Contents
Review.. 1
Balancing moisture elimination and heat loss. 2
Broodrearing in the winter cluster. 3
So let’s do the arithmetic!. 5
Practical applications. 5
Optimal Cluster size. 5
Winter stores ― honey and beebread. 8
Hive placement. 8
Hive insulation. 9
Hive ventilation. 10
Literature cited. 11
The Nosema Problem Part 7c
The Prevention of Dysentery
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in February 2020
Dysentery within the winter cluster may indicate that the colony is suffering from moisture imbalance. Such in-hive defecation can overwhelm the normal colony hygiene that prevents the spread of intestinal parasites, such as nosema and amoeba. Fortunately, the bees ― and the beekeeper ― can take measures to help alleviate moisture imbalance.
Review
Apis mellifera is a tropical insect, which apparently evolved in warm regions of Africa. As the species expanded its range northward, certain races adapted to survive long confinement during the winter by forming a “cluster” that could subsist for months on stored honey. However, the consumption of honey generates water as a byproduct. When it’s warm, bees can fly freely to void that water away from the colony. But that option is not available to bees in a winter cluster.
Trapped in the winter cluster, a bee has four options for dealing with excess water in its system. The first two ― simply trying to “hold it,” or defecation in the cluster ― are problematic. That leaves two other options:
1. Breathing it out (respiratory transpiration), which requires heat generation and ventilation, or
2. Feeding the water (in some form) to other bees.
European races of bees have evolved to use both of the above methods in order to survive long-term clustering when it’s cold. But in order to conserve precious honey stores until the next spring, they have developed behaviors that minimize heat loss (and possibly as a side effect, to offset the loss of “winter bees” to natural mortality).
Balancing moisture elimination and heat loss
Bees have “figured out” ways to vent excess moisture from the cluster with minimal loss of heat (the replacement of which would require more honey consumption).
At cold temperatures, there is apparently scant ventilation in the cluster as evidenced by the observation that there is so little air exchange that oxygen and CO2 concentrations within the cluster can be dramatically different from our ambient air [[1]]. The creation of that hypoxic (low oxygen), reduced-metabolism environment may well have to do with the need for the bees in the core to conserve both energy and moisture in that self-created dry environment, as any air exchange would quickly desiccate them. But as predicted by an elegant model by Omholt, the lower the ambient temperature, the more the bees in the mantle of the cluster accumulate moisture [[2]].
Practical application: the cluster needs to somehow deal with thirsty bees in the core, and water-saturated bees in the mantle ― while minimizing energy consumption and heat loss.
In order to achieve necessary ventilation, bees can create channels within the cluster, as nicely illustrated in a swarm by Bernd Heinrich [[3]]. However, it is not clear whether bees actually use such passive convection currents to any great extent while in winter cluster, as challenged eloquently by Möbus [[4]] ― who questioned whether the bees at the top of the cluster mantle would, or were even able to, vent warm air out the top. Instead, Sachs and Tautz [[5]] concluded that required ventilation is accomplished by the active fanning of only a very few individual bees moving about the cluster:
… the middle section of the hive is rarely ventilated from below. The fanning bees generally move up and down the outer third of the comb alleys. … This behavior actively conveys humidity from the center of the bee cluster to the outer areas of the hive. … In hollow trees, this method of dehumidification has the advantage that virtually no heat is lost. The heat is not fanned outside but rather remains in the hive and rises once more as time goes by.
For the bees to fan ventilation air downward, rather than up, makes sense for two reasons:
· It would minimize water condensation above the cluster, and
· It would maximize heat recovery as that water vapor condensed.
Practical application: Toomema [[6]] placed condensers in the tops and bottoms of hives during winter to determine where moisture actually condenses in the hive. Over 97% was recovered in the lower condensers ― supporting Möbus’ thesis. Control of humidity in the winter cluster is of paramount importance, since it is the main way in which the colony controls water loss. I thank researchers such as Michael Ellis and Kalle Tomemaa for investigating this, and hope that we can eventually incorporate Möbus’s observations into a better understanding of the most conducive hive design for wintering.
But the bees have yet one more trick up their sleeve:
Broodrearing in the winter cluster
This brings up another question for which I’ve long looked for an answer ― why do colonies often engage in broodrearing in the middle of the winter? Indeed, one of Lloyd Harris’ shed-wintered colonies came out of winter confinement stronger than it went in [[7]]. Such stop-and-go broodrearing is energetically expensive, and could result in colony starvation, yet many colonies do it. It doesn’t appear to have anything to do with daylight or the winter solstice, since colonies wintered in pitch-black sheds still initiate broodrearing. Indeed, I’ve found zero evidence to support the claim that cessation or initiation of broodrearing has anything to do with day length. Just ask any Australian beekeeper on the winter solstice as their colonies actively rear brood during the bloom of White Box [[8]], or a keeper of Russian bees that go broodless during the August dearth.
One interesting data set regarding broodrearing during winter comes from an old study by Jeffree in Aberdeen, Scotland. Winters there are cold enough for colonies to go into fairly tight cluster, but it doesn’t get much below freezing (Fig. 1).

Figure 1. Weather averages for Aberdeen, Scotland. Factoring in rain and wind (not shown), bees wouldn’t be expected to do much foraging between late November and early April, nor to break winter cluster. Image from https://weatherspark.com/
But despite the chilly temperatures and lack of incoming pollen, broodrearing appeared to occur intermittently in colonies throughout the winter (Table 1).
|
Table 1. Results of 367 examinations made during September to March from 1945 to 1953. Data from Jeffree [[9]].
|
|
Month
|
No. of colonies examined
|
Percent with brood
|
Square inches of brood (average)
|
|
September
|
45
|
78%
|
76
|
|
October
|
106
|
14%
|
2
|
|
November
|
114
|
25%
|
2
|
|
December
|
31
|
58%
|
10
|
|
January
|
18
|
50%
|
14
|
|
February
|
10
|
100%
|
48
|
|
March
|
43
|
91%
|
50
|
So why would colonies rear brood, even when it was cold outside? As pointed out by Möbus [[10]]:
Even among bees the old saying applies: “Every baby costs its mother a tooth.”
There are two main costs associated with winter broodrearing: Not only must the colony expend precious energy and protein reserves to rear brood, but “winter bees” appear to lose their longevity once they initiate the rearing of brood, as elucidated by Mattila’s analysis of Lloyd Harris’ data [[11]]. Thus, from an evolutionary standpoint, we must assume that the benefit of midwinter brood rearing outweighs the cost. One plausible explanation for the benefit of on-off winter broodrearing was offered independently by two very sharp and experienced observers ― first by Möbus in 1980 [[12]], then by Omholt in 1987 [[13]], and again by Möbus in this very journal in 1998 [[14]].
Practical application: Despite their once-a-decade efforts to bring this explanation to the attention of the beekeeping community, Möbus’ and Omholt’s well-reasoned and observationally-supported papers surprisingly seem to have been largely ignored or forgotten. So I’m trying to bring them back into the light in 2020 ― some 40 years later. As eloquently explained by Omholt:
Möbus suggested that the phenomenon of brood rearing in the winter cluster has a definite survival value for a colony with a water problem, as the production of liquid, glandular brood food will remove some of the individually embarrassing surpluses from the bees, and that the increase in core temperature that follows brood rearing will make efficient evaporation possible.
Establishing a broodnest requires ramping up of the temperature of the center of the cluster (which helps with transpiration loss), and requires the feeding of huge amounts of moisture-rich jelly (67% water) to the larvae. Additionally, there is the need for the bees to then increase the humidity of the core of the cluster enough to prevent the eggs and larvae from desiccating. The three above factors result not only in increased water loss due to respiratory transpiration, but also a massive transfer of water from the nurse bees to the brood.
So let’s do the arithmetic!
According to Alfonsus’ measurements:
Dysentery appears when the fecal accumulations reach 33% of the total body weight of the bees. General defecation does not take place until the accumulation reaches about 45%.
· That 33% accumulation occurs when a bee is holding around 35 mg of water in its rectum.
· A single worker larva at time of pupation weighs around 160 mg, 74% of which consists of water [[15]].
· That works out to at least 118 mg of water required to rear each worker to pupation.
· Thus the rearing of a single larva would easily allow more than three workers to completely dispose of the excess water in their bodies.
· In order for the cluster to transfer the water produced from a weekly consumption of a pound of honey, the colony would need to rear less than half a frame of brood (both sides of the comb) that week.
Omholt’s calculations suggest that at an ambient temperature of 32°F (0°C), a cluster of 15,000 bees (around 8 frames) would be expected to initiate broodrearing at about 43 days after their last cleansing flight ― a figure supported by a number of observations.
Möbus tested the hypothesis experimentally by caging queens in their winter cluster (to prevent broodrearing) ― most colonies then developed dysentery within 3-4 weeks and grew weaker.
Practical application: Dysentery may result from the failure of a colony to initiate winter broodrearing (perhaps due to an aged queen). This is a beautiful and elegant explanatory hypothesis. If true, the practical application is that it would perhaps help to explain why entering winter with a young queen and abundant stores of beebread helps a colony to survive the winter. More research on this subject is clearly needed!
Practical applications
I’ve now covered at least some of the ways that honey bees naturally deal with moisture balance in the winter cluster. This understanding then suggests ways that beekeepers could manage their colonies for optimal wintering success.
Optimal Cluster size
Again we can look at nicely-aged studies by old-school bee researchers, including those of Jeffree [[16]], performed when I was only a child. Jeffree sought to determine the optimal size for the winter cluster, hypothesizing that:
[Small colonies] will have more bees in the cold, outer shell, all making great efforts to stay alive by converting honey into heat ― and accumulating more and more ‘waste water’ within the totality of the cluster. The center being small, no movements in or out of the cluster center can cope with the situation and, only cleansing flights can ― theoretically ― bring relief. When these are not possible, it seems that dysenteric conditions must come about, forcing bees to defecate in the hive, on combs.
… but very large clusters are at a disadvantage in that they either tend to cool too much at the outside or else overheat in the centre. Somewhere between these two extremes would presumably lie an optimal wintering size.[[17]]
In one study, Jeffree and Allen intermittently measured the strengths of 153 colonies in total, divided over the course of two winters in Scotland, in order to see how late-season starting strength correlated with colony strength the following spring ― for colonies with or without nosema infection. They concluded that:
The percentage loss of bees over winter was found to be significantly greater for very large and for very small colonies than for colonies of medium size. In this sense, the fact of a theoretically optimum size for wintering was thus established.
I found the manner in which their data was presented a bit difficult to interpret, so I graphed it differently below (Fig. 2).
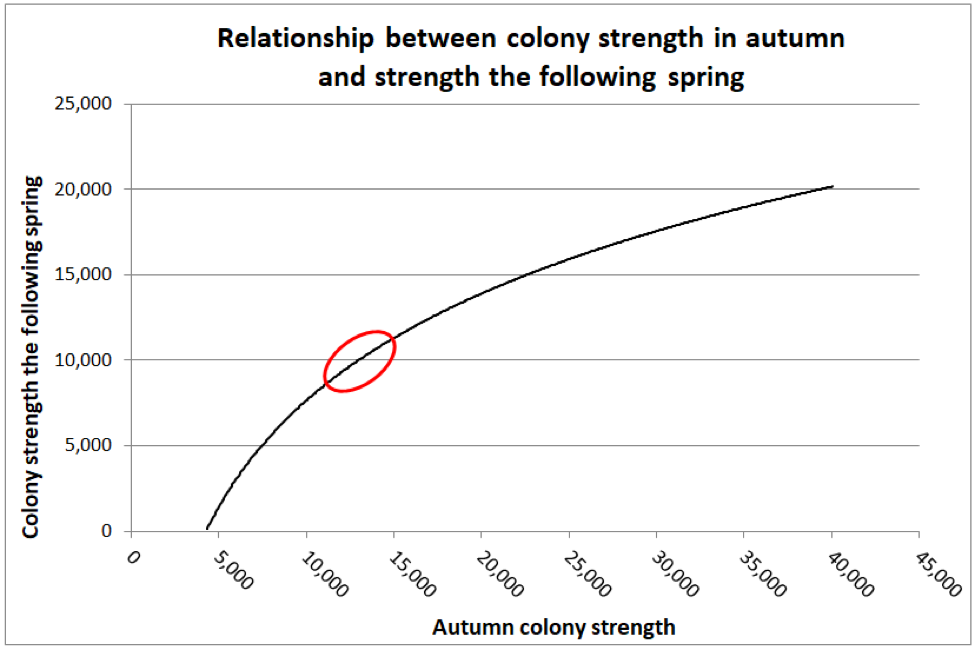
Figure 2. Jeffree and Allen found that as far as predicting colony strength in April, that there was a diminishing return from increased strength going into winter, as indicated by the diminishing slope of the curve. I’ve circled the authors’ suggested “sweet spot” for optimal autumn strength (at least for what I’m assuming were Apis mellifera mellifera in Aberdeen’s moderately-cold winters). The researchers also found that if a colony was infected with nosema, that there was a benefit to starting with a few thousand extra bees, in order to account for the reduced survivorship of the infected workers.
Practical application: Jeffree’s sweet spot works out to be the equivalent of around 6 deep Langstroth frames fully covered tightly with bees [[18]] ― what we’d call an 8-frame (or better) cluster. But “optimal” is likely relative to how cold it gets, as well as one’s goal in spring (having large colonies for almond pollination, as opposed to reducing swarming before the honey flow).
But based upon some data that I published some years ago [[19]], it appeared that my own Italians tended to shoot for an 8-frame cluster in December, with those that started much larger in October exhibiting an apparent shedding of excess bees during November (Fig. 3).
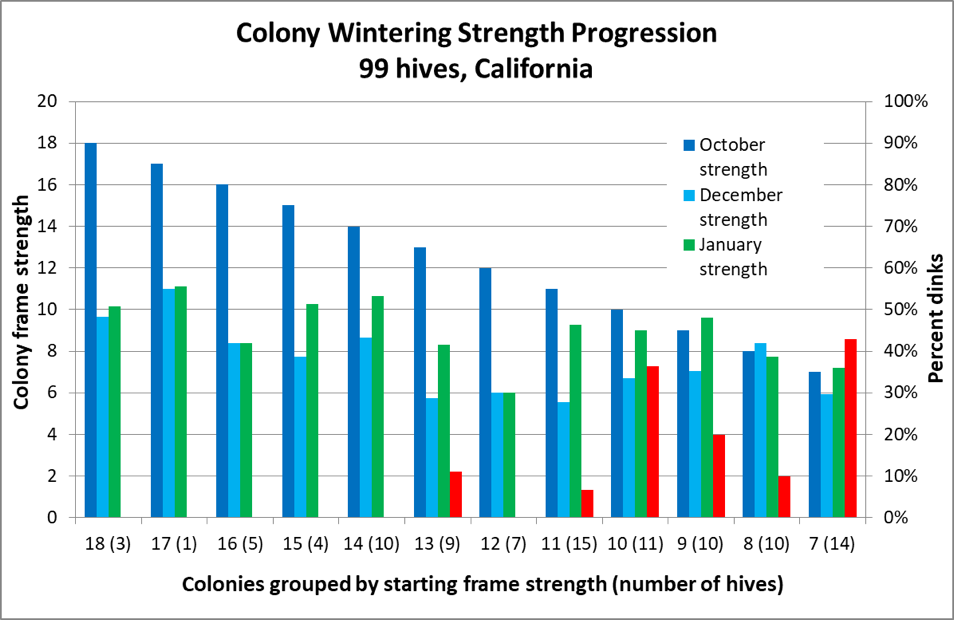
Figure 3. The dark blue columns represent colony strength in October; the turquoise columns strength in December ― note how the difference between the two diminishes with starting strength. This data appears to confirm Jeffree’s conclusion that there is a diminishing return for very large autumn strength ― note the amount of growth by weaker colonies between December and late January (green columns). However, also note for hives going into winter at less than 14-frame strength in October, there was an increased occurrence of them later being too small to take to almonds (the red columns indicating the percentage that turned out to be “dinks”) ― perhaps due to existing nosema or viral issues (which I did not measure).
So why would those strong colonies reduce their winter cluster size to such a great extent? Möbus ran an experiment by combining two colonies into one ― in order create “super colonies.” He found that when there are too many bees in the winter cluster, or if the hive is too well insulated in a mild climate, that the cluster couldn’t deal with its own generated heat, which resulted in both the overheating of the cluster core, as well as forcing “thirst-crazy” bees to fly out to seek water, resulting in them quickly chilling and dying outside of the hive.
Practical applications: The optimal cluster size for wintering is likely dependent upon the ambient temperature. At temperatures around freezing, undersize clusters may be more prone toward dysentery (this assumption needs to be confirmed by research), whereas oversized clusters may suffer from overheating and thirst. This situation may be reversed if the bees are forced to deal with subzero (°F) temperatures ― Omholt’s model suggests that large clusters under those conditions would accumulate moisture.
And then there is the question of bee genetic stock. Cold-adapted races (e.g., Carniolans or Russians) may winter well in a 6-frame or smaller cluster, yet still build up rapidly once pollen becomes available in the spring [[20]].
Optimal cluster size may also be different for a beekeeper intending to go to almond pollination with strong colonies to be split immediately after bloom, as opposed to one who aims for minimal honey consumption during long confinement, and lack of swarming before a later-occurring honey flow.
Winter stores ― honey and beebread
I’ll quote from Farrar’s timeless advice [[21]]:
Whether a colony survives the winter in good condition is determined more by its make-up than by the kind or amount of protection. A good colony normally requires 60 or more pounds of well-ripened honey and the equivalent of 3 to 6 frames of pollen.
Unripened honey contains an excess of moisture. And with honey that crystallizes in the comb, a high-moisture supernatant may form as the glucose precipitates out. Bees feeding upon unripened honey or dilute supernatant may develop dysentery. Beekeepers in areas in which their colonies go into winter with crystallization-prone honey reserves (such as canola or ivy) report that their colonies winter better on sugar syrup (perhaps containing inverted sugar). I’m not at all clear on the effect of local honeydews. As far as stored pollen (beebread), this is necessary to provide the protein necessary for broodrearing.
Practical application: A few natural honeys are tough for colonies to winter on; colonies sometimes do better on stored sugar syrup converted to “honey.” But keep in mind that it’s important to give the bees time to ripen any late-fed sugar syrup to remove excess moisture. Feeding syrup during the winter can overload a colony with moisture, thus the recommendation to feed fondant, granulated sugar, or sugar bricks.
Clarification: I am not suggesting that converted sugar syrup is “better” for the bees than most natural honeys, which contain trace amounts of protein and minerals, which may have some benefit to “winter bees” that do not have access to stored beebread.
Many beekeepers around the world harvest most of their colonies’ honey for sale, and then feed back less-valuable sugar syrup, and their colonies seem to winter well.
Disclaimer: I have a bias towards “naturalness,” in my own life, as well as for my bees. For the first 25 years of my beekeeping career I eschewed any sort of artificial feeding. But then one day a friend who was a professional beekeeper said “I don’t know whether feeding sugar syrup is good or bad biologically, but I can tell you that it’s often like waving a magic wand over a hive.” This observation forced me to rethink whether I was letting my own bias prevent me from practicing optimal bee husbandry.
There is no lack of strong opinions among beekeepers, and one reader questioned my suggestion that the feeding of sugar syrup can be beneficial to a colony, especially with regard to winter stores. So I recently reviewed the literature.
Back in 1977, Dr. Floyd Moeller of USDA summarized the results of their studies on winter prep. His recommendations indicated that honey bees winter well on cured sugar syrup [i].
Guler [ii] more recently found that in their study, the feeding of sucrose or sucrose/invert blend resulted in better wintering than in control colonies feeding upon their honey (I would not extrapolate this finding to suggest the syrup is necessarily better than honey).
Szawarski [iii] found no apparent differences in winter performance of colonies consuming honey or sucrose syrup.
Wang’s findings [iv] suggest that sucrose maintains adequate gut microbial community over winter.
Lastly, Papežíková [v] observed no substantial effect of sugar type upon nosema infection.
Conclusion: The scientific evidence confirms beekeeper experience around the world – bees (similar to hummingbirds) are well-adapted to making use of refined sugar.
I now have many years of experience in feeding sucrose syrup and sucrose/HFCS blends (as well as various sugar fondants and dry forms). We feed judiciously (since it is labor intensive), but don’t hesitate to use sugar to benefit our colonies when indicated. We still winter our colonies mainly on natural honey.
After learning to use sugar syrup, I agree with my friend’s observation that sugar (in either syrup or solid form) is one of the most useful tools available to the beekeeper.
[i] Moeller, F (1977) Overwintering of honey bee colonies. USDA Production Research Report #169.
[ii] Guler, A, et al (2018) Effects of Feeding Honey Bees (Hymenoptera: Apidae) With Industrial Sugars Produced by Plants Using Different Photosynthetic Cycles (Carbon C3 and C4) on the Colony Wintering Ability, Lifespan, and Forage Behavior. Journal of Economic Entomology11(5): 2003–2010.
[iii] Szawarski, N, et al (2019). Effect of abscisic acid (ABA) combined with two different beekeeping nutritional strategies to confront overwintering: Studies on honey bees’ population dynamics and nosemosis. Insects 10(10): 329.
[iv] Wang, H, et al (2020) The different dietary sugars modulate the composition of the gut microbiota in honeybee during overwintering. BMC Microbiol 20 : 61.
[v] Papežíková, I,et al (2020). Effect of feeding honey bee (Apis mellifera Hymenoptera: Apidae) colonies with honey, sugar solution, inverted sugar, and wheat starch syrup on nosematosis prevalence and intensity. Journal of Economic Entomology 113(1): 26-33.
It’s difficult for the beekeeper to control how much beebread is stored, but perhaps the feeding of supplemental protein at the right time may be a management tool for helping colonies to deal with moisture balance. Again, this subject is crying for more research.
Hive placement
It helps a colony greatly to be able to take advantage of the occasional warm winter day, which allows it to break cluster, move to honey stores, and for the bees to take cleansing flights (with sick bees being less likely to return).
Practical application: Experience can teach a beekeeper a great deal about locations suitable for wintering. It helps the colony greatly to avoid being in a cold pocket, and to enjoy a warming southern exposure (indeed, insulation on the south side may perhaps be detrimental). Moving hives even a few dozen yards may make all the difference in the world. One may consider providing a dark south-facing surface to absorb sunlight (with minimal insulation on that side), and making sure that the colony enters the winter with the cluster close to a lower entrance (so that workers needing to defecate don’t need to traverse cold abandoned combs on their way out). To get around these (and other) issues, indoor wintering has long been popular in Canada, and is becoming increasingly common in the U.S.
Hive insulation
A number of researchers have run field trials to determine whether there is a benefit to insulating hives during the winter. The results and conclusions drawn are confusing. There may be a benefit to side insulation where winter temperatures are extreme, but top insulation and making sure that the hive is free of air leakage due to wind appears to be more important. Adding a trapped air space or polystyrene insulation under or over the hive cover helps greatly to minimize colony heat loss, and to prevent moisture from condensing under the lid. Top insulation (without top ventilation) also helps to keep the top of the cavity warmer than the lower parts, thus benefitting the colony by helping to recapture the heat of condensation of vented moisture below the cluster.
During a visit to Chile in 2015, where the winter clusters that I observed were very small, beekeeper Vincent Toledo showed me how some beekeepers there swear by placing a plastic “poncho” over the frames containing the cluster, expanding the poncho over adjacent frames once the cluster starts to grow (Fig. 4).

Figure 4. A plastic “poncho” lifted to expose the cluster that it had been draped over. I have not tested ponchos myself, but such a waterproof blanket may help a small cluster to conserve heat and control moisture. Note: this practice was not universally used in Chile.
Boston beekeeper Fatih Uzuner tells me that he is currently experimenting with using inexpensive 65-gallon plastic bags (black or clear) to trap an air space around the hive (of course leaving the hive entrances clear) ― such an arrangement provides a bit of insulation and draft resistance, yet allows solar gain. I’m eager to see his results.
Practical application: Some beekeepers confuse top insulation and moisture absorption. In general, if a filling absorbs moisture, it is unlikely to serve as insulation. I fail to understand the reasoning for absorbing moisture above the cluster ― why not get it to condense below the cluster? It may also be that too much honey or other enclosed space above the cluster could be a problem, since moisture may condense on the cold surfaces [[22]].
Hive ventilation
If you want to get into a fruitless debate, ask some beekeepers their opinions on providing hive ventilation during winter. As noted by Southwick and Moritz [[23]], perhaps we should listen to the “opinion” of the bees:
Many experienced beekeepers know about the value of a top ventilation hole and maintain such an opening throughout the year. However such extra entrances are usually closed with propolis by the bees.
Möbus [[24]] explains:
How often do we read about the need for top ventilation as the answer to all problems? It is supposed to be essential to let the moisture get away from wintering clusters and out of the hive. … Many of the arguments given to back up any recommendations for providing more and more top-ventilation are based on reasoned considerations or anthropomorphic thinking rather than on sharp-eyed observations of bee behaviour. For those who observe behaviour without preconceived ideas it is obvious that the bee has arranged its home to suit its own experiences within the constructed set of combs and the chosen home. … When levels of carbon-dioxide or humidity rise, bees begin to fan and relieve the situation along the line of least resistance: downward and away from the brood nest.
Practical application: bees have been wintering in cavities far longer than humans have been building hives. If they want to close the door to prevent drafts, perhaps it’s for a reason.
I’ve reviewed a ton of studies, and am not convinced that under most circumstances top ventilation is of benefit to the colony. Toomemaa [[25]] says it well:
However, provision of too much ventilation also removes heat vital for bees and forces them to increase their metabolic rate to compensate for the heat loss. Increased food consumption results in increased production of metabolic water and increased moisture in the hive.
He also cites studies (in Russian) that found that decreased ventilation in hives during the winter resulted in less honey consumption, less bee mortality, and better brood rearing in the spring.
Practical application: If indeed the natural instinct of bees is to fan moisture-laden air out of the bottom of the cluster in order to recycle heat, then the beekeeper creating an air convection current via top ventilation may be counterproductive. Again, we need more good practical research on the subject of moisture regulation in the winter cluster.
Literature cited
[1] van Nerum, K, and H Buelens (1997) Hypoxia-controlled winter metabolism in honeybees (Apis mellifera). Comparative Biochemistry and Physiology 117(4):445-455
[2] Omholt, SW (1987) Why honeybees rear brood in winter. A theoretical study of the water conditions in the winter cluster of the honeybee, Apis mellifera J. Theor. Biol. 128: 329-337.
[3] Heinrich, B (1981) The mechanisms and energies of honeybee swarm temperature regulation. J. Exp. Biol. 91: 25 ― 55. Heinrich’s fascinating papers and books are must reads for those wishing to gain a deeper understanding of bee biology.
[5] Sachs & Tautz (2017) Op cit.
[6] Toomemaa, K, et al (2013) Determining the amount of water condensed above and below the winter cluster of honey bees in a North – European Climate. Journal of Apicultural Research 52(2): 81-87.
[8] Thanks to Trevor Weatherhead.
[9] Jeffree, EP (1956) Winter brood and pollen in honeybee colonies. Insectes Sociaux 3(3): 417―422.
[10] Möbus, B (1990?) op cit.
[11] Mattila H, et al (2001) Timing of production of winter bees in honey bee (Apis mellifera) colonies. Insectes Sociaux. 48: 88―93.
[12] Möbus, R (1980) Proceeds 27th International Congress of Apiculture
[13] Omholt, SW (1987) Op cit.
[14] Möbus, B (1998b) Brood rearing in the winter cluster. ABJ July 1998: 511-514.
[15] Rembold, H & J Kremer (1980) Characterization of postembryonic developmental stages of the female castes of the honey bee, Apis mellifera L.. Apidologie 11(1): 29-38.
Ghosh, S, et al (2016) Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. Journal of Asia-Pacific Entomology 19: 487―495.
[16] Jeffree, E & D Allen (1956) The influence of colony size and of nosema disease on the rate of population loss in honey bee colonies in winter. J. Economic Entomology 49(6): 831-834.
Jeffree, EP (1956) Op cit.
[17] Jeffree, EP (1959) The size of honey-bee colonies throughout the year and the best size to winter. Central Association of Bee-Keepers, Essex.
[18] Burgett, M. & I. Burikam. 1985. Number of adult honey bees (Hymenoptera: Apidae) occupying a comb: a standard for estimating colony populations. J. Econ. Entomol. 78: 1154-1156.
[20] Thomas Rinderer, pers. comm.
[21] Farrar, CL (1944) Productive management of honeybee colonies in the Northern States. USDA Circular No. 702. Free download at Google Books.
[22] Toomemaa, K, et al (2013) Determining the amount of water condensed above and below the winter cluster of honey bees in a North – European Climate. Journal of Apicultural Research 52(2): 81-87.
[23] Southwick, E & R. Moritz (1987) Op cit.
[24] Möbus, B (1990?) Op cit.
[25] Toomemaa, K, et al (2013) Op cit.
Bee Sales by Golden West Bees
2024 Announcement: We are now collaborating with Olivarez Honey Bees to produce queens from our chosen breeders, mated in a dedicated isolated mating yard stocked with our own drone mother colonies. Please contact them for large orders. For nuc sales, please contact Eric at oliverhoneysales@gmail.com
We are a small short-staffed family business, not nearly as polished as the “big boys,” but we do proudly produce and sell top-quality nucs, and a limited number of packages and queens.

