more toxic to the bee!
Mullin and Ciarlo [54, 55] found that:
Formulations usually contain inerts at higher amounts than active ingredients, and these penetrating enhancers, surfactants and adjuvants can be more toxic on non-targets than the active ingredients. For example, we found that the miticide formulation Taktic® was four time more orally toxic to adult honey bees than the respective active ingredient amitraz. Impacts of ‘inerts’ in pollen and nectar alone or in combination with coincident pesticide residues on honey bee survival and behavior are unknown.
The researchers also found that:
Learning was [rapidly] impaired after ingestion of 20 µg of any of the four tested organosilicone adjuvants, indicating harmful effects on honey bees caused by agrochemicals previously believed to be innocuous.
One of the common adjuvants is a solvent NMP, described by BASF [56]:
NMP can be used as a solvent or co-solvent for the formulation of insecticides, fungicides, herbicides, seed treatment products and bioregulators where highly polar compounds are required. NMP is given preference over other highly polar solvents because it is exempt from the requirement of a tolerance when used as a solvent or co-solvent in pesticide formulations applied to growing crops, and it possesses a favorable toxicological and environmental profile.
The key words above are that these toxic solvents are “exempt from tolerance” [57], so they are sprayed all over crops along with the active ingredients of pesticides (including imidacloprid). Yet Zhu [58] recently reported that NMP can rapidly kill bee larvae. The authors conclude that:
Our study suggests that fungicide, the inert ingredient and pesticide interaction should be of high concern to honey bee larvae and overall colony health. None of these factors can be neglected in the pesticide risk assessment for honey bees.
Choosing To Ignore The Obvious
There is no doubt that neonics have the potential to harm bees, but the question is, do they really cause as much problem in the real world as we’ve been led to believe? This is not a matter of convincing the masses; this is an investigation of fact and evidence. For a pesticide to cause harm to a colony of bees, two necessary elements must occur:
- The bees must be exposed to the pesticide. Evidence for this is best determined by chemical analysis of the pollen in the combs, since residues in the bodies of dead bees may be degraded, and because water-soluble insecticides such as the neonics are not absorbed into the wax (residues in the wax do document the history of exposure to lipophilic pesticides).
- The pesticide must be present at a concentration above a trivial level.
When we take the time to determine which pesticides bees are actually found in the combs of hives, neonicotinoids are seldom present, or if detected are often at biologically irrelevant concentrations. Imidacloprid was detected in fewer than 3% of Mullin’s 350 samples, and clothianidin not at all! Similarly, there were zero detects for clothianidin in the 99 USDA samples; imidacloprid was only present in 9%. Likewise, a number of European studies have shown similar results (reviewed in [59]).
In a recent study, the Fraziers [60] looked at hives placed in cotton, corn, alfalfa, apples, pumpkins, almonds, melons, blueberries, or wild flowers, and identified the residues in collected pollen, in returning foragers, and in dead or dying bees near the hives. Again, the only neonic noted was thiamethoxam in alfalfa (in which dying bees contained residues of ten different pesticides). However, there were alarmingly high detects of fungicides, the insecticide acephate, and the metabolite of the beekeeper-applied miticide amitraz.
The latest data comes from Dr. Jeff Pettis [61], whose group determined the pesticides in bee-collected pollen from six crops: apple, blueberry, cranberry, cucumber, pumpkin, and watermelon. Of the 35 pesticides detected, beekeeper-applied miticides and ag fungicides predominated (sometimes at alarming levels), followed by common organophosphate, pyrethroid, and cyclodiene insecticides (again sometimes at alarming levels). In the 17 samples tested, residues of neonics were only found in the samples from the apple orchards, and only one was found at a biologically-relevant concentration.
So my question is why the heck are so many activists pursuing the single-minded focus upon the neonics, when the clear evidence is that neonics are not commonly found in bee-collected pollen, and if present, are generally at levels that do not appear to negatively affect colony health [62]?
There is a lot more to pesticide issues than the neonics alone, and by focusing our attention solely upon them, we ignore the often far more serious effects of other pesticides.
Blinded By Bias
During the intense focus upon neonicotinoids the past few years, we’ve learned that exposure of bees to these insecticides can result in all sorts of sublethal effects. Unfortunately, many researchers appear to be wearing blinders as to the effects of other pesticides. The resulting narrowness of these studies skews our perspective—if we only look for effects from the neonics, we don’t know how to rank the biological relevance of those effects relative to the effects of all the other toxins to which bees are exposed, generally to greater extent.
A practical complaint to researchers: if you are going to look for sublethal effects of neonics, please include positive controls of some other pesticides, so that we can learn whether the neonics are better or worse than the alternatives!
I commend one group that recently decided to take a look at the effects of a common herbicide upon the development of bee larvae [63]. The results of this straightforward and meticulous study are an eye opener!
The researchers found that exposing bee larvae to even infinitesimal amounts of the herbicide paraquat prevented them from fully developing their critical oenocyte cells (see box).
| Oenocyte cells are not only involved in the production of lipids and lipoproteins, but they also appear to play a role in the constitution of external cuticle in both larvae and adults. In addition, they are involved in intermediary metabolism and synthesize hydrocarbons to waterproof cuticle or to make beeswax. Furthermore, oenocytes secrete hormones, especially those involved in larval and adult development. They are also described as the major cells expressing cytochrome P450 reductase, which is involved in detoxification of toxins [information paraphrased from the cited paper]. |
Exposure to even a part per trillion of paraquat suppressed the development of these extremely important cells. The authors conclude:
This study is the first which reports an effect of a pesticide at the very low concentration of 1 ng/kg, a concentration below the detection limits of the most efficient analytic methods. It shows that chemicals, including pesticides, are likely to have a potential impact at such exposure levels.
Who woulda thunk? Paraquat isn’t included in the standard screening for pesticide residues, so we don’t even know how prevalent it is in hives! The above findings should make it clear that we need to go back to the beginning if we are to understand the sublethal effects of pesticides (and adjuvants), even at perhaps undetectable levels.
We do know that here were 812,000 lbs of paraquat applied in California in 2010, as opposed to only 266,000 lbs of imidacloprid. Paraquat shows strong adverse effects upon bee larvae at a part per trillion, as compared to imidacloprid, which is so minimally toxic to bee larvae that no one has even been able to determine an LD50! So the amount of paraquat applied has far greater potential to cause problems to bees in agricultural areas (Fig. 7).
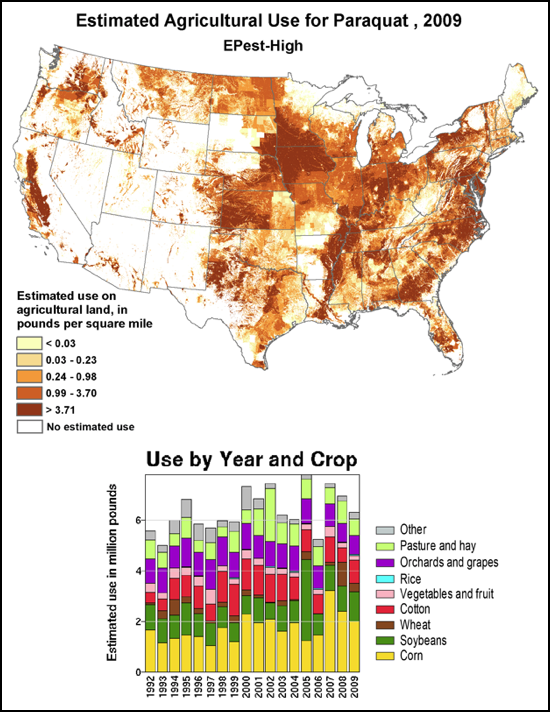
Figure 7. The herbicide paraquat appears to be harmful to bee larvae at levels as low as 1 part per trillion. Note the wide variety of crops, and the extensive areas to which it is applied.
So here we have clear scientific data from a well-designed laboratory experiment that a commonly-applied pesticide has the ability to cause immune suppression and other adverse effects in developing bees, yet these results have been virtually ignored by beekeepers and environmental groups. I just don’t understand it!
No More Safe Home To Return To
Out of their protective hive, honey bees live in a hostile world, full of predators, deadly weather, and toxic agents (both natural and manmade). But the bees of old could generally return to a “safe” home, in which the transmission of natural toxins was largely minimized by the behavior of foragers, and by the processes of the conversion of nectar to honey, and of pollen into jelly (via the digestion of beebread by nurse bees). Both of these processes help to prevent the transmission of toxins from the foragers to the queen and the brood.
With the advent manmade pesticides, bees may no longer have that “safe” home to return to. Beebread and the wax combs nowadays are often contaminated with any number of pesticides (in addition to natural plant toxins and industrial pollutants). But this is not a “new” problem:
A Historical Artifact
Even before we had the ability to detect pesticide residues in combs to the parts per billion level, pesticide analyses often found easily-detectable levels of insecticides in bee hives. As a frame of reference, I sought out a historical artifact—the residues in the beeswax that had been rendered by beekeepers and reprocessed into a sheet of “clean” foundation. I was lucky enough to find that such a sample had recently been analyzed by the Tucson Bee Lab. Dr. Diana Sammataro forwarded me the results of the analysis of an undated “very old” piece of wax foundation from the Northeast (Table 3).
THIS IS THE TABLE !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
|
Pesticides in an old piece of beeswax foundation.
|
|
Positive residue detect
|
ppb
|
| Pendimethalin |
13.1
|
| Endrin |
156
|
| Dieldrin |
160
|
| Trifluralin |
3.6
|
| DDT p,p’ |
32.7
|
| Heptachlor |
35.1
|
| Malathion |
4.3
|
| Chlorpyrifos |
4.6
|
| Dicofol |
6.8
|
| PCB’s |
8190
|
| Chlorothalonil |
84.6
|
Table 3. We can narrow down the foundation’s date of manufacture by the residues present. Pendimethalin was first registered in 1972 (the same year that DDT was banned), and since there were no residues of fluvalinate, the foundation was clearly produced prior to the arrival of varroa around 1990. Thanks to Dr. Diana Sammataro and the Tucson Bee Lab.
Clearly, pesticide-contaminated combs are hardly a new phenomenon. In the above example, the beeswax batch used to produce the foundation came not from a single hive, but rather from the combined wax from many hives, likely from many beekeepers, and thus would represent an average sample of the degree of contamination somewhere in that 1972-1990 time frame. And that doesn’t take into account whether the raw wax came mainly from cappings (which would have been minimally contaminated), or whether it went through the common practice of being filtered through activated carbon. But any colony started on such foundation purchased from a beekeeping supply house would clearly have had to deal with at least the residues of these lipophilic toxins from the get go!
An aside: perhaps of interest is something that I noticed years ago when I switched from dipping my own wax queen cell cups to using plastic cups. My “take” rate became better and more consistent. Was that because the beeswax at the time was contaminated with residues?
The Beekeeper Contribution To Shifting The Tip Point
One thing that is “new” is that since the arrival of varroa, we’ve upped the ante—all commercial beeswax is now contaminated with residues of beekeeper-applied synthetic miticides. The three most prevalent synthetic chemicals found in combs today all get there by being applied by beekeepers for mite control.
Practical note: And although there is no reason to be concerned about the tainting of honey by the legal use of these miticides, the beekeeper/applicator should be aware that both amitraz and tau-fluvalinate make California’s list of “chemicals known to the State to cause reproductive toxicity,” and coumaphos is of concern because it is a “cholinesterase-inhibiting pesticide.” No varroacide is harmless to bees [64]—but the benefits of mite control generally (but not always) outweigh the adverse effects due to the miticide residues.
We beekeepers have clearly shifted the baseline for pesticide contamination of combs, which increases the total toxic load even before the contribution by agricultural pesticides.
Stop Right There!
Although it is a very attractive hypothesis to blame our problems on miticide or pesticide residues, let’s do a reality check. On good forage in good weather, plenty of beekeepers see their colonies thrive even on old, dark, seriously-contaminated combs; but under stressful conditions those same residues might contribute to poor colony performance or even mortality.
No study has yet found support for the hypothesis that miticide residues are the cause of our current bee problems (although one would have every reason to suspect that they may contribute). In fact, vanEngelsdorp [65] found that surprisingly, higher levels of coumaphos residues negatively correlated with colony survival. How could this be? One possible explanation is that those beekeepers who used it experienced better mite control. But there is also another intriguing possibility—hormetic effects.
Undetectable Levels And Hormesis
Is your head spinning yet? I’ve presented evidence that undetectable levels of some pesticides could harm bees, that “inert” adjuvants can do the same, and that combs are often chock full of all sorts of pesticide and varroacides residues. Criminy, it’s a wonder that bees survive at all! Or is it?
Bees have long been exposed to all sorts of natural, and recently, manmade toxins, and survived. Toxicity is a complicated subject. The only thing that separates a medicine from a poison is the dose. In general, if a pesticide has been tested upon adult and larval bees and found to have no observable adverse effects at a certain concentration, we would not expect to see adverse effects at lower concentrations. However, there are exceptions to this general rule—toxicity may vary up or down depending upon the dose [66]!
I’ve previously mentioned the term hormesis [67]— the paradoxical effect of toxins at low concentrations. The paradox is that although most chemicals are toxic at high concentrations, the majority are likely beneficial at low concentrations. For those interested in this fascinating phenomenon, I suggest Dr. Chris Cutler’s excellent and thought-provoking review [68]. It is not only possible, but actually probable that lose doses of pesticides may exert a beneficial effect upon a colony! (Don’t be ridiculous—I’m not suggesting that bees are better off for the presence of pesticides!).
Wrap Up
Toxins, whether natural or manmade, are clearly a potential issue in colony health. To what degree pesticides contribute to colony morbidity or mortality is dependent upon exposure, the dose, and a host of associated factors. Beekeepers have long noticed that their bees often do better if allowed to forage on pesticide-free land. But many beekeepers today tell me that their bees do just fine in the middle of intense agricultural areas—so this is not a black or white situation.
In recent years beekeepers themselves have greatly added to the degree of contamination of their combs. Introductions of novel pesticides and adjuvants keep changing the picture. And now we’re finding that pesticides that we formerly assumed were harmless to bees (fungicides and herbicides) may actually be quite toxic to larvae! Then there is the scary finding that undetectable levels of some pesticides might cause health issues, countered by the fascinating subject of hormesis.
I certainly do not profess to understand all this, but I have come to the following conclusions:
- That bees have had to deal with toxins for a long time,
- That pesticides will be with us for the foreseeable future,
- That varroacides have likely added to the problem,
- That pesticides can cause lethal and long-term sublethal effects in the hive, but
- That many beekeepers in agricultural areas no longer consider pesticides to be a serious issue, whereas,
- That colonies may go downhill after being exposed to some agricultural chemicals, or combinations thereof,
- That toxicology in the hive is complex, and that there are few simple answers,
- That it is unlikely that any single pesticide is to blame for our current colony health issues,
- That we still have a lot to learn!
Next month I will look at the distribution of both managed colonies and of pesticide applications in the United States, and their relationship to bee health problems.
Acknowledgements
As always, I could not research these articles without the assistance of my longtime collaborator Peter Loring Borst, to whom I am greatly indebted. I also wish to thank Drs. Jim and Maryann Frazier, Chris Mullin, David Fischer, Eric Mussen, Thomas Steeger, and Roger Simonds for their generosity in taking the time to discuss pesticide issues with me.
References
[2](Broken Link) http://perc.org/articles/everyone-calm-down-there-no-bee-pocalypse
[4] http://thebreakthrough.org/index.php/journal/past-issues/issue-1/an-environmental-journalists-lament/
[5] vanEngelsdorp and Meixner (2010) op. cit.
[6] Rucker (2012) op. cit.
[16] Wilson, WT and DM Menapace (1979) Disappearing disease of honey bees: A survey of the United States. ABJ March 1979: 185-186.
“Certainly with both pesticide-related and [Disappearing Disease]-caused bee losses, the adult population of a colony may be reduced rapidly to a “handful” of bees or, in some cases, the entire population may be lost.
“However, in the case of pesticide poisoning, there is usually evidence of pesticide application…the worker bees either die in the field or in or near the hive depending on the type of pesticide. When the field force is killed and they “disappear,” many dead or dying bees may be seen on the ground in the field or on the ground between the treated field and the apiary…If the foraging bees bring poison into the hive, then the nurse bees either die in the hive or at the entrance so one can see many crawling and tumbling adults and large amounts of neglected brood. Exposure to pesticides over an extended period results in very weak colonies, and some die out.
“In the case of [Disappearing Disease], the situation is quite different. The colonies frequently have gone through a period o nectar and pollen collection with active brood rearing [as in typical CCD]. Then the weather has turned unseasonably cool and damp and remained adverse for from about 3 to 14 days…During the inclement weather, the bee populations dwindle because the worker bees disappear from the hive leaving a “handful” of bees and the queen. Often these small populations recover and increase in size during hot weather and a long nectar flow or, or occasionally, the entire population absconds…”
[17] Johansen CA and DF Mayer (1990) Pollinator Protection: A Bee & Pesticide Handbook. Wicwas Press.
[21] Medici SK, Castro A, Sarlo EG, Marioli JM, Eguaras MJ (2012) The concentration effect of selected acaricides present in beeswax foundation on the survival of Apis mellifera colonies. J Apic Res 51: 164–168
[22] Eric C. Mussen, Julio E. Lopez, and Christine Y. S. Peng (2004) effects of selected fungicides on growth and development of larval honey bees, Apis mellifera L. (Hymenoptera: Apidae). Environmental Entomology 33(5):1151-1154.
[23] Frazier, J.L., M.T. Frazier, C.A. Mullin & W. Zhu – Does the reproductive ground plan hypothesis offer a mechanistic basis for understanding declining honey bee health? http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[25] Wu JY, Smart MD, Anelli CM, Sheppard WS (2012) Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. J Invert Path 109: 326–329
[27] Maisonnasse A, et al (2010) E-β-Ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS ONE 5(10): e13531. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0013531 These researchers studied (E)-b-ocimene, a volatile terpene commonly produced by plants to attract predatory mites, but also a critical pheromone produced by the brood and the queen.
[34] Scott-Dupree, CD, et al (2009) Impact of currently used or potentially useful insecticides for canola agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae). J Econ Entomol 102(1):177-82.
[36] Hobbs, GA (1967) Domestication of Alfalfa Leaf-cutter Bees. Canada Dept. of Agriculture. Ottawa: Queen’s Printer and Controller of stationary.
[37] Dr. Jerry Bromenshenk, pers. com.
[38] Abbott, VA, et al (2008) Lethal and sublethal effects of imidacloprid on Osmia lignaria and clothianidin on Megachile rotundata (Hymenoptera: Megachilidae). J Econ Entomol 101(3):784-96.
[40] PMRA (2013) Evaluation of Canadian Bee Mortalities Coinciding with Corn Planting in Spring 2012.
[42] Mullin CA, et al. (2010) op. cit.
[44] Christensen, K.; Harper, B.; Luukinen, B.; Buhl, K.; Stone, D. 2009. Chlorpyrifos Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. http://npic.orst.edu/factsheets/chlorptech.pdf.
[45] Rortais, A (2005) Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 36: 71–83.
[46] Cresswell, JE, et al (2012) Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115: 365– 371.
[47] Frazier (2011) op. cit.
[49] Frazier, et al (2011) Assessing the reduction of field populations in honey bee colonies pollinating nine different crops. ABRC 2011
[51] Mussen, et al (2004) op. cit.
[53] Zhu, W., D. Schmehl & J. Frazier (2011) Measuring and predicting honey bee larval survival after chronic pesticide exposure http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[54] Mullin, C.A., J. Chen, W. Zhu, M.T. Frazier & J.L. Frazier – The formulation makes the bee poison. ABRC 2013
[55] Ciarlo TJ, Mullin CA, Frazier JL, Schmehl DR (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7(7): e40848. doi:10.1371/journal.pone.0040848
[56] (Broken Link!) http://www2.basf.us/diols/bcdiolsnmp.html
[58] Zhu, et al (2011) op. cit.
[60] Frazier, M.T., S. Ashcraft, W. Zhu & J. Frazier – Assessing the reduction of field populations in honey bee colonies pollinating nine different crops http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
[61] Pettis, et al (2013) op. cit.
[62] A recent study confirm that the neonic residues in corn, soy, and canola pollen are at very low concentrations. Henderson, C.B. a, J.J. Bromenshenka, D.L. Fischerb. Clothianidin exposure levels from bee-collected pollen and nectar in seed-treated corn and canola plantings. ABRC 2013 http://bees.msu.edu/wp-content/uploads/2013/01/ABRC-abstracts-2013.pdf
[64] Boncristiani, H., et. al. (2011) Direct effect of acaricides on pathogen loads and gene expression levels of honey bee Apis mellifera. Journal of Insect Physiology. 58:613-620.
[65] vanEngelsdorp, D, et al () Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J. Econ. Entomol. 103(5): 1517-1523. (Broken Link!) http://www.eclecticparrot.com.au/research_papers/VanEngelsdorp%202010%20Weighing%20risk%20factors%20in%20Bee%20CCD.pdf
[66] Cutler GC, Ramanaidu K, Astatkie T, and Isman MB. (2009) Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag Sci 65:205-209
[68] Cutler, GC (2013) Insects, insecticides and hormesis: evidence and considerations for study. Dose-Response 11:154–177 (Broken Link!) http://dose-response.metapress.com/app/home/contribution.asp?referrer=parent&backto=issue,2,11;journal,3,34;linkingpublicationresults,1:119866,1
First published in: American Bee Journal, January 2014
Sick Bees Part 18F9:
Colony Collapse Revisited – The Bee/Pesticide Problem Complex
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in January 2014
Examples Of Improved Technology
The Transition
The “Bee/Pesticide Problem Complex”
Pesticide Hazard Quotients
A More Relevant Figure?
Wrap Up
Acknowledgements
Citations and Footnotes
Part 1
In my lifetime I’ve witnessed a wholesale shift in our attitudes toward pesticides. We’ve gone from the arrogant and unrealistic expectation that we could conquer all of our self-created “pest” problems via chemistry, to realizing that it’s a bit more complicated than that, and that we are going to have to learn to work with Nature. I came of age just as Rachel Carson published Silent Spring, which led to a complete overhaul in the way in which we regulate the poisons that we introduce into the environment. But our transition continues to be a work in progress; we still rely upon an ever evolving arsenal of toxic substances to solve our problems (our own industry being an exemplary case in point).
However, I’m heartened by the fact that society’s 21st century values are shifting toward a more sustainable model of environmental stewardship. Both industry and our government are being reluctantly dragged in that direction; none too soon for those species that are forced to eke out a living in areas of intense agriculture. The populations of many species of life, both plant and animal, are rapidly declining. Our current agricultural practices are often at odds with the needs of pollinators, which must forage in the “wild” for a safe and continuous food supply.
It is clearly time for environmental action; however, we are most effective when we base our arguments and protests upon facts and scientific data. Unfortunately, such facts are often difficult to sort from popular myths. For example, take the story of the unfortunate Chinese pear growers of Sichuan province [1] who are forced to hand pollinate their trees due to all the bees being killed off by pesticides—indeed a dreadfully grim image! In reality, this compelling story turns out to be an exaggeration of the facts, as detailed by Mark Grossman in an engrossing and thought-provoking essay [2] (I’m not going to spoil the story—read it yourself).
But I will quote excerpts from Grossman’s conclusion:
Many articles about declining bee populations have a theme and tone that reminds me of those old sci-fi movies from the 1950’s. Somehow, human technological tampering with nature is punished in some awful (and bizarre) way. You can almost read this theme between the lines of more than a few articles — an echoed suggestion that some technological tinkering has angered Mother Nature… And we are being punished by the disappearance of our bees. Then, domino-like, all of modern civilization will fall to ruins…
The inaccurate impression of the Sichuan Province as the scene of a bee extinction fits almost too neatly into an increasingly pervasive, though less than articulate, mythology — the mythology of the bee apocalypse…the mythology of our current bee die-off as divine retribution from God or Gaea, heaven or earth, conceals the actual problem by confusing it with our own most personal hopes and fears about both our technology and our future. Our bees and our agriculture — our food supply — are in real danger. This should drive us directly toward an understanding of the problem and, then, to a solution. And, most certainly, that solution will be technological and require more technology…
If we are to save our bees, we need to forget the myths and fables and remember the technology. Yes, in some way, almost every technological advance brings with it both a blessing and a curse. So, even if our technology is, in some measure, responsible for the problem of declining honeybee populations, that same technology will most certainly be the source of the solution.
The fact is that technological advances in agriculture have allowed the human population to explode. And it’s going to take more technological advances to deal with the consequences. There is no going back to “the old ways”—it is politically unpopular to allow millions of people to starve. As James McWilliams observes [3]:
Currently, there are two paradigms of agriculture being widely promoted: local and organic systems versus globalized and industrialized agriculture. Each has fervent followers and critics. Genuine discourse has broken down: You’re either with Michael Pollan or you’re with Monsanto. But neither of these paradigms, standing alone, can fully meet our needs.
So should we dismiss organic agriculture outright? Absolutely not. Organic may not be “the” solution to global food demand, but it can certainly be part of it. As Jason Clay, senior vice president of the World Wildlife Fund, writes [4], “I think we need a new kind of agriculture—kind of a third agriculture, between the big agribusiness, commercial approach to agriculture, and the lessons from organic and local systems.” With enhanced investment in agricultural research, there’s every reason to hope that organic yields will improve and that the organic model will become more prominent. The fact that we’re not yet there… doesn’t mean we should abandon the quest for agricultural systems that are both high yielding and as ecologically responsible as they can be.
Amen! By promoting a sustainable mixture of traditional practices and new technology we may be able to halt our indefensible destruction of the environment. And we are slowly making progress. The concept of agroecology is catching on, as well as integrated pest management, eco-friendly advances in biotechnology, and the development of “greener” pesticides.
Examples Of Improved Technology
It’s bad enough when one’s bees get killed by a careful application of pesticides. But it gets a beekeeper’s blood boiling when such a kill is due to carelessness or from the intentional disregard of label restrictions [5]. And this is where new robotic and GPS technology may help (Fig. 1).
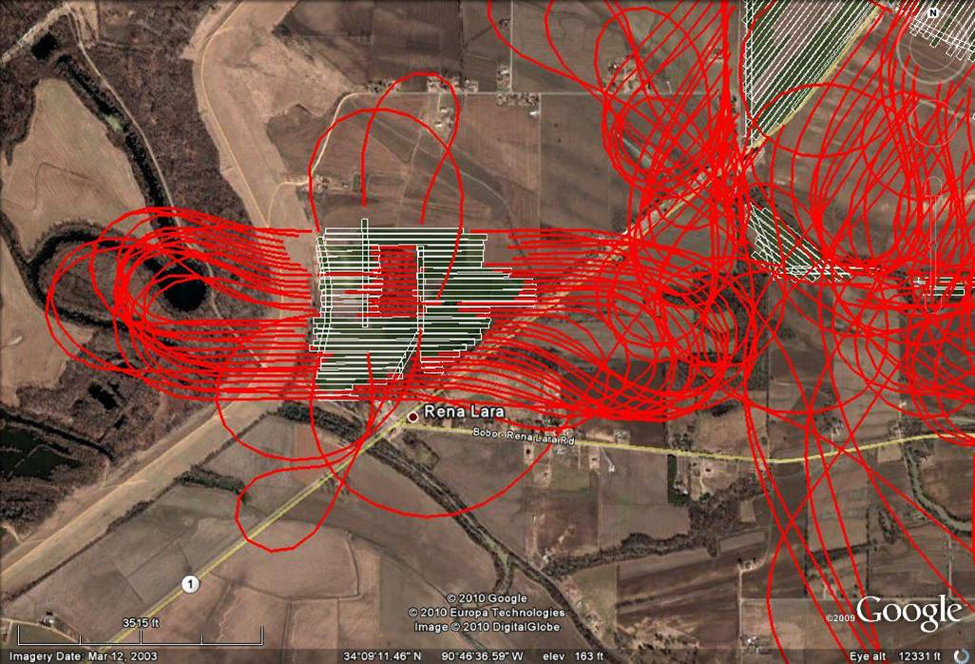
Figure 1. Beekeeper Steven Coy tells me that in Mississippi, cotton receives up to 14 aerial spraying a season! To that State Lead Agency’s credit, all aerial applications of pesticides require GPS tracking to confirm that the pesticide was applied accurately. In the image above, the red lines track the route of the applicator; the white bars where the spray valves were open. Such a record documents what actually took place! Image courtesy Tommy McDaniel, Director, Mississippi Pesticide Division.
But why stop at airplanes? Aerial applications have the great advantage of not needing to drive over the crop, are quick and relatively cheap. But most of an applied insecticide never hits its intended target insect, and any sort of wind results in pesticide drift off the field. The other problem is that pilots don’t want to fly at night, the best time to apply pesticides if one wishes to minimize their impact upon pollinators.
We now have the technology to build GPS-guided robotic sprayers that could accurately spray fields during the night, when there is little wind, and bees are safe in the hive. Such a device would allow growers to spray their orchards while they sleep [6].
This is not pie in the sky—the WeedseekerÒ is already on the market [7]. This device mounts on a tractor herbicide spray rig. It optically recognizes the difference between crop plants and weeds, and then spot sprays only the intended weeds. This reduces herbicide application by up to 90%, and, of more import to the grower, quickly pays for itself.
Despite a frenzy of protests, eco-friendly biotechnology is clearly the wave of the future [8]. Genetically-engineered crops got off to a bad start in the public’s eye due to their association with giant agribusinesses and the fact that the two flagship GM crops both have something to do with pesticides [9]. Engineered plant cultivars already in development stand to save the orange juice industry from citrus greening disease [10], and the world’s wheat production from a devastating fungus [11]—both would greatly reduce pesticide applications.
And all the major [12], and number of startup, pesticide companies are jumping on the “biorational” pesticide bandwagon. California-based Marrone Bio Innovations, Inc. produces state of the art naturally-derived biopesticides [13], including the bee-friendly insecticide GrandevoÒ and fungicide RegaliaÒ.
With all this talk of agricultural innovations, I can’t help but comment that other than the addition of motors to my vehicles and extractor, my own bee operation runs on technology that hasn’t changed since the 1850’s.
The Transition
The transition to agroecological practices won’t be easy—the chemical companies are very good at selling pesticides to farmers, and they have much larger budgets than do the extension agronomists who are doggedly attempting to promote IPM. American consumers have gotten used to cheap meat and grain, and cosmetically-perfect produce. Few farmers would willingly take the chance of losing their crop to some bug. The powerful farm lobby rewards those politicians who write the rules. I’ve come to accept that pesticides are going to be part of beekeeping for the rest of my lifetime.
A thoughtful perspective on pesticide issues can be found at [14], from which I’ll share a couple of excerpts:
…many people today think that pesticides are unacceptably dangerous to the environment or to man. Citizens want to know more about pesticides, their benefits, their risks, and the ways government regulates them. With good information, citizens are better able to analyze the arguments of both opponents and supporters of pesticide use. Pesticide policies should be formulated based on facts and reason instead of false perceptions and hysteria. Any rational approach to pesticide use should include a risk-benefit comparison…Pesticides are poisons and can be hazardous. Fortunately, research, education, and government agencies are constantly reducing the risk of using pesticides by producing “safer” chemicals, pest-specific pesticides, better application methods, and tougher pesticide laws. The result is a constantly improving risk-benefit ratio.
The above is good news for both bees and beekeepers. Beekeepers from every region tell me that pesticide issues have improved since the Bad Old Days of the 1960’s and ‘70’s. But that’s not to say that pesticides aren’t still widely applied, nor that they are not still a problem for bees in some areas. What is devilishly difficult to figure out is exactly why it is, that despite such exposure, bees can thrive in some ag areas, yet perish in others. Perhaps by carefully examining the kinds of pesticides to which bees are most exposed in each area, we can start to make some sense of it all.
The “Bee/Pesticide Problem Complex”
Although the bulk of chemicals are applied to the environmentally-devastating vast corn and soy monocultures in the Midwest, pesticides are often more of a problem for those beekeepers in other areas; such as for those being paid for pollination services, whether that be for melons in California, seed crops in Oregon, apples in New York, or pumpkins in Pennsylvania. These pollination-dependent crops are often grown in areas consisting of a patchwork of different crops, many of which are not dependent upon bees, but upon and around which one’s bees may nevertheless forage (Fig. 2).
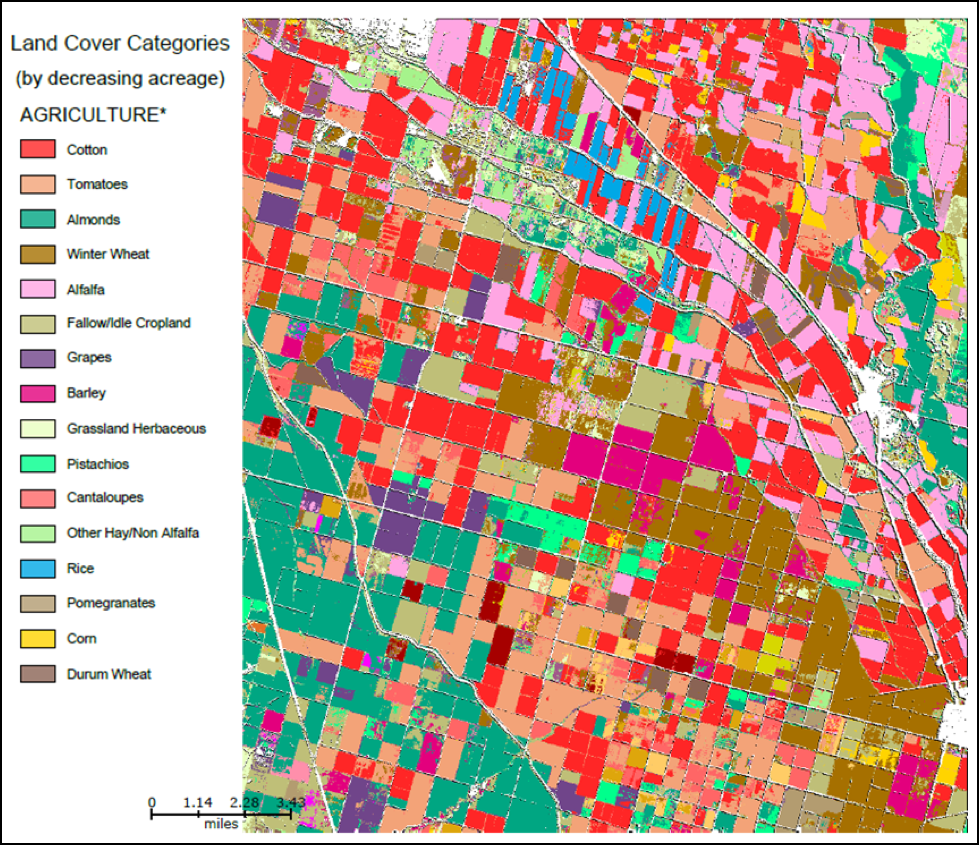
Figure 2. Look at this color-coded patchwork of different crops in California’s San Joaquin Valley. Note the scale of the map, and just how many different crops may be within flight range of any hive. When I ask beekeepers in the Valley which crops are most problematic, it is often not the crop for which they are being paid to pollinate (such as vine crops)–their bees instead get hit by pesticide applications on nearby corn, tomatoes, cotton, and alfalfa. You can download a similar map for any agricultural area from the NASS CropScape website [[i]].
[i] http://nassgeodata.gmu.edu/CropScape/
Since bees don’t pay attention to property lines, the beekeeper has no control over where his bees will actually forage. A number of times I’ve watched a grower write me a pollination check while he was watching the bees fly out of my hives and in a direction away from his orchard. Bees go wherever they get the best return on investment. If attractive flowers are blooming anywhere within flight range, then that’s where the bees will go. It is nearly impossible for the beekeeper to keep track of all the different pesticides that may be applied within that area, which could easily encompass anywhere from 12 to 50 square miles.
The result is that colonies are typically exposed to a greater diversity of pesticides than that applied to the crop upon which they are placed. The Ericksons [16] termed this stew of toxin exposure, coupled with environmental factors, “the bee/pesticide problem complex” (complex: a group or system of different things that are linked in a close or complicated way; emphasis on the word “complicated”).
The diversity of pesticides in pollen is staggering. The Fraziers [17] documented 52 pesticide residues from colonies placed on nine different crops; Pettis [18] found 35 in pollen taken from hives in 7 different crops; Stoner [19] detected well over 60 pesticides from 5 apiaries in Connecticut. An important observation is that two colonies side by side may bring back entirely different pollen loads, with different pesticide residues, none associated with the crop upon which they are placed!
And keep in mind that the Fraziers [20] found that residues may be detected to a greater extent in dead or dying bees as opposed to in the hive matrices. This finding suggests that colonies are losing their field force without leaving residues in the hive (the lesser of two evils), but suggesting that the impacts of some pesticides may be underestimated by samples taken from the combs–they found field forces to be significantly reduced in colonies pollinating cotton, corn, and alfalfa.
Dr. Jim Frazier explains that it is impossible for us to figure out how all the different combinations of pesticides interact with colony health [21], especially when we throw in any contribution from the unlabeled adjuvants, which can be even more toxic to bees than the pesticide active ingredient itself [22].
This is likely the reason that despite beekeepers clearly being able to see that colonies suffer after certain ag exposures, that no one has been able to link any specific pesticide as being the cause of colony losses (other than in the case of outright bee kills). This is of course extremely frustrating to beekeepers who want the EPA to “do something” (and to the EPA, which does want to do something!).
Pesticide Hazard Quotients
Even more frustrating to the beekeeper is that when he is given the results of pesticide analyses, all that he sees are a bunch of nearly meaningless numbers. In order to make any sense of the detect results, he must then search for published LD50 (lethal dose) figures for oral, contact, and chronic exposure (unfortunately, these figures are all over the page, depending upon who did the lab testing; the figures may be in µg/kg, ppm, nanograms/g, ppb, or ppt; and for many pesticides, published figures are either nonexistent or highly suspect). Then he must calculate/guesstimate how much nectar, pollen, or dust a bee actually consumed or to which it was exposed. I’ve done these calculations time and again—believe me, it’s a pain!
Drs. Kimberly Stoner and Brian Eitzer [23] propose that researchers save beekeepers from all that work and confusion by reporting detected concentrations not only in ppb’s, but also as “hazard quotients” [24]. This would really help the beekeeper to put the numbers into context.
The way that they calculated their Pollen Hazard Quotient (PHQ) was to take the concentration of the pesticide residue in the sample (in ppb), and then divide the result by the most appropriate available oral LD50 figure. For example, the maximum residue of phosmet in their samples was 16,556 ppb, and the published oral LD50 is 0.37 µg/bee, resulting in a pollen hazard quotient of 44,746 (they could also calculate nectar hazard quotients).
How About A More Meaningful Figure?
Unfortunately, the above figure (PHQ of 44,746) is, in my book, still just another big, scary, confusing number to the beekeeper. So let’s go a step better…
Regulatory Terms
Pesticide regulators use the term Acceptable Daily Intake (ADI) to quantify the amount of a pesticide that can be ingested daily over a lifetime without appreciable risk (they typically throw in a 10x safety factor to be on the safe side). If the concentration of that pesticide in food exceeds the ADI, then it is said to pose a “consumption hazard.” In the above study, Stoner and Eitzer not only calculated the Pollen Hazard Quotient, but also what I consider to be an even more meaningful figure—the percentage of the oral LD50 consumed per day [25]:
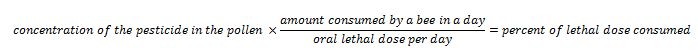
I suggest that we give this figure a catchy name such as the “Daily Consumption Hazard” (DCH). The DCH would be eminently simple to interpret: a DCH of 1 would mean that a bee would consume 100% of a lethal dose over the course of a day; a DCH of 0.50 would mean that it would consume 50% of a lethal dose.
For example, at the highest concentration of phosmet found in their pollen samples, the Daily Consumption Hazard would be calculated thusly [26]:
Now that’s a figure that anyone can understand! A typical nurse bee, if eating the phosmet-contaminated pollen, would be consuming an alarming 42% of the lethal oral dose of phosmet per day. This would clearly be a figure of concern to the beekeeper!
Although they didn’t use that term, the authors calculated the DCH to nurse bees for the highest detect of each pesticide found in their pollen samples (Table 1):
| Pesticide |
Maximum residue (ppb) |
Percentage of oral LD50
(“Daily Consumption Hazard”) |
| Phosmet |
16556 |
42.5 |
| Imidacloprid |
70 |
17.1 |
| Indoxacarb |
417 |
2.0 |
| Fibronil |
3.5 |
0.8 |
| Thiamethoxam |
4.1 |
0.8 |
| Dinotefuran |
7.6 |
0.3 |
| Chlorpyrifos |
25.2 |
0.1 |
| Diazinon |
18 |
0.1 |
| Methomyl |
24 |
0.1 |
| Dimethoate |
4.2 |
0.1 |
Table 1. The “Daily Consumption Hazard” to nurse bees consuming beebread containing the maximum residue concentrations of various pesticides in pollen samples collected over several years from 5 apiaries in Connecticut. The concentration of phosmet approached half the oral lethal dose. For most pesticides, the DCH was less than 1% of the lethal dose, so likely not of serious concern. After Stoner and Eitzen, cited above.
It is important to note that in order to save costs, the authors omitted performing analyses for two pesticides of concern—the pyrethroids and the fungicide chlorothalanil. I suspect that those two would have ranked high on the list.
Curious, I calculated Daily Consumption Hazards from the data of the aforementioned study by Pettis [[i]], in which the researchers collected beebread samples from hives placed in seven major crops. The pesticides with the highest DCH’s were, in order, the organophosphate phosmet, six different pyrethroids, followed by the neonicotinoid imidacloprid.
Note that the above rankings for toxic risk are based upon the maximum levels of residues detected, not the averages. I suspect that it is this sort of sporadic high-but-not-quite lethal exposure that may help to explain some of the colony morbidity in agricultural areas. In none of these studies did the authors mention observing overt bee kills.
Practical application: the main value to calculating Daily Consumption Hazards is that it quickly brings to our attention those pesticides that are most likely to be affecting bees due to residues in the pollen. On the other hand, it also allows us to judge which pesticide residues are not likely to be of biological significance.
Limitations of Hazard Quotients
Although readily understandable, calculations of daily consumption hazards are limited by our lack of knowledge and data. The meaningfulness of any hazard quotient is based upon any number of assumptions about the actual toxicity of a substance. It is troubling that researchers have found that, “All in all, it seems that there is no clear correlation between acute and chronic toxicity” [28]. Unfortunately, until recently, regulators have not been calling for 10-day chronic toxicity data, so we simply do not have those figures at our disposal for many pesticides [29]. Heck, for many pesticides, we don’t even have oral toxicity figures, only contact LD50’s!
- The quotients based upon residues in pollen would only apply to toxicity to nurse bees, since foragers typically do not consume pollen. For older bees we would need to calculate “nectar toxicities,” based upon feeding studies with spiked syrup. And then for nurse bees we would need to add this figure to the toxic load that they receive from their consumption of pollen.
- The quotient does not take into account environmental factors (such as temperature or humidity), colony nutritional stress, or parasite loads.
- The quotient does not automatically calculate the additive risk of pesticides with similar modes of action, such as that from any pyrethroid exposure on top of the “foundation” of fluvalinate contamination of most beeswax.
- Nor does the quotient take into account potential chemical synergies between pesticides or other toxins (including beekeeper-applied miticides). Such synergies would greatly increase the DCH of certain pesticides.
- Do not expect the actual testing labs to calculate hazard quotients, since such interpretation would by necessity be based upon the research and assumptions of others (for food consumption rates, LD50’s, and LC50’s).
Luckily, regulatory agencies across the world are adopting more stringent testing requirements for pesticides, so we will likely have better toxicity figures to work with in the future.
Wrap Up
Beekeepers are drowning in data that makes little sense to them. I commend Drs. Stoner and Eitzen for formally proposing that researchers present their pesticide residue data in a more “user-friendly” way. I feel that the most meaningful figure to beekeepers would be what I’ve termed the “Daily Consumption Hazard” (there may well be a better name), as this would let them know which pesticides in their area would most likely be affecting their bees.