Eric, Randy, and Ian
CONTACT AND ORDERING
Eric has now taken over handling all sales.
Email: In general, contact Eric at oliverhoneysales (at) gmail.com (address protected from spambots).
Phone: If you need to call in order to coordinate pick up please contact Eric at 530 277 5004.
I find that many of the best management resources for professional beekeepers interested in best management practices for their managed livestock were written long ago, before all the hoopla about “The Decline of Bees.” Those practices still apply — other than varroa mite, little has changed about beekeeping. So the update is to keep varroa infestation rate below the 2% level at all times of the year, otherwise the old recommendations are just as good now as then.
The following USDA report is one of the best, applicable to areas with extended winters (not necessarily California):
Farrar 1944 Productive-management-of-honeybee-colonies
Overwintering of honey bee colonies
This subject has long generated endless debate among beekeepers. There are a few excellent resources by those who have collected hard data:
Dr. Floyd Moeller was a USDA researcher who performed extensive field research to test various beekeeping management practices.
This excellent publication covers practices to improve overwintering success, and although written in 1977, most of what he wrote is still relevant.
Updates would be:
- Nosema apis has now been largely replaced by N. ceranae, which doesn’t appear to cause as much problem over winter; his recommendation to prophylactically fed fumagillin is now questionable.
- We’ve now learned that prophylactic feeding of antibiotics against brood diseases results in the development of bacterial resistance. And sulfathiozole is no longer allowed in hives.
- There is debate as to upper ventilation of hives during winter. The issue centers on whether the benefit of the induced convection current outweighs the cost of the additional heating load placed upon the colony. In most areas, simply placing dry insulation at the top of the hive, with a winter wrap in windy areas, seems to be adequate. Moisture accumulation may be the result of the cluster size being out of balance with the cavity size (too small a cluster in too large a hive).
Read Moeller’s report at: Moeller 1978 Overwintering of Honey Bee Colonies
Two other articles of great interest were by Bernart Mobus (umlaut above the o), published in ABJ back in 1998.
Mobus 1998 brood rearing in winter cluster
Mobus 1998 winter cluster part 2
I’ve also covered the subject at:
The Physics of the Winter Cluster
The Winter Continued
The Winter and Hive Design
Contents
The bees’ need for water. 1
Water and the winter cluster. 1
Water Balance. 2
Water homeostasis and buffering in the winter cluster. 3
Water in the gut. 3
Atmosphere and Humidity within the winter cluster. 3
Evaporation via respiration. 5
Defecation/Dysentery. 6
Literature cited. 8
The Nosema Problem: Part 7b
The Causes of Dysentery in Honey Bees: Part 2
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in January 2020
Finally ― it’s time to get to what actually does cause dysentery in a hive, and (in my next article) what the colony (or the beekeeper) can do to minimize its occurrence. In investigating this subject, I was surprised by how much hard-earned knowledge, published years ago by astute researchers, seems to have been forgotten.
The bees’ need for water
During a strong nectar flow, the colony is awash in the excess moisture that needs to be evaporated to ripen that nectar into honey for storage. But for a bee to later use the honey as an energy source, it needs to add back water to dilute it for uptake through its proboscis [[1]], as well as for digestion. The preferred sugar concentration for consumption appears to be in the 40-60% range [[2]]. For much of the year, workers forage for the water necessary to dilute the honey ― if necessary, even flying at temperatures as low as 40°F (4°C) [[3]]. But the focus of this article is about what happens when it gets too cold to fly, and the bees are stuck in a winter cluster with no outside source of water.
Water and the winter cluster
In the winter cluster, bees are in a tricky situation ― if there’s not enough liquid water available, they will desiccate and die, due to the unavoidable water loss from breathing. But on the flip side, too much moisture in the cluster can lead to the growth of mold, fermentation of honey, and dysentery (Fig. 1).

Figure 1. This hive was inadvertently tipped slightly backward, preventing rainwater from draining out. In my experience, water pooled on the bottom board is extremely stressful to a colony during our California winter ― often leading to dwindling or death.
Water Balance
The “winter bees” in the cluster have little need for protein, due to their well-developed fat bodies, and can survive for a long time on a diet of honey (sugar) alone. The weight loss of a hive in winter cluster (not rearing brood) is in the ballpark of around a pound a week ― presumably mainly due to the consumption of its honey. That honey consists of roughly 83% sugars (mostly glucose and fructose) and 17% water. The bees metabolize only the sugars, according to the following equation:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
When you do the math, the metabolism of the sugar in that pound of honey produces 6/10 of a pound of water. Add to that the 17% of liquid water already present in the honey, and you wind up with that pound of honey turning into 2/3 of a pound (1¼ cups) of water (initially held within the bees’ bodies). The bees in the cluster cannot allow that 1¼ cup of water to accumulate, and must deal with it in some manner.
Practical application: The bees recycle roughly ¾ cup of that excess water into saliva to dilute the next pound of honey for consumption, but that doesn’t affect the net 1¼ cups of water gained each week that still needs to be dealt with in some way.
Water homeostasis and buffering in the winter cluster
Bees in the winter cluster have only a few options for what to do with the water in their bodies resulting from the consumption of honey; they can either:
- Hold it in their hind gut (up to a point), or
- Defecate it (not a desirable option in the cluster), or
- Exhale it through their spiracles via “respiratory transpiration,” or
- Feed it out through their proboscis (generally to another bee).
In this article, we’ll look at Options 1 and 2 (The Problem). I’ll cover Options 3 and 4 (The Solutions) in the final installment of this series.
Water in the gut
As explained in an excellent paper on the winter cluster by Johansson [[4]]: Water not absorbed in the midgut is retained in the hindgut, where it forms a reserve that is utilized by osmotic diffusion when the water content of the haemolymph becomes too low.
The bees need a reservoir of water, since with every breath they exhale, they lose some water from their body. This is a serious issue for insects that can’t access liquid water, so they control how often they open their spiracles to breathe. Thus, it’s advantageous for a wintering bee to hold some water in reserve in its hindgut to replace that lost by evaporation during respiration. But that evaporation due to breathing is entirely dependent upon the relative humidity of the intake air, and if the humidity around the bee is too high, it won’t be able to evaporate the water that it gains from consumption of honey. So we need to understand the humidity within the winter cluster.
Atmosphere and Humidity within the winter cluster
The bees in a winter cluster can be quite tightly packed, taking up only a fraction of the volume that they occupy when it’s warm [[5]]. The cluster is surrounded by a “mantle” of bees facing inward, with their bodies squeezed so closely together that they can control any air movement in or out of the cluster.
A fascinating study by van Nerum and Buelens [[6]] found that unlike humans, whose urge to breathe is determined by the CO2 content of their blood, it appears that bees will tolerate a very high CO2 concentration before initiating ventilation, and instead control their respiration and metabolism in response to the oxygen level. The authors found that in the center of the cluster, the oxygen content is allowed to drop to as little as 15% (down from 21% in the air that we breathe), and carbon dioxide is allowed to rise to up to 5-6% (up from 0.04%) (Fig. 2). The researchers found that the lowered oxygen content (hypoxia) in the winter cluster results in the bees in the core decreasing their resting metabolic rate, thus reducing food consumption and minimizing heat loss from needed ventilation. It also conserves water within the cluster.
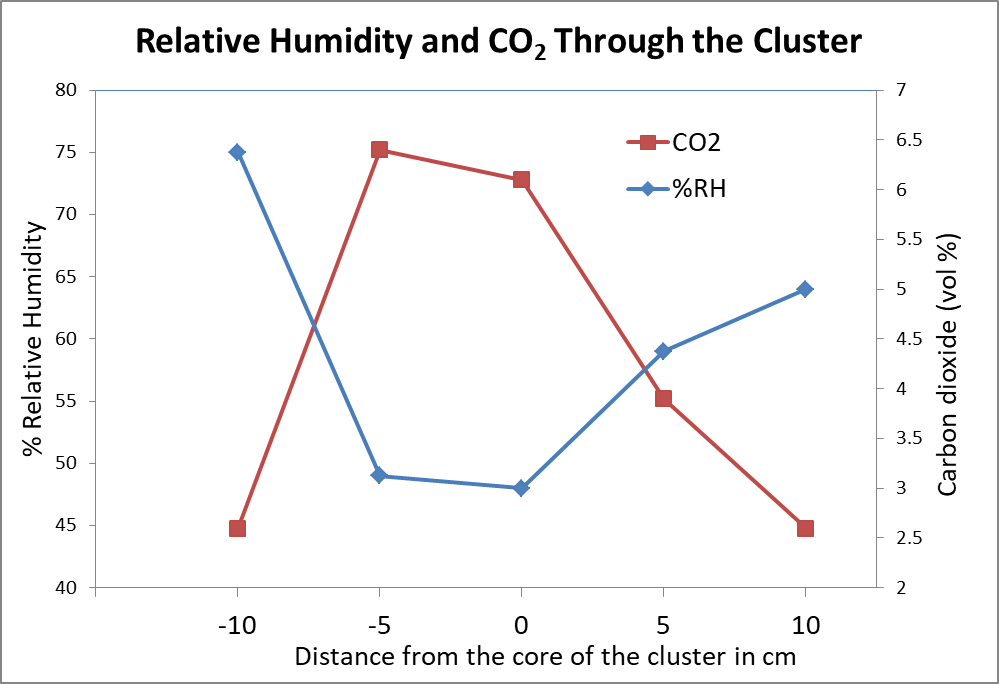
Figure 2. Relative humidity (blue line) is high in the mantle of the cluster, but low in the core. Carbon dioxide (red line) inversely increases greatly in the core. These means of 10 measurements also indicate that there is nearly no air circulation in the cluster when the bees go into this “hypoxic” (low oxygen, low metabolic) state. Chart after van Nerum & Buelens [[7]]
The winter cluster of bees is akin to a warm blooded mammal with a cool skin temperature. But unlike a mammal, the cluster lacks a common bloodstream to transport O2, CO2, water, or heat ― so the bees instead depend upon air flow to do so. A number of researchers have measured humidity in the cluster, finding that it oscillates up and down in the range of 45-70%, but without a concurrent change in temperature, suggesting that the bees are able to ventilate out moisture while at the same time conserving heat. Ellis [], noting that much of the cluster is typically on drawn comb, points out that dark comb full of silk cocoons can absorb up to 11% of its own mass in water when exposed at high humidity. Thus, such combs can exert a substantial buffering effect on humidity within the hive.
Practical application: Dark drawn combs may help to buffer humidity in the winter cluster. Some experienced beekeepers suggest that colonies winter better on old comb containing cocoons, but there is a paucity of research on the subject ― another question calling for investigation.
The control of humidity within the cluster appears to be of utmost importance in the winter cluster, since the relative humidity determines the rate at which bees lose water during respiration.
Evaporation via respiration
As explained in the Johanssons’ “The Honeybee Colony in Winter” [[9]]:
… Since honeybee faeces are liquid, the diffusion of water through the wall of the hindgut into the haemolymph, and subsequently through the tracheal wall where it evaporates into the atmospheric air within the cluster, prevents an excessive accumulation of water in the hindgut.
But as I mentioned previously, that evaporation of water from the tracheal wall is dependent upon the relative humidity of the air that the bee breathes in. A wonderful “old-school” study by Woodrow in 1935 [[10]] demonstrated how humidity affects a bee’s ability to purge itself of excess water via respiratory transpiration. Woodrow placed bees in small cages, all at around 71°F, but held at different humidities, feeding them 50:50 sugar syrup, and carefully observed their bodies and behaviors over time, as well as their longevity (Fig. 3).
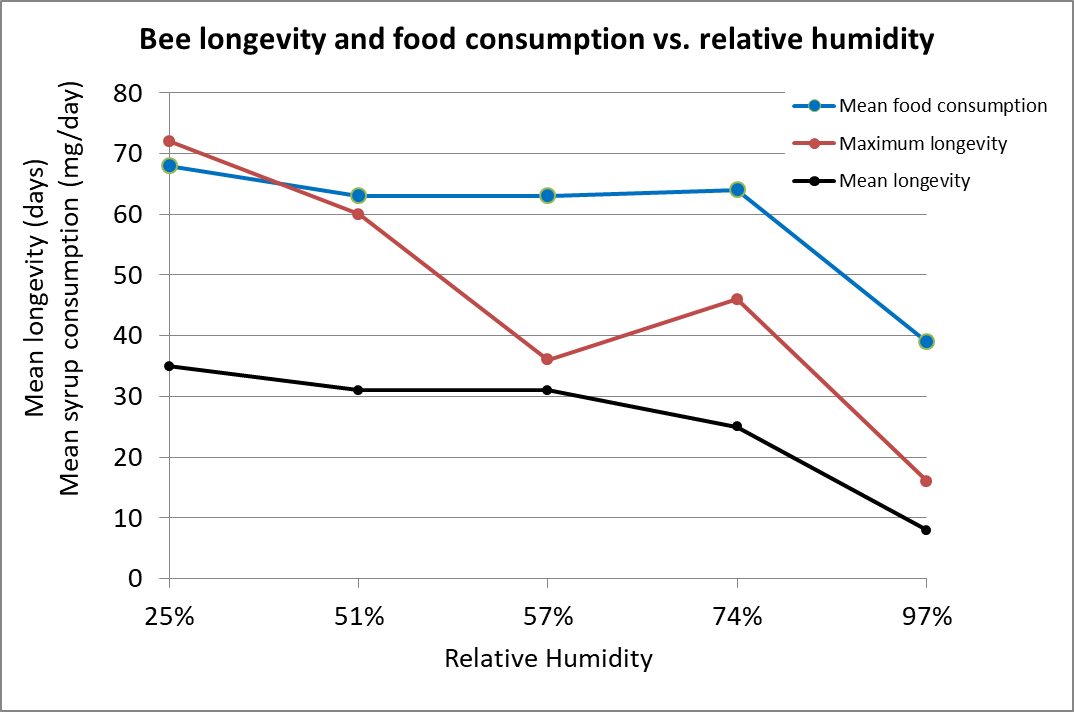
Figure 3. Caged worker bee consumption of 1:1 syrup, and maximum and average longevity at various humidities. Woodrow’s data suggests that at above around 70% RH, a bee is unable to maintain water balance via respiration (compare that to the humidity figures of the mantle in the previous figure). Chart after data from [[11]]
Woodrow found that bees would apparently sometimes die rather than to defecate excess water in their hindgut:
The first evidence of feces accumulation at the lowest relative humidity was noticed about the twentieth day and this condition slowly became more pronounced as the experiment progressed. As the bees in the different cages became more sluggish due to fecal accumulations, it is probable that some of them actually starved to death. The progressively smaller food consumption per bee per day no doubt is due partly to the fact that some of the bees were unable to feed. It appears that accumulation of feces is one cause of the death of the bees exposed to the higher ranges of relative humidity
Practical application: In the high relative humidity present in the cluster mantle, bees there will tend to accumulate moisture in their hindguts, to the point that they can no longer feed.
Free & Spencer-Booth [[12]] on the other hand varied the temperature, but did not measure humidity, offering caged bees both 67% sugar syrup and pure water separately ― measuring how much they consumed of each. They found that:
Very little water was drunk at environmental temperatures of 25°C. or lower but, at 35°C. and above, relatively enormous quantities were taken.
Note that they weren’t sure whether the increased water consumption was solely to replace moisture lost through respiration, or whether at the higher temperatures some transpiration occurred through the cuticle covering their bodies [[13]].
Practical application: At broodnest temperature, bees need additional water to prevent dessication.
Möbus [[14]] put this all together to explain that the bees in the center of the winter cluster will experience desiccation, whereas the bees in the outer shell will accumulate more metabolic water than they can evaporate.
Practical application: The bees in the core of the cluster suffer from thirst, whereas the bees in the mantle are experiencing moisture abundance.
To rectify this situation, it appears that the bees in a winter cluster from time to time exchange positions — those on the inside move outward to consume honey diluted by its absorption of condensing water, while those bees in the outer mantle move to the center to “dry out” (perhaps by sharing dilute saliva, as well as to allow their enzymes to warm up for optimal functionality).
Omholt [[15]] also points out that the bees from the periphery (that were clustered over cool, moisture-rich honey) would likely bring a crop full of that diluted honey to the bees within the cluster. This mechanism would answer a question that I’ve long had about how the bees on empty drawn comb in the core of the cluster get fed).
Practical application: I’ve yet to find any research on the actual movement of honey or water from the bees in contact with honey at the periphery of the winter cluster, to workers elsewhere in the cluster (that are not in contact with honey). Another avenue of research calling for investigation!
Defecation/Dysentery
The easiest way for a bee to get rid of excess moisture in its gut is to simply defecate, and during warm weather, they readily fly out to do so. But that option is off the table when it’s cold, although they will take advantage of any warm days to take cleansing flights. The last thing that a bee “wants” to do is to defecate within the hive. So the key question is, “Then what causes them to do so?”
Over the course of two winters, Erwin Alfonsus at the University of Wisconsin addressed “The Cause of Dysentery in Honeybees,” published in 1935 [[16]]. Alfonsus was a keen observer of bees, and performed the sort of “old-school” detailed and meticulous experiment that I so enjoy reading. His introduction read:
Dysentery, a winter disease of honeybees, has been known since Aristotle’s time. Normal defecation in the honeybee takes place on the wing during the flight season. The wintering bee, confined to the hive, is deprived of this opportunity, and the fecal material accumulates in the rectum. An over-accumulation of feces may lead to a forcible discharge in the hive or on the alighting board; this occurrence is called dysentery.
Over two winters, Alfonsus tested various feeds in order to see whether they would induce dysentery. He fed shed-wintered colonies either natural stores of honey (including honeydew), 1:1 sugar syrup, dilute sugar syrup, fall honey, syrup inoculated and fermenting with yeast, syrup boiled until brown, autoclaved syrup, syrup with dextrin (hydrolyzed starch), crystallized honey, aged honey, crystallized sugar syrup, sugar candy, or candy with honey and pollen.
His conclusions:
The only factor showing any relation to the amount of accumulated feces was the moisture content. The dry matter in feces did not increase fast enough to be considered a causative factor of dysentery. The dilution of feces, however, is very conspicuous and increased as the season advanced and as dysentery became more apparent. The first bees left the cluster to discharge their feces around the entrance when the mean fecal accumulations amounted to 33% of the total body weight of the bees and when the feces contained approximately 80% moisture…The occurrence of dysentery is similar in protected colonies interrupted during the honey flow by rain. The bees are shut in and forced to eat unripe honey. Within a short time, the rectum expands and defecation may take place.
SUMMARY AND CONCLUSIONS
- Dysentery of honeybees is caused by excess moisture in the feces.
- This excess moisture is due to the consumption of dilute food or water. It is generally produced by crystallization of the stores; this divides the honey or syrup into a solid crystalline portion and a liquid portion. The liquid portion contains an excess quantity of moisture.
- Pollen, dextrin, minerals, burned sugar, and fermenting syrup do not produce dysentery.
- Chilling and disturbing honeybees may cause defecation, but do not produce dysentery in a healthy colony.
- Long confinement of bees during the winter, as well as a short confinement on unripe honey, produce dysentery.
- Water alone or dilute syrups produce dysentery in bees if absorbed during confinement.
- Dysentery appears when the fecal accumulations reach 33% of the total body weight of the bees. General defecation does not take place until the accumulation reaches about 45%.
Practical application: Alfonsus’s paper did not mention nosema. Dysentery, rather than being a symptom of nosema infection, appears to be due to unmanageable moisture accumulation in the guts of wintering bees. I’ll wrap this series up in the next installment, in which I’ll cover the things that bees, and their keepers, can do to solve this problem
Literature cited
[1] Simpson, J (1964) Dilution by honeybees of solid and liquid food containing sugar. Journal of Apicultural Research 3(1): 37-40.
[2] Eyer, M, et al (2015) No spatial patterns for early nectar storage in honey bee colonies. Insect. Soc. DOI 10.1007/s00040-015-0432-4.
Kim, W, et al (2011) Optimal concentrations in nectar feeding. PNAS 108(40):16618-16621.
[3] Chilcott, A & T Seeley (2017) Cold flying foragers: Honey bees in Scotland seek water in winter. American Bee Journal 158(1):75-77.
[4] Johansson, T & M Johansson (1979) The honeybee colony in winter. Bee World (60:4): 155-170.
[5] Severson, DW & EH Erickson (1990) Quantification of cluster size and low ambient temperature relationships in the honey bee. Apidologie 21: 135-142.
[6] van Nerum, K, and H Buelens (1997) Hypoxia-controlled winter metabolism in honeybees (Apis mellifera). Comparative Biochemistry and Physiology 117(4):445-455
[7] Ibid.
[8] Ellis, M, et al (2010) Brood comb as a humidity buffer in honeybee nests. Naturwissenschaften 97:429–433. Also see:
Ellis, MB (2008) Homeostasis: Humidity and water relations in honeybee colonies (Apis mellifera). MS Thesis, University of Pretoria. Ellis has written a number of more recent papers, detailing water dynamics in various hive configurations.
[9] Johansson, T & M Johansson (1979), op cit.
[10] Woodrow, AW (1935). Some effects of relative humidity on the length of life and food consumption of honeybees. J. Econ. Ent. 38: 565-568.
[11] Ibid.
[12] Free, J. B. and Spencer-Booth, Y. (1958) Observations on the temperature regulation and food consumption of honeybees (Apis mellifera). J. Exp. Biol. 35: 930 -937.
[13] Wigglesworth, V (1945) Transpiration through the cuticle of insects. Journal of Experimental Biology 21: 97-114.
[14] Möbus, B (1998) Rethinking our ideas about the winter cluster; Part II. ABJ August 1998: 587-591. Eugene, can you fill in the volume number? It would be 138.
[15] Omholt, SW (1987) Why honeybees rear brood in winter. A theoretical study of the water conditions in the winter cluster of the honeybee, Apis mellifera J. Theor. Biol. 128: 329-337
[16] Alfonsus, E. C. (1935). The cause of dysentery in honeybees. Journal of Economic Entomology, 28(3): 568-576.
I Googled the word “beekeeping” today, which came back with 39 million results — far too many for most of us to read!
The other issue is that with the advent of the Internet, those with strong opinions are free to upload their thoughts, without anyone first checking them for supportive evidence. And in beekeeping, there has never been a lack of strong opinions.
Planting Pollinator Gardens
Thanks to Girl Scout Jenna Miller for suggesting that I add this information.
If one wants to help “save the bees,” you don’t need to become a beekeeper. Perhaps the best way is to plant and maintain pollinator-friendly pasture, which will benefit not only honey bees, but also other pollinators and wildlife in general. What you should plant depends upon your ecoregion, and the amount of long-term care that you wish to spend on the garden.
In general, pollinator-friendly trees and shrubs provide the most resources and require little maintenance. Otherwise, planting native flowering plants (including groundcovers) will benefit native pollinators. There are also non-native ornamental and landscape flowers that may be attractive.
It helps pollinators to plant forage plants in blocks, so that they don’t have to fly far between blossoms. And of course, avoid applying most insecticides.
I can’t list planting guides for all ecoregions, but recommend that you check with Xerces Society and regional universities.
Xerces regional pollinator plant lists: https://xerces.org/pollinator-conservation/pollinator-friendly-plant-lists
Pollinator Partnership has a great guide for California at https://www.pollinator.org/PDFs/Guides/SierranStepperx7FINAL.pdf
Also for California: https://anrcatalog.ucanr.edu/pdf/8498.pdf
Northeast: https://pollinator.cals.cornell.edu/resources/planting-pollinator-habitat/
Midwest: https://www.beeandbutterflyfund.org/
Beginner’s texts:
Here’s a list of resources for the beginner that I feel are both readable and informative.
Of course, I’d start with one of my own: https://scientificbeekeeping.com/first-year-care-for-your-nuc/
First Lessons in Beekeeping, Dadant
The Beekeeper’s Handbook by Diana Sammataro & Alfonse Avitabile
Storey’s Guide to Keeping Honey Bees, 2nd Edition. Malcolm T. Sanford, Richard E. Bonney
Beekeeping Basics This used to be a freebie from Penn State.Homegrown Honey Bees by Alethea Morrison
Beekeeping for Dummies. Howland Blackiston
Honey Bee Hobbyist by Norm Gary—good overall understanding, rather than how-to-do-it.
Beautiful prose: A Book of Bees, Hubble. This is the book to share with your family to help them understand your passion.
Let me know if there are others that I should add to the above list!
References
The Biology of the Honey Bee, Winston. As the title says — relatively short and to the point reference book, nicely written by an expert.
The next two are the go-to textbooks:
The Hive and the Honey Bee, Dadant
ABC and XYZ of Bee Culture, Root
Contents
What the beekeeper can do. 1
seasonality. 2
detection and Sampling. 3
Queens And Nosema. 6
Deadout equipment. 7
citations and notes. 8
The Nosema Problem: Part 5
Monitoring and Disinfection
First published in ABJ October 2019
Randy Oliver
ScientificBeekeeping.com
Some beekeepers are concerned about the current unavailability of fumagillin. Although the Canadians are working to get it back on the market, there remains the question as to just how important it actually is to apply a prophylactic treatment to suppress nosema.
Back in the day, I followed the commonly-accepted practice of applying prophylactic treatments of Terramycin® to my hives in order to prevent AFB, Fumidil B® for nosema, grease patties for tracheal mite, and then Apistan® to every hive for varroa. But as our operation grew, and as we learned what was really necessary for colony health, we now rarely use Terramycin, no grease patties, only “natural” treatments for varroa as needed, and no “hive health” products. Yet our colonies thrive with minimal losses, and we sell 1000 healthy nucs every spring. This change in management was not for idealistic reasons, but rather based upon business decisions as to what gave us the best returns on investment.
That said, our “new” nosema still remains something of an enigma, and I’m not about to make any suggestions as to how you “should” best manage it in your apiaries. But I’ve now had over a decade of experience with tracking nosema in my own operation, as well as following research from around the world. I’m happy to share my confusion with you.
What the beekeeper can do
The first question that I ask beekeepers who approach me about a nosema issue is, “Have you checked with a ‘scope to confirm that nosema is actually a problem in your hives?” The usual answer is “No, but I saw dysentery.” I hope by now that I’ve made clear that nosema does not appear to cause dysentery, but that dysentery in the hive will likely increase transmission of the parasite (if it is indeed present) within that hive.
Practical application: A brief bout of dysentery in the spring does not mean that the colony is infected with nosema. However, if you observe dysentery, you may be able to put your mind at ease by checking some bees under a ‘scope. On the other hand, if you observe a colony that is inexplicably not exhibiting a normal rate of buildup in the springtime, an assessment for nosema would be wise.
seasonality
The first thing to keep in mind is that nosema, whether apis or ceranae, is mainly an autumn or springtime issue, and typically causes serious disease only when the workers are constrained by weather from being able to take defecation flights. The seasonality of ceranae was elucidated clear back in 2008, when Spanish researcher Dr. Mariano Higes [[1]] published illustrative charts of the progression of N. ceranae prevalence in the house and field bees over the course of two years. Since then, other researchers have confirmed that ceranae infection tends to follow the same track as that of apis. However, ceranae, unlike N. apis, may sometimes be found during summer.
In 2012, Chen [[2]], in Taiwan, found that the infection intensity of N. ceranae over the course of a year closely correlated with the local ambient temperature ― with greater infection when it was colder, then disappearing during the warm days from July through September. This finding surprised me, since Traver [[3]] had reported the year before that ceranae was prevalent in Virginia during the summer. So I questioned Chen at length to confirm his methodology, and then, in one of my own apiaries in the California foothills, tracked N. ceranae spore counts and prevalence (the percentage of house bees infected) over time [[4]]: It was the same in California ― nosema infection was clearly related to temperature (Fig. 1).
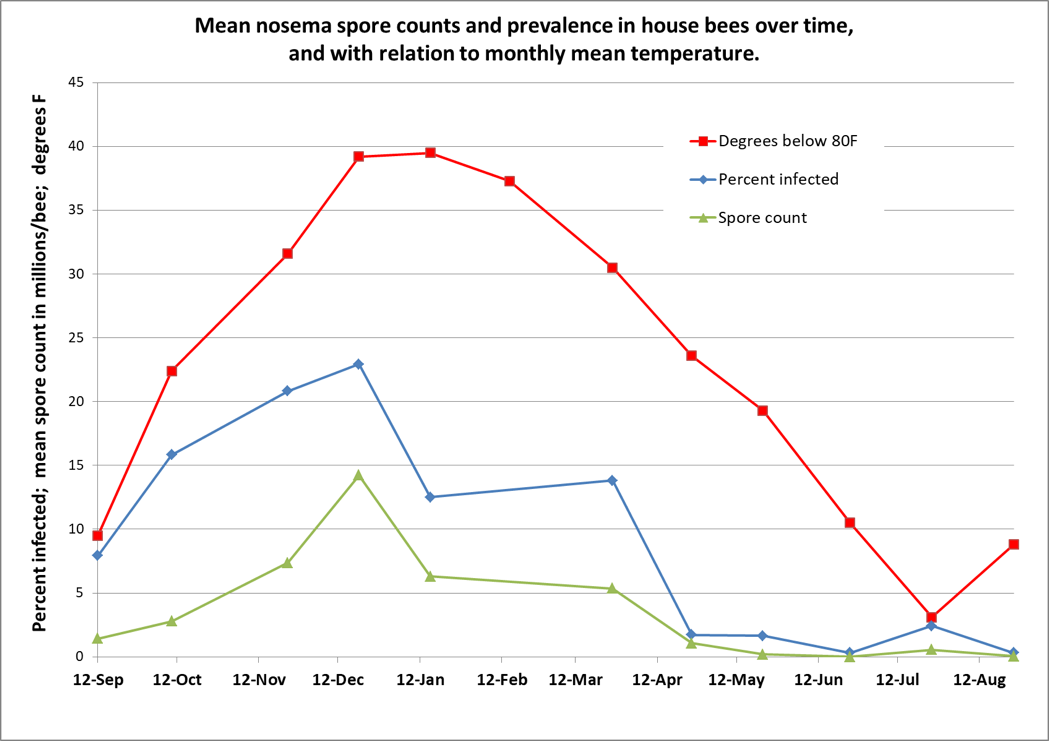
Figure 1. I tracked nosema spore counts and prevalence in an apiary for a year. I started with 36 colonies, and ended with 31, but there was no apparent correlation between colony survival and nosema levels. However, there was a clear relationship between nosema and the average monthly temperature (which I flipped upside down to show the correlation). In my area, colonies enjoy their first pollen flow in early January, and then build rapidly, largely clearing themselves of the parasite by May. Not shown is that the median value for percent of house bees infected never exceeded 20%, although one sample had 80% of the bees infected in December (surprisingly, that colony eventually completely purged its infection without treatment).
Practical application: Seasonally speaking, there’s likely little reason to be concerned about nosema other than during pollen flows in the autumn or spring, and only then if there is poor flight weather.
So that now brings us to the subject of how best to determine the level of infection by nosema in the hive.
detection and Sampling
I’ve written about this subject previously [[5]]. What most surprised me, after being scared to death about the invasion by N. ceranae, is that after I went back in time to read the open-access deep dive into N. apis by GF White, written in 1919 [[6]], that it appears to me that there is little difference between the two species as far as impact upon the colony. Allow me to quote directly:
The prognosis in Nosema-disease varies markedly and is dependent upon the conditions present. Of these conditions the percentage of Nosema-infected bees in the colony, the strength of the colony, the season of the year, and the environment of the apiary are among the most important factors which determine the outcome of the disease….As a rule colonies which in spring of the year show less than 10 per cent of Nosema-infected bees gain in strength and the losses are not detected…When the number of infected bees approaches 50 per cent the colonies become noticeably weakened and in many instances death takes place…As a rule, the stronger the colony, the more favorable is the prognosis.
Everything that I’ve observed or read agrees with what White wrote back then. So the question then is, how to determine what percentage of the bees are infected? White’s method of dissecting individual bees was tedious, so a shortcut was later developed by others to simply grind up 10 bees and count the number of spores in a hemocytometer to obtain an “average” spore count. Thus, we got hooked on “spore counts,” and when people (including myself) started taking spore counts from bees taken from the entrances of hives, those counts often left us gasping in alarm.
Practical application: I’ve given up on spore counts as being very meaningful. What I’ve also learned over the years is not to sample bees from the entrance, as it will just scare you, and doesn’t tell you what’s happening inside the hive.
Many researchers now sample “house bees,” typically taken from under the lid or from an outside frame. This gives you a “more accurate and representative sample of the whole bee population” of the hive [[7]]. And even then, spore counts can be misleading. Retschnig [[8]] points out that if you happen to sample a bee ready to take a defecation flight, that it will skew the spore count upwards (due to all the spores passively sitting in its hindgut).
Mulholland [[9]], using molecular analysis, found only a weak correlation between the degree of infection of a bee’s midgut tissue (where nosema replicates) and the number of spores counted. He also noted that:
Composite samples with only one or a few highly infected individuals can present a skewed infection level for a colony. For example, a few heavily infected bees in a composite sample could generate the same spore count as a sample with many moderately infected bees.
My point is, that I don’t bother any more with spore counts. Instead, I first determine whether any bees in the hive are infected by doing a quick crush of 10-25 bees in a sandwich bag [[10]]. Only if spores are clearly present do I then move to the next step and check for prevalence ― the percentage of infected house bees (rather than the intensity of infection of those bees) [[11]]. N. ceranae, at least, makes this easy, since in general, most any bee is clearly “infected” or “uninfected.”
Practical application: Although in my graph above, spore counts roughly tracked prevalence, that is not always the case. What I observe time and again is that the spore count from an entrance sample may scare you, but the prevalence of infection of the house bees from the same hive may be quite low [[12]].
Cameron Jack and collaborators published an enlightening study in 2016 [[13]], and concluded that:
These results further indicate the risk of using spore counts from composite samples (samples containing multiple bees) for making inferences regarding N. ceranae infection in colonies… Hence, it appears that analysis of individual bee samples would be ideal to accurately diagnose a colony’s infection level even though it is more time-consuming and expensive…
Practical application: I’ll be the first to admit that performing individual gut squashes of bees takes a little effort, but it actually isn’t difficult (and I’d rather do it than hemocytometer counts). Using disposable cover slips, I can personally check 10 bees for the presence of infection in less than 5 minutes. Five minutes of my time at the table with a ‘scope is well worth saving the cost of unnecessary treatments, and sure keeps me from wondering whether nosema is actually an issue in my hives. You’ll also (if you review the step-by-step photos at [[14]]) be able to diagnose if there is something else wrong with the bees’ guts ― especially if you are observing dysentery. I’ve personally found it to be quite illuminating.
the biological relevance of Prevalence vs. intensity
An important difference: “prevalence” indicates the proportion of the sampled bees found to be infected – this is what is biologically relevant to colony health, since nosema generally isn’t a problem to a colony until at least 20% of the house bees are infected (2 infected bees out of 10 in a sample). “Intensity,”on the other hand, only indicates the average number of spores per bee in a sample (in millions). It is thus only a very rough proxy for the proportion of bees actually suffering from disease, and, especially in the case of N. ceranae, can often misrepresent the degree of infection of the colony as a whole.
The above said, let’s compare some spore counts to prevalence counts for samples taken and analyzed both ways ― the first graph is of my data; in the second I plotted out Jack’s data (Figs. 2 & 3).
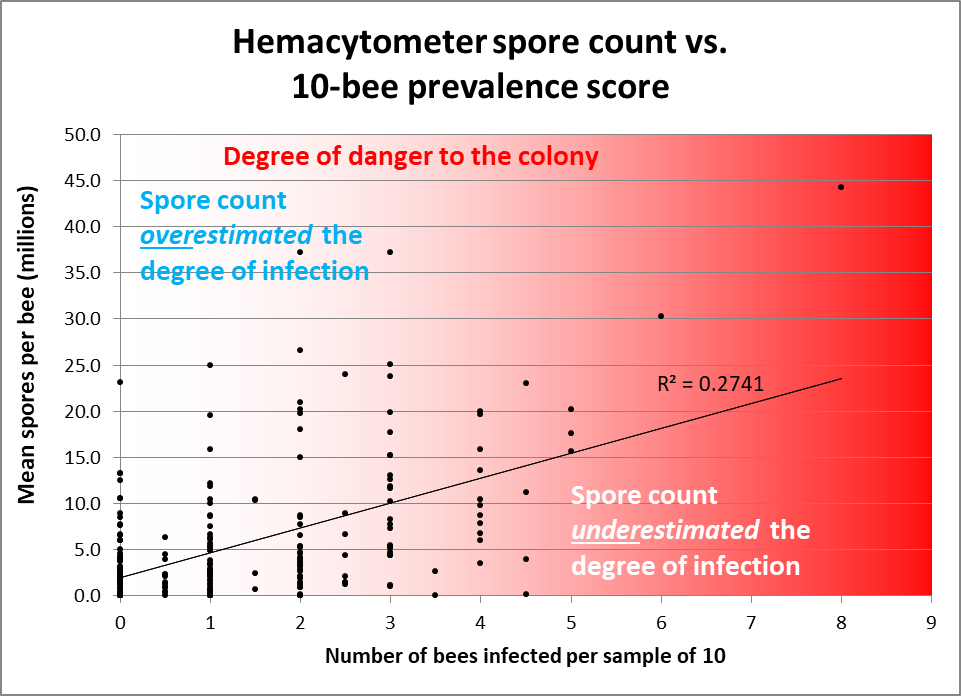
Figure 2. Note how often spore counts miss the mark as to the actual percentage of bees in the hive infected, and the very weak overall correlation (data points from divided samples of house bees — 10 individually inspected, 25 homogenized for a spore count [[15]]). I shaded with red in order to indicate the actual degree of danger to the colony. Colonies having 2 or fewer infected bees out of 10 would likely be nothing to worry about, but often exhibited spore counts in the tens of millions. Conversely, spore counts often underestimated the degree of prevalence of infection in hives having 30-50% of the bees infected.
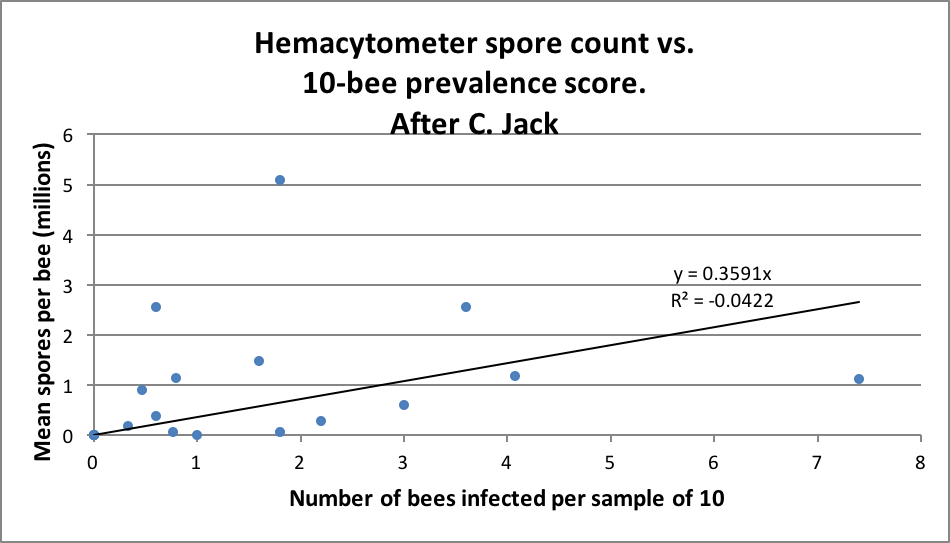
Figure 3. Jack’s data (from his thesis) largely reflect mine ― spore counts often overestimated the degree of infection, and sometimes seriously underestimated the same. Note that there was a much lower rate of intensity of infection overall in Jack’s samples than in mine above.
It’s worthwhile to read Jack’s entire thesis [[16]], in which he suggests:
My results demonstrate that optimal pollen nutrition increases Nosema ceranae intensity, but also enhances the survival or longevity of honey bees. The information from this study could be potentially used by beekeepers to formulate appropriate protein feeding regimens for their colonies to mitigate Nosema ceranae problems.
Practical application: Go into winter with strong colonies, feeding high-quality pollen sub if necessary. Sample some for nosema prevalence before giving perhaps unnecessary treatments. Check for nosema prevalence again in springtime to either assuage your fears, or to confirm that treatment is indeed indicated.
Queens And Nosema
Running young queens in one’s hives is always a good idea, due to their typically more vigorous egg laying and pheromone production. Those attributes also appear to help colonies to fight nosema. Botías [[17]] ran an experiment to see how replacing the queen affected N. ceranae levels in colonies in Spain:
Indeed, the removal of the queen and the subsequent replacement with a younger queen decreased the proportion of Nosema-infected forager and house bees, which maintained the overall infection at a level compatible with colony viability.
The above findings were confirmed by Simeunovic [[18]], who also indicated that older queens may be infected by N. ceranae.
Practical application: Colonies with young queens may be able to handle N. ceranae better than those with older queens.
A common question raised by beekeepers is whether nosema is causing the premature supersedure of purchased queens. Furgala [[19]] found most, but not all, queens to be highly susceptible to Nosema apis. It’s not so clear that queens have as much problem with N. ceranae. I’ve checked a number (as did Cameron Jack and Botías), but none of us found an infected queen.
Deadout equipment
Dr. White [[20]] found that dysentery was not necessary for the transmission of N. apis, and Smith [[21]] determined the same for N. ceranae. But if you’ve ever watched a bee defecate within an observation hive, it’s clear that the mess is quickly consumed by other bees — a sure way to infect that bee. But when I’ve inspected combs from colonies laden with N. ceranae, I couldn’t detect any spores on them [[22]]. But another researcher sent me a comb that did have spores on its surface, so I’m not clear on comb contamination.
White performed an experiment to determine whether Nosema apis could be transmitted via infected brood combs from heavily-infected colonies, by inserting them into nosema-free healthy hives. He did so with 14 hives, inserting the infected frames at various times from April through July. None of the hives got infected. Note, however, the date range in which he inserted them — during the time period in which colonies can typically purge the parasite.
Of interest is when I first became aware of N. ceranae, I sent samples of spores to mycologist Dr. Rob Cramer at the University of Montana. We were greatly surprised that most of the spores became nonviable after an overnight refrigeration. I brought this observation up with the late Dr. Ingemar Fries, who then ran an experiment that found that either refrigeration or freezing appeared to greatly reduce the viability of the spores of N. ceranae [[23]].
But it wasn’t quite that simple. One of Dr. Steve Pernal’s students, Courtney MacInnis, measured the viability of N. ceranae spores stored under different conditions [[24]]:
Practical application: Most spores on beeswax died within a few weeks at broodnest temperature or if frozen; but at room temperature some survived for up to a year. Surprisingly, although freezing quickly kills spores on beeswax, it helps them to survive longer if they are immersed in honey, in which they can survive frozen for well over a year. If the honey, on the other hand is kept at broodnest temperature, the spores only remain infective for several months. So either freeze your contaminated combs or keep them warm, but don’t feed back honey that’s been stored frozen.
Combs without honey can also be decontaminated by fumigation with acetic acid (I’m curious as to whether formic might also do the trick). And Dr. Frank Eischen found that they could be disinfected with Phostoxin [[25]]. The spores are also easily killed with bleach, but I don’t have hard data on the best application method.
Practical application: In our operation, we routinely reuse the boxes of combs from deadouts without performing any disinfection whatsoever (other than to check for AFB). But we typically reuse those combs later in the springtime when colonies are able to purge the parasite.
So much for contaminated combs and disinfection. Now how about treatment? Oh my gosh, I’m out of space. I’ll pick up again next month with treatments.
citations and notes
[1] Higes, M, et al (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environmental Microbiology, 34(10): 2659-2669.
[2] Chen, Y-W, et al (2012) Nosema ceranae infection intensity highly correlates with temperature. J Invertebr Pathol 111(3):264-7.
[3] Traver, B (2011) Prevalence and infection intensity of Nosema in honey bee (Apis mellifera L.) colonies in Virginia. Journal of Invertebrate Pathology 107: 43 ―49.
[4] https://scientificbeekeeping.com/the-seasonality-of-nosema-ceranae/
[5] https://scientificbeekeeping.com/sick-bees-part-15-an-improved-method-for-nosema-sampling/
https://scientificbeekeeping.com/sick-bees-part-14-an-update-on-the-nosema-cousins/
[6] White, GF (1919) Nosema-Disease. USDA Bulletin No. 780. Available from Google Books.
[7] van der Steen, J, et al (2012) How honey bees of successive age classes are distributed over a one storey, ten frames hive. Journal of Apicultural Research 51(2): 174-178.
[8] Gina Retschnig, et al (2017) Cold ambient temperature promotes Nosema spp. intensity in honey bees (Apis mellifera). Insects 8(1): 20. doi:10.3390/insects8010020
[9] Mulholland, GE, et al (2012) Individual variability of Nosema ceranae infections in Apis mellifera colonies. Insects 2012(3): 1143-1155.
[10] https://scientificbeekeeping.com/sick-bees-part-13-simple-microscopy-of-nosema/
[11] https://scientificbeekeeping.com/sick-bees-part-16-the-quick-squash-method/
[12] Martin-Hernandez, R, et al (2009) Nosema Diagnostic. https://coloss.org/publications/Proceedings_Nosema_Workshop.pdf
[13] Jack, C, et al (2016) Colony level prevalence and intensity of Nosema ceranae in honey bees (Apis mellifera L.). PLoS ONE 11(9): e0163522. doi:10.1371/ journal.pone.0163522
[14] https://scientificbeekeeping.com/sick-bees-part-16-the-quick-squash-method/
[15] Thus some positive spore counts despite not catching any infected bees in the matching sample of 10 bees inspected individually.
[16] Jack, C (2015) Colony level infection of honey bee gut pathogen, Nosema ceranae and role of pollen nutrition in Nosema ceranae infection and bee survival. M.S. Thesis, Oregon State University.
[17] Botías, C, et al (2011) The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environmental Microbiology 14(4): 845-859.
[18] Simeunovic, P, et al (2014) Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony. Journal of Apicultural Research 53(5): 545-554.
[19] Furgala, B (1962) The effect of the intensity of Nosema inoculum on queen supersedure in the honey bee, Apis mellifera Linnaeus, J. Insect Pathol. 4, 429 ―432.
[20] White, GF (1919) op. cit.
[21] Smith ML (2012) The honey bee parasite Nosema ceranae: transmissible via food exchange? PLoS ONE 7(8): e43319.
[22] https://scientificbeekeeping.com/nosema-ceranae-kiss-of-death-or-much-ado-about-nothing/
[23] Fries, I., Forsgren, E ( 2009) Nosema ceranae fungerar inte som Nosema apis. Bitidningen 107, Juni, 20-21.
[24] MacInnis, C (2017) Nosema ceranae: A sweet surprise? Investigating the viability and infectivity of the honey bee (Apis mellifera L.) parasite N. ceranae. M.S. Thesis, University of Alberta.
[25] Eischen, F.A., R.H. Graham & R. Rivera (2011) Impact of nutrition, Varroa destructor and Nosema ceranae on colonies in southern Louisiana. Proceedings of the American Bee Research Conference 2011.
Contents
Background. 2
The 2019 field test. 2
Results. 4
Those danged outliers!. 6
Overall efficacy. 7
Could there have been mite immigration from other colonies?. 8
Fate of the applied towels. 9
progression of action of treatment over time. 13
Results from others. 14
Mexico and uruguay. 14
Southeastern States with high humidity. 14
Quebec. 14
What if you lay towels on the bottom boards?. 15
Questions Remaining: 16
Question #1: is there a better delivery matrix?. 16
Question #2: How does this Compare to alternative treatments?. 18
Question #3: do we need to repeat the treatment?. 18
Question #4: Will this application method work in high humidity areas?. 19
Wrap up. 19
EXTENDED-RELEASE METHOD (FOR SUMMER APPLICATION, PERMITTED WITH HONEY SUPERS ON) 19
References. 19
Extended-Release Oxalic Acid Progress Report #5 -2019
First published in ABJ December 2019
Randy Oliver
ScientificBeekeeping.com
I’m working with the USDA to get an extended-release application method for oxalic acid (dissolved in glycerin) to be approved by EPA. If approved, it would give us a treatment that could be applied as we put on the honey supers, to control varroa during the critical midsummer period.
Disclaimer: I’m collaborating with the USDA-ARS to register this application method for oxalic acid, and have a Pesticide Research Authorization from the State of California. The method described here is not yet registered in the U.S. But since my research is funded by donations from beekeepers, I feel that I owe a progress report to those donors. I in no way encourage the unregistered application of any pesticide—please wait until this method is approved by the EPA and your State before using it in your own hives.
Background
Practical application: Our industry is begging for a summertime varroa treatment that can be safely and effectively applied in hot weather, while honey supers are on the hive. The two treatments currently approved for use during a honey flow are either of low efficacy when colonies contain brood (hops beta acids) or limited by summer temperatures (formic acid products).
So when in 2015, the editor of the Argentinian beekeeping journal Espacio Apicola, Fernando Esteban, brought to my attention that beekeepers there were testing an extended-release application method for oxalic acid (“OA”) — by dissolving it in glycerin and applying it on cardboard strips – I immediately experimented with it.
Results were encouraging, so I obtained a Pesticide Research Authorization from the California Department of Pesticide Regulation, and contacted the registrant of oxalic acid – the USDA – to collaborate on requesting EPA to add a generic additional application method to the existing label for oxalic acid. I thank Dr. Jay Evans for agreeing to take on this project, and Dr. Geoff Williams and Jennifer Berry for agreeing to collaborate in the high-humidity state of Georgia. Here I will only discuss my own research; my other progress reports are available online [[1]].
In 2017 I ran a field trial to collect data to present to EPA on possible adverse effects, residues in honey, and efficacy against varroa. All results were very promising in my environment. So in 2018 I dug more deeply, including running a large-scale field trial to determine the optimal ratio of OA to glycerin. Then this summer I tested what appeared to be the best formulation (when applied via Scott Shop Towels).
The 2019 field test
Last year’s results suggested that a 1:1 w:w ratio of OA to glycerin gave optimal results. So I played with the formula and found that a half roll of fully-saturated towels would absorb a heated mixture of 505 g each of OA and glycerin, resulting in easily-dispensable towels with good handling texture, that contained an 18-gram total dose of OA when two half towels were applied to a hive.
Note: the 18-g dose is solely due to the absorptive limitation of this brand of towel, and does not necessarily reflect the optimal dose (balancing efficacy vs. adverse effects), but is a dose that I’ve previously found to be effective.
We intentionally withheld spring treatments from several hundred hives in order to have high-mite colonies to test. We took starting alcohol-wash mite counts, and chose 222 double-deep strong hives in 16 different yards in the low-humidity California foothills to run in the trial (intentionally excluding abnormal or weak colonies, or any hives exhibiting extremely low or high mite counts). We immediately applied two half shop towels across the top bars, between the brood chambers as shown in Figure 1.
Update: We now prefer absorbent matrices other than shop towels, which have many disadvantages. Please refer to my more recent reports on extended-release oxalic acid.