In fact, that is such a good idea, that I may do so myself in the next article, in which I will look at which pesticides are applied in which parts of the U.S., and their known effects upon bees.
Acknowledgements
As always, I’m deeply indebted to my partner in the mission to get accurate scientific information to beekeepers, Pete Borst. I also appreciate James Fischer’s research skills in his posts to Bee-L. And of course, my hat is off to the scientists whom I cite, most of whom have taken the time to share their thoughts with me.
Citations and Footnotes
1 http://thebeephotographer.photoshelter.com/gallery/-/G0000M46TBQX4Odc/
2 http://marklgrossmann.wordpress.com/2013/09/26/thursday-bees-who-needs-em-the-sichuan-sentence-the-bee-apocalypse/
3 McWilliams, JE (2011) Organic Crops Alone Can’t Feed the World. http://www.slate.com/articles/health_and_science/green_room/2011/03/organic_crops_alone_cant_feed_the_world.html
4 http://dotearth.blogs.nytimes.com/2011/03/03/a-hybrid-path-to-feeding-9-billion-on-a-still-green-planet/?_r=1
5 http://grist.org/news/farm-kills-millions-of-bees-with-illegal-pesticide-spraying-gets-slap-on-wrist/
6 http://www.kgw.com/lifestyle/Robot-pesticide-sprayer-future-of-farming–219504211.html,
(Broken Link!) http://www.crophuggerreport.com/2011/02/micothon-developes-new-pesticide.html
7 (Broken Link!) http://www.cropoptics.com.au/weedseeker.html
http://www.cals.arizona.edu/pubs/general/resrpt2008/article3.pdf
8 http://www.forbes.com/forbes/2010/0301/opinions-gmos-crops-genetics-monsato-ideas-opinions.html
9 http://www.slate.com/articles/life/food/2013/07/a_hippie_s_defense_of_gmos_why_genetically_modified_food_isn_t_necessarily.single.html
10 http://www.nytimes.com/2013/07/28/science/a-race-to-save-the-orange-by-altering-its-dna.html?_r=1&
11 http://www.k-state.edu/media/newsreleases/jun13/sr3562713.html
12 http://www.cropscience.bayer.com/en/Products-and-Innovation/Brands/Biologicals.aspx
1 http://www.marronebioinnovations.com/agriculture/products/, http://www.marronebioinnovations.com/biopesticides
14 Pesticide Usage in the United States: History, Benefits, Risks, and Trends http://ipm.ncsu.edu/safety/factsheets/pestuse.pdf
15 http://nassgeodata.gmu.edu/CropScape/
16 https://scientificbeekeeping.com/historical-pesticide-overview/
17 Frazier, M.T., S. Ashcraft, W. Zhu & J. Frazier (2011) – Assessing the reduction of field populations in honey bee colonies pollinating nine different crops http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.UhDTZX-aucw
18 Pettis JS, et al. (2013) Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 8(7): e70182. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0070182
19 Stoner KA, Eitzer BD (2013) Using a hazard quotient to evaluate pesticide residues detected in pollen trapped from honey bees (Apis mellifera) in Connecticut . PLoS ONE http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0077550
20 Frazier, M.T., et al. (2011) Op. cit.
21 Presentation at the Monsanto Honey Bee Health Summit.
22 Mullin, CA, et al (2011) A primer on pesticide formulation ‘inerts’ and honey bees. 2011 American Bee Research Conference. http://www.extension.org/pages/58650/proceedings-of-the-american-bee-research-conference-2011#.Ulq6D1Mgvd5
23 Stoner KA, Eitzer BD (2013) Op. cit.
24 http://www.epa.gov/R5Super/ecology/erasteps/erastep2.html#hazquot
25 In the right-hand column of their Table 3, with the heading “Percentage of oral LD50.”
26 I simplified the calculation in the text. The actual calculation would be:
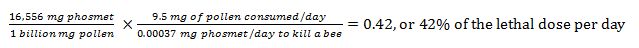
For the first term, I converted ppb to a rate. For the second, I used the authors’ reasonable estimate that a nurse bee would consume 9.5 mg of pollen per day, and converted the published LD50 of 0.37 µg/bee to 0.00037 mg/bee. The final resulting “Daily Consumption Hazard” is in essence the inverse of the “margin of safety,” or the Toxicity Exposure Ratio (TER)—the smaller the TER, the more toxic the exposure, approaching lethality as the TER approaches a value of 1.
27 Pettis JS, et al. (2013) Op. cit.
28 Simon-Delso, N, et al (2011) Risk assessment of pesticides on bees: evaluating risk coefficients for assessing acute and chronic toxicity. 11th International Symposium of the ICP-BR Bee Protection Group, Wageningen (The Netherlands). (Broken Link!) http://pub.jki.bund.de/index.php/JKA/article/view/1934/2310
29 What we need are figures for the chronic LC50—the lethal concentration of a pesticide in pollen or nectar that causes mortality or morbidity in 10-day feeding studies.
First published in: American Bee Journal, September 2013
The Slaughter of the Innocents
Why the Current Hoopla on Pesticides?
Ecosystems and Agriculture
Our Creation of “Pests”
The Slaughter of the Innocents
Pesticide (Mis)use Today
The Nitty-Gritty
The Killing Fields
Chronic Exposure
Hitting the Colony Where it Really Hurts
Progress in Reducing Pesticide Use
A Brighter Future
References
Sick Bees Part 18f6: Colony Collapse Revisited
the Slaughter of the Innocents
First published in ABJ September 2013
Randy Oliver
ScientificBeekeeping.com
The Slaughter of the Innocents
I’ve just learned something that helps me to understand what drives me to write these articles. Scientists have recently discovered that some people’s brains are wired for wanting to share useful information with others [1]. I’ve always been this way—I love learning about things and then sharing what I’ve learned with others. Learning about pesticide issues with bees has certainly been an educational experience for me, taking me down roads that I’d never imagined, and forcing me to rethink many of my previous assumptions. So please allow me to continue into my investigation of pesticides and their relationship to colony mortality.
Why the Current Hoopla on Pesticides?
The pesticide situation for bees has clearly improved since the “bad old days” of the 1960’s and ‘70’s [2], and doesn’t seem to be much different than when the Ericksons wrote about the subject in ABJ back in the 1980’s (a “must read” for anyone interested in this subject; copies at my website [3]). Yet today a media circus has convinced the public that certain pesticides are suddenly driving bees to extinction, and our industry has come together to bring a test case against the EPA to see whether we can finally get more bee-friendly restrictions in the labeling of new pesticide registrations. So why the current renewed interest in pesticide issues?
The simple answer is that beekeepers are hurting! Winter colony losses are averaging an excessive 30 percent, which makes beekeeping less profitable. It is simply more difficult and expensive to keep bees these days. Everyone is looking for something simple to blame, and pesticides have a long history of causing problems for bees. The mass media love to spin public fears of into doomsday stories, and they know that this story has all the necessary elements to sell to a largely urbanized population far removed from the realities of agriculture, nature, and beekeeping, with an innate distrust of any sort of chemicals or the companies that make them, and a renewed environmental consciousness (a good thing).
Then add the circumstantial widespread adoption of a new class of insecticides (Fig. 1), with scary-sounding words like “CCD,” “neonicotinoid,” “neurotoxin,” “lethal dose,” and “systemic,” and activists who have adopted our beloved honey bee their cause célèbre. Anyone can go to the internet to enjoy the current theatre of the absurd in which bloggers, advocates, and petitioners spin legitimate science, imaginative speculation, and outright misinformation into a frenzied free for all. Yet the question still remains, to what extent are pesticides truly to blame?
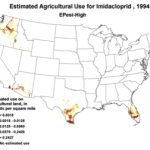 1994 |
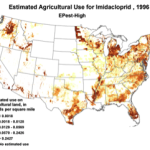 1996 |
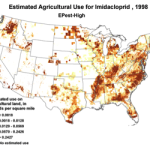 1998 |
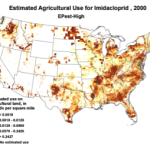 2000 |
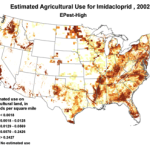 2002 |
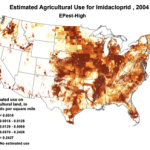 2004 |
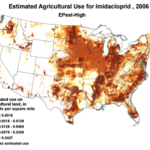 2006 |
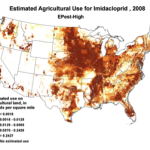 2008 |
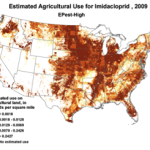 2009 |
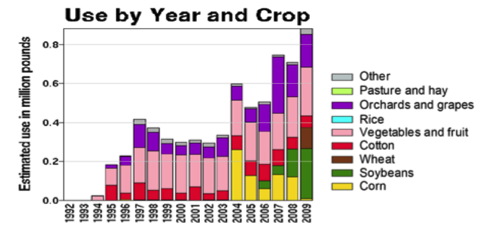 Use of the neonicotinoid insecticide imidacloprid over time. USGS data |
Figure 1. Use of the older classes of insecticides is being supplanted by newer (and ostensibly more eco-friendly) compounds such as the neonicotinoids. The widespread adoption of one of them has garnered the most attention—there were 284 published scientific studies with the word “imidacloprid” in the title in 2012 through the first half of 2013 alone! Yet we are still trying to determine whether any adverse sublethal effects of these insecticides are related to the generally increased rate of winter colony losses since the winter of 2004/2005. Source of maps [4].
Yet for all this interest in pesticides and bees, no one has yet produced a smoking gun that conclusively shows that pesticides are actually the major problem. I’ve previously listed studies from every continent that failed to make any clear link between pesticides and increased colony mortality [5]. But that doesn’t necessarily mean that they aren’t contributing to colony morbidity—a “weakening” or state of unhealthiness. Beekeepers and toxicologists have long known that pesticide residues can cause slower colony build up, poor brood patterns, decreased honey production, increased queen supersedure or failure, small populations in fall, or poor wintering.
But it’s silly to blame all beekeeping problems on one thing. I’ve no doubt that pesticides are a serious issue for some beekeepers (especially the large operators with thousands of hives in the middle of intense agriculture), but I also have seen that they are also a non issue for the majority of beekeepers elsewhere. What I’m trying to figure out is exactly where pesticides fit into the larger picture of the elevated rate of colony mortality of late. Since most exposure of honey bees to pesticides is in either agricultural or landscape settings, let me start my investigation into this subject from the ecological perspective of agricultural systems.
Ecosystems and Agriculture
Every form of life on Earth is part of a local ecosystem, in which the plants, animals, and microbes exist in some sort of “dynamic equilibrium”—commonly referred to as the “balance of Nature” (which in fact can tip wildly one way or the other). The more mature an ecosystem, the greater the diversity of species, the more complex the “food web,” and the more stable and resilient that balance.
One of the main factors that keeps any single plant species from dominating the landscape are the herbivorous insects that can quickly adjust their populations to exploit such a food resource. In response, through the process of “coadaptation,” the plants fight back against those insects with constantly-evolving chemical warfare. As beekeepers, we should keep in mind how insects (including honey bees) perceive the environment very differently than the way in which we humans do—insects are far more attuned to the chemical attractants, deterrents, and toxic metabolites of plants.
When we humans practice agriculture, we greatly disrupt or destroy the preexisting natural ecosystem and replace it with an artificial system designed to favor certain domesticated plants—“high-yielding” cultivars which generally possess less innate resistance to herbivorous insects.
In traditional agricultural systems, farmers grew locally-adapted (“heirloom”) cultivars of crops that often displayed some degree of resistance to the local insects. In addition, farms used to be small family affairs, and tended to be biologically diverse, containing a wide variety of domesticated and wild plants, grazing livestock, insect-eating fowl, cropland rotated with pasture, and a scattering of woodlots and hedgerows. This diversity helped to maintain a balance of food sources and natural predators which helped to keep any single insect species from taking too large a bite out of the farm’s total production (not to say that there weren’t problems with pests—especially with generic feeders such as locusts). But diversified farms are resilient in that they tend to limit pest populations. This traditional model of farming would be considered to be “sustainable,” since prior to synthetic fertilizers and pesticides, a farm’s viability from one generation to the next depended upon maintaining soil fertility and functional ecosystems.
On the other hand, in today’s “get big or get out” conventional agricultural model (in the U.S., 85% of farm sales are produced by less than 10 percent of farms, which control 44 percent of farm acreage [6), farmers now plant hundreds or thousands of acres to a single cultivar of domesticated plant. Such a system is ecologically unstable, since it essentially offers an unlimited buffet to whichever species of insects are adapted (or adapt) to consume that particular variety of plant. This sort of agricultural hubris then creates a demand for poisons to kill those plant-eating insects.
Our Creation of Pests
Keep in mind that every plant and insect that we now consider to be a “pest” was originally a part of some natural ecosystem. It is only if such an insect has the audacity to feed on our favored plant that we deem it a “pest.” But remember that such a value judgment is a human construct—that herbivorous insect is innocent in intent and certainly feels no malice towards us! Take the case of the Western Corn Rootworm (WCR), a close relative of the familiar 12-Spotted Cucumber Beetle (also called the Southern Corn Rootworm). The beetle, in its original ecosystem (which did not include corn until Native American farmers brought it north from Mexico) lived unnoticed on native gourds and prairie grasses [7]. But being an opportunistic species, it adapted to feeding on cultivated corn when that crop began being grown in vast monocultures.
In 1909 the WCR was first noticed to be damaging corn in Colorado, but wasn’t a problem in the Midwestern Corn Belt until the 1940’s [8]. Since then it has become one of the most economically-important pests of corn, accounting for about a billion dollars worth of lost revenue a year. It’s important to remember that for a hundred years, farmers minimized WCR damage by managing their farm ecosystem to disfavor the pest. This was done by the simple practice of rotating corn with pasture or legumes—non-host plants for the WCR—and by providing habitat for the parasites and predators of the rootworm—tachinid flies, parasitic wasps, nematodes, lacewings, etc.
But when government price supports made the growing of corn more and more profitable, farmers began to plant “corn on corn.” In doing so, they also grew huge populations of corn rootworms—an insect blindly fulfilling its “role” of bringing an artificial ecosystem back into balance by consuming an overabundant plant species. By working against Nature, farmers create their own “pest” problems.
Every pest also has natural enemies—predators, parasitic wasps, viruses, etc.—that tend to bring excessive populations back into “balance.” Relying upon pesticides rather than cultural practices often messes up this dynamic system, resulting in an even greater resurgence of the population of the pest. As Dr. Patricia Muir explains in her excellent syllabus on pesticides [9]:
One clear example of this effect involves corn in the US. In the 1940’s, little or no insecticide was applied to corn, and losses to insects were 3.5% of production. Since then, insecticide use on corn has increased more than 1000-fold, whereas losses due to insects (especially to the corn rootworm complex) have increased to 12%. This is largely attributed to reductions in crop rotation. When corn is rotated with another crop on which corn pests (such as corn rootworms) cannot survive, the pest population declines during the non-corn years. However, when corn is grown continuously (as it is today on 20 – 40% of US acreages depending on the year and on who you read!), populations of these pests are well fed every year and can increase immensely.
The Slaughter of the Innocents
Rather than reevaluating how to better farm their land in an ecologically-friendly and sustainable model, conventional farmers are pushed by market forces and wooed by pesticide salesmen to “control” these newly-created pests by the extensive application of synthetic insecticides (Fig. 2). In the process of applying these insecticides, vast numbers of “innocent” off-target species (such as honey bees and other “beneficials”) are often slaughtered (see a visual account of this at [10]).
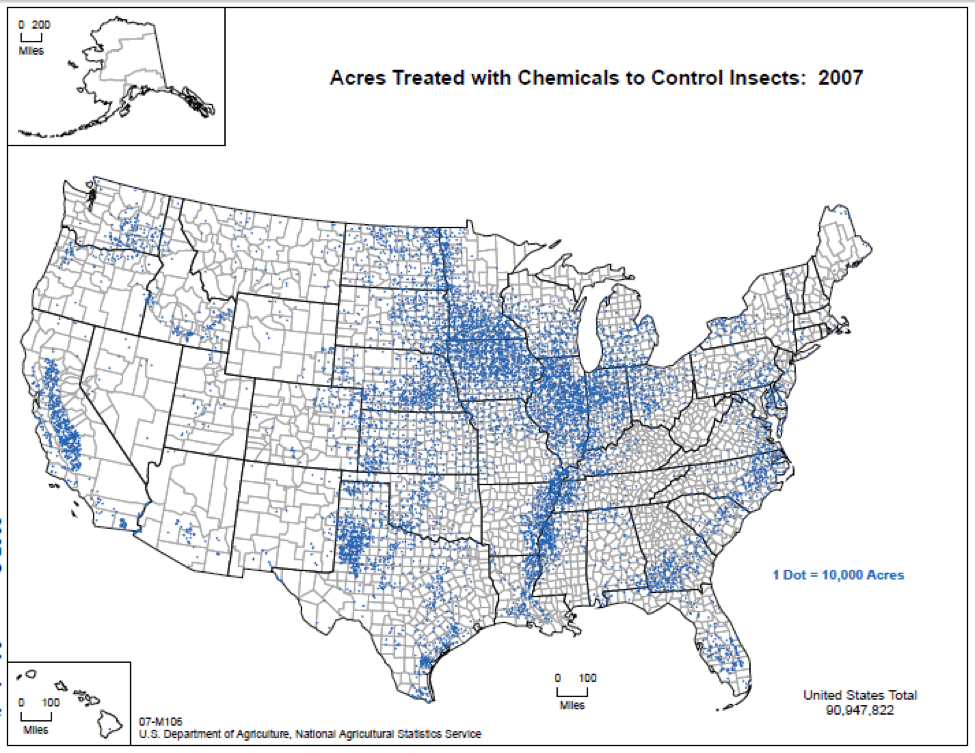
Figure 2. If you add up all the blue dots (each representing 10,000 acres treated with insecticides), it’s easy to see why in some areas it’s hard for beekeepers to find “safe” places for their hives. Source USDA.
Pesticide (Mis)use Today
In today’s factory farming model, one prepares the soil bed, plants the seeds, and controls pests with poisons. It does not have to be this way. Over 50 years ago, entomologists at the University of California laid the foundation for many of the most important concepts in Integrated Pest Management (IPM) to reduce our dependence upon pesticides. Integrated pest management can be defined as the practice of preventing or suppressing damaging populations of insect pests by the application of comprehensive and coordinated control tactics. IPM emphasizes the least possible disruption to agro-ecosystems and encourages natural pest control mechanisms.
IPM has time and again been demonstrated to be both practical and cost effective—greatly reducing the need for pesticide applications by helping to maintain a more natural balance between herbivorous insects (what we call “pests”) and their natural predators.
Despite the long and successful history of IPM [11], it is unfortunate that chemically-based pest control still predominates in U.S. agriculture (the reader may note that IPM also applies to beekeeping, yet is practiced by only by a minority of beekeepers). A recent lament by prominent bumblebee researcher Dr. David Goulson (in his excellent review of neonic issues) describes the situation well [12]:
When I was at University in the 1980s, I read Rachel Carson’s Silent Spring, and was taught about the terrible mistakes made in agriculture in the 1950s and 1960s when indiscriminate use of persistent, broad-spectrum insecticides, and the abandonment of traditional cropping practices such as rotations, led to huge pest outbreaks. The pest insects had all become resistant, while their natural enemies had largely been eradicated. As a result, an approach called integrated pest management (IPM) had been developed, and we were taught that this was the future of pest control. IPM is predicated on minimizing pesticide use: farmers monitor their crop pests, and only take action when necessary; they encourage natural enemies as far as possible, use crop rotations and other cultural controls to suppress pests, and only use the insecticides as a last resort. Even then, they avoid those that persist in the environment.
Whatever happened to this philosophy? Why are we now applying pesticides prophylactically to more or less all crops? Did we learn nothing from our past mistakes?
It is disheartening to hear that Agriculture is not embracing IPM to the extent that it could. But who is to blame? As one researcher wryly notes:
It seems that a new form of IPM has become commonplace across many commercial corn and soybean fields–insurance pest management. The increased use of crop production inputs without scouting or use of economic thresholds is being exacerbated by high commodity prices, and it will probably continue under the current crop production parameters. However, there are likely to be unintended and unforeseen long-term negative consequences, such as insecticide resistance and reductions in natural enemy populations–the same potential undesirable outcomes mentioned more than 50 years ago by the University of California entomologists–when fundamental ecological principles of pest management are largely ignored [13].
And those long-term consequences are exactly what will make overdependence upon pesticides unsustainable. At some point, all of agriculture will be forced into sustainable IPM. Farmers are willing to go along—they certainly don’t like spraying dangerous pesticides on their land and around their families. But in the current economic climate, the consumer clearly votes with their pocketbook by rewarding farmers for producing cheap, cosmetically-perfect food. That apple in the supermarket may have come from orchard sprayed as many as 20 times in a season! Pesticide salesmen are very effective at marketing their products, giving lip service to IPM, but winkingly suggesting that it might be a good idea to minimize one’s risks with just one more chemical application.
On the other hand, university researchers and publicly-funded extension agents are trying to educate growers on more eco-friendly methods, while at the same time the EPA applies pressure for farmers to phase out the more ecologically-damaging pesticides. And like it or not, the adoption of genetically-engineered Bt cultivars has led to decreased pesticide use in some commodity crops. Overall, pesticide use appears to have been declining a bit since the late 1990’s, and beekeepers nationwide tell me that things are better than they used to be (Fig. 3).
Figure 3. The EPA’s data show that insecticides (which generally exhibit the most direct impact to bees) constitute about a tenth of total pesticide application. However, recent research indicates that some herbicides, fungicides, and surfactants may also harm bees. But judging insecticide use by pounds applied does not account for the higher toxicity of some of the newer insecticides, which although used at lower rates, may have equal or greater environmental impact. Source of chart [[i]].
[i] http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf
Some of the newer classes of insecticides are more targeted, having less impact on bees and other beneficial insects. Keep in mind, though, that all pesticides are mere Band-Aids, with a limited effective life until the insects evolve resistance. Nature always bats last, and resorting solely to the development and application of new classes of synthetic insecticides is a fool’s errand in the long run. But for now, pesticides are the name of the game, and we beekeepers should make an effort to truly understand how they fit into the big picture of bee health.