Figure 1. Towels were applied as above, with a space between them for bee movement up and down between the boxes. The trial ran from early July through early September, for roughly 60 days in each test yard.
Practical application: the towels with this 1:1 formulation were very easy to apply, since the OA crystallizes and leaves the towels with a “dry” and slightly stiff texture.
Results
I felt that box plots and mean values wouldn’t be the best way to illustrate the data to beekeepers wishing to make practical management decisions, so I’m trying something different. Here’s an introductory graph to get you used to a format that I hope may better visually present the data in a meaningful way (Fig. 2).
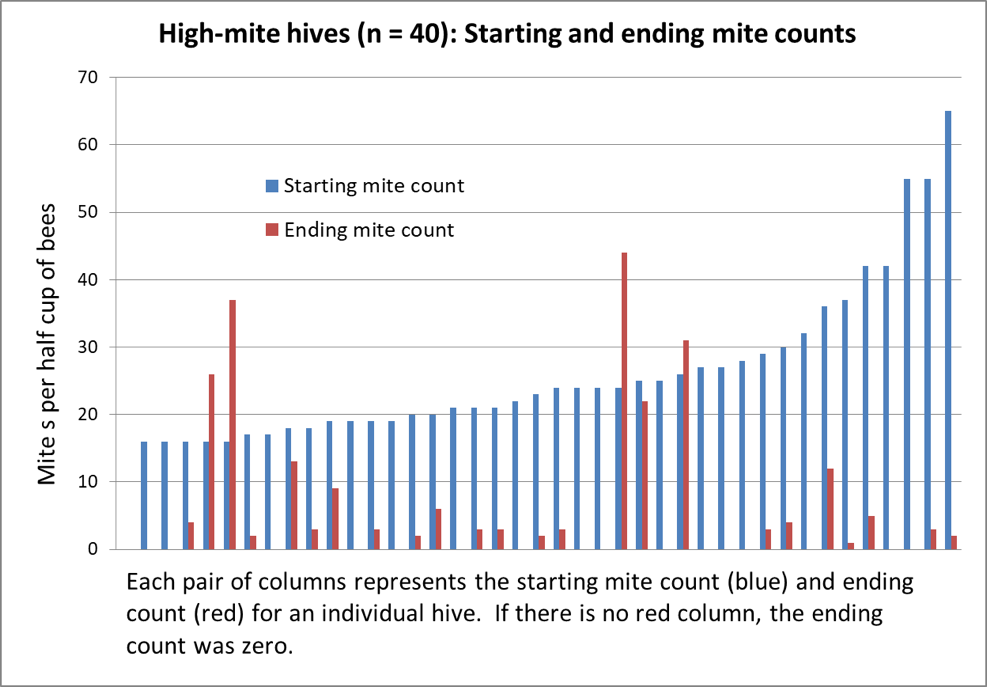
Figure 2. Above are the results from the 40 hives with the highest starting mite counts, sorted left to right by starting count. 40% of the hives wound up with counts of zero; 82% at or below the 3% infestation level. Note the surprising lack of correlation between starting and ending counts.
OK, now that you understand how to interpret this form of graph, allow me to present the data for the full 222 hives in Fig. 3.
Figure 3. In the graph above, the mite counts for all 222 hives are sorted left to right by ending count after treatment (the red columns) [[2]]. The blue columns indicate each colony’s starting count, immediately to the left of its corresponding ending count. There are no red columns for the third of the colonies to the left, since they ended up with mite counts of zero, despite some of them starting with sky-high counts (note that 50% of the hives ended up with counts of either zero or 1 per half cup of bees). The horizontal red line indicates the “safe” level for varroa in late summer, to be followed by a treatment after removal of the honey supers.
The median starting mite count was 8; the median ending mite count was 1; the median reduction in mite count was 88%. Note the lack of correlation between starting and ending mite counts – many of the highest-mite hives went to counts of zero.
Practical application: my sons and I consider the results to be pretty impressive, and can see a place for this treatment in our California operation once it gets approved.
Another interesting way to look at the results would be to look at the absolute change in mite counts – by how many mites per sample the count changed in each hive (Fig. 4).
Figure 4. Note that in the hives to the far left, mite wash counts dropped by as many as 60 mites between the starting and ending counts. In 7/8ths of the hives, mite counts stayed the same or went down during a time in which they’d be expected to explode. I have no explanation for why the counts went up in an eighth of the hives.
Those danged outliers!
One consistent thing about field trials with honey bees is the inconsistency of colonies in any test group – that is, there are nearly always the danged aberrant colonies that don’t perform like the rest of their group. In a breeding program, we look for the outliers exhibiting extra-high honey production, or outstanding resistance to varroa. But when testing for the efficacy of a varroa-control product or method, there are often a few anomalous hives in which, for some reason or another, the mite population just keeps on increasing. This is one reason that I present median values rather than means (in which the outliers can greatly skew the “average”).
At Apimondia, Dr. Jim Masucci gave a fascinating presentation entitled “Lessons learned from developing an RNAi-based varroa control product.” I was involved in the early stages of this research [[3]], and spoke with him afterward. One of the frustrations that he experienced is that even in his “positive control” group — treated with the well-proven, highly-effective synthetic miticide Apivar® (amitraz) — that in a percentage of those colonies mite levels continued to rise despite being continuously treated. This is exactly what I see again and again in my own field trials.
| PRACTICAL APPLICATION: OUTLIERS AND AVERAGES
When we speak of giving a “center” or “average” value for a data set, there are three ways used to calculate it:
1. The “mean” is the arithmetical “average” that we’re all used to, where you add up all the values and then divide by “n” (the number of values). The mean is useful for calculating things such as “average” honey production per hive. The problem with means is that even a single outlier can skew the “average” to the extent that it may have little practical meaning (like the effect of one extremely high outlier mite or nosema spore count). In this trial, the mean ending mite count was 4.
2. The “median” is the middle value of a data set – with half above, half below. For practical bee management decisions, the median generally best represents the “average,” since it is barely influenced by extreme outliers. In this trial, the median ending mite count was 1.
3. The “mode” is the value that occurs most often – the most common observation. The mode may be useful for things such as “average” colony strength for a pollination contract. In this trial, the most common ending mite count – the mode – was 0. |
Practical application: I minimize the distraction from the outliers by looking at the median values of the results, and at the overall distribution curve of ending mite counts.
And this brings us to the concept of “efficacy of treatment”: meaning the comparison in mite buildup between the treated colonies and untreated controls
Overall efficacy
We included some untreated moderate-mite control hives, but their mite counts jumped so much by a midpoint check, that we treated them in order to save the hives. So although I cannot claim to have run a formal control group, of interest are the data from the untreated hives, in the same yards, that had exhibited starting counts of 0 or 1, which we then tracked as potential mite-resistant breeders. Those in which mite counts increased during the course of the trial went from a median value of less than 1 to a median count of 15 (range of 7 – 35), which would be a median 15x increase in the mite infestation rate over the course of the trial.
Although I wish to be clear that one could question whether those hives could legitimately serve as controls, when I run the Henderson-Tilton formula on the median values for mite counts, it suggests an efficacy due to the treatment in the 99% range. If instead, I use last season’s increase in the controls (5x instead of 15x), the formula still indicates an efficacy of 98%.
Practical application: so far, I have not been running a positive control group with a “known” effective miticide to which I could compare the efficacy of extended-release OA. The only two treatments that are registered for use while honey supers are on are Hopguard and formic strips [[4]]. I intend to test them next season against OA/gly.
Could there have been mite immigration from other colonies?
The question arises as to whether the increase in mite counts in the outlier hives was due to bee drift or the robbing out of collapsing hives. Serendipitously, I had been asked by a U.C. Davis grad student to supply hives for another project, which involved us placing 24 hives in each of three high-elevation (6500 ft) wet-meadow locations (Fig. 5). Before we moved the hives in, she had confirmed that the locations were free of honey bees (either naturalized or managed), since her crew had not observed any honey bees working the flora. Thus there would be no immigration of mites from hives other than my own.

Figure 5. Grad student Maureen Page was studying the impact of honey bees on the pollination of the blue Camas lilies in high-elevation wet meadows. In this photo, she’d bagged a portion of the flowers just before we placed the dark-colored hives in the background.
The placed hives included a few very-high-mite hives, which we did not include in the trial, some of which unfortunately collapsed during the course of the trial (1-2 per yard), and thus could have contributed to some mite immigration. The data for the isolated hives (which are also included in the previous graphs) is shown in Figure 6.
Figure 6. The high-mite outlier to the right was odd – it had quickly removed most of the applied towels. We checked the ending count twice to confirm. But then we checked it again a week later (with 6 samples to confirm)– it had dropped to a median count of 8.5 mites! But the one next to it at 22 had by then jumped to a median count of 65. Inspection indicated that its brood had all recently emerged. These high-mite outliers can apparently really jump around!
Interpretation: in this group we can rule out mite immigration from outside of each group of 24 hives. Unfortunately, there were a few colonies that did collapse from varroa, so we can’t completely rule out the possibility that they were involved. The wild fluctuation in mite count over a 1-week period in the colony exhibiting the highest ending count is certainly confounding.
Fate of the applied towels
With the 1:1formulation that I tested this year, there was large variation in how the bees dealt with the applied towels (Figs. 7-9).

Figure 7. Most colonies removed a portion of the towels over the two month treatment. By this point in time, although the towels still clearly tasted of acid, they may no longer be dispersing enough OA to the bees to further prevent the mite counts from starting to climb again, and may call for a second application. Or perhaps I should use a higher initial dose on a matrix that is not chewed out as quickly.

Figure 8. On the other hand, some colonies chewed out most of the towels and propolized the remainder. This may give the colony a high initial dose of OA, but very little would be being distributed later on.

Figure 9. A few colonies quickly removed every trace of the towel from the hive; some of these did not exhibit good mite reduction.
Practical application: Shop towels may not be the ideal matrix for consistent extended distribution of OA/gly in a hive.
progression of action of treatment over time
With these large field trials, it takes a lot of time to perform mite wash counts on a weekly basis, so I haven’t done that to any great extent. Instead I simply take ending counts when it appears that we’ve reached maximum efficacy, based on spot checks. Below are the results of some of my spot checking this summer (Fig. 10).
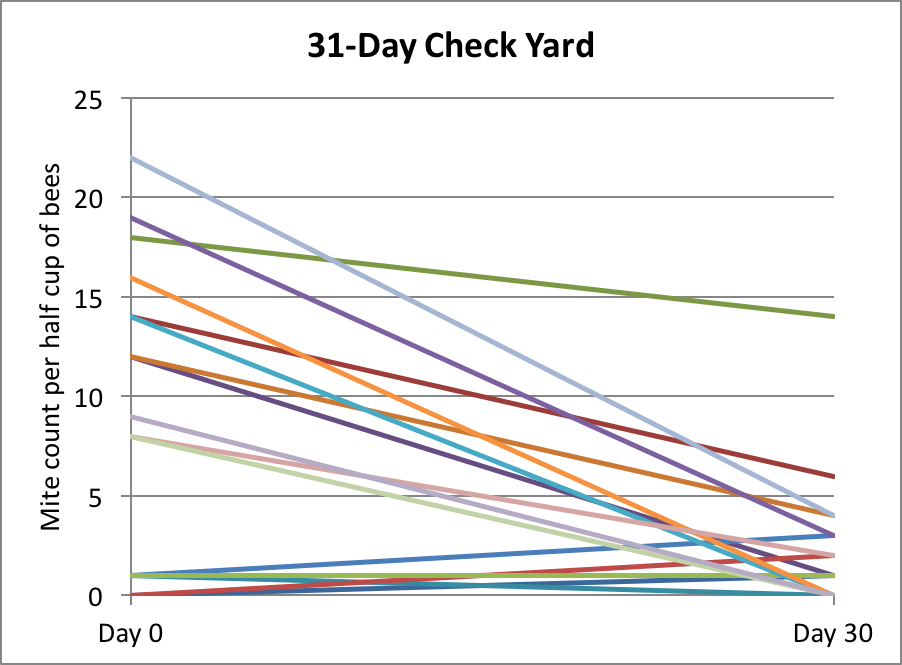
Figure 10. In this yard, we took mite counts at 31 days, but then due to a miscommunication, all the hives got treated (these counts are not included in the data sets above).
We later took spot counts at 40 days from 27 hives in various yards — the results are shown in Figure 11.
Figure 11. Since a number of counts were still above zero on Day 40, I ran the trial for around 60 days. Given the benefit of final data, it appears that at some point between 40 and 58 days the treatment resulted in the greatest mite reduction.
Practical application: Note that had I taken final counts at Day 40, that our efficacy calculation would likely have been even better. Next year, I plan to take weekly counts in a group of hives to clarify the progression of mite reduction in the hives over time.
Results from others
Mexico and uruguay
At Apimondia 2019, there were two posters of interest. In Michoacán, Mexico, Rueda, et al [[5]] tested the Shop Towel application method and calculated 87% efficacy at 94 days in the 5 hives tested. In Uruguay, Diaz-Cetti & Mendoza [[6]] using impregnated cardboard strips obtained 95% efficacy in 41 days.
Southeastern States with high humidity
Results from Georgia, Virginia, and some other states suggests that this application method may not work as well in areas with high humidity.
Quebec
A meticulous beekeeper from Quebec sent me this exemplary data sheet of his test of OA/gly towels.
| Application time: nest in expansion, before the main nectar flow |
|
| Two half towels between the two brood chambers |
|
| 505 g OA + 400 mL glycerin |
|
| One stationary beeyard (average queen age : 2 years) |
|
|
|
|
|
|
Varroa Mite Count (number/300 bees) |
|
|
Initial count |
Final count |
|
| Hive |
June 7 |
Jul 26 |
Notes |
| 1 |
0 |
0 |
Sick, chalk brood |
| 2 |
1 |
0 |
Very strong |
| 3 |
0 |
0 |
Weak, supersedure |
| 4 |
2 |
0 |
Weak, supersedure |
| 5 |
2 |
2 |
Strong |
| 6 |
0 |
1 |
Strong |
| 7 |
4 |
1 |
Strong |
| 8 |
1 |
2 |
Extra propolis |
| 9 |
2 |
2 |
Very strong |
| 11 |
1 |
2 |
Young queen |
| 12 |
1 |
0 |
Sick, chalk brood |
| 13 |
1 |
0 |
Strong |
A request: I am happy to receive data on this application method from beekeepers over the world (of course only if you have an experimental permit), especially since I’m curious as to whether humidity is a factor. Please send data in a format similar to that above, which clearly explains exactly what you did on what dates, and has the data clearly organized and labeled.
What if you lay towels on the bottom boards?
A beekeeper asked me what would happen if I simply laid OA/gly towels on the bottom board. There were a couple of high-mite hives sitting next to my house, so I did so (Figure 12).
 Figure 12. I covered the bottom boards of two hives with towels for a quickie test in order to see whether simply exposing the bees’ feet to the towels would result in any mite control. The towels looked like this after the 68-day treatment (you can certainly see the main travel path).
Figure 12. I covered the bottom boards of two hives with towels for a quickie test in order to see whether simply exposing the bees’ feet to the towels would result in any mite control. The towels looked like this after the 68-day treatment (you can certainly see the main travel path).
Surprisingly, this 2-hive test indicated that even this small amount of exposure to only one side of OA/gly towels may be enough to obtain some degree of mite control (Table 1).
| Table 1. Results from a single layer of towels laid on the bottom board. |
| Mite counts |
| Hive |
29-Jun |
5-Sep |
| A |
11 |
13 |
| B |
17 |
9 |
Questions Remaining:
I’m not trying to sell a product, and have limited funding from the beekeepers who support my research, so I can only test a few things each year. We’re going at this step by step, learning as we go. That said, I’ve discussed with my collaborators a number of questions that we need to address before this application method is ready to roll.
Question #1: is there a better delivery matrix?
My sons and I felt that using the chipboard strip method of application was too time consuming, and required an excessive dose of OA. My initial testing of saturated chipboard across the top bars suggested that I should use a different matrix. I chose to work with Scott Shop Towels due to commercial beekeeper familiarity with the towels, their low cost, ease of preparation and application, the fact that bees readily chew and remove them from the hive, and their biodegradability.
I’m not in any way stuck on shop towels – there may be other matrices that hold a larger dose, that disperse the OA over a longer period of time, or that are more easily removable after treatment. I do prefer a biodegradable matrix. I’m looking for a matrix that will deliver more than the 18 g maximum that a shop towel can hold. It would also be nice if the matrix was easier to remove from the hive after it had done its work.
A beekeeper from Europe suggested cellulose kitchen sponge cloths made in Sweden (Fig. 13).

Figure 13. I was able to find two brands of these cellulose sponge cloths online [[7]]. Compared to a shop towel, which can hold 18 g of OA, one of them holds 40 g (another brand 51 g). I’m currently running a late-season test in 5 hives.
I just ran out to give a quick check at 25 days after application, and these sponges look very promising (Fig. 14).

Figure 14. At 25 days after application, you can see enough dead mites to warm the heart of any beekeeper, and the little ring of propolis around the sponge. I’m impressed by the liquidity and strength of the OA/gly solution remaining in the sponge, and the ease with which the towel could still be removed from the top bars in one piece.
Update: I fed each hive a couple of pounds of pollen sub at the midpoint check to encourage continuation of broodrearing, so that I could check for adverse effects due to treatment. I took final alcohol wash mite counts for the hives at 51 days after applying the sponges. Mite reduction looked pretty good. So did colony strength and the health of the brood. Here’s the data:

The above sponge would bump the cost of a treatment up by a dollar, but the savings in labor, and greater dose of OA may well be worth it. I’m excited to test them next season.
Question #2: How does this Compare to alternative treatments?
We need to run positive controls of the two approved treatments for when honey supers are on: Hopguard and Formic Pro® strips. We can then compare efficacies and outliers to the OA/gly treatments.
Question #3: do we need to repeat the treatment?
We may not need to do so if we use something like the sponges. For any test, we should track mite infestation rates of at least some colonies on a weekly basis to see how long the matrix sustains OA delivery.
Question #4: Will this application method work in high humidity areas?
Glycerin is highly hygroscopic, meaning that it absorbs moisture out of the air. This may be an issue in areas of high humidity. I’m hoping to find additional collaborators to test it under those conditions.
Wrap up
I have no financial interest in registering this application method, but see its great potential to fill a current hole in our mite management strategy – at least in California. I’m hoping that we can convince EPA to add an additional approved application method to the OA label something like this:
EXTENDED-RELEASE METHOD (FOR SUMMER APPLICATION, PERMITTED WITH HONEY SUPERS ON)
Add equal weights of Oxalic Acid Dihydrate and vegetable glycerin into a saucepan, Place over heat and stir constantly to dissolve the acid crystals completely, being careful not to exceed a temperature of 160°F (71°C) (above which the solution will start to form bubbles).
To the heated solution, add precut cellulose towels, sponges, chipboard strips, or similar carrier matrix to absorb the solution. Then allow the carrier to drip dry.
I updated a typo in the following sentence (8 Feb 2020) — thanks to beekeeper Rex Roberton for pointing it out!
Apply not more than 50 g [[8]] oxalic acid dihydrate in saturated carrier to the lower brood box. The towels, sponges, or strips can be laid across, or hung over the top bars. The treatment must be separated by at least one chamber from any honey to be extracted.
Full efficacy is not obtained until roughly 40 days. The application may be repeated once per season.
This is a work in progress, and I thank California DPR, Jay Evans at USDA, Jennifer Berry and Geoff Williams, and the EPA for collaborating. I also thank beekeepers throughout the world for their own test results (assuming of course that they obtained permission from their local regulatory agency). My own research is funded by donations from beekeepers — I thank you all.
Practical application: in our commercial operation, achieving 98% efficacy in preventing mite counts from building up during the honey flow makes us very happy. And this for a treatment that is very safe to handle, takes seconds to apply, and does not contaminate the honey or beeswax. The cost would be about 32¢ per dose via shop towel (not counting the labor involved in preparation), or $1.30 with the cellulose sponges (which are easier to prepare and apply). In case you haven’t guessed by now, I’m pretty excited about this method, but feel that we have at least another year of testing before we’re ready to submit to EPA.
References
[1] https://scientificbeekeeping.com/beyond-taktic/
https://scientificbeekeeping.com/extended-release-oxalic-acid-progress-report-2/
https://scientificbeekeeping.com/extended-release-oxalic-acid-progress-report-3/
https://scientificbeekeeping.com/extended-release-oxalic-acid-progress-report-4/
[2] There are fewer than 222 columns, since some mite counts overlapped, which results in some of the columns being darker than others.
[3] I worked with Beeologics on the early development of RNAi products before they were purchased by Monsanto, and later by Bayer. https://scientificbeekeeping.com/sick-bees-part-2-a-model-of-colony-collapse/
[4] MiteAway Quick Strips or Formic Pro.
[5] Rueda, H, et al (2019) Evaluation of two methods of treatment with oxalic acid for the control of Varroa destructor in honey bee colonies Apis mellifera L. Poster at Apimondia 2019
[6] Diaz-Cetti, S & Y Mendoza (2019) Control of Varroa destructor by oxalic acid in cardboard strips. Poster at Apimondia 2019
[7] Amazon: “Sponge Cloth, Swedish Dishcloth, Cellulose, Reusable, 10-sponges in Pack.”
[8] The suggested 50-g limit is subject to further field research.
Contents
Why would a colony exhibit signs of dysentery?. 3
honeydew.. 4
Microbial dysbiosis or parastism.. 5
The link between pollen and nosema. 7
How about those “winter bees”?. 8
Literature cited. 10
The Nosema Problem: Part 7a
The Causes of Dysentery in Honey Bees Part 1
First published in ABJ December 2019
Randy Oliver
ScientificBeekeeping.com
Okay, I’ve called into question whether nosema of either species actually causes dysentery. But since dysentery in the hive can clearly spread nosema spores, we should do our best to help our bees to avoid it. To do so, we should understand what factors do contribute to dysentery.
The terms “diarrhea” and “dysentery” are often used interchangeably, but as best I can tell, diarrhea is the name for the symptom of producing frequent, loose stools, whereas dysentery indicates an infection of the intestinal tract. For the purposes of this article, I’ll use the term “dysentery” as it is commonly applied to beekeeping ― the field sign of voided feces dripping down the hive near an exit point (Fig. 1).

Figure 1. Given a chance to fly during a warm spell in winter, some of the exiting bees appear to be so eager to relieve themselves that they do so the moment they take flight. But does this mean that they are diseased?
The photo above suggests the importance of flight opportunities during the winter. Should those ready-to-burst bees have instead let loose inside the hive, they might have spread nosema spores. Luckily, nosema-infected bees are prone towards flying out of the hive even when it’s cold, perhaps due to an ingrained behavior that helps the colony to remove infected individuals [[1]]. Note the number of dead bees in the snow. Since nosema hampers energy production in a bee, infected individuals may not be able to return to the hive, thus helping to prevent them from infecting their sisters.
So nosema may induce suicidal winter flight by infected workers, but that doesn’t mean that it caused dysentery. I’m open to the possibility that it could, but my current overall assessment is that we should refrain from repeating the apparently unsubstantiated claim that nosema causes dysentery until Koch’s Postulates have been fulfilled by peer-reviewed research — meaning that someone needs to demonstrate that infection by nosema actually does induce bees to exhibit dysentery.
That said, I’ve looked at a fair amount of dysentery scrapings under the ‘scope — there are sometimes nosema spores present, but as far as I can tell, no more often than in bee samples taken from nearby hives not exhibiting dysentery. But I do often see other indications of gut dysbiosis (Fig. 2).
Figure 2. I’m no microbiologist, but I often see abnormal things in bee guts. Here’s a field of view of diluted gut contents (at 400x) full of nosema spores (the very small pill-shaped objects), and what may be some pollen grains (the dark objects). But there are also what appear to be rust fungus spores (orange centers), and perhaps some yeast cells, amoeba, or other organisms (the grayish oblong, round, or irregular objects).
Practical application: Seeing signs of dysentery is likely never a good thing, but keep in mind that it could be caused by any number of things. If you’re worried about nosema, get a microscope and confirm that the pathogen is indeed prevalent in the hive(s).
Why would a colony exhibit signs of dysentery?
By nature, honey bees are fastidiously clean within the hive. This is extremely important for a colony of eusocial insects, what with so many bodies packed into a small cavity for long periods of time. Therefore, there has been strong evolutionary pressure for bees to avoid defecating within the hive. Thus even young nurse bees take short “cleansing flights” to relieve themselves — but generally not on the hive itself. In general, it’s only when the bees have been constrained by weather from flying out to void (or when a colony is really sick), that you see the telltale signs.
Experts have blamed dysentery on any number of factors. Allow me to quote Dr. White in 1919 [[2]]:
When discussing nosema and dysentery, one thing to keep clear is that the nosema parasite reproduces and grows in the bee midgut (ventriculus) — only its inert spores show up in the hindgut (rectum). Dysentery (diarrhea), on the other hand, is a sign of a disorder of the hindgut, apparently due to chemical irritation, microbial dysbiosis, a poorly-digested diet, a sudden switch to a pollen that is irritating, or to which the bee gut microbiota were not adapted, consumption of a honeydew containing complex sugars, minerals, or other phytochemicals, or an infection by yeast or Malpighamoeba mellificae.
Wow, that’s quite a list! Nowadays you can add poorly-digested pollen subs, contaminated or fermented sugar syrup (containing yeasts or other fungi, molasses, flavorings, or floor sweepings), or some of the homebrew potions that beekeepers feed their colonies. Unfortunately, most authors that list causes of dysentery fail to provide citations of supportive evidence to back those claims, so let’s go over what I could find …
honeydew
In 1914, E.F.Phillips [[3]], when measuring temperatures in overwintering colonies, observed that:
Honeydew honey is a poor food for winter and is so recognized. It contains the same sugars as honey, but contains in addition a considerable amount of dextrin [as well as other indigestible complex sugars and perhaps a high mineral content]… From the evidence at hand it appears that dextrin cannot be digested by bees and, whether or not this is the explanation, honeydew honey causes a rapid accumulation of feces which usually results in the condition known as dysentery, in bad cases of which the feces are voided in the hive. In the case of [a colony wintered on honeydew] the whole hive inside and out, as well as the frames and combs, were spotted badly, the inside of the hive being practically covered. Even with fine honey stores such a spotting is usually noticed after a prolonged confinement, especially in severe weather (or during brood rearing). It therefore appears that the accumulation of feces acts as an irritant, causing the bees to become more active and consequently to maintain a higher temperature. We are therefore justified in believing that the cause of poor wintering on honeydew honey is due to excessive activity, resulting in the bees wearing themselves out and ultimately in the death of the colony…
It therefore follows that excessive activity causes the consumption of more food, resulting in turn in more feces, so that colonies on poor stores are traveling in a vicious circle, which, if the feces can not be discharged, results in the death of the colony. In the work here recorded no attention was paid to the theory that dysentery is due to an infection, since there is nothing in the observations made that lends any support to that idea.
On the other hand, more recently Pohorecka and Skubida in Poland studied the relationship between wintering on honeydew and colony health [[4]], tracking a total of 1400 hives over the course of three winters.
In this study… no increase in mortality rate among bees related to the percentage of honeydew honey in winter stores was found. It shows that wintering performance is significantly influenced by other factors such as duration of the wintering period, colony strength, and the positioning of honeydew honey containing stores in the nest as well as the extent to which the stores are used up.
Unfortunately, they did not record whether dysentery was observed. However, their data suggested that the presence of honeydew in the bees’ diet actually decreased infection by Nosema apis.
Practical application: Some honeydews or other irritating foods may cause dysentery.
Microbial dysbiosis or parastism
Something that I sometimes observe in dysentery samples are round objects that appear to be amoeba cysts (Fig. 3). Prell [[5]] cited Dr. Morganthaler of Switzerland, who described that with amoeba infection “there is often a soiling of the alighting board, and even of the inside of the hive, by faeces containing cysts.”
Figure 3. The round shapes in this image from Wikipedia [[6]] are identified as Malpighamoeba mellificae cysts. Note also the presence of the glowing nosema spores
And then there are yeasts (Fig. 4), whose presence in the bee gut indicates that some form of dysbiosis is occurring, perhaps due to the presence of undigested sugars or excessive water in the hindgut. I’ve observed dysentery to be associated with the feeding of fermenting syrup.
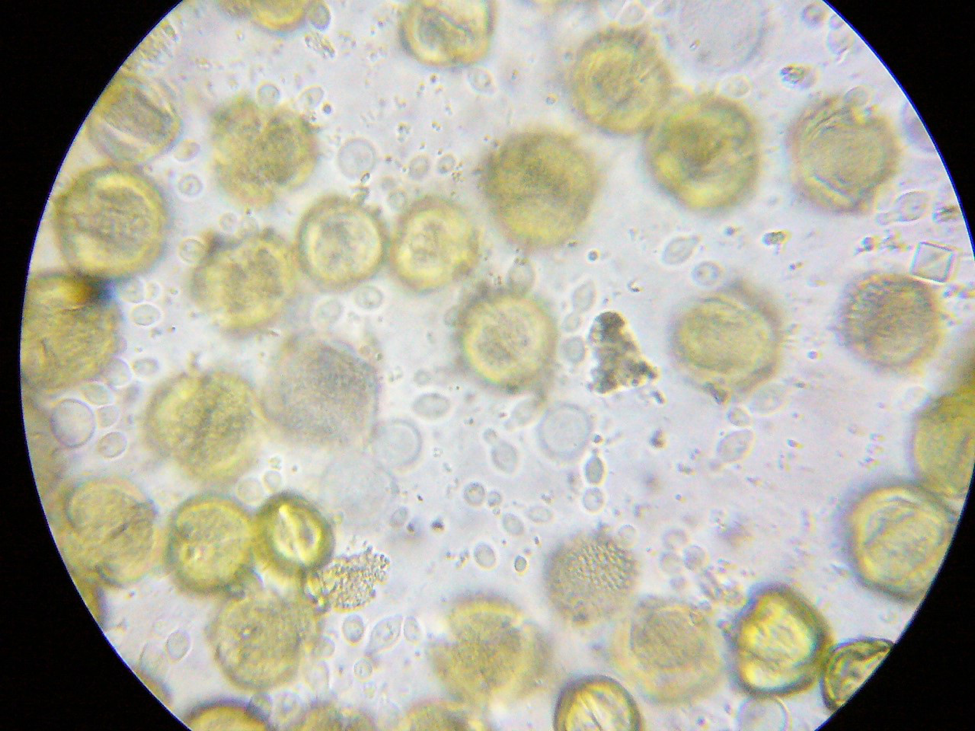
Figure 4. Here is beebread that I mixed with dilute syrup and allowed yeast fermentation to start. You can see what appear to be large grayish yeast cells (I have no idea what the little square object is), and I’m far from certain as far as my identification of the gray cells as yeast.
Update: Beekeeper Georges Prigent questioned me on the identification of yeasts. So I grew some more cultures. Thanks to readers such as Georges for feedback!
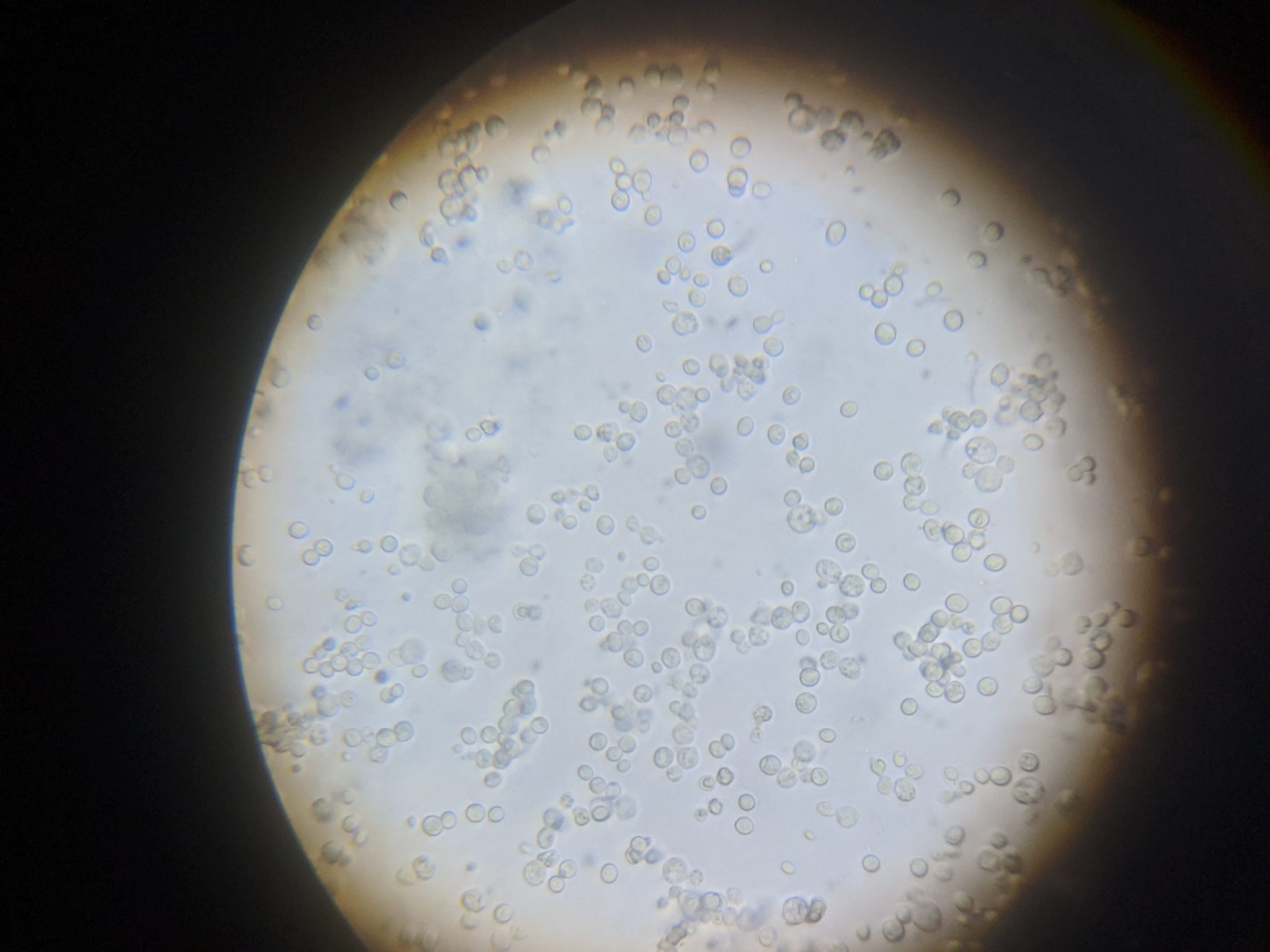 Above is bread yeast grown in diluted honey. 400x
Above is bread yeast grown in diluted honey. 400x
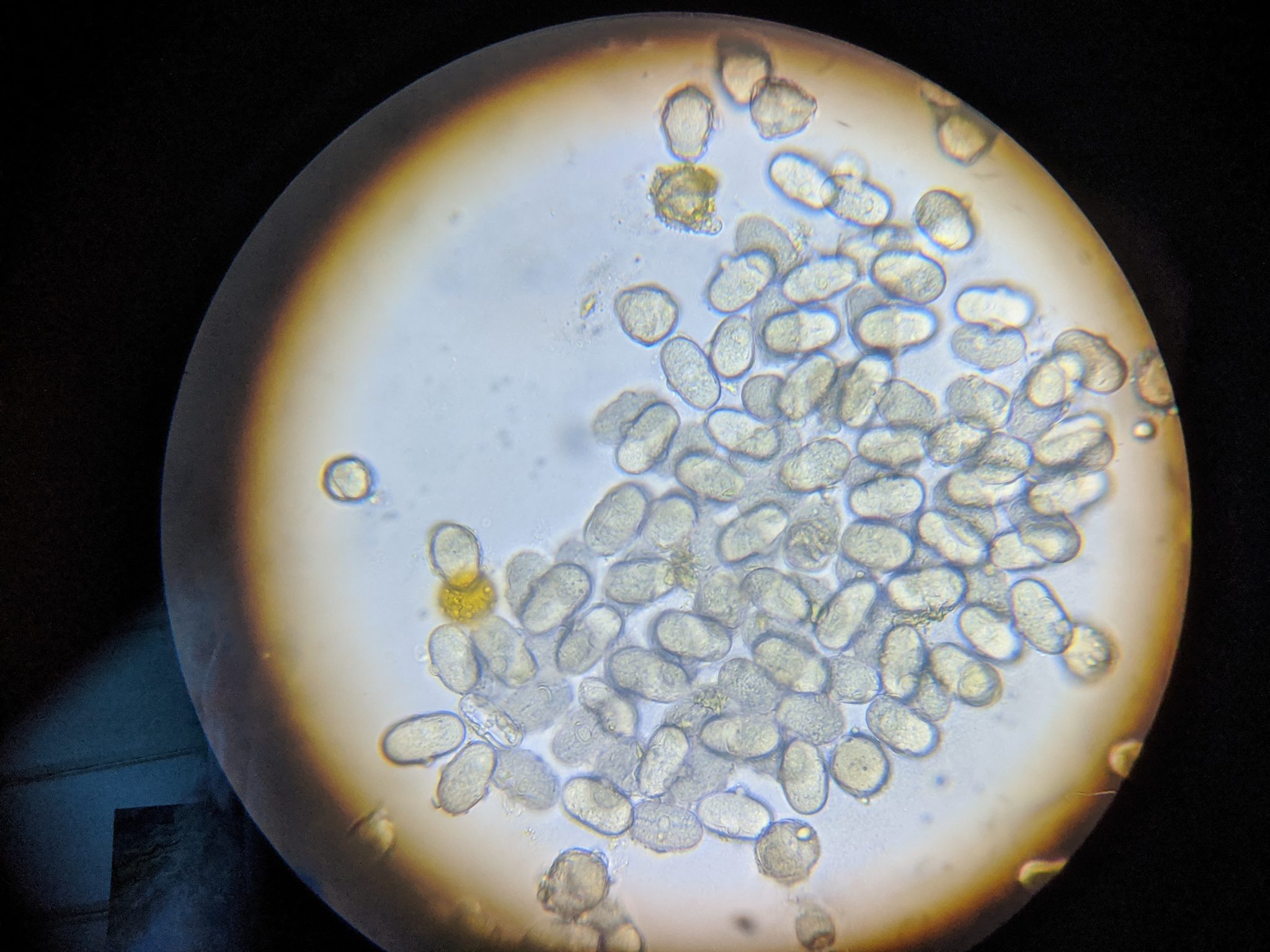 Above is a pollen/sugar culture at 400x, which I first suspected the gray ovals to be some sort of organism, but which Georges pointed out were actually pollen grains (which I later confirmed). These grains could be from vetch (Viscia).
Above is a pollen/sugar culture at 400x, which I first suspected the gray ovals to be some sort of organism, but which Georges pointed out were actually pollen grains (which I later confirmed). These grains could be from vetch (Viscia).
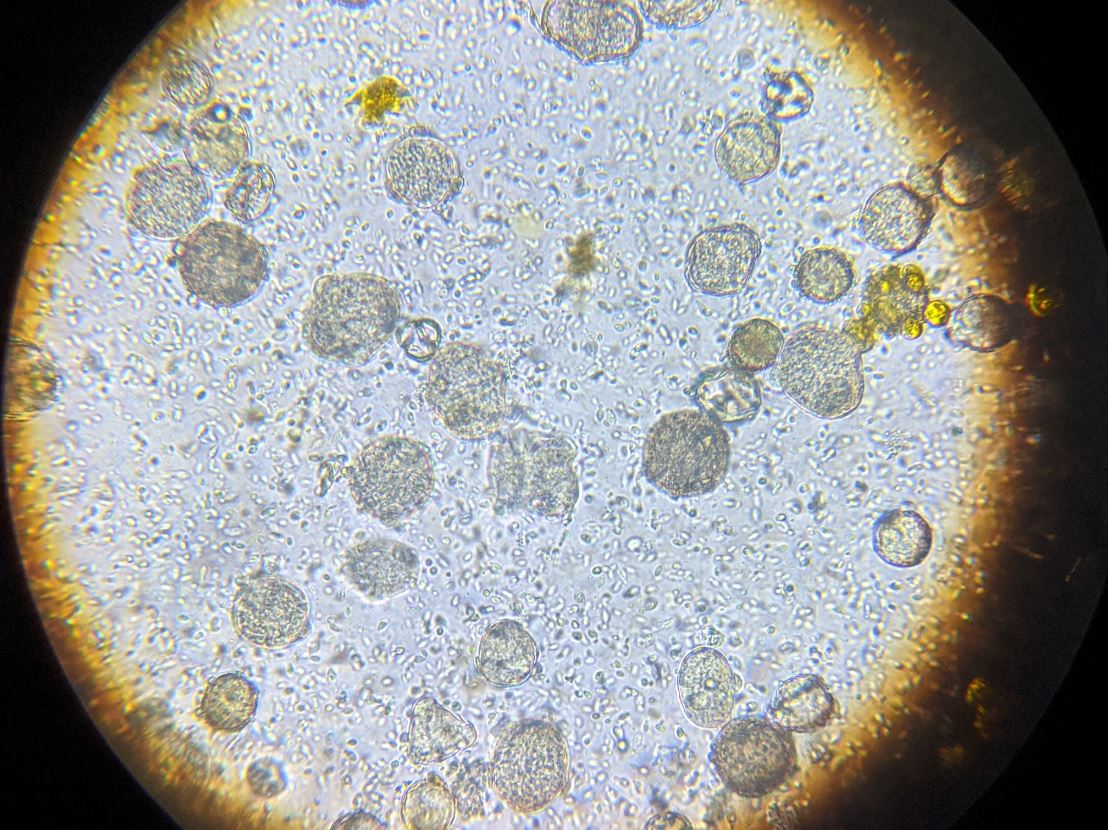 Here I grew bread yeast in a mixture of pollen grains and sugar water in order to show their relative sizes. The small ovals that look like nosema spores are the bread yeast. Unfortunately for us microscope-viewing beekeepers, the yeast cells are about the size and shape of nosema spores.
Here I grew bread yeast in a mixture of pollen grains and sugar water in order to show their relative sizes. The small ovals that look like nosema spores are the bread yeast. Unfortunately for us microscope-viewing beekeepers, the yeast cells are about the size and shape of nosema spores.
Dang, now I was curious. So I crushed some nosema-laden bees to make a spore-rich slurry, and put a tiny drop on a slide. Then I put a tiny drop of yeast culture immediately to the left of it, and pressed on a cover slip. I’ll tell you right now, the untrained eye could easily mistake yeast cells for nosema spores! See below:
 In this photo, yeast culture is to the left; the nosema suspension to the right. I’ve labeled the numerous yeast cells, and a group of nosema spores to the right.
In this photo, yeast culture is to the left; the nosema suspension to the right. I’ve labeled the numerous yeast cells, and a group of nosema spores to the right.
Tips:
- The nosema spores sink, so they come into focus below the level of the yeast cells.
- The outlines of the yeast cells aren’t as sharp as those of the nosema spores, and the centers of the nosema spores glow a little more brightly.
- But the key difference is that the nosema spores are more regularly shaped than the yeast cells — smoother, more uniform, and consistently sized and shaped.
I now wonder how often yeast infection is misdiagnosed as nosema???
The literature on the impact of yeasts, and their interactions with other microsymbionts is confusing, with some findings suggesting that nosema may either promote or restrict yeast replication [[7]]. Others report seeing sperm-like trypanosomes in bee guts, such as Crithidia, but I’ve yet to notice them, so don’t have a photo to share.
Practical application: The hindgut contents of a healthy bee generally contain only pollen grains in some stage of digestion and pieces of degrading peritrophic membrane. But in dying bees, or those from sick colonies, I often observe all kinds of other organisms or unidentified objects. I do not know whether they induce dysentery, or whether the excess moisture in distended bee hindguts simply allows them to proliferate.
The link between pollen and nosema
There are several ways in which pollen and nosema are linked:
- Pollen brought back by foragers is often contaminated with nosema spores.
- Nosema appears to reproduce best in a bee’s midgut if that bee is consuming pollen.
- A bee’s rectum can quickly fill with the indigestible exines of consumed pollen.
- There is often a lot of pollen available in early spring and fall ― exactly when bees may be confined by cold or wet weather, and thus unable to take cleansing flights.
So it’s easy to understand why nosema is typically an issue only in autumn or springtime. So why does it sometimes become prevalent during winter? Could it be due to the consumption of pollen? A deep and detailed study by Whitcomb & Wilson [[8]] on the digestion of pollen by the honey bee did not find any association between the feeding of pollen and dysentery (Fig. 5).
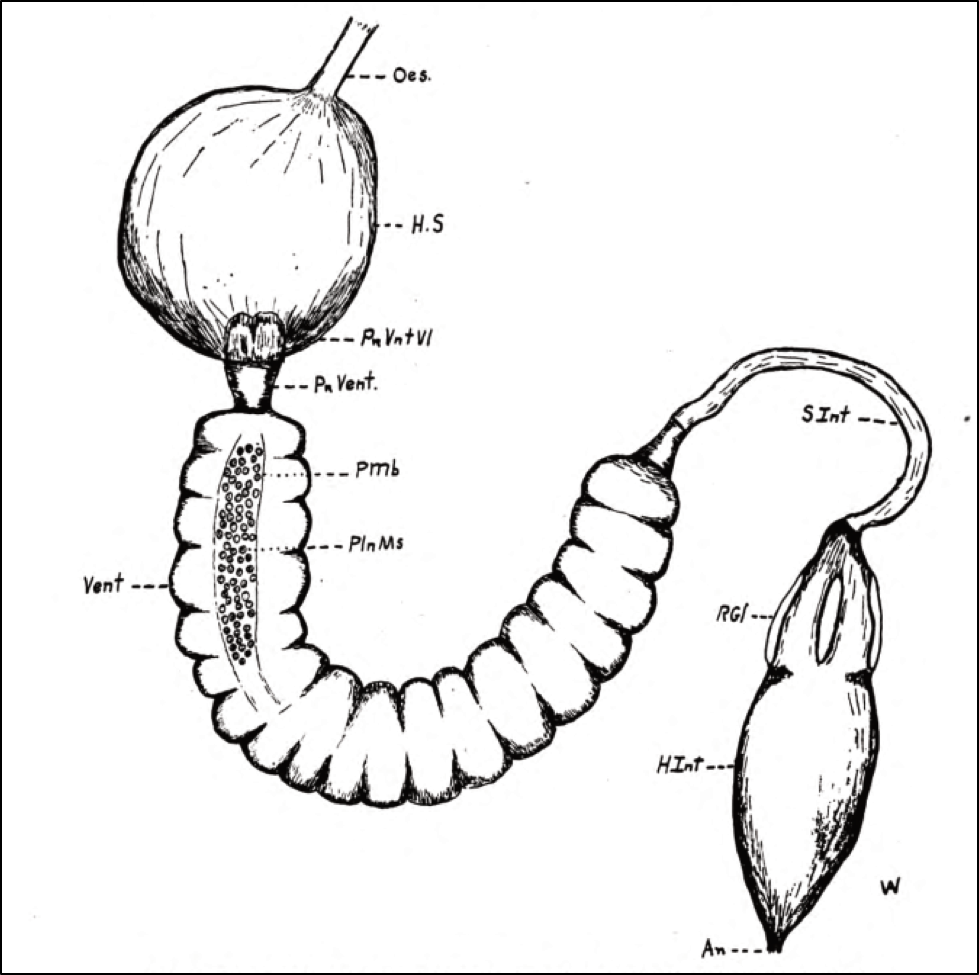
Figure 5. Whitcomb made detailed drawings of the process of pollen digestion by the bee, by staining pollen grains mixed in sugar syrup bright red, and the syrup itself blue. Here he illustrated a bolus of ingested pollen grains (enclosed by the peritrophic membrane (PMb) shortly after entering the midgut (ventriculus) from the crop (HS for “honey sac”) It only takes a few hours for the process of digestion to take place, culminating in the largely-emptied exines of the pollen grains being deposited into the hindgut (HInt), where they remain until defecation.
How about those “winter bees?”
Adult bees, if not rearing brood, need very little protein in their diet. Thus, bees can live a long time on a diet of easily-digestible honey alone, and have little need to defecate (such as during winter, or in incubator trials when they are fed sugar syrup alone). By now most of us understand that the long-lived “winter bees” store enough protein and lipids in their fat bodies to survive until spring. So why would winter bees have pollen in their guts?
Some years ago I used fluorescent tracers to track consumption of pollen sub by the bees by freezing samples of bees from the combs, and then crushing them en masse on a grid of 50 crosslines. Normally, only a portion of the bees (the nurses) would contain pollen. But to my surprise, when out of curiosity I crushed a sample of bees in November, every single bee had a gut chock full of pollen (Fig. 6).

Figure 6. Why would every bee in the hive in November (as broodrearing ceased) have a gut full of pollen?
Come winter, it appears that the long-lived “winter bees” not only load up their fat bodies, but also fill their guts with pollen. It’s not yet clear whether this phenomenon is simply to store as much protein as possible, or whether it has to do with the pollen exines functioning as a water reservoir in the rectum [[9]]. In any case, this may be why we sometimes see a spike in nosema in fall. But more importantly, could those guts full of pollen later be a cause of dysentery during winter?
Dang, I was just getting on a roll! But I’m out of space. In my next installment I’ll cover what appears to be the main cause of dysentery in bees, and what the bees may do to deal with it.
Literature cited
[1] Węgrzynowicz, P, et al (2014) Causes and scale of winter flights in honey bee (Apis mellifera carnica) colonies. J. Apic. Sci. 58(1): 135-143. DOI: 10.2478/jas-2014-0014
Wolf S, et al. (2014) So near and yet so far: harmonic radar reveals reduced homing ability of nosema infected honeybees. PLoS ONE 9(8): e103989.
Dosselli, R, et al (2016) Flight behaviour of honey bee (Apis mellifera) workers is altered by initial infections of the fungal parasite Nosema apis. Scientific Reports 6:36649 DOI: 10.1038/srep36649
[2] White, GF (1919) Nosema disease. U.S. Dept Agric Bulletin 780, 59 pp. Available in Google Books.
[3] Phillips, EF & GS Demuth (1914) The temperature of the honeybee cluster In winter, Bull. 93, U.S. Dept. Agr.
[4] Pohorecka, K & P Skubida (2004). Healthfulness of honeybee colonies (Apis mellifera L.) wintering on the stores with addition of honeydew honey. Bull Vet Inst Pulawy 48: 409-413.
[5] Prell, H, et al (1926) The Bee Laboratory. Bee World, 8(1): 10-14.
[6] Image open access, from Institut für Saat- und Pflanzgut, Pflanzenschutzdienst und Bienen, Abteilung Bienenkunde und Bienenschutz.
[7] Ptaszyńska, A, et al (2016) Nosema ceranae infection promotes proliferation of yeasts in honey bee intestines. PLoS ONE 11(10): e0164477. https://doi.org/10.1371/journal.pone.0164477
Tauber, J, et al (2019) Effects of a resident yeast from the honeybee gut on immunity, microbiota, and nosema disease. Insects 10: 296.
[8] Whitcomb, W & H Wilson (1929) Mechanics of digestion of pollen by the adult honey bee and the relation of undigested parts to dysentery of bees (Vol. 92). Agricultural Experiment Station of the University of Wisconsin. https://babel.hathitrust.org/cgi/pt?id=wu.89098844962&view=1up&seq=5
There’s also an excellent review of pollen digestion by Clarence Collison at https://www.beeculture.com/a-closer-look-7/
[9] Dr. Kirk Anderson, pers comm.
Contents
First a return to queens. 1
Treatments for nosema. 2
fumagillin. 3
Alternative treatments. 3
What else is on the market?. 6
Nozevit®. 6
Hive alive®. 9
Acids in syrup. 9
Possibilities on the radar. 9
What if you don’t want to treat?. 9
citations and notes. 10
The Nosema Problem: Part 6
Treatment
First published in ABJ November 2019
Randy Oliver
ScientificBeekeeping.com
OK, so you’ve checked under a ‘scope to confirm that some of your hives actually exhibit nosema prevalence levels of concern ― so what can you do about it?
2023 Update: see https://scientificbeekeeping.com/field-trial-of-several-nosema-treatments/
But First, let’s revisit queen failure
Root in 1940 [[1]], observed:
During the past few years, or since the shipping of package bees has come to be such an enormous industry, there has been not a little complaint that the queens in these packages, even from reputable breeders, are superseded either before they get to laying at all, or sometimes after they have a nice lot of good brood in all stages of growth.
I still hear this complaint today, despite lots of work by Pettis, Tarpy, and the queen producers to figure out why. Yet the Tarpy lab’s webpage suggests that nearly 30% of newly-installed queens die in the first 6 months.
I was curious as to how that figure compared to back in the old days. Farrar, from 1938-1946, kept track of the survivorship of 1540 package-bee queens over the first two months after installation (Table 1).
Table 1. Queen losses in a total of 1540 packages over a period of nine years, data reworked from Farrar [[2]] (packages presumably shipped to Wisconsin).
|
Percentage of queen losses due to abnormal supersedure |
| Time of loss |
First 2 weeks |
Second 2 weeks |
Second month |
Total losses |
|
5% |
8% |
6% |
18% |
| Percent of losses suspected to be due to nosema |
17% |
Farrar’s data suggest that early queen failure rates may have been substantially less in the old days than those of today. Of even more interest is that Farrar blamed most of the failures that he observed upon Nosema apis (although he didn’t associate nosema with colony mortality).
But now that N. ceranae has nearly completely replaced N. apis, is that still the case? Tarpy [[3]] purchased 10 queens each from seven California queen producers in 2011 and analyzed them for the presence of nosema 3-5 weeks after being introduced into nucs (which may have already been infected). His technicians didn’t observe any spores in the queens, and only three of the 59 queens tested had detectable levels of nosema as determined by PCR analysis [[4]].
An early failure rate of some queens is to be expected ― we observe it often if poor weather had contributed to poor mating, but such nucs typically quickly requeen themselves; Root suspected that early supersedure may be due to the aging package bees not getting the right signal that enough young bees were emerging, thus inducing them to replace the queen. Anyway, something other than nosema appears to be at play.
But the real question is the very high rate of colonies dying due to queen issues nowadays ― surveyed beekeepers put the blame for 25-40% of their colony losses on “queen failure” [[5]]. I’ve had trouble finding historical records of the rates that colonies used to go queenless, but it didn’t appear to be a common phenomenon. Langstroth [[6]] wrote in 1878:
Queens sometimes die of disease or old age, when there is no brood to supply their loss. Few, however, perish under such circumstances; for either the bees build royal cells, aware of their approaching end, or they die so suddenly as to leave young brood behind them. Queens … are usually the last to perish in any fatal casualty. As many die of old age, if their death did not ordinarily occur under favorable circumstances, it would cause, yearly, the loss of a very large number of colonies.
And then in 1940 Root wrote:
As a rule, the average colony will supersede its own queen in two years without any attention on the part of its owner. The process is a perfectly natural one and goes on in the best regulated yards year after year [Fig. 1).