The Nitty-Gritty
There is nothing new about pesticide problems with honey bees. Reading the Erickson’s 1983 articles on pesticides in this magazine is like déjà vu all over again! In their section with the above title, they explain:
Whether we like it or not, pesticides are an important part of world agriculture and they are here to stay for the foreseeable future. Our task is to learn to live with them and keep bees in their presence. Our success will be largely measured in economic terms… [we] must deal within the real world of dollars and cents, business survival and compromise. As Dr. Eva Crane…has pointed out “the best that beekeepers can hope for, in the light of the great need to kill pest insects, is an “acceptable level of mortality among their bees.”
Beekeepers realize that in order to get locations, that they gotta get along with the landowners, who are often farmers (or friends of the farmers). If the beekeeper raises a stink, he may lose his welcome. So in general, commercial beekeepers accept the occasional bee kill as a normal cost of doing business.
The Killing Fields
We must acknowledge that the EPA and the agricultural community have made great strides in cleaning up the environmental insult due to pesticides by phasing out the worst offenders, yet we still seem to be stuck in the same place—beekeepers still live in fear of having an apiary wiped out by some pesticide application (or misapplication) (Fig. 4).

Figure 4. A bee kill in an almond orchard this spring. Surprisingly, no insecticides were involved! These bees were killed by a tank mix of herbicides, spray oil, and liquid fertilizer. A number of colonies were killed outright and others were weakened. Fortunately the beekeeper was present when the spraying took place and was able to stop the spray rig before more colonies were damaged.
Some pesticides are more acutely toxic to bees than others (for a ranked list, see [15]). And there are more pesticide problems associated with some crops than with others—a recent survey of beekeepers indicated that cotton, corn, alfalfa, vine crops, and berries were most problematic [16]. A pesticide kill can be less predictable than an Oklahoma tornado—timing, temperature, humidity, stage of bloom, the repellency of the product to bees, where the bees are foraging that day, or a shift of the wind can make all the difference in the world!
One thing to keep in mind is that a pesticide that is acutely toxic to individual bees may actually be safer to the colony as a whole. A product that kills foragers before they return to the hive may decimate the field force, but have relatively little long-term effect on the hive so long as it isn’t repeated.
Practical application: a number of beekeepers have found that it is necessary to give their bees a recovery period on pesticide-free forage between pollinations. Good forage or the feeding of a protein supplement may also help the colony to rebuild.
I am not discounting the impact of serious acute bee kills—I know plenty of beekeepers who have gotten hit hard time and again, losing entire yards of strong colonies when the crop that they are working is sprayed in full bloom.
However, in some cases even a dramatic kill with piles of dead bees in front of the hive may not be the end of the world. For example, the seed planting dust from corn (containing fungicides and a neonic) readily adheres to foragers, but is relatively non toxic to them unless the humidity is quite high. Unfortunately, this property then allows them to bring the toxic dust back into the interior of the hive, where it may even kill the queen. More insidiously, the poison gets incorporated into their pollen loads and packed into the beebread, where it subsequently kills pollen-hungry young workers and drones.
Luckily, since neonics are water soluble, after that thin layer of pollen is consumed, the colony generally recovers (provided that the queen wasn’t killed). If there is then abundant “clean” pollen available, the colony may quickly rebuild to full strength.
Acute pesticide kills are ugly, but obvious, since there are generally piles of dead bees at the entrance. But how about the impact upon colony health from those pesticides that don’t kill the foragers outright, but that are then inadvertently carried back into the hive?
Chronic Exposure
Once in the hive, there are several routes of exposure by which pesticide residues can be transferred from the returning foragers to other members of the colony:
- External transfer of dust or oily residues from bee to bee in the crowded cluster.
- Transfer via nectar shared between bees.
- Storage of tainted honey.
- Transfer of residues in pollen to nurse bees and drones.
- Contamination of the beeswax combs.
The first two modes of exposure would likely be short term. We should be more concerned with chronic exposure via the honey and pollen.
Via the honey
Only pesticides with some degree of water solubility can dissolve into honey. The most common residues found in honey in the U.S. are those from the beekeeper-applied miticides (coumaphos, fluvalinate, and amitraz), although I suspect that any effects from such residues would normally be insignificant due to their very low concentration. It may surprise some that as of 2007 (the last year of USDA testing), the neonicotinoids, which are far more water soluble, were not detectable in any tested honey sample at the 20 ppb lower limit [17].
Via the pollen and combs
A more significant problem is due to the unusual nest of honey bees—it is built of beeswax and then reused year after year. The combs of a hive have the property of readily absorbing environmental contaminants, and then shielding them from any water of sunlight that might wash them away or degrade them. So unlike other insects, honey bees return each night to a nest that may become more and more toxic over time!
This is especially a problem since most insecticides, some herbicides, and common adjuvants [18] are fat-soluble (“lipophilic”)–a property that allows them to better penetrate the waxy cuticle of insects and leaves. When chemically-stable lipophilic toxins are brought back with pollen, they readily diffuse into the combs, from which they can then transfer back into the fatty acids of subsequent pollen loads, into worker jelly, or directly through the skin of larvae, pupae, or adult bees–for years afterwards. The beekeeper-applied miticides fluvalinate and coumaphos are notable for falling into this category.
Hitting the Colony Where it Really Hurts
The “legacy effects” of these residues in the combs are a vexing problem, since they hit the colony precisely where they have the potential to do the most harm—in the nursery. Various researchers have developed models of colony population dynamics [19], which invariably find that anything that negatively effects either larval survival or the age of initiation of foraging will suppress the normal rate of colony population growth. This concept should be self evident, since the intrinsic rate of growth (r) of a colony is the result of the simple equation r = birth rate – death rate. If either larval survival or worker longevity is decreased to any extent, the colony simply cannot grow, or may dwindle or collapse.
The problem is that the beekeeper would have a hard time pinning the cause of the poor performance or slow death of his colonies after a particular pesticide exposure (or combination thereof), due to the stew of residues found in combs these days, the main ingredients usually being the beekeeper-applied miticides. Please be clear that I’m not saying that those miticide residues are the sole problem, but they are likely contributory factors. Some circumstantial credence is lent to this hypothesis by the common observation that “treatment free” beekeepers in areas of intense agricultural exposure often do not appear to suffer from the sorts of fall and winter losses as do beekeepers who routinely apply synthetic miticides. I am not saying that foregoing mite treatments will reduce winter losses; nor am I saying that miticide residues are the cause of losses.
A few years ago I was reviewing the data from a trial in which a group of Dave Hackenberg’s hives had been tracked for the course of nearly a year. At one point, Dave had split the hives between several apple orchards, and then brought them back together for other pollinations. What struck me was the legacy effect of one of the apple orchards, which appeared to be the eventual kiss of death for any colony that had been placed there during bloom—not immediately, but later in the season. This legacy effect haunts beekeepers who plan on taking strong colonies to almonds in February—those hives that had foraged in some agricultural areas the previous summer simply dwindle or die later in the season.
This dismaying observation has led a number of beekeepers to take a pass on summer pollination contracts, since the rental income does not cover their later colony losses. The situation is reaching the point that growers who continue to create killing fields for bees may be forced to pay a “rental rate” equal to the replacement cost for what would essentially be a “disposable pollination unit” (similar to the cardboard bumblebee nests supplied to greenhouse growers). These would be one-time-use colonies sacrificed to pollinate the crop, with full knowledge that they would be expected to succumb afterward. To me, this would be a sad commentary on our agricultural system—rather than fixing the problem, that growers would be throwing money at it instead, showing no concern for the survival of pollinators. I truly hope that it never comes to this!
Progress in Reducing Pesticide Use
We must acknowledge that great strides have been made in recent years towards cleaning up our act with regard to the environmental insult of pesticides. Unfortunately, it’s often difficult to get accurate and relevant data on actual pesticide use. The EPA and the USGS do some tracking of sales and application, but run several years behind in reporting. However, in recent years, the trend has been to phase out the most human-toxic and environmentally destructive insecticides, as evidenced by these figures from California (Fig. X).
Figure X. Reduction in use of organophosphate and carbamate insecticides in California over time—this is a good thing for bees, wildlife, and humans! Unfortunately, the top three insecticides (chlorpyrifos, aldicarb, and acephate) applied to farmland nationwide in 2007 (the most recent data) were still all in this group. Sources [[i]].
[i] http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf http://www.cdpr.ca.gov/docs/pur/pur11rep/chmrpt11.pdf
A Brighter Future
On the negative side, commodity prices are high, encouraging farmers to indiscriminately apply pesticides without regard to actual need. My heart goes out to those beekeepers (and their bees) who experience devastating pesticide kills or delayed late-season losses due to pesticides, and feel that it is completely unfair that they are not compensated for those losses. Even more so, it is a sad commentary on our agricultural system that pollinators cannot survive in some areas.
On the brighter side, public sentiment for the environment and pollinators is growing, and our congressional representatives are well aware of this. The agricultural industry and the chemical companies are under pressure from the public and the EPA, and are diligently working to develop “reduced risk” insecticides and biopesticides (microorganisms, pheromones, or compounds essentially identical to naturally occurring compounds). New technologies and IPM advocacy by extension agents offer great promise for reducing the need for pesticide applications. Beekeepers, through the National Pollinator Defense Fund, are pushing for better pesticide labeling and enforcement of existing law (please donate). And of course Mother Nature will eventually force farmers to embrace integrated pest management and sustainable agroecological practices [21].
For the large commercial beekeepers in areas of intense agriculture, pesticides are clearly often still a problem. But when I speak to beekeepers all over the country, for most, pesticides are no longer a major issue. The system is not perfect by any means, but we appear to be heading in the right direction.
I need to wrap up this article (at this moment I’m pounding away at the keyboard 35,000 feet above the surreal checkerboard of Midwestern cornfields, in which it’s hard to imagine how any honey bee colony could find enough to eat). I’ll continue next month with an assessment of our current understanding of the contribution of various pesticides to colony health problems.
References
[1] Wolpert, S (2013) How the brain creates the ‘buzz’ that helps ideas spread. http://newsroom.ucla.edu/portal/ucla/how-the-brain-creates-buzz-247204.aspx
[2] https://scientificbeekeeping.com/sick-bees-part-18f-colony-collapse-revisited-pesticides/
[3] https://scientificbeekeeping.com/historical-pesticide-overview/
[4] http://water.usgs.gov/nawqa/pnsp/usage/maps/compound_listing.php
[5] https://scientificbeekeeping.com/sick-bees-part-18f-colony-collapse-revisited-pesticides/
[6] 2007 figures http://www.census.gov/compendia/statab/2012/tables/12s0835.pdf
[7] Oyediran, IO, et al (2004) Prairie grasses as hosts of the Western Corn Rootworm (Coleoptera: Chrysomelidae). Environ. Entomol. 33(3): 740-747. (Broken Link!) http://web.missouri.edu/~hibbardb/CV_files/prairie%20grasses%20as%20hosts%20of%20the%20western%20corn%20rootworm.pdf
[8] Meinke, L, et al (2009) “Western corn rootworm ( Diabrotica virgifera virgifera LeConte) population dynamics” . Faculty Publications: Department of Entomology. Paper 244. http://digitalcommons.unl.edu/entomologyfacpub/244
[9] http://people.oregonstate.edu/~muirp/whynot.htm A great online pesticide syllabus by Dr. Patricia Muir.
[10] http://gardenbees.com/cotton%20spray/cottonspray.htm
[11] http://ipmworld.umn.edu/ipmchap.htm
[12] Goulson, D (2013) Neonicotinoids and bees. Significance. 10(3): 6–11. One of the best objective reviews on neonic issues.
[13] Gray, M (2012) Insurance pest management the norm in many corn and soybean fields of the Midwest. http://bulletin.ipm.illinois.edu/article.php?id=1597
[14] http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf
[15] Protecting Honey Bees from Pesticides http://extension.entm.purdue.edu/publications/E-53.pdf
[16] http://pesticideresearch.com/site/?page_id=24
[17] USDA (2008) Pesticide Data Program Annual Summary, Calendar Year 2007. http://www.ams.usda.gov/AMSv1.0/getfile?dDocName=STELPRDC5074338
[18] Mullin, CA, et al (2012) Pesticide formulation adjuvants may impact honey bee health. http://www.americanbeejournal.com/site/files/828/79344/436207/598079/2012_Proceedings_ABJ.pdf
[19] E.g., Zhu, WT, et al (2012) A stage-structured model of honey bee colony population dynamics assessing impacts of pesticides and other stressors. Ibid.
[20] http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf http://www.cdpr.ca.gov/docs/pur/pur11rep/chmrpt11.pdf
[21] De Schutter, O (2010) Report submitted by the Special Rapporteur on the right to food, http://www2.ohchr.org/english/issues/food/docs/A-HRC-16-49.pdf
First published in: American Bee Journal, June 2013
Part 1: Environmental and Biotic Factors
Setting the Stage
The Lead Up
The Drought
Lack of Good Forage
Varroa
Diseases
Other Indicators of Impending Collapse
An Unexpected Chill
Feedback from Brokers
The Silent Majority
Beekeeper Management
Part 2: The Contribution From Pesticides
The Lynch Mob
Debunking The Myths
The Precautionary Principal
See For Yourself
Be Careful What You Ask For!
The Effect Of Drought
Actions To Take
Bottom Line
References
What Happened To The Bees This Spring?
Part 1: Environmental and Biotic Factors
Randy Oliver
ScientificBeekeeping.com
First published in ABJ June 2013
By now, most everyone has heard that honey bee colonies died in massive numbers this winter. Reporter Dan Rather, in his newscast Buzzkill [1], showed unfortunate beekeepers, some of whom had lost half or more of their colonies, predicting gloom and doom for the bee industry. What were the causes of this year’s bee shortage? As Rather says, “Everyone has an opinion.” The question is whether those opinions are based upon fact! So let’s go over the events leading up to the bee supply debacle.
Setting the Stage
Nearly 800,000 acres of almond trees in California came into bloom this winter—the trees typically start flowering about Valentine’s Day, and the bloom lasts for only about two weeks. Almonds require cross fertilization between adjacent rows of varieties (Fig. 1), and honey bees are trucked in from all over the country to do the job (roughly a million and a half colonies). Many large commercial beekeepers move their hives into California in November to overwinter in holding yards; others build them up on winter pollen flows in Florida or Texas, or hold them in temperature-controlled potato cellars until shortly before bloom. The hives are generally placed into the orchards about a week before the first flowers appear. There is virtually no forage in the orchards prior to, or after bloom in many areas.

Figure 1. An almond orchard in late February, showing the flowering of rows of different cultivars required for cross pollination. The bare “late” varieties have not yet bloomed; the green “early” pollenizers have finished bloom. Grading of colonies is normally done during the bloom of the main crop (usually Nonpareil).
The Lead Up
Two seasons ago there was also a shortage of bees in almonds, following the coldest January (2011) in 17 years (cold being a major stressor of wintering bee colonies). Beekeepers then replaced their deadouts with package bees and splits, thus starting a new generation of colonies, which tend to have lower varroa mite levels than established colonies. These colonies entered autumn 2011 in pretty good shape, and then enjoyed the fourth warmest January (2012) on record! As a result, there was the lowest rate of winter mortality in years, and plenty of bees for almonds in 2012 (Fig. 2).
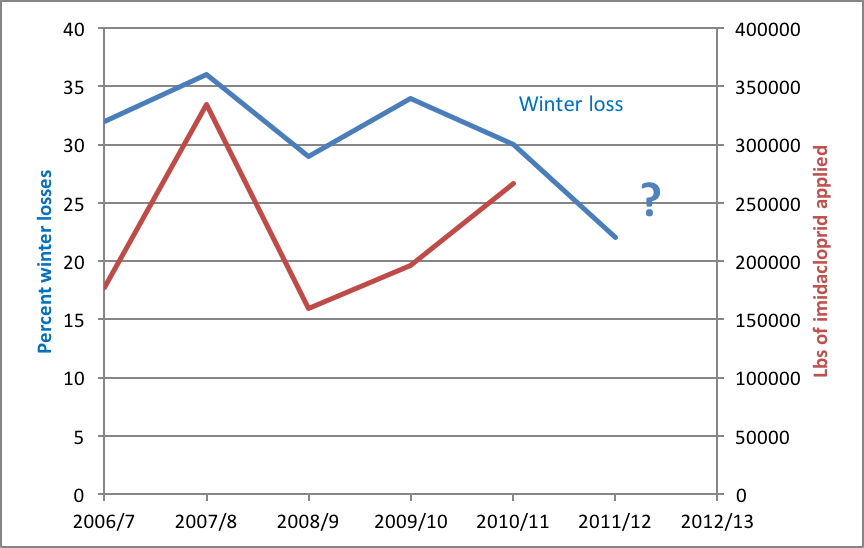
Figure 2. Percent winter losses since the beginning of the national survey—the data is not yet in for 2012/13. Note that there has been a general downward trend, suggesting that whatever caused the high losses in 2007/8 has not been such a problem in recent years. Note also the cyclical nature of colony winter losses, with high losses in 2004/5, 2007/8, 2009/10, and 2012/13 (some data not shown) Data from [[i]].
[i] http://www.ars.usda.gov/is/pr/2012/120531.htm and California DPR.
I was curious as to whether the colony loss rate was linked to the use of neonicotinoid insecticides. There is no recent USDA data, so I went through the California Pesticide Use Reports (data available through 2010). I plotted the amount of imidacloprid applied to crops in California in the preceding year in red (the seed treatment clothianidin didn’t even make the top 100 list of pesticides applied). Although there appears to be a possible correlation from 2006 through 2009, the trends were reversed for 2010. I will be curious to add the 2011 data when it becomes available.
In March of 2012 I received a phone call from a California queen producer who had a prescient insight as to a potential brewing disaster. He was receiving calls for queen bees from Northern beekeepers whose bees had already grown to swarming condition due to the unseasonably warm spring weather (Fig. 3).
Figure 3. Last year’s warm spring in much of the country lead to early broodrearing, and as a result, early buildup of varroa levels. Note the record warm spring in the Midwest.
The queen producer noted that such early brood rearing also meant early mite buildup, and predicted that since most Midwestern beekeepers treat for mites by the calendar, that they would unknowingly allow mites to build to excessive levels before treatment. This was strike one against the bees.
The Drought
Then it didn’t rain–by midsummer, it was clear that the continental U.S. was in serious drought, including California, whose beekeepers supply nearly half the bees for almond pollination. The only ways that we kept our colonies strong was to either feed expensive pollen supplement and sugar syrup, or to move them to elusive better pasture out of state. By late summer, 60% of the U.S. was in drought, meaning that unless your bees were next to soybeans or irrigated crops, there was little forage for them. This lack of good nutrition was strike two against the bees (Fig. 4).
Figure 4. The severe drought in the Midwest really put the hurt to bee pasture in those states in which the majority of commercial hives spend the summer. Source [[i]].
[i] http://www.ncdc.noaa.gov/temp-and-precip/maps.php
Drought not only dries up nectar and pollen sources, but also forces bees to fly further and more frequently for water. Plus it concentrates ag chemicals and pesticides in the few sources of surface water available to bees. The bees started to show the hurt.
Beekeepers tried to move their hives to areas of better forage, sometimes overstocking an area with too many hives, which led to excessive competition for resources, and the spreading of parasites. Others desperately chased less desirable crops such as sunflowers. Colonies in holding yards in California found little to eat, due to our record dry weather. Some beekeepers with winter eucalyptus locations found them crowded with other hives.
Lack of Good Forage
In Buzzkill, Bret Adee brought up the fact that bee pasture in the Midwest is disappearing under the plow, largely due to our environmentally-irresponsible taxpayer-subsidized policies that encourage farmers to plant every square foot of land into corn (Fig. 5). Bee brokers told me that colonies coming to almonds from the Midwest were in generally poorer shape this year than those coming from the southern states.
Practical application: some Midwestern beekeepers split their operations, hauling some to the South to rebuild over winter, and the rest directly to California–there was a night and day difference as to how the colonies looked in February!
Figure 5. Grasslands and wetlands in the Corn Belt are rapidly being converted to monocultural, heavily herbicided corn/soy, which eliminates virtually all bee and wildlife forage. A new study found that between 2006 and 2011 there was a net loss of 1.3 million acres of grassland. This affects not only bees—the authors [[i]] state that “As a consequence, populations of grassland nesting birds are declining faster than any other group of birds in North America.”
[i] Wright, CK & MC Wimberly (2013) Recent land use change in the Western Corn Belt threatens grasslands and wetlands. https://www.motherjones.com/files/pnas201215404_nwow5w1.pdf
To put this loss of bee pasture into perspective, I asked some Dakota beekeepers for estimates of how many acres of CRP grassland are needed to sustain a colony of bees. In recent years, the overall hive density in North Dakota has been more than 10 hives per square mile (less than 64 acres per hive, including wastelands).
Practical application: the best guess by those beekeepers was that each colony of bees requires about 5-15 acres of productive land for forage (late summer forage being the critical factor). If we use the figure of 10 acres per colony, then the conversion of 1.3 million acres of grassland to herbicided cropland suggests that forage for 130,000 colonies of bees has been eliminated in the past five years in the Corn Belt alone! This figure represents nearly 9% of all colonies needed for almond pollination.
Varroa
An excellent window into the causes of colony health problems is the USDA National Honey Bee Pests and Diseases Survey Report [5] (the latest data have not yet been released). It is worrisome that varroa levels appear to be steadily climbing year after year.
And if the drought and forage problems weren’t enough, the favored miticide of commercial beekeepers became unavailable for a time last summer, and mite levels built to killing levels in a number of operations. By late July, some of us were already predicting a disaster for the upcoming almond pollination season. Although many beekeepers finally got mite levels down with late-season treatments, the damage had already been done, and there was no turning the colonies around. Strike three for the bees!
In November semi loads of hives started moving into California, or had been placed in potato cellars. Some of the colonies that arrived from the Midwest were in poor shape, or crawling with mites. Oddly, few beekeepers at the time owned up to having problems, despite the reports that I kept hearing of mite and forage issues! I’m not sure whether this was due to denial, wishful thinking, simple lack of lifting the lids, or something else.
Diseases
Nosema infection also runs rampant across the country—70% of colonies were infected in June of last year. The stressful factors leading up to almond bloom apparently put a lot of hives close to the “tip point” at which pathogens can overwhelm the colony immune system and start it going backwards, or initiate the slide into sudden depopulation (detailed at [6]). Few seem to be mentioning signs of CCD–it is unfortunate that the media keep using that term as a catch-all for all hive problems!
One should keep in mind that the winter collapse issue appears to be cyclical, similar to flu or other pathogen epidemics. I have strong reason to suspect that the constantly-evolving viruses are involved in these colony collapse epidemics.
There has also been a strong resurgence of European Foulbrood and other unidentified brood diseases [7] (Figs. 6, 7, and 8). Unlike EFB of old, the new forms don’t go away with a nectar flow.

Figure 6. “Shot brood” due to EFB. Note the fat queen near the center. Despite her vigorous egglaying, this colony is unable to pull ahead due to excessive brood mortality. Lots of beekeepers reported EFB symptoms this winter.

Figure 7. You really have to look hard in some colonies with spotty brood to see the cause! Two larvae in this photo show signs of EFB infection.

Figure 8. Dying brood from one of my sick colonies this spring with EFB-like symptoms. Note the “shot” pattern, the twisted larvae, and the dried larval remains. There is also some AFB-like coloration, but lack of roping or AFB odor (this odor is distinct and sour), nor a positive Holst milk test. In this colony, even pupae were dying. I observe these symptoms independent of whether the hives went to almond pollination or not. Colonies with this (or similar) infection cannot grow. Treatment with oxytetracycline generally clears it up.
One thing that I noticed in Buzzkill was the uneaten pollen supplement patties in many of the crashed hives. I’ve mentioned before [8] that I’ve found a colony’s failure to consume pollen supplement to be a reliable predictor that that colony will later collapse.
Another strong predictor of winter collapse is weak strength in fall (upcoming article), again strongly suggesting that those colonies already have some sort of health issue going into winter. I heard reports from all over the country that bees went into winter in poor condition.
An Unexpected Chill
The final blow to hives in California was a blast of icy weather (Fig. 9). This unexpected chilling compounded all the existing problems! I’ve previously pointed out that colony collapse often follows unseasonable chills, since it shifts the tip point for virus and nosema epidemics. Clusters that had expanded for broodrearing contracted, resulting in chilled brood and dead young bees on the ground. My own colonies simply shut down broodrearing completely, losing about two weeks of buildup.
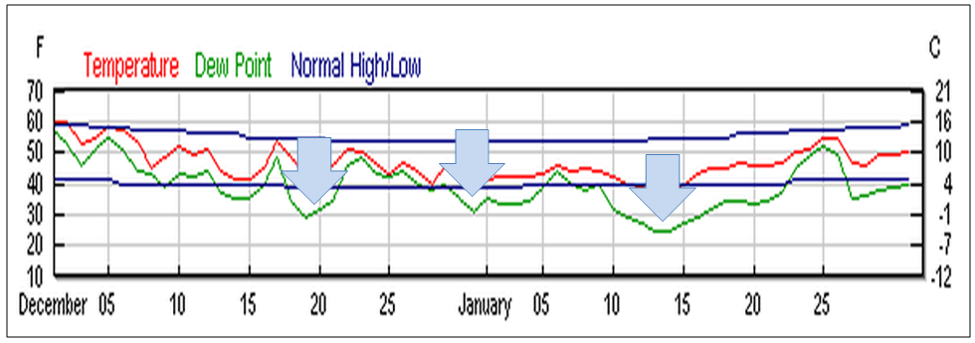
Figure 9. Chilling events (blue arrows) in Modesto, California this winter (the dark blue lines represent normal highs and lows). The unusual chilling in late December and early January (hitting the ‘20’s in a number of areas) came at the time when colonies normally begin to build up for almonds. This severe (for California) cold set the already-stressed colonies back hard, and may have allowed nosema and viruses to gain the upper hand. Graph from wunderground.com.
At the national convention in January, the first reports of beekeepers with collapsing operations were heard. But still, the industry was in denial, with an apparent glut of promised bees as late as the end of the month (two weeks before start of bloom)! But when the rubber finally hit the road in mid February, that illusory supply quickly evaporated, with desperate growers and brokers scrambling to obtain bees—some offering obscenely high prices for substandard colonies.
And then, due to the cool spring, the trees held off on blooming for an extra 10-14 days [9]–colonies placed in anticipation of normal start of bloom just sat there starving and shivering on the cold orchard floors.
Practical application: the biology here is that this is the time of the “spring turnover” in bee populations in California, during which the old overwintered adult bees must rear their replacements for the spring buildup of population. The conditions in the almond orchards prior to bloom are miserable for smaller colonies—it is warm enough to encourage them to break winter cluster and expand the broodnest, but overnight frosts on the Valley floor can cause serious chill stress. Furthermore, it is often warm enough to fly at midday, but there is virtually nothing to forage upon until the trees start blooming! Such fruitless foraging further wears out the workers, and allows sick bees to drift to adjacent hives. Worse yet, the desperate foragers rob out any dead or dying colonies in adjacent orchards, rapidly and effectively transmitting mites, nosema, viruses, and anything else harmful in the deadouts.
Many colonies went backwards during this excruciatingly long wait. Some beekeepers told me that hives graded at placement scored better than those graded at bloom (just the opposite of normal)!
I’ve been carefully observing spring turnover in my “dinks” (weak colonies) in February (Fig. 10). What I find is that the problem is generally not the queen; rather, the colonies are infected with some pathogen– most commonly nosema [10], the paralytic viruses [11], or EFB (or EFB-like brood disease). Those colonies that are able to successfully emerge one solid round of brood are often able to “clear” the infection and completely rebound by April. Those that get hit by frost in February often collapse.

Figure 10. An example of an unsuccessful spring turnover. This colony is in the middle of typical February collapse from nosema or IAPV. You can easily see the outline of the area recently covered with brood, delineated by the crescents of freshly-packed pollen. Colonies undergoing this sort of depopulation tend not to forage for nectar, and do not respond well to supplemental feeding. This colony continued to collapse quickly, and finally died in a cold snap a week later—with only silver-dollar sized patch of dead bees remaining.
Feedback From Brokers
I asked a few of the major pollination brokers for their observations on the colony shortage this season. Their feedback suggested that the causes for the bee shortage were varied and many.
Summary:
- Most were able to eventually fill their contracts. Beekeepers often hold colonies in reserve “just in case,” or gambling that in “short” years they can rent those last hives at an elevated price. Also, when the offered price went up, hives not originally intended to go to almonds were loaded up at the last minute and shipped to California (I was in Florida at the start of bloom, and had an inspector tell me of certifying colonies for shipment after the bloom had already begun!).
- A number of hives received in November were already headed downhill. Some exhibited the symptom of bees not clustering properly (a typical sign preceding sudden colony depopulation/CCD). Some arrived crawling with mites, or with recent mite treatments in place (suggesting that they were treated too late).
- Some graders saw piles of dead bees in front of hives—cause unknown. There were reports of some herbicide tank mixes killing bees.
- Many of the placed colonies were below standard grade— growers paid for less than they expected!
- Graders told me that there was a huge variation in hive strength from beekeeper to beekeeper. Many hives were strong (12-16 frames of bees) and healthy; other operations graded at zero to three frames of bees (some of the deadouts had spider webs inside, suggesting that they hadn’t been occupied by bees for some time).
- The unusual winter chill was tough on colonies that had been stimulated into early buildup, and then forced to contract their broodnests. Some colonies kicked out chilled brood and dead bees afterwards.
- Many beekeepers watched their colonies go “backward” prior to bloom.
- Colonies from the Southern states (especially those delivered in February) were generally in better shape than those from the Midwest.
- Midwestern beekeepers blamed drought, mites, poor nutrition.
- Several beekeepers said that their best bees came from remote areas, and their worst from ag areas.
- A number of beekeepers admitted inadequate mite treatment; mites were a recurrent theme.
- There were a number of reports of EFB hitting colonies.
- Some had gotten hit last summer with pesticide sprays, and their colonies didn’t recover.
- “There were good bees and bad bees from every state. They all seemed to have different problems depending on location/state.”
- Many good beekeepers simply didn’t know what happened to their hives; there were lots of lifeless hives delivered. The atmosphere was ripe with speculation as to the actual causes.
- “The shortage was also created by beekeepers that chose not to come to California for a variety of reasons. They can make more money with honey, didn’t get paid for what they have brought in the past, bees come back home with mites, beetles and whatever else takes a ride on the hives. Beekeepers don’t want to risk bee health to chase the dollar.” Many out-of-state beekeepers have had bad experiences going to almonds, and simply don’t feel that it’s worth it. The supply of bees will largely depend upon the price that growers offer for renting them!
The Silent Majority
Buzzkill leaves one with the impression that the entire bee and almond industries are on the verge of collapse. Of course, the news media focus on fear and disaster, so we may consider taking such dire projections with a grain of salt. In the case of Dan Rather, the focus was on the beekeepers with troubles, not upon those who successfully filled their pollination contracts.
So just how severe was the problem? Let’s say that there was an overall shortage of 100,000 hives (a figure that I heard floated)—that would represent only about 6% of the total number of hives placed into almond pollination. The other 94% were successfully delivered (although a proportion of those were weak due to the poor season).
Since the debacle, I’ve heard from plenty of beekeepers whom I’ll refer to as the “silent majority,” who experienced “normal” colony winter losses in the 5-25% range, and who successfully filled their pollination contracts. Although the hearts of all beekeepers go out to those who suffered severe colony losses, many felt that some of those losses could have been prevented if the afflicted beekeepers had been more proactive than reactive.
And don’t forget those upon whom the rest of the industry depends to supply bees for restocking their deadouts! The California package producers, who have been pollinating almonds for decades, are routinely counted on to consistently take strong hives to almonds, and to then shake over a hundred thousand packages of bees for sale afterwards. Few of these major producers experience severe unexplained colony losses.
Beekeeper Management
By no means am I suggesting that those beekeepers who suffered losses engaged in poor beekeeping practices, but I can’t help but notice that not all beekeepers were equally affected—a great number provided strong, healthy colonies to almonds. I’ve spoken to some of them–the common thread is that those who recognized the problems of poor nutrition and mites in August, and took remedial action for the rest of the season, had acceptable winter losses.
Some beekeepers who really put serious effort and money into bee husbandry were even able to sell “shook bees” from their colonies to others in February! For example, watch Keith Jarrett feeding substantial quantities of pollen supplement to very strong colonies in January [12]—Keith consistently brings very strong colonies to almonds every year, and this year was no exception!
Practical application: I’m here to tell you, that one lesson that I’ve learned during our intense California drought, is that those yards that I fed with protein in late summer before they started going downhill went to almonds much stronger than those that I didn’t feed until fall! Proactive is better than reactive—if you wait until colonies are already going downhill, it is much more difficult to turn them around!
I’ve often been accused of being politically incorrect for speaking frankly. I’d like to make amends at this point by retiring the rude and unsympathetic term “PPB” (Piss Poor Beekeeping). The fact is that the average wintering loss for the past few years has hovered around 30%. So if you experience 30% losses, you can now proudly call yourself an “Average” beekeeper!
But what about those beekeepers who consistently manage to enjoy lower rates of winter loss? I propose that we call them “Lucky” beekeepers, and the best of them, “Consistently Lucky.”
Practical application: the harder those beekeepers work, the luckier they get!
But there were clearly “unlucky” beekeepers this year—especially the “big boys” who brought tens of thousands of hives from the drought-ravaged, and corn-converted Midwest to California. California beekeepers are used to summer drought. We have learned to either move our colonies to better (often irrigated) pasture, or to feed expensive pollen supplements. This would be a very expensive proposition to the larger operators, with hives spread all over the place—a cost not covered by current pollination prices.
What Happened To The Bees This Spring?
Part 2: The Contribution From Pesticides
Randy Oliver
ScientificBeekeeping.com
First published in: American Bee Journal, July 2013
It’s pretty straightforward to attribute the majority of colony losses this winter to the usual and aforementioned causes, but a number of beekeepers are also pointing the finger at pesticides. There is no doubt that in certain areas pesticides were a serious issue to beekeepers. Colonies set back by pesticide kills may not fully recover over the season, and those going into winter with pesticide residues may go downhill. There is also reason to suspect that pesticides and miticides have something to do with today’s high rates of queen failure.
The bees in some drought-stricken areas were forced to forage on irrigated and pesticide-laden crops—the only place in which there was anything to eat. This changes the entire dynamics of pesticide exposure, since residues would no longer be diluted by the pollen and nectar of non crop plants. The lack of good natural forage also suppresses the ability of colonies to deal with the insult of those pesticides. And colonies may be forced, by necessity, to forage upon one treated crop after another, resulting in multiple exposures.
Practical application: under drought conditions, bees may suffer more from pesticides than when times are good.
Due to the current high prices for agricultural commodities, farmers are often applying pesticides indiscriminately as “risk insurance” rather than due to actual need. A chilling recommendation from an extension entomologist reads:
I encourage you to be risk averse and to make an investment that will pay dividends for your valuable crop. Consider applying [flubendiamide, indoxacarb, or spinosad] for corn earworm. If you have stink bugs and are in the [mature plant] stages, you might want to tank mix one of these products with a pyrethroid. A tank mix of a pyrethroid and acephate are an option, but will wipe out all beneficials [13].
The first three insecticides mentioned are considered to be “reduced risk” to bees if residues are allowed to dry for a few hours, but no mention was made to spray at night. Of the five insecticides recommended above for spraying on corn in tassel, at least four are highly toxic to bees if sprayed during the day! No farmer wants to kill bees, but with recommendations like this from state extension agents, well-meaning growers may unwittingly be hurting pollinators.
Bees in agricultural areas are exposed to a vast array of insecticides, miticides, fungicides and surfactants—many of which have clear links to colony health problems. And applications of new mixes of chemicals are up. For example, in addition to the neonicotinoid seed treatments, granular insecticide soil treatments for corn in the Midwest were up by 30% over the previous year [14]. These treatments consist of combinations of organophosphates and pyrethroids.
But I’m not hearing either the bird groups or beekeepers even addressing these treatments! It is scary to read the sales literature for Counter insecticide, the organophosphate terbufos [15]. Growers are encouraged to apply it at planting time, despite the facts that:
- “Terbufos is highly toxic to birds, fish, and aquatic invertebrates [and bees]. [It] shows significant acute mortalities of birds, mammals, reptiles, and fish resulting from broadcast application…In the same study, the application of terbufos as a soil-incorporated treatment to corn…resulted in acute mortalities to birds and reptiles” [16].
- Terbufos is strongly systemic, meaning that it is absorbed by the plant roots and could be expected to be expressed in the pollen and nectar.
- It can synergize with other pesticides since it ties up the critical CP450 enzymes used in detoxification, to the extent that growers are cautioned that it can cause problems to corn from herbicides [17].
During drought, certain insect pests become more problematic, perhaps resulting in increased exposure to insecticides by bees. For example, drought encourages corn leaf aphids. Read this chilling recommendation for aphids on corn during tasseling (when bees are actively foraging):
If less than 50% of pollination has occurred, aphids and honeydew are covering tassels and plants are stressed, an insecticide may be necessary to ensure adequate pollination, but treatments need to be made within 48 hours of tassel emergence. Asana XL, Brigade, Capture, Cobalt, Dimethoate, Lannate, Lorsban, or Malathion may be used for control [18].
Or this:
Prolonged drought always raises the specter of two-spotted spider mite outbreaks in soybeans and corn. As the 2012 drought intensifies in Minnesota, infestations are reaching treatable levels…The only products that are recommended for spider mites in soybean include insecticides containing chlorpyrifos, dimethoate and bifenthrin[18].
The names of the recommended insecticides above strike fear into the hearts of beekeepers!
Practical application: many “consistently lucky” beekeepers go to great effort to allow their colonies to recover after exposure to pesticides—moving them to unsprayed areas or natural forage, or by immediately feeding protein supplement to stimulate increased broodrearing. Unfortunately, such “recovery” areas are getting harder and harder to find.
The Lynch Mob
Despite the fact that a wide range of bee-toxic insecticides are being applied (often during bloom) to corn, soy, sunflowers, alfalfa, cotton, and other major crops, if you Google anything about insecticide use, you’ll quickly find that the blogosphere focuses only upon the putative link between a single class of insecticides—the neonicotinoids–and the demise of pollinators [19].
People look at me incredulously when I point out that there is zero firm evidence to date that the neonic seed treatments are a serious problem! But the notion that all honey bee problems are caused by an insidious new insecticide resonates with a distrustful public [20], and has firmly established itself as “common knowledge.” But repeating something does not make it true!
“It’s easier to fool people than to convince them that they have been fooled”–Mark Twain
Practical application: the question is, “Are the neonic seed treatments being railroaded into a guilty verdict in the media’s kangaroo court of public opinion?”
One group recently brought suit against the EPA to ban the use of the seed treatments clothianidin and thiamethoxam [21], neither of which even make California’s top 100 list of pesticides applied [22], nor that have ever been demonstrated to harm colonies feeding on the pollen or nectar of seed-treated plants! A number of people have made up their minds that the neonics are the main cause of colony collapse, and it appears that no amount of facts to the contrary will cause them to reconsider!
Debunking The Myths
As anyone who knows me will tell you, I am a stickler for honesty, accuracy, and factuality. I am concerned about the amount of misinformation and speculation going around about the neonics. So let’s look at some of the claims vs. the actual facts.
| Arguments Against Neonic Seed Treatments |
Actual Facts
|
| The neonicotinoids have been “linked” to increased colony mortality. |
In actuality, such a “link” is merely an urban legend, and has never been demonstrated or confirmed in any study.
On the other hand, the residues of other classes of pesticides are more suspect for causing increased brood or adult bee mortality [24]. |
| The timing of CCD coincides with the introduction of the neonic seed treatments in 2004. |
CCD started in California bees in the winter of 2004/2005, prior to them ever being exposed to seed-treated crops. |
| But what else could have changed at that time other than the introduction of neonics? |
In California, Dr. Eric Mussen [25] determined that the increased colony losses were due to poor summer forage and failure of mite control products (just as this last winter).
There is actually a much stronger association between the incidence of the novel gut parasite Nosema ceranae and increased colony mortality [26].
But the main thing that has changed is the dynamics of the varroa/virus complex, which coincidentally occurred at about the same time that the neonics came into use. |
| European countries banned the neonics, and the bees recovered after those bans. |
A few countries placed temporary suspensions on certain seed treatments until planting dust issues were resolved [27]—only Germany has one suspension still in place. The foliar applications were not suspended. The suspensions did not resolve bee health problems. |
| The European Food Safety Authority recently decided that neonics pose a threat to bees. |
“The Center for Regulatory Effectiveness (CRE) has recently completed a Data Quality Act (DQA) Alert on the … (EFSA) report on neonicotinoids which found that neonicotinoids pose a risk to bees. The DQA Alert outlines the serious deficiencies of the EFSA report and demonstrates why the EFSA report violates the DQA…In particular, the EFSA report failed to maximize the objectivity of the data by failing to reconcile numerous studies whose conclusions contradicted the findings of the EFSA report” [28]. |
| Several lab studies have found that neonics affect individual bee behavior, longevity, or immunity. |
True — although many studies used unrealistically high doses. The question is whether such artificial studies apply to actual colonies in the field. The numerous field studies to date have failed to find any link between seed treatments and later colony health issues. |
| It is the seed treatments that make corn a problem. |
As Bret Adee points out in Buzzkill, corn is replacing pastureland (Fig. 4). Corn, as grown today, is a virtual “bee desert” (similar to the way in which suburban lawns are green bee deserts). And it’s not only the bees that this is affecting, the populations of birds and other wildlife are plummeting due to loss of favorable habitat (see my blog on birds and neonics [29]).
A recent survey by Dr. Jerry Bromenshenk found that bees actually avoid field corn pollen, and are exposed to very little of the seed treatment residues [30].
Numerous independent studies, and the experiences of stationary beekeepers throughout the Corn Belt, support the conclusion that colonies can thrive when surrounded by corn, provided that there is some alternative forage within flight range. |
| As the use of neonic seed treatments increases, bee mortality goes up. |
In actuality, colony mortality rates go up and down year to year, largely dependent upon weather and varroa mite control. If the neonics were to blame for this winter’s bee losses, why didn’t they cause similar losses last winter, in which the colony mortality rate was the lowest in years? |
| French beekeepers also started seeing problems with the introduction of the neonics. |
I’ve spoken with beekeepers in France whose apiaries are in pesticide-free areas. They tell me that they experience the same sorts of colony mortality problems as do those in areas exposed to neonics. |
| Bees in the U.S. are commonly exposed to neonicotinoids. |
In the most recent USDA survey (100 samples across the country), imidacloprid was only detected in 9% of the samples [31] (although I found some of the residue levels alarmingly high). However, the most common seed treatment, clothianidin (or its degradation products), was not detected at all!
The above real-world data suggests that efforts to ban clothianidin as a seed treatment may be misplaced. It appears that imidacloprid, especially as a foliar application, would be of more concern. |
| Neonics are the most common pesticides that bees are exposed to. |
In the above survey, other serious insecticides were more commonly prevalent: chlorpyrifos (in 20% of samples), cyhalothrin (in 7%), and endosulfan (in 11%).
Notably, there was also a high prevalence of beekeeper-applied miticides: fluvalinate (in 38%), coumaphos (in 87%), amitraz (in 27%), fenpyroximate (in 11%), and thymol (in 27%).
There was even higher exposure to fungicides and adjuvants. |
| It is misleading for the pesticide companies to blame the problems on varroa, nosema, or poor nutrition. |
The above survey (over 1000 samples) found that the average varroa infestation rate in the U.S. in autumn is above the danger level for virus epidemics!
Sixty to 100% of hives are infected with nosema in December.