Figure 1. This colony is rearing a fat supersedure cell, during the “normal” process of replacing their failing queen. Some beekeepers cut such cells out; I figure that the bees likely know what they’re doing. The question is, why do so many colonies these days fail to successfully supersede their queens, thus allowing the hive to go queenless?
Practical application: Back in the day, at least in my operation, I don’t remember colonies going queenless that often ― they’d just replace a failing queen themselves, as described by Dadant and Root. But nowadays, colonies appear to commonly go queenless. Since few of today’s queens appear to be infected with ― much less die from ― N. ceranae infection, it appears that today’s reported high failure rates can’t be blamed on nosema.
More to the point, since a healthy colony would be expected to replace a failing queen by normal supersedure (or emergency cells), perhaps we should instead be asking the question, Why are we seeing so much “failure to successfully supersede” these days?
An interesting study by Butler in 1957 [[7]] indicated that a failing queen, should she continue to produce adequate pheromones to suppress her workers from starting supersedure cells, could put the colony in the position of not being able to replace her once she’s unable to lay any more eggs. Could today’s queens be sending the wrong signals for some reason? Before you point fingers, I see this happening even in my pesticide-free apiaries. I’ve discussed this apparent phenomenon with other researchers; it is currently an open and nagging question towards which we should devote more study.
OK, I’ve perhaps diverged a bit; let’s get back to treatment for nosema.
Treatments for nosema
We are still on the learning curve as far as figuring out how microsporidians such as nosema infect host cells [[8]], and thus largely shooting in the dark as far as how to figure out which directions to take in our search for potential treatments. In today’s backdrop, “antibiotics” are being more closely regulated, and we beekeepers need to keep in mind that the public is concerned about what chemicals get into our honey. So how about fumagillin, which has recently come back on the market?
fumagillin
Back in 1969 Moffett [[9]] tested 24 different compounds against N. apis ― only fumagillin gave evidence of any control. But what we’re now realizing is that all antibiotics come with potential “side effects.” One of the issues with fumagillin is that, as pointed out by van den Heever [[10]], the commercial formulation consists of the dicyclohexylamine salt (DCH) of the product, which is coming onto the radar of those who are concerned about consuming DCH-contaminated honey:
The contribution of DCH present in the commercial fumagillin formulation to the overall toxicity and resulting food safety implications in products destined for human consumption is definitely not negligible.
Not only that, but fumagillin does not appear to be as effective against N. ceranae as it was against N. apis, and may even exacerbate the problem [[11]]:
Fumagillin suppresses reproduction of microsporidia but disease prevalence and hive performance in treated apiaries were similar to untreated apiaries 6 months after treatment. We compared responses of N. apis and N. ceranae to the antibiotic at concentrations that simulated fumagillin degradation in hives during the honey bee foraging season when use of the drug is discontinued. N. ceranae spore production recovered at a higher fumagillin concentration than N. apis. At lower fumagillin concentrations, significantly more infective N. ceranae spores were produced in treated bees than in untreated infected bees.
Practical application: After the application of fumagillin was banned in Europe and illegal applications were also reduced, some expected an increase in colony losses caused by nosemosis. However, this did not happen.
I also find of great interest that when a group of Canadian researchers [[12]] followed up after fall treatments with fumagillin, they concluded that:
Fall treatment with fumagillin of hives in Canada did not appear to decrease either winter mortality nor spring spore counts.
A colleague in Spain, Antonio Pajuelo, tells me that:
Although treatment with fumagillin will generally reduce spore counts of Nosema ceranae, that does not necessarily correlate with improved colony strength or productivity.
Practical applications: Fumagillin used to work well against N. apis; it may not be as effective against N. ceranae. Treatment with fumagillin for Nosema ceranae may not improve winter survivorship, and may actually have negative health effects upon the colony. And ― if buyers start testing for residues ― it may not only be a waste of money, but also affect the salability of your honey.
Alternative treatments
Hey, we’d all love to have a food-grade treatment for nosema. Some years ago, two Canadian beekeepers hired me to determine what doses of Generally Recognized as Safe (GRAS) antifungal food preservatives could be safely fed to bees in syrup, and then to see whether such doses would control nosema. I never published the results, so here’s the short version.
I found that caged broodnest bees fed sugar syrup could tolerate up to 4000 ppm of any of three common food preservatives, although all the preservatives at high doses caused serious defecation of previously-consumed pollen (perhaps due to disturbing the gut endosymbionts) (Fig. 2).

Figure 2. A preliminary run, demonstrating the dysentery caused by feeding a preservative, compared to the sugar syrup controls (the two cages in the upper left). I then chose “safe” doses of each preservative to test for their effects upon nosema.
I then inoculated workers shaken from brood combs of a lightly-infected colony into 6 replicate cup cages per treatment, and fed them a freshly-prepared inoculum of ~20,000 spores each. I maintained the caged bees in an incubator and fed them (based upon my previous findings) sugar syrup laced with either 3000 ppm potassium sorbate, 1000 ppm sodium proprionate, or 1000 ppm sodium benzoate, and then tracked both bee mortality and nosema spore counts of some surviving bees each day (Figs. 3 & 4).
Figure 3. The bees in all the treated groups survived as well as the untreated controls.
Figure 4. Bottom line: unfortunately, although it was a great idea, none of the preservatives appeared to adequately suppress nosema reproduction in the bees.
Since then, researchers (including myself) have tested many other possible treatments, generally with disappointing results. I’ve reviewed some of those treatments previously [[13]]. Thymol and resveratrol have shown some promise [[14]].
What else is on the market?
Nozevit®
For anyone with a deeper interest in the bee gut and pollen digestion, I suggest perusing a great paper by Whitcomb and Wilson from 1929 [[15]], which contains some great drawings of the honey bee peritrophic membrane, such as the one below (Fig. 5).
Figure 5. This drawing from Whitcomb is a side view of a bee’s midgut wall, outer surface to the left, gut lumen to the right, with an infolding of the nutrient-absorbing intestinal villi cells (the darkened structures) in which nosema is able to reproduce. The layers of the protective peritrophic membrane lie to the right.
Whitcomb pointed out that honey bees, compared to other insects, create a more multilayered peritrophic membrane. More recently, other authors have found that the membrane serves several important functions. Within the midgut, the membrane not only compresses any ingested pollen into a bolus for passage, but also traps some toxic chemicals. As the food bolus makes its way down the midgut, the peritrophic membrane allows for a countercurrent flow of fluids inside or outside of the membrane, thus allowing the bee to also better utilize its digestive enzymes.
But none of the above valuable functions explain why honey bees would create so many layers in the membrane. Some researchers suggest that the membrane may help to protect the insect from infection by ingested parasites, such as viruses, bacteria, or (I’m adding this) nosema. And this is where it gets interesting.
Practical application: as far as I can tell, the only place that nosema can infect a bee is in the epithelial (surface) cells of the intestinal villi. In order for a nosema spore to gain entry, it needs to eject its polar body and first penetrate the peritrophic membrane, and then harpoon an epithelial cell. So one possible way to thwart nosema would be to toughen the peritrophic membrane.
Nozevit® is a natural extract of oak bark, following a traditional beekeeper recipe used in Croatia. Oak bark is well-known to contain plant polyphenols commonly called “tannins.” Tannins give plants an astringent taste due to their property of binding with proteins, thus making those proteins unavailable for digestion. Humans use tannins to toughen up the collagen proteins in animal skins during the process of “tanning.” We’re also learning that some tannins may have beneficial effects when consumed in small quantities.
As found by Tlak Gajger [[16]]
Nozevit induces the production and secretion of mucous from the epithelial layer of treated bees, and additionally coats the peritrophic membrane to form a firm and resilient envelope. Thus, the preparation may ensure protection from new invasion with Nosema sp. spores and also from normal physiological processes.
Thus the mechanism of action of Nozevit may be due to containing infective nosema spores within the peritrophic membrane, and/or preventing them from harpooning an epithelial cell.
Nosevit can be applied by any of three methods, or some combination thereof. Since I found application methods hard to find, I asked the manufacturer directly:
- Drenching: Mix 1.5 ml in 8 oz. thin syrup solution. Apply this mixture by drenching 4 times 4 days apart. Apply drench when bees have at least 4 hours of flying time left in the day and above 60 F.
- In syrup: Mix Nozevit PLUS in feeding syrup at the rate of 1.5 gallons Nozevit Plus in 1000 gallons normal feed syrup.
- In patty: Mix Nozevit or Nozevit PLUS in protein patty mix at the rate of 1.5 ml per 1# finished weight patty. 200# final weight batch of patties needs 300 ml or 1 1/4 cup Nozevit or Nozevit PLUS.
Practical application: I’ve reviewed a number of lab studies and field trials of Nosevit [[17]]; in general, researchers have found some reduction in spore counts.
Update: A small SARE study on caged bees found Nosevit to reduce spore loads, but not to improve the survivorship of the caged bees.
Hive alive®
Another product on the market is Hive Alive, which contains seaweed extracts. The product makes a number of claims, the ability to suppress nosema among them. But careful inspection of the supportive studies suggest that the claims may be a little bit wishful. The most supportive study was run in Greece by Charistos [[18]]; however, the results are somewhat “polished” in the sales brochures. What beekeepers are really interested in is the effect of treatment upon colony strength and survival ― despite application of the treatment, Charistos’ study found no appreciable differences in colony strengths between the test and control groups during the first eight months (November through June). By the next November the colonies had dwindled to only 3-4 frame strength ― too small for me to consider any subsequent results to be meaningful. I’d need to see stronger supportive data before making any further assessments of the product. So until I can review further supportive data on Hive Alive, I am unable to provide an assessment.
Acids in syrup
Beekeepers just love to dump this, that, and the other thing into sugar syrup, often without any supportive evidence that it is of any benefit to the bees. Many add acid, in the hope that it will help with nosema. Swedish researchers Eva Forsgren and Ingemar Fries found otherwise [[19]]:
No effect from altering the pH by addition of acetic acid, or from benzoic acid could be found, neither on the quantitative disease development of the parasite, nor on the infection rate of individual bees. The results from the field experiments support the laboratory results: addition of acetic or benzoic acid to the feed of honey bees has no influence on nosema prevalence or development.
Possibilities on the radar
There is now renewed interest in the development of products to take the place of fumagillin. Dr. Boris Baer has devoted a number of years studying how queen bees manage to keep sperm viable in their spermatheca. His lab recently found that the seminal fluid contains chitinases and proteases (enzymes that degrade the chitin and protein in the nosema spore’s protective “shell”) that “kill” the spores [[20]]. It appears that drones surround their semen in protective agents. Further study may come up with something that we can use as bee medicine.
Another group of compounds of interest are porphyrins, pigments commonly found in nature. Ptaszyńska [[21]] found that feeding some porphyrins in syrup increased the longevity of nosema-infected bees, and reduced spore counts.
Practical application: Researchers continue to test substances against nosema. Perhaps some will get lucky.
Note: I’m happy to update this page if anyone comes across additional studies, or new products come on the market. Let me know!
There is a nice review of the state of the art of nosema control prodcts in: The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits. https://www.mdpi.com/2076-0817/10/2/234/htm
I’ve also personally tested zeolite, but didn’t find any benefit.
What if you don’t want to treat?
Then just don’t worry about nosema (and focus upon varroa instead). Most colonies survive nosema infection, and one can argue that those that don’t should be removed from the gene pool anyway. I’ve previously mentioned studies that indicate that having young queens, a large winter cluster, a south-facing sunny winter location (to allow cleansing flights, Fig. 6), and abundant pollen allow colonies to hold their own against nosema.

Figure 6. This colony is clearly suffering from dysentery. Nosema-infected bees tend to chill during cold-weather cleansing flights, thus eliminating themselves from the hive, which is a good thing.
Allow me to end this section with a quote from USDA researcher Floyd Moeller [[22]]:
The results … suggest that two natural factors can help to reduce the level of Nosema infection: emergence of brood allows replacement of infected bees with healthy young bees; winter flights reduce the amount of defecation in the hive, and help to eliminate diseased bees by chilling them in flight.
The resistance of colonies to this infection should, therefore, be increased by encouraging the production of brood (e.g. by having vigorous productive queens, and abundant pollen or pollen supplements as appropriate), and by allowing winter flight whenever weather permits.
Aknowledgements
Thanks to Peter Borst, Dave Tarpy, Jeff Pettis, the late Ingemar Fries, Mariano Higes, Antonio Pajuelo, and the other researchers and beekeepers who’ve helped me.
citations and notes
[1] Root, AI & ER (1940) The ABC and XYZ of Bee Culture
[2] Farrar, CL (1947) Nosema losses in package bees as related to queen supersedure and honey yields. J. Econ. Ent. 40(3): 333-338.
[3] Tarpy, D, et al (2012). Assessing the mating ‘health’ of commercial honey bee queens. Journal of Economic Entomology 105: 20-25.
[4] I’ve cited other studies indicating that N. ceranae is not normally a problem for queens in Part 4 of this series.
[5] https://bip2.beeinformed.org/survey/
[6] Langstroth, L (1878) Langstroth’s Hive and the Honey-Bee. Dover.
[7] Butler, CG (1957) The process of queen supersedure in colonies of honeybees (Apis mellifera Linn.). Insectes Sociaux 4(3): 211―223.
[8] Franzen C (2004) Microsporidia: how can they invade other cells? Trends in Parasitology 20(6): 275-279.
[9] Moffett, J, et al (1969) Compounds tested for control of nosema in honey bees. J. Econ. Ent. 62(4): 886-888.
[10] van den Heever, J, et al (2014) Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 62(13): 2728-2737.
[11] Huang W-F, et al (2013) Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog 9(3): e1003185.
[12] Williams, G, et al (2010) The microsporidian Nosema ceranae, the antibiotic Fumagilin-B®, and western honey bee (Apis mellifera) colony strength. Apidologie 42: 15-22.
[13] https://scientificbeekeeping.com/the-nosema-twins-part-5-alternative-treatments/
[14][14] Costa, C, et al (2010) Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 41: 41―150.
[15] Whitcomb, W & H Wilson (1929) Mechanics of digestion of pollen by the adult honey bee and the relation of undigested parts to dysentery of bees. Vol. 92 Agricultural Experiment Station of the University of Wisconsin. Available at https://babel.hathitrust.org/cgi/pt?id=wu.89098844962&view=1up&seq=4
[16] Tlak Gajger, I, et al (2011) Effect of the herbal preparation Nozevit on the mid-gut structure of honeybees (Apis mellifera) infected with Nosema sp. spores. Veterinarni Medicina, 56(7): 344―351.
I’ve discussed Dr. Gajger’s detailed and methodical research regarding Nozevit with her over the years, and find the results compelling. She’s published twice in this journal:
Tlak Gajger I, Petrinec Z, Pinter Lj, Kozaric Z (2009a): Experimental treatment of nosema disease with “Nozevit” phyto-pharmacological preparation. American Bee Journal 149, 485―490.
Tlak Gajger I, Vugrek O, Pinter Lj, Petrinec Z (2009b): “Nozevit patties” treatment of honeybees (Apis mellifera) for the control of Nosema ceranae disease. American Bee Journal 149, 1053―1056.
[17] Rhoades, P (2009?) Testing the efficacy of three new alternative treatments for Nosema disease of honey bees in Tennessee. https://projects.sare.org/project-reports/gs09-086/
Higes, M, et al (2014) Preliminary effect of an experimental treatment with “Nozevit®”, (a phyto-pharmacological preparation) for Nosema ceranae control. Journal of Apicultural Research 53(4): 472-474.
van den Heever, J, et al (2016) Evaluation of Fumagilin-B® and other potential alternative chemotherapies against Nosema ceranae-infected honeybees (Apis mellifera) in cage trial assays. Apidologie 47(5): 617―630.
Michalczyk, Maria, et al (2016) Evaluation of the effectiveness of selected treatments of Nosema spp. infection by the hemocytometric method and duplex PCR. Acta Veterinaria-Beograd 66 (1): 115-124. Open access review of other studies to date.
[18] Charistos, L, et al (2015) Long term effects of a food supplement HiveAlive™ on honey bee colony strength and Nosema ceranae spore counts. Journal of Apicultural Research 54(5): 420-426.
[19] Forsgren, E & I Fries (2005) Acidic-Benzoic Feed And Nosema Disease. Journal of Apicultural Science 49(2): 81-86.
[20] Peng Y, et al (2016) Seminal fluid of honeybees contains multiple mechanisms to combat infections of the sexually transmitted pathogen Nosema apis. Proc. R. Soc. B 283: 20151785. It’s a bit more complicated than just “killing” the spores.
[21] Ptaszyńska, A, et al (2018) Porphyrins inactivate Nosema spp. microsporidia. Scientific Reports 8: 5523.
[22] Moeller, F (1972) Effects of emerging bees and of winter flights on nosema disease in honeybee colonies. J. Apic. Res 11(2): 117-120.
Pesticides in the News
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in August 2019
Our beleaguered Environmental Protection Agency, tasked with protecting man and the environment, is caught between an unsupportive Administration and a public that is becoming more and more concerned about the effects of pesticides and climate change.
I plan to return to my series on pesticides, but our extreme weather this spring was disastrous for our bee operation, since we were unable to rear queens for splitting our hives after almonds. As a result, we were also unable to control swarming. We’ve since been working overtime to try to recover our numbers in time to build our colonies up for next year’s almond contracts. I recently congratulated my son Eric for now being able to claim the title for having the honor of running the operation during the worst season in memory.
That said, in recent months, a few scientific papers and news items of interest have caught my attention. We may look back on some of them as watershed events that changed the courses of our future.
Revocation of some neonic labels
OK, there was a lot of hoopla about the recent final settlement of a lawsuit by Center for Food Safety (CFS) that has been ongoing for six years, representing plaintiffs CFS, Sierra Club, Beyond Pesticides, Center for Environmental Health, Pesticide Action Network, and four commercial beekeepers, Steve Ellis, Jim Doan, Tom Theobald, and Bill Rhodes. The settlement brought headlines such as:
|
Center for Food Safety wins in case to force EPA to ban 12 neonicotinoids.
***
Center for Food Safety secures legal victory for bees!
CFS scored another huge legal victory! As a result of our lawsuit against EPA, 12 toxic, bee-killing “neonic” pesticides will soon be withdrawn from the market!
|
Note how the neonics are invariably labeled as “bee-killing pesticides” — it just sorta rolls off the tongue, doesn’t it? This “victory” actually meant that EPA would accept the voluntary requests from Bayer, Syngenta, and Valent to withdraw their registrations of 12 products containing one or more neonics: 5 containing thiamethoxam, 6 containing clothianadin, and 1 containing imidacloprid. All were turf or seed treatments, other than Bayer’s “Flower, Rose & Shrub Care” and one foliar product.
None of the neonicotinoid insecticides were banned from use — only a few formulated products, representing a drop in the bucket of neonicotinoid applications, were being allowed to have their registrations expire (and stock on the shelf could continue to be sold for another year).
There are extreme views on both sides of pesticide issues. I’m more interested in rational discussion as to how farmers can best control pests via sustainable and eco-friendly methods. For the foreseeable future, pesticides will remain part of that equation. The neonics live up to many of their promises, but as one would expect from any neurotoxin, must be applied with caution, and the EPA must follow research findings that indicate that there may be unforseen adverse effects.
Such unforeseen effects are noticed only after a pesticide has been in use for some time, and there are plenty of folk out there looking for any problems due to the neonics. One recent paper suggests that we need to call for more research on the affects of neonics upon mammalian reproductive hormones.
Practical application: I’m glad to see neonics removed from the homeowner arsenal, due to their potential for problems to pollinators visiting excessively-treated plants. The lawsuit didn’t change much for agricultural uses, but the public attention helps to keep the pressure on the EPA (although it likely falls upon deaf ears in our current Administration).
Neonics and mammalian reproduction
In a recent study run in South Dakota [[1]], the researchers divided 20 captive pregnant female deer into four groups, and fed them a range of doses of imidacloprid in their drinking water, up to 15 ppb, covering a realistic range for field exposure for the lower doses. I’ll quote their discussion directly:
Our study provides the first overview of effects of imidacloprid on white-tailed deer. We documented that deer in our experiment avoided imidacloprid-contaminated water. Moreover, we discovered that fawns that died during our experiment had greater concentrations of imidacloprid in spleens compared to those that survived. Fawns with relatively high concentrations of imidacloprid in spleen and genital organs also tended to be smaller and less healthy than those with relatively low concentrations of imidacloprid in these organs. Finally, our study provides support for reduced activity of adult and fawn white-tailed deer with relatively high concentrations of imidacloprid in spleens.
I reviewed the study carefully, including reading their supportive citations and beyond. I have a number of questions about the study and their analyses; the author to whom I submitted them declined to answer. So although I can’t yet accept their conclusions wholeheartedly, this study calls for follow-up research.

The question is whether some of our pesticides are causing long-term reproductive harm to wildlife, and perhaps us humans as well [[2]].
The reason is that some of the pesticides used nowadays are neurotoxins or may inadvertently act as endocrine disruptors — interfering with hormones involved in sexual development, cancerous tumors, birth defects, and other developmental disorders.
Practical application: The problem is that we may not notice subtle adverse effects until we’ve caused harm to generations of wildlife or humans. Hence, although I feel no need for alarmism, we do need to keep a close eye on studies such as the one above.
The Phase out of Chlorpyrifos
One class of chemicals that environmental protection agencies are united in trying to phase out are the acetylcholinesterase inhibitors — the organophosphates and carbamates. These have been clearly linked to causing problems in childhood brain development (yes, my generation grew up with a lot of it). Oddly, EPA made a special case for the registration of coumaphos for beekeepers to use in their hives against varroa and Small Hive Beetle.
Chlorpyrifos (an organophosphate, commonly sold as Lorsban® and Dursban®) used to be widely used for home insect control and outdoor residential pest management, and remains one of the most widely used insecticides in the world. It is often the most common insecticide found in bee hives (after beekeeper-applied miticides) [[3]].
EPA has been trying to eliminate its use, but the Trump Administration has appealed. California is nevertheless charging ahead with a ban. The ag community is alarmed, since there are a number of pests for which chlorpyrifos is the only effective control product. We’ll see how this plays out.
Practical application: Once a pesticide has been used for a while, it’s not uncommon that there were unforeseen adverse effects to man or the environment. Chlorpyrifos started to be used in 1965 — five years before the creation of the EPA — so it’s not surprising that it’s got some issues. Beekeepers have long dreaded bee kills due to Lorsban, so we can hope that growers replace it with something less harmful to bees.
When a regulatory agency declares that it is going to restrict any product or practice, you can count on there being a loud protest that says that change is impossible. We taxpayers pay the EPA to weigh the evidence for us, although unfortunately of late, politics has trumped science.
Fipronil Cumulative Effects
Beekeepers got it wrong and right
Back in the late 1990s beekeepers in France claimed that Gaucho, a seed treatment containing the systemic neonicotinoid insecticide imidacloprid, which was being applied to sunflowers, was causing massive colony mortality. But the case against imidacloprid was weak, since the low levels of the pesticide in nectar or pollen would be rapidly metabolized in a bee’s body (~92% is gone within 48 hours), and thus it appeared unlikely that it could kill an entire colony from foraging on the treated crop. Nevertheless, public protests against Gaucho occurred, and the registration of its use was revoked.
But there was another seed treatment also being applied to sunflowers at the time (Regent) which contained a different systemic insecticide (fipronil). The beginning of colony deaths actually better coincided with the use of Regent on sunflowers [[4]]. The colony deaths continued. Despite the claims by the manufacturers that the treatments were safe for bees, Regent was also banned [[5]]. I searched the Web and found that Regent insecticide appears to still be sold in the U.S., but only for use as a furrow treatment on potatoes, and a treatment for corn seed to be exported outside of the U.S. or its territories.

When I walked in to shoot this photo of hives being used to pollinate sunflowers, the smell of pesticide on the tomatoes in the foreground was intense. Sunflower pests unfortunately often get a foothold when the flowers open, thus calling for insecticide applications at exactly the worst time for pollinators.
A recent study by Holder [[6]] found that the beekeepers were likely wrong about imidacloprid, but were right about a pesticide causing the deaths of their hives. Of interest is that fipronil takes a while to kill an insect that has consumed it, thus allowing ants, yellowjackets, and honey bees to take it back to the nest, where it eventually kills the entire colony — it’s actually a preferred bait treatment for those three insects.
Holder found that unlike imidacloprid, fipronil exhibits “time-reinforced toxicity,” meaning that its metabolites bioaccumulate in an insect’s body tissue, resulting in a lethal cumulative dose after a few days’ feeding.
Practical application: we beekeepers must demand that EPA require long-term feeding trials as a requisite for pesticide registrations.
Glyphosate in Your Groceries?
I’m not about to take an opinion on the recent concerns about glyphosate/Roundup® ― I’ll let our regulatory agencies review the evidence. To date, none have been able to make a compelling case against the herbicide, which has transformed farming in the U.S., allowing growers to reduce erosion and improve soil structure by practicing “no till.” And when I review the prevalence of Non-Hodgkin’s Lymphoma since glyphosate came on the market, I don’t see a huge spike. Those applicators that claim that Roundup caused their disease, generally also admit to not following label instructions to wear protective clothing. It’s unfortunate that the debate, instead of being conducted scientifically, is taking place in the courtroom, with attorneys and plaintiffs hoping for huge judgements in their favor. That said, a recent study may explode both the science and the number of lawsuits:
Transgenerational effects of glyphosate?
A new paper in the journal Nature may, if it gets traction, shift the entire way that the chemical companies and EPA would need to assess any chemicals for allowed use. Currently, registrants must submit data for short-term toxicity, and if indicated, for longer-term trials over perhaps multiple generations of test animals. But these methods were arrived at before we understood transgenerational epigenetic inheritance, by which environmental exposure to temperature, diet, calamity, or any other stress or strong benefit can affect how the genes of the exposed individual’s children, grandchildren, or great-grandchildren may be expressed.
And that is exactly what Deepika Kubsad of Washington State University’s Michael Skinner lab, did with glyphosate [[7]]. Their results were stunning. Smithsonian Magazine headlined its coverage with:
Biologist Michael Skinner has enraged the chemical community and shocked his peers with his breakthrough research.
The rest of the Smithsonian article is well worth reading [[8]]. What Skinner has shown is that a number of common chemicals can not only act as “endocrine disruptors,” but that exposed individuals can pass those effects on to their offspring without any change to their DNA, but rather by epigenetic (meaning “on the outside of genes”) changes that may be passed on for generations. This is called “transgenerational epigenetic inheritance.” Skinner’s team found that the great-grandchildren of the rats exposed to glyphosate exhibited disease and pathologies associated with the exposure of their great-grandparents.
Before you jump to conclusions, be aware that the researchers had “exposed” pregnant female rats to extreme doses of glyphosate, which was injected under their skin, rather than consumed. By my quick calculations, each pregnant female rat was injected with the amount of glyphosate equivalent to what a 140-lb pregnant human being would receive if injected with a full cup of the recommended dilution of Roundup to be sprayed upon weeds — and injected for six days in a row (to be clear, this is far more glyphosate than any human being, pregnant or otherwise, would ever be exposed to).
Despite the high dose of glyphosate (which did not appear to adversely affect the pregnant females): “There was no effect on litter size or sex ratio observed for any generation…”
But in subsequent generations there were some differences in weights and other abnormalities.
Thus, my worry level about glyphosate has not appreciably increased until we see this experiment replicated with more field realistic doses. But that doesn’t mean that the study hasn’t gotten a lot of attention in principle.

The use of glyphosate and Roundup Ready® crops has revolutionized agriculture. Glyphosate has been touted as one of the safest chemicals used in agriculture; but could it have insidious effects? [[9]]
Practical application: There’s a possibility that these findings may directly apply to honey bees, but more importantly, the chemical industry, chemical users in manufacturing, the ag industry, and the world’s regulatory agencies are in shock. This study could disrupt the status quo, and signal a new beginning for how modern human societies — which have embraced the thousands of chemicals that we all use in everyday life — would need to test every new, and previously-registered, chemical for “safety.” We can expect serious pushback and delaying tactics, but we may be entering a new age of chemical regulation.
Climate change and the “new Normal”
Speaking of new ages, we are now in the Anthropocene — the geological age in which Homo sapiens is the major cause of environmental and biological change on our planet.
One of those changes is in weather patterns (a.k.a. “climate”). The Earth has been coming out of its current ice age for some tens of thousands of years (during which time human civilization developed). But that change has accelerated in recent years, and many species on this planet are likely going to be unable to adjust. Depending upon one’s politics, humans appear to be responsible for this rapid change, due to our unquenchable demand for the burning of fossil fuels.
One effect of the resulting higher CO2 content of the atmosphere is warming, resulting in both droughts (as we suffered through for several years in California), or massive rainfall (due to greater evaporation of water into the atmosphere) — as many areas of the Midwest and East are enjoying this season. Farming in the U.S. and much of the world may never again be the same.