Summer drought has historically been associated with high winter mortality. |
| But didn’t the planting dust from corn seeding kill colonies in Ontario? |
Planting dust is separate issue that clearly needs to be remedied. It does on occasion cause bee kills, for which beekeepers are rarely compensated. This situation must change! All parties are actively working on solutions [32]. |
| Bees in certain agricultural areas tend to go downhill later in the season. |
This has been observed for a long time—long before the neonics. The question is, which chemicals, chemical synergies, or chemical/nutrient interactions are responsible? The Frazier/Mullin team at Penn State has developed a protocol for helping to figure this out. I strongly support its adoption by the EPA for pesticide risk analysis. |
| Colonies foraging upon nectar or pollen of seed-treated crops get poisoned. |
Ask yourself this: if neonic residues were actually so harmful to bees, how is it that the Canadian beekeepers, whose bees forage largely on seed-treated canola, feeding solely upon a diet of canola nectar and pollen with well-documented residues of clothianidin, experience very low winter losses, despite the long Canadian winter (so long as they control varroa and nosema)?
And how is it that the vast majority of beekeepers in the U.S. Corn Belt report that their colonies thrive and that they have far fewer pesticide issues these days than in the past? |
| The neonicotinoids are “systemic,” meaning that they are in the plants all the time! |
True, but this property is not unique to the neonics—a number of other insecticides also go systemic. In any case, with seed treatment, the concentration of the insecticide in the plant is only high when the plant is young—it gets diluted as the plant grows (e.g., clothianidin in canola is at a level high enough to kill aphids for only about the first 30 days of growth).
The only time that residues in the plant matter to pollinators is when the mature plant flowers. The amount of seed treatment is carefully calibrated so that the residue in the pollen and nectar are below the level that causes demonstrable harm to bees.
In the case of foliar, drench, or chemigation applications prior to bloom, there are greater possibilities for bees to be exposed to toxic levels. |
| There are fewer butterflies and pollinators in the fields these days. |
Not surprising, since the new push for “clean farming” has removed the host plants upon which the butterfly larvae feed. Pollinators are forced to subsist upon the stretches of weeds growing along roads at the edges of fields. But surprisingly, pollinators may be abundant there, suggesting that even though populations as a whole are reduced by habitat conversion, it is that, rather than the use of seed treatments, that causes the population declines. |
| The evil pesticide companies want to kill honey bees. |
Give me a break! Does anyone truly believe that anyone wants to kill honey bees? What pesticide company would want the bad press of being associated with killing bees? The chemists and biologists on their staffs earnestly work to develop insecticides that are bee friendly. |
| The EPA is being derelict in their duty to protect pollinators. |
I have spoken at length with EPA staff, and reviewed their risk assessments, as well as those by, DEFRA, EFSA, PMRA, and other regulatory agencies. I find that the risk assessors have not overlooked any evidence, are well-informed on the subject of neonics, and are justified in their assessments that the on-the-ground evidence (to date) indicates that neonic seed treatments pose acceptable risk to pollinators. |
| We must all remember that the tobacco industry tried to hide the fact that nicotine was addictive [33]. |
Spare me! Does anyone seriously think that the EPA is unaware that industry executives may stretch the truth? Of course the EPA is skeptical of any reassuring claims by the pesticide industry—that’s why they go over all studies with a fine-toothed comb! |
| This winter’s losses spell the end to commercial beekeeping. |
The fact of the matter is that many observers note that the bee supply for almonds often follows a boom-bust cycle. Although losses were high this year, the trend for the last decade has been for beekeepers keep ramping up the supply of bees for almonds. So long as growers are willing to pay a profitable rental rate for colonies, market forces will encourage the bee industry to meet the demand (for a detailed analysis, see [34]). |
The Precautionary Principal
“But,” you say, “shouldn’t we exercise precaution due to the lab studies that find adverse effects from the neonics?” Look, I make my living as a beekeeper, I’m not out to sell insecticides, and am as concerned as the next person about the environment and the safety of the food I eat. I’ve researched the neonics exhaustively, and addressed them in several articles [35]. I am acutely aware that there are suggestions that the neonics may be causing insidious effects in the environment, and I’ve studied the excellent environmental document Late Lessons from Early Warnings [36], which hammers the message that we should use the “precautionary principle” when dealing with chemicals. The problem is, there is nothing without risk—for example, you have a 1 in 83 chance of being killed in an auto accident in your lifetime. But most people still take the risk of getting into cars, since they feel that the benefit outweighs the clearly high risk!
My practical perspective as both a scientist and a beekeeper: if researchers perform lab studies on any insecticide, they will find that there are all kinds of negative effects upon bees—this should be pretty obvious, since insecticides are specifically designed to harm insects! However, the majority of these studies are taken out of the context of full colonies under field conditions, where bees fly free and choose the flowers upon which they forage. The evidence to date supports the contention that the neonics, properly used as seed treatments, are indeed an improvement over other insecticide options.
As Dr. Eric Mussen succinctly notes:
Nobody’s really been able to show that [the neonicotinoids] are more problematic than the rest [of the pesticides to which bees are exposed] [37].
Far be it from me to suggest that the neonics (or any other pesticides) are harmless! But consider this—if the neonic seed treatments were indeed as harmful as some make them out to be, you’d think that after a decade of intense study that at least one researcher could have come up with a single solid piece of field evidence against them!
Let’s do a thought experiment. Why doesn’t someone simply put a bunch of healthy hives into the middle of seed treated crops and see whether they die afterward? Oh, I forgot—this experiment has already been run by thousands of beekeepers year after year in the Corn Belt and the Canadian prairie! And those beekeepers have invited me to look at their colonies, sent me photos of colonies stacked head high with honey supers, and bragged about their high winter survival!
Some will argue ’til they’re blue in the face, but the fact remains that virtually every beekeeper that I’ve spoken with in the Corn Belt and in canola areas feels that the seed treatments are not a problem [38]. In fact, most tell me that this is the best it’s ever been as far as bees and pesticides!
Common sense: I just don’t get what is so hard to understand about the reality that there are thousands of colonies thriving year after year in areas of intense seed treatment? To any reasonable person it would suggest that the treatments are causing little noticeable harm other than the occasional planting dust kill, which I have repeatedly stated is a problem that needs to be corrected!
See For Yourself
Let’s look at actual independent (from the manufacturer) data from corn and canola areas:
Corn
I asked friends in the Corn Belt if they had any data on winter losses. It so happens that the Michiana Beekeepers Association has been collecting exactly that since the spring of 2010 (Fig. 11).
Figure 11. Percentage of winter losses by the “Michiana” hobby beekeepers. The 2013 figure is as of mid March; it may eventually go down a bit due to a prolonged cold spring. Note that the winter survival rate appears to be linked to average winter temperature. Thanks to beekeeper Danny Slabaugh for sharing the data; temp deviations from [[i]].
[i] http://www.ncdc.noaa.gov/temp-and-precip/maps
How could the above be? Eighty percent winter survival despite sitting in the middle of seed-treated corn and soy? So of course I did a fact check to confirm that those beekeepers were indeed sitting in corn/soy areas (Fig. 12).
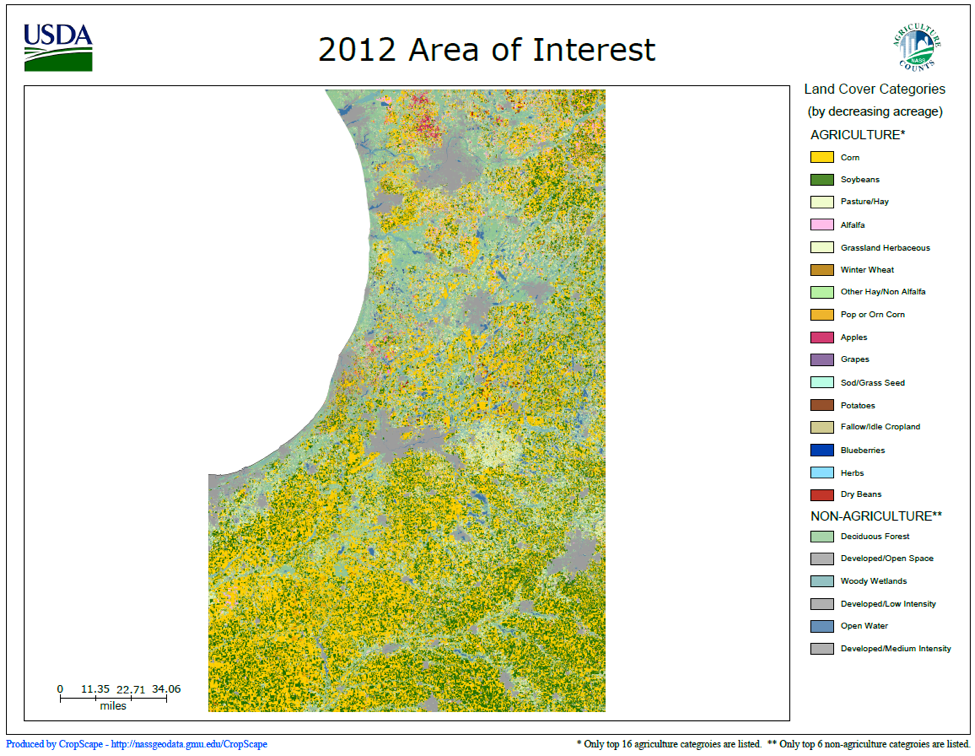
Figure 12. USDA land cover categories for the region in which the Michiana hobby beekeepers keep bees—corn and soy acreage is color coded yellow and green, respectively. The selected area is the top half of Indiana and bottom of Michigan, with Lake Michigan at the left. Clearly, these apiaries were exposed to seed-treated corn and soy! I created the map at [[i]].
[i] http://nassgeodata.gmu.edu/CropScape/
The above figures suggest that colony winter survival for stationary hobby beekeepers in the above corn/soy region is higher than the national average, despite the fact that about half of them don’t even treat for mites! They also suggest that the neonics or other pesticides used in corn/soy in that region do not cause excessive winter loss. Finally, the data indicate that a main factor for winter loss rates is the winter temperature.
Canola
I’ve heard some beekeepers saying that their bees crashed after working canola, suspecting that the seed treatments were the problem. So as a reality check I called a Dakota beekeeper who has been running bees to canola for over a decade—some 10,000 hives last season. He tells me that colony strength after canola varies from year to year, but that he sees no problem with the seed treatments. He did point out that beekeepers should be aware that colonies can plug the broodnest on intense canola flows.
The biology: The plugging out of the broodnest during an intense bloom means that three weeks afterward, there will be few emerging workers to take the place of the worn-out foragers, and the colony population will temporarily plummet. Even worse, the remaining mites are then concentrated onto fewer bees—which can initiate virus epidemics. These colonies must then attempt to rebuild from scratch, starting in August, meaning that the weakened, mite-infested colonies faced three long months of drought last summer for that rebuilding process.
Every field study that I’ve seen for canola also supports the conclusion that the seed-treatments are safe for bees. I joined other beekeepers and regulators in observing a large-scale study of seed-treated canola in Canada [41]. Canola (or rapeseed) is likely the best test crop, since bees eagerly (and virtually exclusively) forage upon it for both pollen and nectar, meaning that every bit of their food supply contains contain easily verifiable residues of the insecticides. The preliminary results indicate that the clothianidin seed treatment did not harm the colonies [42].
Another recent independent long-term field study in Poland [43] came to the same conclusion. In it, the researchers followed 50 colonies for more than two years under field conditions as they foraged on five different large fields of oilseed rape treated with various combinations of five different neonicotinoids applied by seed treatment and spraying. Pollen and nectar samples were taken, and demonstrated that the bees were clearly exposed to normal residues of the insecticides (there was also additional exposure to other common agricultural pesticides). The colonies were monitored for health, brood, strength, nosema, viruses, and winter survival, and compared to two control apiaries set in an area free of the crop. The results?
During the time from the placing of the colonies on the rape fields until wintering, the colonies developed properly in all groups… All colonies overwintered properly… In both years, during the period of being placed in the oilseed rape fields as well as after being moved to the stationary apiary, none of the groups showed disturbances in development or functioning.
Following a paper that suggested that the seed treatments would impair bumblebee colonies’ ability to rear queens, DEFRA performed a common-sense field study last year [44]. Their findings:
…the study has shown that bumble bee colonies remained viable and productive in the presence of the neonicotinoid pesticides under these field conditions…The study underlines the importance of taking care in extrapolating laboratory toxicology studies to the field, as well as the great need of further studies under natural conditions.
Sunflowers
Some beekeepers report that their colonies later crashed after they chased sunflowers last summer for honey. One must keep in mind that sunflowers are not a natural food for honey bees, and provide only poor-quality, nutritionally-inadequate pollen [45]. But the main problem with putting bees on sunflowers may be related to the fact that sunflowers are a native plant—meaning that there are a number of native insects that evolved to feed upon it:
Maximum seed yields often require the use of insecticides to protect the crop from insect competitors. Unfortunately, many of the major insect pests of sunflower attack the crop when it is flowering. Thus, insecticides used to control the pest also harm pollinating bees [46].
If sunflowers are the only forage available, colonies may eventually go downhill, due to the one-two punch of poor pollen nutrition coupled with insecticide exposure. And which pesticides would those be? One scary list– Asana XL, Baythroid, endosulfan, Furadan , Lorsban , methyl or ethyl parathion, , Proaxis, Scout X-TRA, Sevin, Warrior, Mustang Max, Declare, Cobalt, Yuma, Delta Gold, and Grizzly Z [47]!
Note that none of the above are neonics, other than seed treatments for wireworms. Surprisingly, field evidence indicates that the seed treatments only “stun” the wireworms for a while [48], which certainly raises the question as to how harmful they might be to bees months later when the plants flower! I will return to sunflowers below.
Be Careful What You Ask For!
Allow me to assure you that I am no pitchman for neonics or any other insecticide—the typical farmer practices far too little integrated pest management, and applies far too many pesticides! All insecticides (and several fungicides and adjuvants) cause problems to pollinators—the neonics are no exception. Any systemic insecticide has the potential to harm bees when applied as foliar applications, by chemigation, or to flowering trees, but it there is no compelling evidence that the neonics are any worse than the alternatives in most applications. On the contrary, there is quite a bit of evidence that they may often be “safer” (“reduced risk”).
If the neonic seed treatments were banned, it’s not as though all agriculture is suddenly going to go pesticide free—only about 1% of U.S. cropland is registered as “organic”! We must consider the likely alternatives. The products that farmers would then use to control insects would need to be sprayed all over the cropland—we’d then be back to the problem that the bulk of sprayed insecticides go into the environment without ever hitting the intended pest!
I hear from knowledgeable beekeepers that worse than in previous years, some of the new formulations of the spray-applied insecticides [49, 50, 51] can really knock the snot out of bees! One large beekeeper found his hives already dead before moving them away from the fields. Again, this was not a neonicotinoid issue.
Practical application: no one is saying that the neonics are “harmless.” The question is whether they are better or worse than the alternatives.
The Effect of Drought
Let’s discuss some of the problems (or suspected problems) with the neonics last season. The record warm and dry spring appeared to exacerbate corn planting dust issues (corn seeds are the worst offender due to their non spherical shape). Beekeepers in some areas of the Corn Belt, the East Coast, and in Ontario suffered from confirmed (in at least some of the cases) planting dust kills (although many went on to make good honey crops after their colonies recovered). The final analysis from Ontario is not yet completed, but dry soil conditions and an early clover bloom likely contributed to the problem. Regulators and the seed companies are working on solutions to the problem [52]. Still, IMHO it is unacceptable to ask beekeepers to bear the burden of bee kills without compensation, and no one could blame the affected beekeepers for being pissed!
Drought-stressed plants
There are a number of advantages to the neonic seed treatments. Besides their safety to the farmer and to most wildlife, there is virtually no way for the farmer to misapply them! The timing of application is only at planting time (when bees normally have little interest in the bare fields), and the dose is determined by the seed-treating company. This means that the applicator can’t be tempted to apply at the wrong time, or to over apply too strong a dose (however, their excessive near universal use can be expected to accelerate the development of resistant pests).
That said, beekeeper Bret Adee brought an interesting question to my attention: the dose of seed-applied systemic insecticides (whether neonic or other) is based upon the dilution factor as the plant grows, so that the residues in nectar and pollen will be reduced to below the “no observed adverse effects level.” But what happens during drought, when the water-stressed plants only grow knee high before desperately flowering? There would be far less plant biomass in which to dilute the insecticide (assuming that drought-stressed plants absorb the same amount from the seed treatment).
Certain plants (including sunflowers and canola) are known to “hyperaccumulate” toxic metals [53], perhaps more so during drought. Could this also be the case with systemic insecticides? Something that’s been stuck in the back of my mind is that Bonmantin [54] found that the concentration of imidacloprid first drops in sunflower plant tissue as it grows, and then reconcentrates in the flower heads.
It occurs to me that the translocation of systemic insecticides is generally studied in plants grown under “normal” conditions. I’d very much like to see data for residues in pollen and nectar from seed-treated plants grown under drought. Had we thought of this earlier, we could have collected pollen and nectar samples from drought-stressed plants last summer. I’m currently trying to track down any data or samples from such plants—if any reader has any such sample analyses, please let me know!
Practical application: the above hypothesis is speculative, but we need actual data from drought-stressed plants to see whether such an effect occurs. If so, it would need to be taken into consideration for the registration of seed treatment products!
Once planting was completed and the drought took its toll, the reports that I’ve heard are that soybean honey saved a lot of bee operations this season, right in the middle of treated corn/soy farmland. In this case, seed treatment with neonicotinoids may have been a blessing to beekeepers:
The benefits of [seed treatment] not only include the early-season disease control but also suppression of soybean aphids for quite a ways into the growing season. With it, we typically make only one foliar insecticide application for aphid control, usually in August, instead of two applications when [treatment] isn’t used. In 2012, with the extremely dry conditions in mid-season, there wasn’t as much of an aphid problem, and we treated just 300 acres of soybeans…Last year we sprayed closer to 30,000 acres for aphids [55].
On the other hand, some beekeepers on alfalfa or cotton got hit hard by other classes of insecticides. A hit from a pesticide application can lead to poor subsequent colony performance, queen failure, dwindling, or winter collapse. ABJ published an excellent series of articles on pesticides by Drs. Barbara and Eric Erickson in 1983; Editor Joe Graham has graciously granted me permission to post copies of those articles to my website [56]—I strongly suggest any beekeepers interested in pesticide issues read them! In the second article, the authors discuss both the problems with systemic insecticides and of sublethal effects—note that these articles were written long before the introduction of the neonics!
An anti-pesticide group, along with a handful of beekeepers, recently filed suit against the EPA [57], calling for an immediate ban on the two most common neonicotinoid seed treatments, despite the easily-verifiable fact that hundreds of thousands of colonies thrive in the midst of seed-treated corn, soy, and canola! To me, this suit smacks of being some sort of well-orchestrated publicity stunt, and does not serve the interests of either beekeepers or environmentalism. Worse, it now gives the powerful farm lobby cause to label beekeepers as “radical” enemies.
We don’t want this battle: do we really want to take on the farm lobby by backing them into a corner? The French beekeepers took a similar case against fipronil all the way to their supreme court and lost [58, 59]–worth reading]. Agriculture is already positioning itself for a fight [60, 61, 62]. Think about it—the EPA lives in fear of a conservative congress slashing their funding. Does anyone really think that they are going to go against the agricultural lobby without unimpeachable evidence? We should also think twice before calling for a ban on the seed treatments—the alternatives are not pretty!
It disturbs me to hear industry executives and lawyers stretching the truth or misrepresenting data. It disturbs me even more to hear my fellow environmentalists and beekeepers doing so! If we wish to maintain credibility, we should hold ourselves to a higher standard. The question we must ask ourselves the way in which we wish to have pesticide regulation decisions made:
- 1. By the EPA (the Environmental Protection Agency), whose risk assessors carefully study and weigh all available research and evidence in order to make objective and rational decisions, or
- 2. To have it decided instead by impassioned, fearful, and often misinformed advocacy groups who hire lawyers and pressure politicians who know little about the subject?
We depend upon the EPA to strike a balance between the availability of cheap food and profitability for those who provide it, versus the risks to human and environmental health and safety. It is good to have activists on both sides of the issues (industry and the anti-pesticide groups) to keep the EPA informed. But I don’t feel that either of those groups should be telling the EPA which pesticides to register or to ban! Let the regulators do their job!
Rather than wasting EPA’s funding to fight frivolous lawsuits, there are more productive actions that we can take:
- Help the EPA to do its job by filing “adverse effects incident reports” if you observe a problem due to pesticides [63]. EPA is begging beekeepers to do this! Unless they have documented reports of pesticide problems, their hands are tied as to restricting the uses of those pesticides!
- Support the National Pollinator Defense Fund [64]. Our industry is currently represented by a reasoned and knowledgeable group of (mostly) beekeepers. (Challenge to the pesticide companies: why don’t you stand behind the safety of your products and donate? The NPDF is about ensuring that your pesticides are properly applied, so there would be no conflict of interest).
- If your local state lead agency is not actively investigating bee kills or enforcing pesticide regulations, then use the local media to embarrass them into action!
- Keep pressure on the EPA to resolve corn planting dust problems. Here’s a wild idea: I’m not sure of the exact figures, but let’s say that 90% of the 95 million acres of corn is grown from neonic-treated seed. If the states were to levy a surcharge of 50 cents per acre (neonic seed treatment adds about $12 per acre to seed costs), they could collect over $42 million each year to fund a pool from which to indemnify the occasional beekeeper who suffers a confirmed kill from planting dust!
- Tell Congress that we’d like to see wording added to the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) to specifically protect pollinators. Currently, such protection is nebulous (although the EPA is acutely aware of pollinator issues): “The Administrator shall register a pesticide if… when used in accordance with widespread and commonly recognized practice it will not generally cause unreasonable adverse effects on the environment.” Unless there is specific wording to protect pollinators, bee kills may not be considered to be “unreasonable”!
- We need far more independent field studies to determine which pesticides and application practices are actually causing harm to pollinators. For pesticides in question, keep pressure on the EPA to require additional field trials to demonstrate whether they are indeed safe for pollinators under field conditions. I’d like to see the establishment of monitoring apiaries (and patches of untilled land) in representative agricultural areas nationwide, with the hives in each apiary to be carefully managed by independent parties. Such apiaries and sites could then be closely monitored each summer to see whether honey bees and other pollinators are able to survive local pesticide practices.