I shot this photo of a field in Kansas this spring. Note the center pivot irrigation system at the upper left. Many acres of farmland will not be planted this season, due to excessive moisture.
A recent paper in Scientific American [[10]] helps to explain why we are seeing such extreme weather. It appears that the warming of the poles affects how the jet stream moves the highs and lows that cause what we call “weather” from west to east across the continents. The North Pole is warming more quickly than the lower latitudes, resulting in abnormally wide oscillations in the high- and low-pressure zones that used to progress across our continent. These zones now often stall in place, leading to prolonged periods of drought or heavy rain.
Practical application: These extreme weather events are likely to be the “New Normal” [[11]]. They’ve made beekeeping far more difficult for us in California — our plant phenology is all screwed up, and in recent years we’ve either been parched or drowning in rain. And this year, heavy rainfall is affecting agricultural areas across the country. Independent of one’s political persuasion, there is a strong case to be made for invoking the Precautionary Principle — in this case, since we know that CO2 traps heat in the atmosphere, perhaps it would be wise for us to stop throwing gasoline on the fire.
Wrap Up
The demands of the human population of our planet, coupled with our current chemical-heavy methods of agricultural production and urban life in general, along with our daily burning of incredible quantities of fossil fuels, are finally getting to the point that we will no longer be able to ignore the consequences. Luckily, consumer and voter activism are starting to ignite in response, with the honey bee being a poster child for all pollinators and wildlife. We beekeepers can perhaps help to shift the way we do things to a more sustainable manner, with less harm to man and the environment.
citations and notes
[1] Berheim, EH, et al (2019) Effects of neonicotinoid insecticides on physiology and reproductive characteristics of captive female and fawn white-tailed deer. Scientific Reports 9: 4534. Open access.
[2] Photo by Veledan – https://commons.wikimedia.org/w/index.php?curid=326306
[3] Mullin CA, et al (2010) High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 5(3): e9754. doi:10.1371/journal.pone.0009754
[4] See Figure 16.3 in EEA (2013) Late Lessons from Early Warnings: Chapter 16 – Seed-dressing systemic insecticides and honeybees. https://www.eea.europa.eu/publications/late-lessons-2/late-lessons-chapters/late-lessons-ii-chapter-16/at_download/file
[5] http://www.ipsnews.net/2004/03/environment-europe-alarm-sounds-on-bee-killing-pesticides/
[6] Holder, P, et al (2018) Fipronil pesticide as a suspect in historical mass mortalities of honey bees. https://www.pnas.org/content/pnas/early/2018/11/26/1804934115.full.pdf
[7] Kubsad, D, et al (2019) Assessment of glyphosate induced epigenetic transgenerational inheritance of pathologies and sperm epimutations: Generational toxicology. Scientific Reports 9: 6372. https://www.nature.com/articles/s41598-019-42860-0
[8] https://www.smithsonianmag.com/innovation/the-toxins-that-affected-your-great-grandparents-could-be-in-your-genes-180947644/ Just Google “skinner epigenetics.”
[9] Photo credit: By USFWS Mountain-Prairie. https://commons.wikimedia.org/w/index.php?curid=47582798
[10] Mann, M (2019) The Weather Amplifier. Scientific American, March. http://www.meteo.psu.edu/holocene/public_html/Mann/articles/articles/MannSciAmFeb19.pdf
[11] Francis, J (2019) Rough Weather Ahead. Scientific American 320(6): 46-53.
Contents
Effects of nosema upon an individual bee. 1
Effects of nosema upon the colony. 2
Colonies can fight back. 2
Nosema and almond pollination. 4
Nosema and the queen. 7
How concerned should you be about nosema?. 8
The take-home message. 10
Coming. 11
citations and notes. 12
.
The Nosema Problem: Part 4
Nosemosis
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in September 2019
Nosema is a common parasite of honey bees, and does not cause any obvious signs of disease. But should the prevalence of infection in the hive get out of hand, then the disease “nosemosis” may become apparent at the colony level, resulting in poor performance, or even depopulation.
Since the invasion of N. ceranae worldwide, we’ve learned a lot more about this parasite and its effects upon individual bees, but are still not completely clear as to its effects upon the colony as a whole.
Effects of nosema upon an individual bee
Although infected bees typically do not exhibit overt signs of disease, they may be negatively affected in a number of ways:
- By damage to the cells lining the bee’s midgut, resulting in impaired digestion.
- By poorer development of the hypopharyngeal glands and fat bodies, and reduced ability to produce jelly as a nurse bee, which may affect protein dynamics for the entire colony.
- By general stress due to infection, and immune suppression by the parasite, which may make the infected bee more susceptible to other stressors or pathogens such as viruses [[1]].
- Infected bees may also exhibit an accelerated transition to foraging behavior, leading to reduced overall longevity. And infected foragers, due to energy issues, may not be able to forage as well.
- The bee’s navigational abilities may also be impaired by infection, resulting in drifting and/or failure to return.
Effects of nosema upon the colony
Nosema, due to its impairment of digestion, and the increase in the protein and energy demands of infected bees, means that those bees may be poorer performers overall, especially with regard to their production of the critical jelly when they are fulfilling their role as nurses. In addition, the infected bees’ reduced overall longevity due to their premature transition to foraging, and then less efficient and curtailed foraging abilities [[2]], result in the inability of a colony to build up its population to its full potential. And if the majority of bees in a colony become infected, the colony may start to spiral downhill (Fig. 1).

Figure 1. One of my colonies, photographed as it was rapidly dwindling in early springtime due to a high prevalence of nosema-infected bees. Note the healthy-looking queen top center, but also a supersedure cell, often found in a heavily nosema-infected hive. Image courtesy Kodua Galieti
Colonies can fight back
My own observations of the impact of N. ceranae upon colony performance agree with those of Dr. White for N. apis ― it’s all about the percentage of bees in the hive infected (the “prevalence” of infection) [[3]]. If fewer than 20% of the workers are infected, I observe little effect. By 40%, I see signs of colony buildup slowing down and decreased honey production [[4]]. At 60% infected, the poorer performance is obvious, and when 80% or more of the bees are infected, the colony can suddenly collapse when it can no longer care for its brood (as in Colony Collapse Disorder). Those percentages are of course approximations, since other colony health factors are often involved.
The critical issue for the colony is then to minimize the transmission of nosema to newly-emerged bees. And this is where is gets complicated ― a colony can rear a lot of new workers when pollen is abundant, and pull ahead of nosema if it has a vigorous queen. But if that pollen is contaminated by nosema spores, or if there is defecation occurring in the hive due to poor flight weather, the rate of infection can increase.
Clearly, the vigor of the queen is critical; but she can also become infected herself (more on this later in this article). Colonies that somehow “sense” that their queen is failing (or that the colony is suffering from a high parasite load or some other issue) will summarily replace their dear mother without hesitation, via the rearing of supersedure cells (Fig. 2). Furgala [[5]] showed that this is the case when the queen becomes infected by nosema.

Figure 2. Should the queen become infected by nosema, the workers may attempt to replace her by rearing supersedure cells. Supersedure is a generic response exhibited by colonies struggling with any number of issues. With a new queen, and better flight weather, the colony may be able to break the infection cycle, and purge nosema from the hive.
Practical application: Despite the fact that nosema proliferates during times of pollen abundance (more on this later), a colony with a large enough population and a vigorous queen [[6]] with plenty of room for egg laying, may be able to rear new workers fast enough to stay ahead of the accelerated attrition of infected workers ― provided that the fecal-oral route of spore transmission is reduced due to there being adequate opportunity for defecation flights.
Nosema and almond pollination
Early this winter some beekeepers paid me to test a substance to control nosema. I began the trial at the end of January (after the colonies had been rearing brood for nearly three weeks), by taking samples of bees from an outer comb from 25 hives ― I intentionally chose medium-strength hives for the trial, since I assumed that they would be more likely to exhibit some degree of nosema infection. To my great surprise, we only spotted a few (sometimes questionable) spores in only a few of the bee samples — nosema appeared to have disappeared during our brief winter brood break in the California foothills.
We then gave all the hives pollen sub and moved them to the almonds, where they then suffered through the rainiest bloom in memory, but were able to fly for a short period of time most days. This combination of pollen income coupled with cool weather and greatly reduced flight hours was not favorable for colony buildup, but certainly conducive to nosema buildup. So it wasn’t unexpected that when we tested the same hives 34 days later in early March, the median nosema prevalence had risen considerably (ranging from 0-9 bees/10) (Table 1 & Fig. 3).
We initially scored colonies as 0.5 if we saw the rare spore in a composite sample of 10 bees; if we saw more than a single spore, we then individually crushed 10 new bees from the same sample. The trial was to determine whether the test substance reduced nosema prevalence (unfortunately, there was no apparent effect from treatment); measuring colony strength was not part of the protocol. But at final grading, there was so much difference in colony performance, that I noted whether each colony at that time was of strong, medium, or weak strength by a quick eyeball grading.
Note that even some of the strongest colonies coming out of almonds had reached a prevalence of half the bees being infected by end of bloom, yet both the strong and medium groups largely purged their infections by the end of March. I’ve graphed the results below.
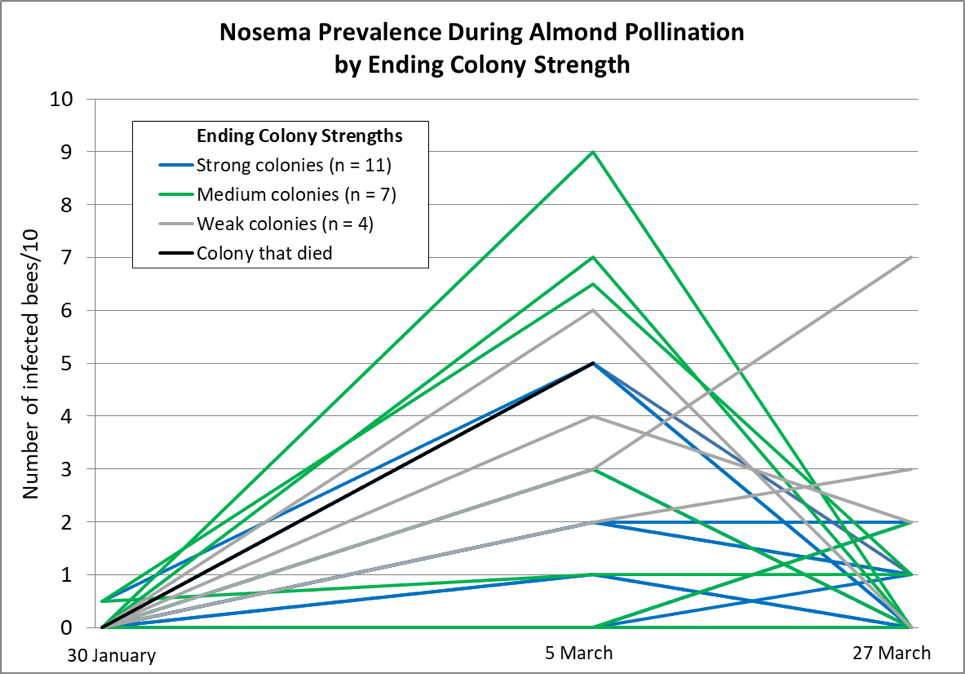
Figure 3. Nosema prevalence rose during the almond bloom, and then tended to decline toward the end of bloom (the bloom extended well into March). The chart does not make clear that a number of colony plots overlap. Note how the weak colonies (gray lines) and dead colony (black line) tended not to get nosema under control.
We took around 1000 hives to almonds. Since I had intentionally chosen weaker hives for the nosema trial, the hives above were fairly representative of the poorer hives that we took. The cool, wet weather definitely set our colonies back during almond bloom, and it continued through March, with the bees confined to the hive for days or weeks at a time — meaning that conditions were favorable for nosema to go wild in the hives. And although our hives returned from almonds weaker than normal, only a handful had died (although a fair number had barely grown). Yet when the weather broke in the second week of April, our main problem was that nearly all of our colonies had built up to swarming strength and were ready to hit the trees, indicating that nosema just didn’t seem to be that big an issue in the long run.
Practical application: Go figure — under the cold, rainy conditions this late winter and spring, with adequate pollen and little opportunity for defecation flights — a situation certainly conducive to nosema reproduction — our colonies did not suffer notable mortality, nor even a major setback in their buildup once good weather returned.
I’ve also in recent years been checking my cell builder colonies when we have trouble rearing queen cells ― again, I do not see nosema as being prevalent. And this brings us to the queens themselves.
Nosema and the queen
What’s difficult to make sense of is the history of nosema. Back in 1947, Farrar [[7]] presented compelling data that N. apis was infecting most package bees, that the queens were easily infected, and that infected queens would soon fail:
The first queens examined for Nosema infection were removed from their colonies in June 1941. They had laid normally for about 2 months, but suddenly stopped laying, became sluggish, and most of their last eggs shriveled and failed to hatch. All were found to be heavily infected with Nosema. Previously, many queens had been observed to manifest similar symptoms, but the superseded queens either were not found or they were not examined for Nosema.
Something that is seldom discussed is that the problem with a queen becoming infected may not only be a decrease in her ability to produce eggs, but that she’s the only bee that routinely defecates within the hive.
Practical application: Since the queen’s feces are consumed by the attendants around her, an infected queen can become a “Typhoid Mary” ― a source of inoculum to her daughters, for as long as that queen continues to survive. This has been studied to some extent with N. apis, but more research regarding the extent that this occurs with N. ceranae would be informative.
One would think that nosema would have continued to be widespread through the U.S. But compare Farrar’s observations to the USDA Diagnostic Lab data that I showed in Part 2 of this series *(July 2019 ABJ), which indicated that during the period from 1984 clear until 2002, nosema was rarely detected in samples of sick bees sent to the lab for analysis. Had our bees developed some degree of resistance to nosema?
It appears to me that if our bee stocks had indeed developed some resistance to N. apis, that such resistance did not appear to apply to N. ceranae, as the invasive wave of this version clobbered our industry from 2004 through around 2009 (during the CCD epizootic). A number of studies have demonstrated that N. ceranae is indeed able to infect queens [[8], [9], [10]]. So why don’t we see the queens rapidly dying in colonies infected with N. ceranae, as did Farrar with N. apis? I can’t cite definitive data, but personal observations and some published studies [[11]] suggest that while N. ceranae may infect queens, that it may not do so as readily as did its cousin (again, we need more research along this line).
Practical application: My sons and I rear thousands of queens each season, and N. ceranae can generally be found in our colonies in spring. Yet we don’t notice any great degree of queen failure, and our nucs typically grow rapidly (although we often see successful early supersedure in some groups). This spring I couldn’t make any connection between laggard nucs and nosema prevalence. Even in their second season, our queens tend to be productive and healthy, until they apparently start running out of stored sperm late in their second summer, as would be expected.
When we have trouble with rearing queen cells, I check my cell builders for nosema ― I rarely see more than minor infection prevalence in the house bees. And when I check what appear to be poorly-performing queens under the ‘scope, I don’t find any infected by nosema. I occasionally hear reports of commercial queens failing at a young age, but have yet to see evidence that N. ceranae is causing the sort of queen and package failure that was previously associated with N. apis.
How concerned should you be about nosema?
In his review on N. apis in 1993 [[12]], Dr. Ingemar Fries concluded that:
- apis infections are frequently present in most apiaries without causing significant damage. Except for the infective agent, contributing factors in the environment decide if the infection develops into an epizootic disease. Thus, nosema disease can be regarded as a factorial disease.
It appears to be the same for N. ceranae, although ceranae may pop up in warm weather. The factors that Fries listed were:
- Colony crowding and stress.
- Disturbance of the hive during winter, or the moving of hives early in the season.
- Lack of flight opportunities leading to defecation within the hive (Fig. 4).
And similarly to others, he noted that the availability of good pollen sources, or supplementary feeding of protein, “reduces the infection level in infected colonies, probably due to a larger number of bees being produced.”
Figure 4. A friend sent me a sample of dead bees swept from the floor of his large winter storage shed. This diluted composite sample was loaded with spores (enlarged from 400x, with a pollen grain for reference). Storing hives indoors at a controlled temperature of around 41°F (5°C) allows infected workers to leave the colonies for defecation flights, although they don’t make it back to the hive. This would prevent them from defecating spores within the hive, and could be one of the major benefits of controlled-temperature indoor wintering.
Practical application: I’ve reviewed studies from all over the world [[13]], and my impression is that nosema is an opportunistic parasite that thrives when colonies are bringing in lots of pollen, and that tends to disappear when they are not, and is not normally the cause of colony mortality. Perhaps surprisingly, the above appears to apply even in cold-winter climates, as evidenced in both Germany and Canada:
The results of our study failed to reveal a relation between N. ceranae infection of colonies and colony mortality, even in seasons with unusually high colony loss rates. Likewise, monitoring of the fate of individual N. ceranae-infected colonies over several years did not show a mandatory link between this infection and failure of the colony [[14]].
No differences in Nosema infection levels were found between colonies that died and those that survived [the winter in Ontario, Canada] [[15]].
The above findings do not let nosema completely off the hook, as it may get out of hand in colonies stressed by lack of flight weather, cold (especially if the colony is weak), poor nutrition, brood disease, or varroa. Other contributing stressors can be a co-infection by viruses or other pathogens [[16]], treatment with an antibiotic [[17]], or perhaps certain pesticides. The major factor appears to be whether for one reason or another, much defecation is taking place in the hive.
The take-home message
At least in California, once the initial invasive wave of Nosema ceranae passed through, nosema no longer appears to be something for me to particularly worry about. When I tracked its prevalence and spore counts in 36 hives over the course of the year, I found only a slight negative correlation with colony performance [[18]]. But as in my experiment in almonds detailed above, I do see that weak colonies can indeed get hammered by nosema during cold or rainy weather. Researchers around the world have now confirmed that infection by N. ceranae, similar to that of N. apis, typically peaks during cool weather [[19]] or early spring, and tends to disappear in summer. Nor is it necessarily correlated with winter loss — even in northern climes. However, infection may reduce colony productivity.
Keep this in mind when you think of nosema: Unlike American and European Foulbroods, which need to kill their host in order to gain transmission of their offspring to new hosts, there is no benefit to nosema to kill or harm its host bee, nor the colony. N. apis appeared to be a relatively benign parasite (unless it infected the queen), rarely killing infected colonies unless they were confined in early spring for long periods by cold or inclement weather (Fig. 5). N. ceranae appears to me to be much the same, other than perhaps being more prevalent during summer (not surprising, since it appears to be adapted to tropical environments).

Figure 5. A rapidly-growing colony with plenty of pollen can shrug off having a small percentage of its workers infected by nosema. However, if the beekeeper were to sample foragers at the entrance, they might freak out at the spore count. I’ll elaborate in my next article.
Nosema, in and of itself, may not normally cause serious disease, but rather it is about external nutritional and weather factors that can tip the scale toward it going epizootic in the hive. For example, in the eastern U.S., a bout of early warm weather along with tree pollens can cause the diutinus “winter bees” to start consuming pollen and shift to nurse physiology. This would tend to kickstart nosema reproduction in those unfortunate geriatric bees. But if that burst of broodrearing was then curtailed by the return of cold weather (which might also prevent defecation flights), then the colony would be unable to continue to replace the infected bees with fresh workers, and nosema could then be problematic.
Practical application: Since nosemosis exhibits no obvious signs of the disease, it behooves the beekeeper to better understand this parasite, its seasonality, and the factors that contribute to it going epizootic in a hive. I’ve seen far too many beekeepers dosing their hives with unnecessary (and possibly harmful) treatments, without having ever spent a few minutes to confirm that nosema was actually prevalent in their hives.
Coming
In my next articles I’ll cover monitoring for nosema, dealing with deadout equipment, treatments, and the causes of dysentery.
citations and notes
[1] Robinson, C & J Pfeiffer (2014). Viruses and the microbiota. Annual Review of Virology 1: 55―69.
[2] Dussaubat , C, et al (2013) Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. Journal of Invertebrate Pathology 113: 42―51.
[3] White, GF (1919) Nosema disease. U.S. Dept Agric Bulletin 780, 59 pp. Available in Google Books.
[4] https://scientificbeekeeping.com/nosema-ceranae-and-honey-production-in-healthy-colonies/
[5] Furgala, B (1962) The effect of the intensity of nosema inoculum on queen supersedure in the honey bee, Apis mellifera Linnaeus. Journal of Insect Pathology 4(4): 429-432.
[6] Botías, C, et al (2011) The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environmental Microbiology 14(4):845-5.
[7] Farrar, C (1947) Nosema losses in package bees as related to queen supersedure and honey yields. J Economic Entomol 40: 333―338.
[8] Higes M, et al (2009) Horizontal transmission of Nosema ceranae (Microsporidia) from worker honeybees to queens (Apis mellifera). Environ Microbiol Rep. 1(6): 495―498.
[9] Simeunovic, P, et al (2014) Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony,.Journal of Apicultural Research, 53(5): 545-554.
[10] Alaux, C, et al (2011) Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). Journal of Invertebrate Pathology 106(3): DOI : 10.1016/j.jip.2010.12.005
[11] Botías, C, et al (2011) The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environmental Microbiology 14(4): 845-59.
[12] Fries, I (1993) Nosema apis — A parasite in the honey bee colony. Bee World 74(1): 5-19.
[13] Genersch, E, at al (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie DOI: 10.1051/apido/2010014
Traver, B & R Fell (2011) Prevalence and infection intensity of Nosema in honey bee (Apis mellifera L.) colonies in Virginia. Journal of Invertebrate Pathology 107(1): 43-49.
Traver, B & R Fell (2012) Comparison of within hive sampling and seasonal activity of Nosema ceranae in honey bee colonies. Journal of invertebrate pathology 109(2): 187-193
Invernizzi, C, et al (2009) Presencia de Nosema ceranae en abejas melíferas (Apis mellifera) en Uruguay. J. Invertebr. Pathol., 101: 150-153.
Williams, G, et al (2010) Effects at Nearctic north-temperate latitudes of indoor versus outdoor overwintering on the microsporidium Nosema ceranae and western honey bees (Apis mellifera). Journal of Invertebrate Pathology 104: 4―7.
Williams, G (2013) Nosema ceranae in western honey bees (Apis mellifera): biology and management. PhD Thesis, Dalhousie University.
Gina Retschnig, et al (2015) Effects, but no interactions, of ubiquitous pesticide and parasite stressors on honey bee (Apis mellifera) lifespan and behaviour in a colony environment. Environmental Microbiology doi:10.1111/1462-2920.12825
Compare the above to the publications of Mariano Higes et al., 2007-2009.
[14] Gisder, S, et al (2010) Five-year cohort study of Nosema spp. in Germany: does climate shape virulence and assertiveness of Nosema ceranae? Applied and Environmental Microbiology, 76: 3032―3038. http://dx.doi.org/10.1128/AEM.03097-09
[15] Guzmán-Novoa, E, et al (2010) Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41: 443―450.
[16] Cornman, RS, et al. (2012) Pathogen webs in collapsing honey bee colonies. PLoS ONE 7(8): e43562. doi:10.1371/ journal.pone.0043562
[17] Li, JH, et al (2017) New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PLoS ONE 12(11): e0187505. https://doi.org/10.1371/journal.pone.0187505
[18] https://scientificbeekeeping.com/the-seasonality-of-nosema-ceranae/
[19] Chen, Y, et al (2012) Nosema ceranae infection intensity highly correlates with temperature. J. Invertebr. Pathol. 111: 264―267.
Contents
Requisites for nosema to gain a foothold. 2
The importance of pollen to Nosema transmission and reproduction. 4
The connection between nosema and dysentery. 6
putting it all together. 7
Coming. 8
citations and notes. 8
The Nosema Problem: Part 3
Seasonality and Effects of Nosema
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in Aug. 2019
Nosema apis had long been considered to be of concern only in the spring and fall; but once N. ceranae showed up, there are reports of it appearing even in summer. An understanding of the reasons for the seasonality of nosema may help us to better understand the parasite as a whole.
Nosema apis was not named until 1909, and was not on U.S. beekeepers’ radar until it was well described by G.F. White [I believe E.B. specialized in spiders] in his seminal publication in 1919 [[1]]. Ingemar Fries reviewed what we knew about N. apis in 1993 [[2]], and described the typical seasonality of nosemosis (disease that may be the result of serious infection by nosema):
The level of infection found in a colony is highly variable. The seasonal trend of typical infections exhibits low levels during the summer, a small peak in the autumn and a slow rise of infection during the winter. In the spring, the level of infection increases rapidly as brood rearing starts and while flight possibilities are limited still. The pattern is similar in both the northern and the southern hemisphere.
But in the early 2000s, in both North America and Europe, bee disease labs recorded a new trend — nosema prevalence in samples sent to diagnostic labs suddenly started to climb, and might be found at any time of the year [[3],[4]]. By 2005, nosema was being detected even in summer — retrospectively indicating that there had been an invasion by N. ceranae. Unfortunately, the graph of prevalence in samples from Spain was widely misinterpreted as indicating intensity [[5]], suggesting that N. ceranae lacked the seasonality of N. apis. This was actually not the case at all, as shown by the same researchers in a subsequent paper [[6]].
Since then, other researchers (including myself [[7]]) have documented that the epidemiology of N. ceranae rather closely follows that of N. apis, but since ceranae’s original host was the tropical Apis cerana, it’s not surprising that ceranae is somewhat better adapted to warmer temperatures, and may occasionally flare up during summer [[8]].
Practical application: During the invasive wave of N. ceranae, it was easy for me to find infected colonies at any time of the year in my apiaries. But by 2012 it was difficult for me to find an infected hive in summer. I suspect that a new host-parasite relationship had developed over that period of time.
Requisites for nosema to gain a foothold
The question of interest is why nosema has a seasonal pattern. As elaborated by White, nosema transmits from one bee to another by the transfer of infective spores via the fecal-oral route. Some observations of interest by White relate to the fact that simply exposing a colony to spores does not necessarily result in an epizootic of nosema within the hive:
Although wintering bees on soiled comb greatly increases the risk of detectable nosema disease the following spring, a detectable disease level does not always result. Thus, spore availability is not sufficient to create an epizootic disease in the honey bee colony [boldface mine].
He also noted that other beekeeper practices may contribute to the transmission of spores:
Other factors that contribute to the spread of N. apis spores within the honey bee colony include all management where bees are crushed. The liquid remains of crushed bees are readily ingested by other bees. [Australian beekeepers have long been cautioned to avoid the crushing bees in hives during winter manipulations [[9]] (Fig. 1).]