- Give farmers workable options! Disseminate and promote bee-friendly agricultural practices that don’t hurt the farmers’ bottom line. For example, by adopting IPM practices, Arizona cotton growers reduced insecticide spraying from 12.5 times a season to only 1.3 times (cutting insecticide use twentyfold), while using more environmentally-friendly insecticides [65]! Another recent study in Iowa found that adding additional clover or alfalfa rotations in corn/soy farmland was equally profitable, improved the soil, used less energy, used far less pesticides, and decreased water pollution [66].
- Business and agriculture respond to consumer demand. Consumer demand stopped most dairymen from injecting their cows with the hormone BST. Consumers could do the same by demanding pasture-fed beef and dairy (which would create more pollinator forage)! I’d also like to see the expansion of consumer choices (other than organic certification) that reward farmers who manage their lands to the benefit of wildlife and pollinators. For ideas, see [67. 68. 69].
Bottom Line
In conclusion, it appears that a perfect storm of a preceding exceptionally warm winter, followed by serious drought across the country, the lack of good mite control, a high prevalence of pathogens, and an unexpected California chill in the orchards prior to bloom, resulted in an unusual degree of colony losses. In other words, rather than one specific cause, there were simply not enough of the good things, and too many of the bad things.
I don’t see evidence that pesticides were the major factor in the shortage of bees in almonds this winter, although, as usual, a number of individual beekeepers on certain crops certainly took serious hits.
And how about the fear that there won’t be enough bees for almond pollination next year? Beekeepers have already told almond growers to expect higher pollination prices next year (especially since California is again going into serious drought, and beekeepers will be forced to invest extra money in feeding their hives). Most every beekeeper I know is madly making increase right now in anticipation of higher pollination prices next season. The fact of the matter is that should conditions allow beekeepers to successfully rebuild their numbers (following the typical swings of our boom/bust cycle), there could possibly even be a glut of bees for almonds next winter!
Feedback and Corrections
- A few countries placed temporary suspensions on certain seed treatments until planting dust issues were resolved [27]—only Germany has one suspension still in place. – Actually there are some more: France (Thiamethoxam in oilseed rape, Imidacloprid in corn and sunflower), Italy (all Neonic seed treatment in corn), and Slovenia (Imidacloprid, Thiamethoxam, and Clothianidin seed treatment in all crops)
- French beekeepers also started seeing problems with the introduction of the neonics; I’ve spoken with beekeepers in France whose apiaries are in pesticide‐free areas. They tell me that they experience the same sorts of colony mortality problems as do those in areas exposed to neonics – This is, by the way, likewise confirmed by monitoring results from the French authorities.
- In the case of foliar, drench, or chemigation applications prior to bloom, there are greater possibilities for bees to be exposed to toxic levels. – This is not the case for foliar application: as Neonics are xylem-systemic, but hardly mobile in the phloem, they can only be distributed in a plant after root uptake, but not be translocated for instance from a leaf to a later developed flower.
Then, on the topic of systemic residues in plants under drought stress: first, I am quite sure that the decrease of concentration in seed-treated plants over time is not only due to dilution, but also to degradation of the compounds – a factor that is not specifically dependent on water availability for the plants (e.g. photodegradation!); second, even if there would be less dilution in plants under drought stress: the concentrations in nectar and pollen of treated crops are normally so low (when we consider average rather than peak concentrations, and when we consider scenarios where colonies have chronically access exclusively to contaminated nectar/pollen over months unlikely in practice), that even an increased concentration due to drought stress-affected plants should not make a significant difference: if we for instance assume an average concentration of let’s say 3-4, or even 5 ppb Clothianidin in corn pollen, and likewise assume a dilution reduced to 50% (which is probably exaggerated), then we would still not end up with excessive residues. And finally, we have residue figures from crops grown in different countries, different climatic conditions, and different agronomic practices; though we have not specifically addressed the drought stress scenario, we have seen that residue figures are quite consistent over all scenarios, and there does not appear to be strong evidence that different environmental conditions would substantially (i.e. by orders of magnitude) and systematically alter residue concentrations.
Dr. Christian Maus
Global Pollinator Safety Manager
Bayer CropScience
/
A ppt on the impact of CRP lands on wildlife in North Dakota
http://www.redriverbasincommission.org/Conference/Proceedings/26th_Proceedings/Kading_RRBC_09.pdf
/
Feedback from a Midwestern apiary inspector:
Just a quick update: This beekeeper who recently told his local newspaper that pesticides were killing his bees, he was making excuses. We examined two of his yards with him yesterday. One yard was showing EFB throughout the whole yard. I think his “mid-summer losses” last year (half of that yard’s hives) were EFB kicking in with the mid-summer dearth. In his other yard, most of his dead outs were obvious starve outs. He harvested all of their stores with the first frost, and then didn’t feed them. So, in my opinion, and from my observations, pesticides are usually being used responsibly, and aren’t killing honey bees. I also think, with the aggressive way bees were on soy fields last summer, the systemic pesticides are not harmful to honeybees. I’m not seeing honey bee problems other than EFB getting the upper hand, due to our cold, late spring. My two cents. Thanks.
References
[1] http://www.frequency.com/video/dan-rather-reports-buzzkill/87705620/-/YouTube
[2] http://www.ars.usda.gov/is/pr/2012/120531.htm and California DPR.
[3] http://www.ncdc.noaa.gov/temp-and-precip/maps.php
[4] Wright, CK & MC Wimberly (2013) Recent land use change in the Western Corn Belt threatens grasslands and wetlands. https://www.motherjones.com/files/pnas201215404_nwow5w1.pdf
[5] http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2011_National_Survey_Report.pdf
[6] https://scientificbeekeeping.com/sick-bees-part-2-a-model-of-colony-collapse/
[7] vanEngelsdorp, D, et al (2013) Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Preventive Veterinary Medicine 108(2-3): 225-233. http://www.sciencedirect.com/science/article/pii/S0167587712002656
[8] https://scientificbeekeeping.com/sick-bees-part-18a-colony-collapse-revisited/
[9] http://almondinsights.com/692, http://agfax.com/almonds/2013/reports/03042013-almonds-web.htm
[10] https://scientificbeekeeping.com/sick-bees-part-18a-colony-collaspse-revisited/
[11] http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2010-2011-Limited_Survey_Report.pdf
[12] http://www.youtube.com/watch?v=y6B5qm2ut18, http://www.youtube.com/watch?v=PYbLbhZXizY
[13] (Broken Link!) http://www.nccrops.com/2012/07/27/insecticide-recommendations-for-corn-earworm-in-soybeans/
[14] http://www.agriview.com/news/crop/corn-soil-insecticide-use-up-dramatically-to-combat-widespread-rootworm/article_5d09decc-5b40-11e2-b485-001a4bcf887a.html
[15] http://www.amvac-chemical.com/products/documents/Counter20G%20Tech-Sell%20Sheet%20-%202013.pdf
[16] http://pmep.cce.cornell.edu/profiles/insect-mite/propetamphos-zetacyperm/terbufos/insect-prof-terbufos.html
[17] http://www.lewishybrids.com/PDF/3-5-2013Agronomic+ALERT+-+Interaction+between+herbicides+insecticides+corn.pdf
[18] http://pest.ca.uky.edu/EXT/Recs/ENT16-Field%20corn.pdf
[19] (Broken Link!) http://www.soybeans.umn.edu/crop/insects/spider_mites.htm
[20] http://www.nytimes.com/2013/04/07/opinion/sunday/calamity-for-our-most-beneficent-insect.html?nl=todaysheadlines&emc=edit_th_20130407&_r=0
[21] http://www.nytimes.com/2013/04/07/opinion/sunday/calamity-for-our-most-beneficent-insect.html?_r=0
[22] http://www.panna.org/press-release/beekeepers-and-public-interest-groups-sue-epa-over-bee-toxic-pesticides
[23] http://www.cdpr.ca.gov/docs/pur/pur10rep/top_100_ais_lbs10.pdf
[24] http://www.extension.org/pages/60318/pesticides-and-their-involvement-in-colony-collapse-disorder
[25] Mussen, EC (2006) Chaotic almond pollination. http://entomology.ucdavis.edu/faculty/mussen/JanFeb2006.pdf
[26] https://scientificbeekeeping.com/sick-bees-part-18e-colony-collapse-revisited-genetically-modified-plants/
[27] http://www.epa.gov/pesticides/about/intheworks/ccd-european-ban.html
[28] http://www.thecre.com/oira_pd/wp-content/uploads/2013/04/DQA-Alert-EU-Commission-Ban-on-Neonicotinoids-4-10.pdf
[29] https://scientificbeekeeping.com/home/news-and-blogs/
[30] Henderson, CB, JJ Bromenshenk, DL Fischer (2013) Clothianidin exposure levels from bee-collected pollen and nectar in seed-treated corn and canola plantings. Proceedings of the American Bee Research Conference.
[31] http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2011_National_Survey_Report.pdf
[32] http://www.ontariograinfarmer.ca/MAGAZINE.aspx?aid=534
[33] http://www.pbs.org/wgbh/pages/frontline/shows/settlement/timelines/april94.html
[34] https://scientificbeekeeping.com/2012-almond-pollination-update/
[35] https://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science/, https://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science-part-2/, https://scientificbeekeeping.com/testing-of-bee-feed-syrups-for-neonicotinoid-residues/
[36] http://www.eea.europa.eu/publications/late-lessons-2
[37] http://www.sciencefriday.com/playlist/#play/segment/9088
[38] https://scientificbeekeeping.com/the-extinction-of-the-honey-bee/
[39] http://www.ncdc.noaa.gov/temp-and-precip/maps
[40] http://nassgeodata.gmu.edu/CropScape/
[41] https://scientificbeekeeping.com/a-new-large-scale-trial-of-clothianidin/
[42] http://www.producer.com/daily/ontario-field-study-finds-no-link-between-seed-treatments-bee-deaths/
[43] Pohorecka, K, et al (2013) Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. Journal of Apicultural Science 56(2): 115-134. http://www.degruyter.com/view/j/jas.2012.56.issue-2/v10289-012-0029-3/v10289-012-0029-3.xml?format=INT
[44] http://www.fera.defra.gov.uk/scienceResearch/scienceCapabilities/chemicalsEnvironment/documents/reportPS2371Mar13.pdf
[45] http://repository.up.ac.za/bitstream/handle/2263/20334/Nicolson_Chemical(2012).pdf?sequence=1
[46] (Broken Link!) http://www.ag.ndsu.nodak.edu/aginfo/entomology/entupdates/Sunflower/a1331sunflowerhandbook.pdf
[47] http://www.sunflowernsa.com/growers/approved-chemicals/insecticides-test/
[48] http://www.mydigitalpublication.com/publication/?i=151958&p=41
[49] http://www.sunflowernsa.com/growers/approved-chemicals/insecticides-test/
[50] http://www.farmassist.com/agriedge/images/Resource_PDFs/Soybean/Warrior_Zeon.pdf
[51] http://www2.dupont.com/Production_Agriculture/en_US/assets/downloads/pdfs/K-09315.pdf
[52] http://www.ontariograinfarmer.ca/MAGAZINE.aspx?aid=534
[53] http://en.wikipedia.org/wiki/List_of_hyperaccumulators
[54] Bonmatin, JM, et al (2005) Behaviour of Imidacloprid in Fields. Toxicity for Honey Bees. In Environmental chemistry: green chemistry and pollutants in ecosystems pp. 483-49. http://www.buzzaboutbees.net/support-files/bonmatin2005behaviour-of-imidacloprid-in-fields.pdf
[55] http://cornandsoybeandigest.com/seed/do-soy-seed-treatments-pay?page=2
[56] https://scientificbeekeeping.com/historical-pesticide-overview/
[57] http://www.centerforfoodsafety.org/press-releases/1911/cfs-beekeepers-and-public-interest-groups-sue-epa-over-bee-toxic-pesticides
[58] http://www.theworldlawgroup.com/files/file/docs/Soulier_health_environment_June_2012.pdf
[59] http://www.soulier-avocats.com/upload/documents/Soulier_health_environment_september_2010_F.pdf
[60] http://westernfarmpress.com/government/pesticide-battle-over-honey-bee-health-under-way?page=1
[61] http://westernfarmpress.com/management/total-ag-pesticide-elimination-sought-radicals
[62] http://www.neonicreport.com/home/project-compass/
[63] https://scientificbeekeeping.com/pesticide-incident-reporting/
[64] http://pollinatordefense.org/site/
[65] http://cals.arizona.edu/apmc/docs/IPM_Delivers.pdf
[66] Davis, AS, et al (2012) Increasing cropping system diversity balances productivity, profitability and environmental health. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0047149
[67] http://www.pcl.org/pcl_files/5_Wildlife_Habitat_Farmland.pdf
[68] http://pfspbees.org/
[69] http://www.nwf.org/CertifiedWildlifeHabitat/UserAccount/SignIn
First published in: American Bee Journal January 2013
Sick Bees Part 18F1
Colony Collapse Revisited
Pesticide Kills
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in January 2013
Pesticides
A Bit of History
Nailing Down the Guilty Party
Keep ’em Honest!
Pesticides and CCD
Making the Link
Acknowledgements
References
As long as I’ve been keeping bees, one of our worst fears has been that we might suffer a serious pesticide kill. Pesticides (especially insecticides) have always been, and will continue to be, a problem for bees and beekeepers.
Jim Doan of New York hadn’t experienced a serious pesticide kill in 25 years of keeping bees in corn/soy/alfalfa farmland. But when he approached one of his yards last spring, he smelled the stench of dead bees. What he saw made him sick to the stomach—piles of dead and rotting bees in front of every hive!
Jim related to me that he called his state’s Department of Conservation to investigate the kill, to no avail. So he contacted his State Apiarist, who sent out an inspector a couple of days later to take samples, which then languished in a refrigerator until at Jim’s request they were sent to Dr. Maryann Frazier, who in turn sent them to the USDA lab for analysis.
Although pesticide residues were found, no investigation was done. No applicator was reprimanded, and no fine imposed. And Jim’s losses weren’t covered by either his insurance policy or by ELAP [1].
Jim’s disaster was hardly an isolated case. I’ve spoken with a number of beekeepers who have suffered recent pesticide kills. Dave Shenefield’s bees were working white clover in Indiana at corn planting time. A farmer drilled treated corn seed directly into a field of flowering clover without first burning the weeds off with herbicide. The planting dust fell directly onto the blossoms being worked by the bees, poisoning his colonies as the foragers returned covered with toxic dust.
Darren Cox’s bees in Utah get hit regularly by applications of pyrethroids or carbamates onto flowering alfalfa hay. These applications done are despite label restrictions that clearly state:
“This product is highly toxic to bees exposed to direct treatment or residues on blooming crops or weeds. Do not apply this product or allow it to drift to blooming crops or weeds if bees are visiting the treatment area.”
Darren related to me a scenario: an aerial applicator, under a contract arranged perhaps two weeks earlier, loads up with insecticide, and flies 50 miles to treat the field. But when he gets there, he’s surprised to see that the alfalfa is purple with bloom. What’s he to do—turn around and unload, or just go ahead and spray anyway, knowing that such an action would be in violation of the label. But this is Utah, where the local primacy partner tends to turn its head to pesticide violations (Fig.1). You can guess the rest–such unnecessary and preventable bee kills frustrate Darren to no end!

Figure 1. A typical insecticide kill in Utah. Simple timely communication between the grower and the applicator as to the stage of bloom could prevent many such kills, as the applicator could then make more appropriate product application choices. Photo courtesy Jared Taylor.
I could go on and on, beekeeper after beekeeper. What I hear is that some states are better at others at enforcing pesticide regulations—it’s tough to be a beekeeper in those states that aren’t doing their job! To make things worse, beekeepers are often justifiably hesitant to pursue investigation, since in a number of states, complaining beekeepers have been fined for having illegal miticide residues in their hives! And if a beekeeper raises too much of a stink he could become persona non grata to the local landowners and lose his locations.
Farmers and applicators could often easily prevent bee kills by simply making sure that they spray before or after a crop comes into bloom, or by spraying after dusk with a product having a short residual toxicity, or by using a less bee-toxic product that is labeled for application during bloom. Such practices would eliminate a large proportion of bee kills, yet some farmers and applicators just don’t give a damn, and worst of all, get away with illegal applications (scofflaw applicators may consider any fines levied for pesticide misapplication as a minor business cost)!
What bothers beekeepers most is the unfairness of it. Ranchers (even of alpacas, reindeer, or emus) receive government benefits for livestock losses due to fire or severe weather [2], and beekeepers may be eligible for benefits for colony losses if they jump through the hoops of ELAP [3]. But neither of those programs cover losses due to pesticide application–either legal or in violation of the law.
Think about it–if someone poisoned a herd of cattle with pesticide overspray, it would make the news! You could damn well bet that the incident would be investigated and the applicator fined, and the cattleman would sue for damages via civil action. But this is generally not the case if your livestock are honey bees. Few damaged beekeepers receive any compensation at all for their losses.
Now I don’t want to give the impression that the pesticide situation is dire for all beekeepers. As I pointed out in a previous article [4], many beekeepers in agricultural areas have little or no problem with pesticides. And many commercial beekeepers simply shrug off the occasional bee kill as a cost of getting good locations in agricultural areas. However, in some areas of intensive agriculture, those commercial beekeepers who provide the bulk of pollination services tell me that pesticide issues are their major problem.
A Bit of History
In order to understand the run up to our current situation, it is helpful to read the engaging “Report on the Beekeeper Indemnity Payment Program” (which was in effect from 1967-1980) [5]. I’ll share a few excerpts:
During the mid-1940’s, [pesticide] damage subsided as farmers shifted from the use of arsenicals to DDT which is less toxic to bees. However, by the late 1960’s, use of DDT was decreased sharply because of insect tolerance to the poison. Finally, use of DDT and other chlorinated hydrocarbons was banned because of environmental concerns. In most cases, the highly toxic [organo] phosphates and carbamates were used in place of the banned sprays. This increased the problem of bee loss to the point of disaster for many beekeepers…
Partial colony losses are not always easy to detect…pesticides may weaken colonies to such a point that they do not survive the winter. This type of loss is often ascribed to winterkill rather than pesticides. Further, this loss may be extended to the replacement bees placed in contaminated equipment the next season. Often, not all losses are discovered soon enough after the chemical application to determine the exact cause of death.
Investigatory clue: these records of the field experiences of beekeepers prior to varroa are important to keep in mind, notably that there were “sublethal effects” from the pesticides that caused later winter mortality. I hear the exact same complaints from beekeepers in agricultural areas today. Clearly, varroa and beekeeper-applied miticides have added to the stress upon bee colonies, but elevated winter mortality due to pesticide exposure was the norm prior to the introduction of varroa.
Colony losses due to pesticides were severe in several states during the 1960’s. There was a “sharp decline in pesticide losses” in California during the early ‘70’s due to the state imposing “strict control of spray application”—only 54,000 colonies were killed in 1974, compared to 89,000 in 1970 (an improvement, but hardly cause for celebration). But then in the mid 1970’s, encapsulated insecticides (Penncap-M) were brought to market, again causing devastating losses when foragers dusted with the time-release particles returned to the hive and stored them in the beebread.
During June 1976, selected beekeepers in California and Washington were contacted to discuss the pesticide situation…Beekeepers in Washington report that there are no safe locations for bee yards. One beekeeper said, “No matter where I place my bees in the Yakima Valley, they will be sprayed at least once within ten days.” A beekeeper in the San Joaquin Valley of California described his efforts to protect his apiaries as “playing musical chairs with 40 loads of bees….” Several beekeepers said that even if they did move their colonies to another location, it could be sprayed the next day.
Practical application: I hear exactly the same words today from commercial operators. We have made great progress with pesticides since the 1960’s, but still not enough!
Beekeepers in Arizona, California, and Washington accounted for a large proportion of claims because they lacked access to “safe” forage areas (these were the early days of using forklifts in bee operations, and moving bees was hard work). It was not unusual for large beekeepers to suffer serious pesticide damage to half their hives each year, and they would likely have been unable to stay in business without governmental help (Fig. 2).
Figure 2. Back when the Agricultural Stabilization and Conservation Service kept records of reported bee kills for indemnification purposes (not all kills were reported), it was easy to see in which states pesticide applications were a serious problem. In recent years bee kills have not been tracked by any agency. Map from Erickson & Erickson 1983 [[i]].
[i] Erickson, EH, and BJ Erickson (1983) Honey bees and pesticides. ABJ 123(10): 724-730.
For nearly a decade, the Indemnity Program compensated beekeepers for pesticide losses. Those in only eight states filed the bulk of claims. As today, a small percentage of commercial beekeepers control the vast majority of colonies, and provide most pollination services. Well less than 1% of beekeepers in the country filed claims in any one year. By contrast, over 90% of the Arizona beekeepers in the program filed claims— not surprising due to the frequent spraying of the vast acreage of cotton suffering from a serious infestation by pink bollworm in the mid 1960’s [7], and the lack of alternative non-agricultural forage in that dry state.
The largest payment to a single beekeeper (name and state not specified) was $225,400 in 1972 (that would be $1 million in today’s dollars), and he filed for $228,000 two years later. You can imagine how this might not have set well with some budget-conscious congressmen!
And of course some crafty beekeepers learned to work the system:
On the other hand, some commercial beekeepers contend the indemnity payments have permitted, and in some cases encouraged, the survival of marginal beekeeping operations. The “marginal manager,” in this context, was characterized as any beekeeper who had become dependent upon indemnity payments as a source of income.
Those alleged “marginal beekeepers” reportedly left their hives in areas that they knew would be sprayed, and managed their colonies only enough to keep them barely alive so as to be able to collect more payments the next year (or kept collecting payments on deadouts). These fraudulent practices also did not play well to the program overseers.
The study also looked at the profitability of beekeeping; I found one of the tables to be of particular interest (Fig. 3):
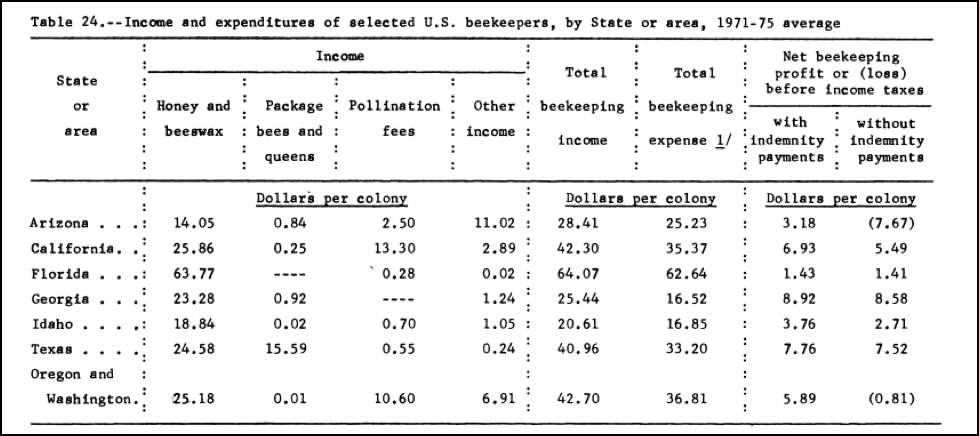
Figure 3. You can roughly adjust these figures into today’s dollars by multiplying them by five. What surprises me is that despite it being painfully costly to maintain colonies today in California (the annual expense being about $190) [[i]], the profit margin is substantially higher now than it was back then–not because of honey (since honey prices have only kept pace with inflation [[ii]]), but rather due to much higher pollination rates in almonds. Also of interest is that in those days beekeepers spent next to nothing on feeding syrup, and pollen supplement isn’t even mentioned!
[i] Mussen, E (2009) How much does it cost to keep commercial honey bee colonies going in California? http://projectapism.org/content/view/83/27/
[ii] http://www.nass.usda.gov/Statistics_by_State/California/Historical_Data/Bees.pdf
It is instructive that the analysts were aware of the cost to the beekeeper of pesticides:
This analysis shows that beekeeping income is affected most by severely damaged and destroyed colonies. Severely damaged colonies may require 6-8 weeks to recover colony strength. If the damage occurs during a major honey flow, the field force will be greatly reduced and honey yields could be lowered 60 percent or more. Severe damage in late summer may weaken a colony preparing for winter and increase the chances for significant winter kill…Beekeepers estimate it takes about one year for a destroyed colony to regain its income earning potential.
The authors conclude that without the indemnity payments, “farmers seeking pollination services would have to pay substantially higher rental fees to obtain bees.” Congress decided to pass that cost onto the farmers anyway, and terminated the indemnity program in 1980 (leaving some beekeepers with still-unpaid IOU’s). Today the almond growers bear the brunt of those higher rental fees; the huge number of colonies produced to meet the demand for high-paying almond pollination ensures that there are plenty of strong hives available for other crops afterwards.
Colonies generally come out of almonds in better shape then when they went in. This is not true for a number of other crops. The combination of poor forage and pesticides in several crops can weaken colonies to the extent that they don’t survive the season.
Allow me to close with some prescient conclusions from the report:
Unless Federal and State governments act ot regulate and caution applicators of toxic pesticides, colony damage will continue to be a major problem for beekeepers. However, most government officials emphasize that farmers and spray applicators are already confronted with enough regulations…the current development of stronger and longer-lasting pesticides…is creating an environment entirely unsuitable for honey bees in many parts of the U.S. These areas will find it harder to maintain the present level of bee population regardless of an Indemnity Program or higher honey and pollination prices.
Remember that the above words were written prior to the invasion of the tracheal mite, the varroa mite, Nosema ceranae, or the Small Hive Beetle—beekeeping hasn’t gotten any easier since their arrival!
Practical application: beekeeping in agricultural settings has always been a tough way to make a living. Fortunately, many beekeepers tell me that things have gotten better in their regions. But in some areas of intensive pesticide application, it’s hard to keep a hive alive from one year to the next.
Nailing Down the Guilty Party
This spring my bee operation suffered from a case of Sudden Forklift Collapse (Figure 4).

Figure 4. Early this spring I suffered from a case of Sudden Forklift Collapse. This was no “sublethal effect” and did not go unnoticed! In a forklift kill like this, it didn’t take Sherlock Holmes to determine that the cause of death was due to a falling oak tree. If only the causes of pesticide kills were so easy to pin down!
In my case of Sudden Forklift Collapse, the cause was evident. Such is often not the case with pesticide kills. You may not even see any dead bees if the field force is poisoned in the field and never makes it back to the hive. Perhaps (as in the case of planting dust) you only see a handful of young bees and drones dying at the landing board. Or maybe the brood turns spotty. If the pesticide disorients the foragers, you may wonder why you didn’t get the normal honey crop. Or maybe there is some sublethal effect from which the colony simply “slows down” for a few months, or doesn’t make it through the winter.
In any of those cases, it may be difficult, if not impossible, to nail down the culprit. You don’t know where your bees were foraging, and any pesticide application within a 3-mile radius is suspect. You may not immediately recognize that there was a pesticide problem at all, so any residues could be degraded or washed off by rain by the time you think to have the dead bees or beebread tested. And even if you happen to visit the yard immediately after the kill, good luck in getting an understaffed and untrained state or county agency to quickly come out and properly collect and freeze a good fresh sample. And even then the analytical tests cost so darned much!
Action item: aggrieved beekeepers often have VERY STRONG FEELINGS! However, in order to change pesticide regulations, the EPA needs incontrovertible evidence that a certain pesticide used according to label restrictions caused adverse effects to honey bees. We need any and all beekeepers who suffer from substantial pesticide kills to file an “incident report.” Such a report is most effective if it contains a photographic record, documentation that rules out other plausible causes for the dead bees (e.g., tracheal mites or starvation due to unusual weather or forage conditions), and chemical analysis of samples of bees and beebread, properly taken by a state agent. If your local primacy partner is unable or unwilling to help, you may report directly to http://www.npic.orst.edu/eco or beekill@epa.gov.
One would think that solving Jim Doan’s kill would have been straightforward, since there were fresh piles of dead bees in front of the hives. He hadn’t previously experienced serious kills in those yards, so something different had happened. There was no apparent change in plantings this year, but with commodity prices at an all-time high, a farmer might have felt that it was worthwhile to apply more or different insecticides as precautionary “risk management.” Surely it would be easy to find incriminatingly-high levels of the offending pesticide in the dead bees or combs.
According to Jim, due to unfamiliarity with the investigation of pesticide kills, the state inspector collected less than an optimal amount of bees for pesticide analysis. Two samples were later sent off to the USDA lab (the cost of analysis was split between Jim and Project Apism)—results below (Fig. 5).

Figure 5. Analysis report of the two samples from Jim Doan’s spring bee kill (column headings added).
Figure 5. Analysis report of the two samples from Jim Doan’s spring bee kill (column headings added).OK, so now Jim had a report. But what did it tell him? As for the dead bees, the 1.6 ppb* of clothianidin insecticide is far too low to have caused bee mortality (1.6 ppb = 0.16 ng/bee; the LD50 for clothianidin lies in the range of 22-44 ng/bee).
* To help with the math, LD50 = median lethal dose; 1 ppb = 1 part per billion = 1 μg/kg = 1 ng/g; μg = microgram (one millionth); ng = nanogram (one billionth); a bee weighs about a tenth of a gram, so for every 10 ppb of residues in a sample of dead bees, any bee on average would contain 1 ng/bee .
So how about the high dose of Captan fungicide? As best I can tell from the literature, “Studies on the honeybee using technical Captan fungicide indicate that the LD50 is greater than 10 μg a.i./bee, and that there is 9.8% mortality at 215 μg a.i./bee.” So let’s do the math: 1290 ppb = 129 ng/bee, or 0.129 μg/bee—so again, it would be hard to make a case that this chemical was responsible for the obvious pile of dead bees.
Maybe the analysis of the pollen sample from the comb might help. I have no idea as to how it was taken, which can make a huge difference (Fig. 6).

Figure 6. These are plugs of beebread that I pulled from a brood frame. Note the layering of the different species of pollen. If a colony suffers from a pesticide kill, any traces of the responsible pesticide residue may only be in the topmost layer of pollen. If the state agent who takes the beebread sample scoops all the way to the midrib, he may dilute the offending pesticide by a factor of 10 or more.
The one pollen sample from the one comb from one colony (get my point?) in Jim’s affected apiary contained 399 ppb of the organophosphate insecticide Phosmet. The contact LD50 for this compound is listed as 0.0001 mg per bee (= 0.1 μg/bee = 100 ng/bee). Surprisingly, there doesn’t appear to be any published oral LD50 for Phosmet to honey bees! By my math, the concentration of Phosmet in Jim’s pollen sample would not be expected to have killed his bees either, although since it is a violation of the label to spray the insecticide on flowering crops, one is left wondering how it appeared in the pollen.
So this is how it can be for a beekeeper and his innocent bees—the suddenly-appearing piles of rotting corpses in front of every one of his hives certainly suggest that his bees were killed by a pesticide application. Unfortunately, due to a lackluster investigation by the primacy partner, and lack of implicating chemical evidence, Jim will never know what or who was responsible for the kill, nor be compensated for his losses, if justified. And he has no idea whether the same thing will happen again next season!
To make matters worse, Jim’s bees apparently got hit again in July, resulting in piles of greasy-looking dead and twitching dying bees in front of the entrances. And as I write these words in November, Jim sent me yet another photo of hundreds of freshly-dead bees once again in front of the hives (despite him confirming low levels of varroa and nosema). Jim is now a justifiably frustrated and angry beekeeper–not only did he suffer considerable financial loss (not to mention the ugly death of his beloved bees), but no one learned anything from the experience! The unwitting farmer(s) have no idea whether their pesticide applications caused the problem, Jim’s state agencies aren’t making any particular effort to prevent the same thing from happening again next year, and EPA didn’t receive any useful adverse effects report. Yes, frustrating!
It is disturbing for me to present these facts. Our managed honey bees function as a conspicuous and charismatic indicator species for the effects of pesticides upon “non target organisms.” Yet some agricultural areas are a “no bees land” due to either inadequate label restrictions or flagrant violation of those restrictions. And keep in mind that the honey bee colony has the capacity to absorb pesticide kills that would exterminate solitary pollinators, such as native bees, butterflies, and beneficial insects.
Practical application: if honey bee colonies are being killed, we can safely assume that the situation is even worse for more sensitive species!
Keep ‘em Honest!
Let me share another quote from the Indemnity Report:
The Beekeeper Indemnity Program itself discourages civil court action…Greater use of the civil court system by beekeepers to seek compensation for pesticide losses could reduce applicator negligence.
There you have it! The sad truth is that it will take the push of lawsuits to ensure that our pesticide laws are actually enforced. Accordingly I’ve studied the judgments for some beekeeper lawsuits. Be forewarned that a successful lawsuit requires unimpeachable evidence and impeccable argumentation—so one should not enter into an expensive lawsuit lightly!
The AHPA has started a legal defense fund to pursue test cases against egregious violations of pesticide law, with the hope of setting legal precedent, as did Jeff Anderson’s successful lawsuit against the state of Minnesota in 2005 [10]. I’m hesitant to step into politics, but I feel that this is probably a good course of action that could help the cause of advancing pesticide regulation. We beekeepers must tread carefully here to avoid pissing off the farmers who allow us to place bees upon their land. In truth, I’d like to see Xerces or some other environmental groups filing such lawsuits, so that they, rather than beekeepers, would take the heat. However, action is preferable to inaction.
Action item: you may join me in contributing to the National Pollinator Defense Fund at http://pollinatordefense.org/site/?page_id=11
I wish that I could present a simple solution to this problem, but there isn’t one—especially since the U.S. is currently locked into the high-input large-scale monoculture agribusiness model. The good news is that EPA is on the side of the beekeepers and the environment [11], and that things are clearly getting better—the worst pesticides are being phased out, new “reduced risk” pesticides and “biological” are put on the EPA fast track in order to get them into the market, plus a new generation of “smart” robotic application systems are being developed. There has never been more public awareness of the plight of the honey bee, and beekeepers are awkwardly basking in the spotlight of being considered as environmental stewards. The bad news is that the process of reducing the damage by pesticides to non target species is hampered by, among other things, ignorance (and lack of enough good scientific data), politics, property rights, consumer demand, and Money (intentionally spelled with a capital M).
OK, that’s enough griping for now–let’s get back to an investigation into any connections between pesticides CCD.
Pesticides and CCD
Biological plausibility: pesticides can weaken the colony by killing or otherwise affecting the foragers, reducing adult bee longevity, having adverse effects upon the queen, brood, or nurse bees, or by affecting bee behavior. In addition, they could react with other toxins, beekeeper-applied miticides, or suppress the bee immune response to pathogens. Any of the aforementioned could conceivably result in colony dwindling, mortality, or collapse.
Residues in the Combs
Let’s narrow down our focus. CCD by definition is not the result of the sorts of acute pesticide kills detailed above. So what we are interested in is colony mortality or morbidity due to sublethal effects that hadn’t already killed bees outright! In the case of winter mortality, since few pesticides are applied at that time of year, and since colonies normally purge any remaining field bees during the “fall turnover” [12], we’d expect any contribution by pesticides to be from residues in the combs, where they should be detectable by analysis.
Making the Link
One would think that it would be a simple matter to make the connection between pesticide residues and winter mortality—simply analyze pollen and beeswax samples from the combs, and determine whether there is a correlation between residues of specific pesticides and colony mortality.
The above sounds so straightforward and easy, but in actuality this is where it gets complicated. My point of going into detail on the analysis of Jim Doan’s apparently obvious bee kill was that if it’s that hard to figure out exactly what caused an acute pesticide kill, imagine how difficult it would be to definitively link colony mortality to any sublethal effects from a specific pesticide!
In fact, I took artistic license in greatly simplifying Jim’s story. In doing my usual fact checking, I found out that the actuality was complicated by personalities, politics, weather (Fig. 7), and a history of indemnity payments. To add further confusion, another beekeeper on the same farm did not observe any dead bees in front of his hives (but did notice that his nucs on that farm did not build up as well as those at other nearby locations).
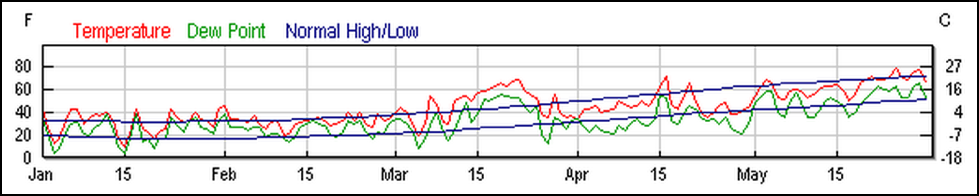
Figure 7. Western New York experienced extraordinarily warm weather (followed by cold) in May. I find that such weather anomalies can result in piles of dead bees in front of hives due to short-term starvation events. Weather graph from www.weatherunderground.com.
However, I’m appreciative of Jim for sharing his observations and analysis report, and feel that it was a good example of the problems that researchers and regulators encounter as they try to figure out exactly how pesticides are affecting colony health.
These complicating factors may be why no scientific study has yet been able to firmly link colony mortality to pesticides. Here are the conclusions of all monitoring and analytical studies that I’ve seen to date:
- Germany: “As expected, the results show that pollen [from 210 hives sampled over 3 years] is contaminated with a plethora of chemical substances originating from the agricultural practice of using pesticides but also from the apicultural necessity of using acaricides… Accordingly, no relation between contamination of pollen and colony development or winter losses could be demonstrated in the course of the project although special emphasis was put into this aspect” [13].
- France: “Several cases of mortality of honey bee colonies (varying from 38 to 100%) were observed in France during the winter of 2005-6. In order to explain the causes of these mortalities, a case control study was conducted on a limited area, together with a larger survey in 18 other apiaries located in 13 sites over the entire country…No pesticide residues of agricultural origin were found in the samples of beebread, beeswax, honey and dead honey bees, with the exception of imidacloprid…found in one apiary [and] not considered to be able to cause honey bee acute mortality” [14].
- France: “A 3-yr field survey was carried out in France, from 2002 to 2005, to study honey bee … colony health in relation to pesticide residues found in the colonies… No statistical relationship was found between colony mortality and pesticide residues” [15].
- Italy: “The data obtained from the winter 2009-2010 inspections were used as the basis for chemical analyses on bee and wax samples, to test for residues of organophosphate, organochlorurate, carbamate and neonicotinoid pesticides, but no significant presence of these substances was detected” [16].
- Spain: “The present data [beebread samples from 12 apiaries] are in agreement with studies showing no negative effects of seed-treated crops. Some pesticide residues were found here, in particular several varroacides and insecticides, but no significant differences were observed between the different sunflower crop samples and those from the sites of wild vegetation. This fact not only implies environmental contamination but also supports the theory that, most of the time, inadequate [read that “unapproved”] treatments are the main source of residues that might weaken bee colonies and make them more sensitive to other factors” [17].
- Spain: “This study was set out to evaluate the pesticide residues in stored pollen from honey bee colonies and their possible impact on honey bee losses in Spain. In total, 1,021 professional apiaries were randomly selected… A direct relation between pesticide residues found in stored pollen samples and colony losses was not evident accordingly to the obtained results” [18].
- Europe (thorough review): “Currently there is no clear evidence from field based studies that exposure of colonies to pesticides results in increased susceptibility to disease or that there is a link between colony loss due to disease and pesticide residues in monitoring studies” [19].
- USA (CCD Descriptive Study): “This study found no evidence that the presence or amount of any individual pesticide occurred more frequently or abundantly in affected apiaries or colonies” [20].
- USA (2012 CCD Progress Report): “When pesticides are viewed in aggregate on a national scale, residues of pyrethroids …pose a threefold greater hazard to bee colonies than neonicotinoids, based on mean and frequency of detection in pollen samples and relative acute toxicity. The synthetic pyrethroid detected in the highest quantity and frequency in honey bee colonies that is used by beekeepers to control Varroa mite is tau fluvalinate” [21].
- USA (Stationary Hive Project) : “We did not find any relationship with any of our measures of pesticide contamination and colony loss rate at the apiary level for either 2009 or 2010” [22].
OK, I’m as puzzled as you are! It defies both common sense and a long history of beekeeper experience that researchers haven’t yet nailed down any link between pesticide residues in the combs and colony mortality! The above were not industry-funded studies, and several of the researchers started with a strong anti-pesticide bias (nearly all researchers suspect that pesticides are involved to some extent). And I’m certainly not about to tell you that pesticides/miticides and winter mortality are unrelated–it’s just, like I said, complicated.
I found that in order to begin to understand the effects of manmade pesticides upon bee health that I first needed to back up and examine some of the complex biology involved in natural bee/plant/toxin interactions. We’ll start in on that next month…
Acknowledgments
I’d like to thank the editor of this journal, Joe Graham, for giving me the latitude, support, and encouragement to write this series of articles. And a special thanks to Dianne Behnke of the publishing department for digging up and scanning archived issues of ABJ for my research.
References
[1] http://www.fsa.usda.gov/Internet/FSA_File/elap_honeybee_11.pdf
[2] http://www.fsa.usda.gov/Internet/FSA_File/lip2011_158c020211.pdf
[3] http://www.fsa.usda.gov/Internet/FSA_File/elap_honeybee_11.pdf
[4] https://scientificbeekeeping.com/the-extinction-of-the-honey-bee/
[5] ERS (1976) Report on the beekeeper indemnity payment program. http://babel.hathitrust.org/cgi/pt?id=coo.31924001799307;seq=8;view=1up
[6] Erickson, EH, and BJ Erickson (1983) Honey bees and pesticides. ABJ 123(10): 724-730.
[7] http://naldc.nal.usda.gov/download/48077/PDF
[8] Mussen, E (2009) How much does it cost to keep commercial honey bee colonies going in California? http://projectapism.org/content/view/83/27/
[9] http://www.nass.usda.gov/Statistics_by_State/California/Historical_Data/Bees.pdf
[10] Anderson v. State Department of Natural Resources Minnesotahttp://www.animallaw.info/cases/causmn693nw2d181.htm
[11] http://www.epa.gov/opp00001/ecosystem/pollinator/then-now.html
[12] Mattila HR, Otis GW (2007) Dwindling pollen resources trigger the transition to broodless populations of long lived honeybee each autumn. Ecol Entomol 32:496–505.
[13] Genersch E, et al (2010) The German bee monitoring project, a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41: 332-352.
[14] Chauzat MP, et al (2010) A case control study and a survey on mortalities of honey bee colonies (Apis mellifera) in France during the winter of 2005-6. Journal of Apicultural Research 49: 40-51.
[15] Chauzat MP, et al (2009) Influence of pesticide residues on honey bee (Hymenoptera, Apidae) colony health in France. Environmental Entomology 38: 514-523.
[16] Mutinelli, F, and C Porrini (2010) Report based on results obtained from the second year (2010) activity of the APENET project. http://ebookbrowse.com/apenet-2010-report-en-6-11-pdf-d189566755
[17] Bernal J, et al (2011) An exposure study to assess the potential impact of fipronil in treated sunflower seeds on honey bee colony losses in Spain. Pest Management Science 67: 1320-1331.
[18] Bernal J, et al (2010). overview of pesticide residues in stored pollen and their potential effect on bee colony (Apis mellifera) losses in Spain. Journal of Economic Entomology 103: 1964-1971.
[19] Thompson, HM (2012) Interaction between pesticides and other factors in effects on bees. http://www.efsa.europa.eu/en/supporting/doc/340e.pdf
[20] vanEngelsdorp D, et al. (2009) Colony Collapse Disorder: A Descriptive Study. PLoS ONE 4(8): e6481.
[21] http://www.ars.usda.gov/is/br/ccd/ccdprogressreport2012.pdf
[22] Drummond, F, et al (2012) The first two years of the stationary hive project: Abiotic site effects. http://www.extension.org/pages/63773/the-first-two-years-of-the-stationary-hive-project:-abiotic-site-effects
 Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.
Table 2. This 2012 survey by the USDA [43] echoes the previous findings—the only pesticides found in at least 10% of the samples were from either beekeeper-applied miticides or chlorpyrifos. The 99 analyzed samples came from Alabama, California, Colorado, Florida, Idaho, Indiana, New York, South Dakota, Tennessee, Texas, and Wisconsin.