Figure 1. One way in which beekeepers can contribute to nosema spore transmission within the hive is by the inadvertent crushing of bees. The workers in the photo above are lapping up the body fluids of a bee crushed moments ago.
Practical application: Don’t contribute to nosema spore transmission. Avoid crushing bees within the hive, especially during winter [[10]]. The stress and confinement involved in the trucking of colonies — again, especially during winter — may also contribute to defecation within the hive [[11]].
But the observations by White that most caught my eye were:
The fact that a colony may contain a small percentage of Nosema-infected bees throughout the year and not become heavily infected at any time furnishes further evidence that Nosema infection does not always spread with rapidity within the hive. [As does his finding that a colony experimentally infected during summer will typically clear itself of infection within six weeks.]
Since merely exposing a colony to nosema spores does not necessarily result in the parasite going epizootic in the hive, to explain the seasonality of nosemosis, we need to look a little deeper. At this writing, it appears to me that the seasonal pattern of nosemosis is largely about two things: (1) the parasite’s success at reproduction in individual bees, and then (2) achieving adequate bee-to-bee transmission of spores within the hive. And then the overall effect of nosema upon colony performance is largely about the percentage of the colony’s bees that are infected (“prevalence”), the impact of that upon both broodrearing, and the shift in the survivorship of those infected workers. To summarize in advance, we need to look at:
Pollen
- The involvement of pollen with nosema reproduction in the bee midgut, and
- The seasonal availability of pollen.
Transmission in the Hive
- The transmission of nosema spores via consumption of pollen,
- The causes of dysentery, and
- The hours per day suitable for defecation flights.
Effect upon Colony Performance
- The percentage of bees infected,
- The effects of infection upon the nurse bees, and
- The effect of nosema-induced acceleration of worker transition to foraging.
I will be covering some of the above in subsequent articles, but first let’s focus upon pollen.
The importance of pollen to Nosema transmission and reproduction
The seasonal peaks of nosema in spring and fall certainly suggest an association with what are commonly heavy pollen flows at those times of the season. It appears that there are at least three reasons for this:
- Within-colony transfer of nosema spores via the pollen loads brought back by the foragers.
- Between-colony transfer of spores at the flowers.
- The greater amount of nosema infectivity and reproduction in the gut when pollen is present.
So let’s take a look at the above one at a time. When a bee defecates in flight, any post-defecation hygiene consists of it grooming itself with its hind legs — the same legs used to pack pollen into its corbicula. Higes [[12]] found infective nosema spores in the pollen loads brought back to the hive — the very pollen that the nurse bees would soon consume. Such transmission of spores could allow a single infected forager to spread the infection to the next generation of young bees in the same hive.
Graystock [[13]] showed that nosema could be surprisingly effectively transferred via the spores left on flowers by visiting foragers, and that bumblebees could vector honey bee parasites without getting infected themselves. The implication is that parasite spores can be readily transmitted from colony to colony via flower visitation (Fig. 2).

Figure 2. This freshly-returned forager may be bringing back a load of nutritious pollen, but that pollen could possibly be contaminated with nosema spores from its own gut, or picked up from a flower.
Practical application: Nosema transmission appears to easily take place via floral visits, and the transfer of spores via incoming pollen loads.
The importance of pollen doesn’t end there. Reproduction of both nosema species appears to be largely dependent upon pollen being present in the bee midgut [[14],[15],[16],[17],[18]] — the more pollen in the diet, the greater the number of spores produced.
In northerly climes, strong pollen flows typically occur in spring and fall, with perhaps some midsummer spikes. In Mediterranean climes, nosema tends to disappear during the late-summer pollen dearth. In tropical Mexico, N. ceranae levels correlate with the rainy season from April through December [[19]].
A couple of very nice studies [[20], [21]] have shown a strong correlation between pollen or pollen sub in the diet, and the number of nosema spores produced. Of interest is that at least in cage trials, it appears that although the consumption of pollen increases nosema reproduction in a bee, that pollen in the diet largely offsets the reduction in bee longevity (provided that those bees don’t engage in precocious foraging) [please explain—do you mean “precocious foraging”?] due to being parasitized by nosema.
Practical application: The reproduction and transmission of nosema in a colony appears to be largely dependent upon the bees foraging upon, and consuming, pollen. Expect nosema spore counts to go up if there’s a pollen flow on, or if you’re feeding pollen sub.
The connection between nosema and dysentery
It would make complete sense to expect that nosema would cause diarrhea (dysentery) in infected bees; but I’ve yet to see any evidence in support of that premise. But that doesn’t mean that dysentery is not a critical component for understanding the seasonality of nosema.
Workers typically become infected as nurse bees, likely due to their ravenous consumption of pollen. The parasite then rapidly reproduces within their midgut epithelial cells during times of heavy pollen consumption (via “vegetative spores”), and then later produces the “environmental spores” found in the hindgut and feces. It is thus the mid-age bees, and especially the foragers, that exhibit the highest spore counts [[22]].
Practical application: Although I’ve found no supportive evidence that nosema causes dysentery in bees, that discharged fecal matter can clearly act as a transmitting vehicle (fomite) of nosema spores within the hive. And that doesn’t mean that you need to see signs of dysentery all over the face of your hive. We like to think that bees never poop in the hive, but if you’ve spent much time watching an observation hive, you’ve likely noticed that occasionally a bee does (Fig. 3) — and that other workers then immediately “clean it up” with their tongues.

Figure 3. The occasional defecation “accident” in the hive may be enough to ensure low-level transmission from the older workers to younger ones. The droplets on the top bars above have obviously not yet been ingested by the janitors, but it’s safe to assume that any accidents on the faces of the combs would have been “cleaned up” immediately by ingestion.
Practical application: The occasional “accident” within the hive may be enough to ensure the continued transmission of nosema from one generation of bees to the next. This could explain how a low-level prevalence could continue even during summer — especially during confinement due to rain, or if hives get trucked.
putting it all together
As best that I can tell, the reason that nosema peaks in fall, may slowly climb during winter, and then spike again in spring, is that reproduction and transmission of the parasite strongly correlate with two factors: flight hours and pollen availability. And this then brings us back to dysentery and in-hive defecation.
The fewer warm days in which nosema-infected bees can freely fly for cleansing flights, the greater the chance that some of them are going to slip up and defecate within the hive. Such in-hive defecation is a highly effective basis for the fecal-oral route of transmission, and may largely explain why nosemosis is more likely to occur when cool weather prevents bees from freely flying outside the hive to defecate, and why infection tends to disappear during the summer months.
During winter, nosema can persist in the long-lived winter bees, but doesn’t appear to correlate with winter colony mortality [[23]]. It is only when the colony begins to rear brood again (often in mid-winter) that nosema again begins to increase— likely due to the combination of the consumption of nosema-contaminated stored beebread, or of honey or honeydew stores that cause dysentery, or increased in-hive defecation due to water balance issues coupled with lack of opportunity for defecation flights (I’ll cover the causes of dysentery in a following article).
Once springtime comes, then nosema is most effectively transmitted and infective due to the colony actively gathering pollen and rearing brood [[24]]. All colonies likely get exposed to nosema spores via colony-to-colony transmission of spores when the foragers of different hives visit the same flowers. And then the nurse bees would tend to get infected from the consumption of nosema spores inadvertently added to pollen loads by infected foragers. The nurses’ hindguts, which are often packed full of the remains of digested pollen, may contain very large numbers of infective spores — young workers defecate during their first orientation flights, before they begin foraging, and they then “wipe themselves” with their hind legs before they come back to the hive. And when the weather does not allow those bees to take cleansing flights, there is even more chance for in-hive transmission of spores via accidental defecation. I’ve seen little research on whether highly-infected forager bees defecate within the hive, but even if they defecate on the wing, they would still be expected to inadvertently transfer at least some spores to their pollen loads.
Once the weather warms, in-hive defecation rarely occurs, and the colony can then again break the infection cycle and largely purge itself of the parasite — which in the case of N. apis, largely disappears other than for dormant spores hidden in the combs, and in the case of the more heat-tolerant N. ceranae, smolders along in only a few bees in the hive.
Coming
The effects of nosema infection upon the colony, the causes of dysentery, sampling for nosema, how concerned you should actually be about this parasite, and what you can do about it.
citations and notes
[1] White, GF (1919) Nosema disease. U.S. Dept Agric Bulletin 780, 59 pp. Available in Google Books.
[2] Fries, I (1993) Nosema apis—A parasite in the honey bee colony. Bee World, 74(1): 5-19.
[3] Martín-Hernández, R, et al (2007) The outcome of the colonization of Apis mellifera by Nosema ceranae. Applied and Environmental Microbiology http://aem.asm.org/cgi/content/abstract/AEM.00270-07v1
[4] https://scientificbeekeeping.com/sick-bees-17a-nosema-the-smoldering-epizootic/
[5] The study documented only whether nosema was present in a sample, not the intensity of the infection. N. apis tends to completely disappear during the summer, whereas N. ceranae may often be found at a very low level during the warm months.
[6] Higes, M, et al (2008) How natural infection by Nosema ceranae causes honeybee colony. Environmental Microbiology 10(10): 2659–2669.
[7] https://scientificbeekeeping.com/the-seasonality-of-nosema-ceranae/
[8] Traver, B.E., Fell, R.D., Prevalence and infection intensity of nosema in honey bee (Apis mellifera L.) Colonies in Virginia, Journal of Invertebrate Pathology (2011), doi: 10.1016/j.jip.2011.02.003
[9] Hornitzky, M (2005) Nosema disease: Literature review and survey of beekeepers. RIRDC Publication No 05/055
Hornitzky, M (2008) Nosema Disease: Literature review and three year survey of beekeepers, Part 2. RIRDC Publication No 08/006.
The two above reviews are worth reading; both are free downloads.
[10] https://scientificbeekeeping.com/does-the-crushing-of-bees-affect-colony-health/
[11] Bailey, L (1955) The epidemiology and control of nosema disease of the honey-bee. Annals of Applied Biology 43(3): 379-389.
[12] Higes, M, et al (2008) Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. Journal of Invertebrate Pathology 97: 76–78.
[13] Graystock, P, et al (2015) Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. B 282: 20151371.
[14] Rinderer TE, Dell Elliott K (1977) Worker honey bee response to infection with Nosema apis: influence of diet. J Econ Entomol 70: 431–433.
[15] Porrini, MP, et al. (2011) Nosema ceranae development in Apis mellifera: influence of diet and infective inoculum. J Apicult Res 50: 35–41.
[16] Stevanovic J, et al (2013): Characteristics of Nosema ceranae infection in Serbian honey bee colonies. Apidologie 44: 522-536.
[17] Mendoza, Y, et al (2012) Incidence of Nosema ceranae during winter in honey bees colonies removed from Eucaliptus grandis plantations. Veterinaria (Montevideo) 48(188): 13-19. In Spanish.
[18] Fleming JC, et al (2015) Characterizing the impact of commercial pollen substitute diets on the level of Nosema spp. in honey bees (Apis mellifera L.). PLoS ONE 10(7):e0132014.
[19] Guerrero-Molina (2016) op. cit.
[20] Porrini, MP, et al (2011) Nosema ceranae development in Apis mellifera: influence of diet and infective inoculum. Journal of Apicultural Research 50(1): 35-41.
[21] Jack C, et al (2016) Effects of pollen dilution on infection of Nosema ceranae in honey bees. J Ins Physiol. 87:12–19.
[22] Jack, C, ibid.
[23] Even in Manitoba, Canada; Les Eccles, pers comm.
[24] Dr. Shimanuki, in his coverage of nosema in the 1992 edition of The Hive and the Honey Bee, notes that European researchers had pointed out the connection between active broodrearing and nosema.
Below are links to some classic beekeeping publications:
Farrar 1944 Productive-management-of-honeybee-colonies
USDA 1967 Beekeeping in the United States Scroll down to Moeller’s “Managing Colonies for High Honey Yields”
Beekeeping-in-California_1987
I also recommend (or have had recommended):
Storey’s Guide to Keeping Honey Bees, 2nd Edition: Honey Production, Pollination, Health, Malcolm Stanford. Malcolm has an easy-to-read writing style.
Honey Bee Hobbyist by Norm Gary—good overall understanding, rather than how-to
First Lessons in Beekeeping, Dadant
The Beekeeper’s Handbook by Diana Sammataro & Alfonse Avitabile. This is a well-loved textbook.
Homegrown Honey Bees by Alethea Morrison. Very down-home book by a beekeeper’s learning experience.
The Honey Revolution: Restoring the Health of Future Generations by Ron Fessenden
Almond Pollination Handbook Traynor
A Book of Bees, Sue Hubble. Beautiful prose about a beekeeper’s love of bees and beekeeping.
Bumblebee Economics, Bernd Heinrich. This book was instrumental to my understanding of the biology of the bee.
Beekeeping for Dummies. I haven’t yet read, but a number of beginners have told me that they found it helpful.
If there are others that you feel that I should add to this page, please let me know!
Contents
The invasion of Nosema Ceranae. 2
How did ceranae get into the U.S.?. 5
Signs of nosema infection. 5
A key finding. 8
Next. 9
citations and notes. 9
The Nosema Problem: Part 2
The Enigma of Nosema
Randy Oliver
ScientificBeekeeping.com
First published in ABJ July 2019
I’ve recently read Raquel Martin-Hernandez’s excellent review of our state of knowledge of Nosema ceranae [[1]]. The conflicting results and interpretations from the many studies cited can make one’s head spin. And with fumagillin currently off the market, what’s a beekeeper to think or do? Last month I addressed the confusion about the association between nosema and dysentery. I’ll now continue with my own retrospective look at what I and others have learned about nosema since we discovered in 2007 that our bees had surreptitiously been invaded by a new version of this gut parasite.
As a California beekeeper, I’ve long been involved in almond pollination. So it came as quite a surprise when, in the winter of 2004/2005 there was a severe shortage of bees available to fill pollination contracts — driving the offered rental price from $45 to $155 overnight. But it didn’t really hit us just then that something may have changed in the biology of the beehive. That offered pollination fee never went back down, as beekeepers continued to struggle in the following years to keep their colonies alive. And then in 2007, beekeeper Dave Hackenberg — clear across the country in Pennsylvania, and unable to fulfill his almond contracts — brought our attention to the fact that many hives were collapsing from a strange malady subsequently referred to as “CCD” (Colony Collapse Disorder).
CCD had specific “clinical signs”: colonies going off feed, clustering strangely, and ultimately having the majority of the worker bees in a colony suddenly disappear, perhaps leaving behind a queen and a handful of nurse bees attempting to care for the remaining brood. We beekeepers were frantic in trying to figure out the cause. There were many suspects for CCD (as I’ve detailed in previous articles), but since in my own operation I could rule out the influence of pesticides, GMOs, cell phones, and likely aliens, I suspected that a new pathogen was involved.
A Red Flag: When I searched the literature for pathogens that were known to cause the dwindling or sudden depopulation of colonies without other signs, I found that nosema was the only one known to do so. So when Dr. Joe DeRisi discovered in 2007 that a new species of nosema had flown beneath the radar and invaded our colonies, it was clear that we needed to learn more about this parasite, as well as nosema in general.
Being a California beekeeper, I’d never worried about nosema. So I got a new microscope and learned how to look for it. Wow, my colonies were loaded with gazillions of spores! Could nosema be part of the CCD problem? Curious, I downloaded the data from Dave Hackenberg’s collapsed colonies from Dr. Diana Cox-Foster’s groundbreaking study on CCD [[2],[3]], and saw that his hives were also chock full of both species of nosema — so perhaps their collapse was not so mysterious after all.
The invasion of Nosema Ceranae
Nosema apis has long been considered as a cause of poor colony buildup in springtime, often associated with queen failure or signs of dysentery [[4]]. But nosema was generally only considered to be a serious problem in package bees, or in regions where cold winter and springtime weather restricted bee flight. California beekeepers (other than the package producers) tended not to worry much about nosema, perhaps in light of the survey by Doull and Eckert in 1962 [[5]], which, after finding fewer than ten percent of hives to be infected (and even then only lightly), concluded that “nosema disease was of little economic importance to the package-bee industry in California in 1961.”
Then in 1974, Eric Mussen asked beekeepers across the country to send bee samples for nosema detection, and found that it was widespread, even in California, as indicated by the map published at the time in ABJ (Fig. 1).
Figure 1. The average spore counts (in millions), and percentage of samples exhibiting infection (nearly 2/3 of the hives), in springtime of 1974 [[6]]. The authors found a positive correlation between latitude and nosema (the further north, the more nosema). Surprisingly, the percentage of colonies infected in California appeared to be much higher than in 1961. But look at the spore counts overall — in few cases did they exceed an average of 4 million spores per bee — lower than what is often observed in spring today with N. ceranae.
Practical application: Although determining the average spore count per bee in a sample can be quickly determined, it is not as biologically relevant as is the prevalence of the infection within the hive — that is, the percentage of bees infected [[7]]. I’ll elaborate later, since this difference became apparent with the invasion of N. ceranae.
Early in the CCD epizootic, Dr. Mariano Higes made a well-supported claim that N. ceranae was causing similar “colony depopulations” in Spain [[8]]. We immediately struck up regular correspondence, comparing our observations.
Curious as to where I might find further records of nosema prevalence in the U.S., I contacted Bart Smith at the USDA Bee Diagnostic Lab, since with each sample of sick bees sent to the lab, he did a quick check for the presence of nosema (not knowing at the time that there might have been two different species of the parasite present). He sent me his data; keep in mind that these samples were all from colonies that beekeepers had sent to the lab because their colony was deemed “sick.” When I graphed out Bart’s data, it stunned me (Fig. 2):
Figure 2. Plot of nosema prevalence (present or not) in samples received by the USDA Bee Disease Diagnostic Lab. Keep in mind that this data is not from random sampling, but rather from samples of sick bees sent by beekeepers to the lab, which biases the sampling toward apiaries that are having problems. This graph strongly suggests that the “invasive wave” of N. ceranae occurred between the years 2002 and 2010 — which intriguingly corresponds exactly with beekeeper reports of unexplained colony depopulations.
Oddly, this host jump (from Apis cerana to A. mellifera), and subsequent timeline of invasion by Nosema ceranae appears to have simultaneously occurred in North and South America, Europe, and Australia — all at around the same time [[9]].
Practical application: When a novel parasite invades a naïve host population, it is normal for the host population (in this case honey bees) to exhibit serious mortality during the “invasive wave” of the parasite, as natural selection weeds out those host bloodlines that are unable to mount an adequate immune response against the new parasite. The question then is, could the invasion of N. ceranae have been a player in CCD, or was its invasion merely coincidental?
Although the Higes lab published convincing research that N. ceranae was responsible for massive colony losses in Spain, I couldn’t make the case in my own mind that nosema alone was the sole culprit in the U.S., since I watched colonies of my own with mean spore counts of 5 million grow and thrive. In addition, N. ceranae didn’t appear to cause serious problems when it invaded Australia (which was free of varroa and its associated viruses), nor necessarily even in all of Spain [[10],[11]].
How did ceranae get into the U.S.?
Of interest is the detection of nosema in Mexico — a country with a relatively warm climate. Back in 1980, Wilson [[12]] took 84 samples of bees from hives in the country (some samples were composites from multiple hives). They took the samples in February and March, when nosema prevalence would be expected to be the highest, and individually dissected and inspected 10 bees from each sample. They detected nosema (presumably N. apis) spores in only 3.8% of those 840 bees — and those few infected bees had come from the mere 11% of the hives in which nosema was detected at all. Bottom line —nosema appeared to be an insignificant parasite in Mexico in 1980. But by the year 2004, N. ceranae was found to be present in 94% of 99 hives sampled [[13]]. Further investigation confirmed that by 1995 N. ceranae was already well-established in Mexico [[14]].
Practical application: Although N. ceranae didn’t really “catch hold” in the U.S. until the beginning of the century, retrospective analysis of bee samples indicates that it had been introduced somewhat earlier. We don’t know how it first got here, but it may well have invaded via Mexico. Or, back then, some beekeepers were buying unsterilized pollen from China and feeding it to their bees, which could have easily introduced the parasite (especially into the almond “mixing pot”). In any case, between 2001 and 2010, the prevalence of nosema across North America appears to have exploded.
The question then is, what does this mean to us beekeepers?
Signs of nosema infection
When my own colonies were struggling with collapse in the early 2000s, it was clear that mites, viruses, poor nutrition, and EFB were involved. But that still didn’t explain some of the sudden dwindling, and collapses down to a few bees and the queen (Fig. 3).

Figure 3. Note the recent size of the brood area, as outlined by the band of beebread, on this frame taken from a collapsing colony in February in California, when we still have frosty nights. When more than half the bees in the hive are infected with nosema, the colony may not be able to successfully complete its “spring turnover” (during which the overwintered bees must replace themselves with a new generation of workers). This may lead to the brood getting chilled overnight, and then removed by the bees the next day. What’s left are some aged and infected “winter bees” with no chance of rebuilding the colony. In a few days, there’ll be no bees left in this hive.
The above signs are similar to those noted by White for N. apis: “At times, indeed, the brood was in excess of the amount that could properly be cared for by the diminishing number of bees present.”
Practical application: A poor bee-to-brood ratio remains the field sign that makes me suspect nosema — plenty of healthy brood, but a dwindling adult population, generally occurring when the daytime temperatures are just barely warm enough for bee flight. But most of the time, I no longer see much of a problem from nosema in my hives. So yes, a high prevalence of nosema in the hive can result in poor buildup, dwindling, or even sudden collapse. But in most cases, in my climate that high prevalence does not normally occur, and the colony just “deals with” the parasite.
What puzzled me was when I carefully tracked my nosema-infected colonies in spring and early summer, most of them didn’t collapse, but went right on doing fine. Dr. Higes and I couldn’t figure out why we observed such a difference, since he had detailed how infection could eventually lead to collapse [[15]]. And then in 2010 I was asked by the Israeli startup, Beeologics, to run field tests on suppressing Israeli Acute Paralysis virus (IAPV) by applying an experimental treatment of virus-suppressing double-stranded RNA. In one impressive field trial of 72 hives that we experimentally inoculated with a nasty strain of IAPV, Dr. Eric Mussen and I, after going through the collapsing hives frame by frame at the final assessment, put our thoughts together about the stages of collapse that we had observed that day [[16]]. I went home and started putting together a diagram of how a combination of nosema, virus, and other stressors could result in sudden colony depopulation (Fig. 4).

Figure 4. In 2010, I published this hypothetical model of what occurs during colony collapse due to positive feedback loops resulting from colony stressors, viruses, and nosema. Note how the loops play into one another (asterisks), and can result in colony recovery, slow dwindling, or even rapid depopulation.
Practical application: It generally takes more than one stressor or pathogen to cause colony collapse — some combination of interactions, immune suppression, and feedback loops are generally involved. Once a colony is stressed by something else, then nosema may, due to its reductions of worker efficiency and longevity, deliver the coup de grâce to the colony by causing rapid depopulation, since the workers no longer live long enough to replace themselves. As we found with Beeologics, the combination of nosema and a virus can result in the field signs of CCD. I’ve also observed that nosema and EFB are a bad combination.
Once it came to light that we were dealing with a new species of nosema, researchers worldwide jumped into both laboratory and field investigations, and a slew of scientific papers followed. It quickly became clear that infection by nosema (of either species) causes immune suppression [[17]], metabolic stress, and reduced longevity.
Side note: Although some have suggested that the “new” species of nosema may be more pathogenic than the “old” nosema, a USDA analysis of CCD colonies by Cornman [[18]] suggests that N. apis might be the more problematic of the two.
A key finding
A number of studies have now shown that nosema-infected bees typically initiate “age of first flight” at an earlier age, and then quickly wear themselves out from increased (but inefficient) flight activity during foraging. When a bee gets infected by nosema, it becomes “energy challenged,” and may not be able to sustain long periods of flight, thus leading to more flights of shorter duration [[19], [20] ], needing to take breaks during its foraging trips, and having less chance of return from each trip [[21]] (Fig. 5). This earlier initiation of flight activity, coupled with the metabolic issues, then leads to a considerable decrease in overall worker longevity and productivity.

Figure 5. Another sign of nosema in the hive in springtime is when some foragers don’t quite make it back into the hive, and can be seen resting or crawling in front of the hive (often with pollen loads). In my experience, these bees frequently have high loads of the parasite.
Next
The more that we understand each of the parasites and pathogens that can affect our colonies, the more informed our decisions can be as to how to better manage them. In my next installment, I’ll go deeper into the reasons for the seasonality of nosema, how colonies “fight back,” and how much we should be concerned about this parasite.
citations and notes
[1] Martίn-Hernández, R, et al (2018) Nosema ceranae in Apis mellifera: a 12 years postdetection perspective. Environmental Microbiology 20(4): 1302–1329.
[2] Cox-Foster, DL, et al. (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318(5848): 283-287
[3] http://science.sciencemag.org/content/sci/suppl/2007/09/06/1146498.DC1/Cox-Foster_SOM.pdf Table S1.
[4] Farrar, C.L. (1947) Nosema losses in package bees as related to queen supersedure and honey yields. J Economic Entomol 40: 333–338.
[5] Doull, K & J Eckert (1962) A survey of the incidence of nosema disease in California. Journal of Economic Entomology 55(3): 313-317.
[6] Mussen, E, B. Furgala, & R Hyser. (1974) Enzootic levels of Nosema disease in the continental United States. American Bee Journal 115(2):48-50.
[7] White, GF (1919) Nosema disease. U.S. Dept Agric Bulletin 780, 59 pp. Available in Google Books.
[8] Higes, M, et al. (2005) El síndrome de despoblamiento de las colmenas en España, Consideraciones sobre su origen, Vida Apícola 133: 15-21.
[9] Klee, J, et al (2007) Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera, J. Invertebr. Pathol. 96: 1–10.
[10] Pers. comm. Antonio Pajuelo in Spain.
[11] Fernández, J, et al (2012) Asymptomatic presence of Nosema spp. in Spanish commercial apiaries. J. Invertebr. Pathol. 111, 106–110.
[12] Wilson, WT & RA Nunamaker (1983) The incidence of Nosema apis in honeybees in Mexico. Bee World, 64(3): 132-136.
[13] Guzman-Novoa, E, et al (2011) Nosema ceranae has parasitized Africanized honey bees in Mexico since at least 2004. Journal of Apicultural Research 50(2): 167-169.
[14] Guerrero-Molina, C, et al (2016) Nosema ceranae is an old resident of honey bee (Apis mellifera) colonies in Mexico, causing infection levels of one million spores per bee or higher during summer and fall. Journal of Invertebrate Pathology 141: 38-40.
[15] Higes, M, et al (2008) How natural infection by Nosema ceranae causes honeybee colony collapse, Environmental Microbiology, 34(10): 2659-2669.
[16] https://scientificbeekeeping.com/sick-bees-part-2-a-model-of-colony-collapse/
[17] Li, W, et al (2018) Chronic Nosema ceranae infection inflicts comprehensive and persistent immunosuppression and accelerated lipid loss in host Apis mellifera honey bees. Int. J. Parasitol. https://doi.org/10.1016/j.ijpara.2017.11.004
[18] Cornman, RS, et al. (2012) Pathogen webs in collapsing honey bee colonies. PLoS ONE 7(8): e43562. doi:10.1371/ journal.pone.0043562
[19] Dosselli, R, et al (2016) Flight behaviour of honey bee (Apis mellifera) workers is altered by initial infections of the fungal parasite Nosema apis. Scientific Reports DOI: 10.1038/srep36649
[20] Dussaubat,C, et al (2013) Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. Journal of Invertebrate Pathology 113(1): 42-51.
[21] Wolf, S, et al (2014) So near and yet so far: harmonic radar reveals reduced homing ability of nosema infected honeybees. PLoS ONE 9:e103989.































































