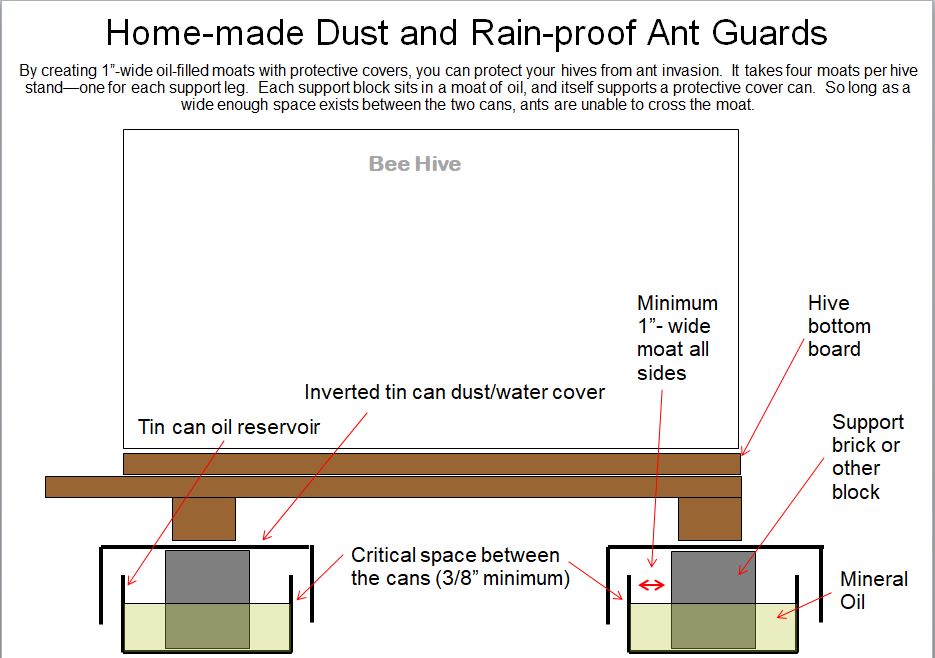
Hi,
I registered these three domain names in the hope that some regenerative farming nonprofits might wish to use them.
I’d be happy to give them to an organization promoting regenerative farming that, unlike “certified organic,” accepts precision-bred (Crispr-edited) plant cultivars, and synthetic eco-friendly pest control methods.
earthfriendlyfarming.org
workwithnature.org
backtonatural.org
If you’re interested, you can contact me at randy@randyoliver.com
Selective Breeding Progress Report 2023
Randy Oliver
ScientificBeekeeping.com
First published in ABJ September 2023
In 2017 I proposed a simplified method for commercial queen breeders to select for varroa-resistant stock, and then undertook a demonstration project to see if it would work. After six years of strong selection, we appear to be making substantial progress!
In 2017 I published the article “Bee Breeding for Dummies,” [[1]] lamenting that we’d been battling varroa for over three decades, yet most queen producers were still not offering mite-resistant stock. I described “Randy’s K.I.S.S. Recipe For Commercial Queen Producers,” and followed up in 2018 with “Selective Breeding for Mite Resistance: 1000 hives, 100 hours,” [[2]] in which I kept track of the amount of labor involved, the costs, and an evaluation of our results during the first year.
The Premise for Selective Breeding
Selective breeding is a direct way to determine if a specific trait can evolve in response to selection [[3]]. I like the way that E.W. Hill [[4]] describes breeding:
The basis of genetic improvement programs in any organism is selective breeding, where individuals are chosen that are expected to have offspring with desirable properties. This is directed evolution: fitness is defined by the breeder rather than by the individual’s ability to survive and reproduce in nature.
For selective breeding to be effective, there must be genetic variation present in the population, a way of identifying individuals for selection that are likely to transmit the desired properties to the descendants, and sufficient spare reproductive capacity so that the population can be bred from only the chosen individuals. For most traits there is considerable variation at the observed or phenotypic level, thus providing plenty of selective opportunity.
The premise for our own selective breeding program for varroa resistance is based upon several things (key words in boldface):
- That in any breeding population of honey bees, there are likely a few colonies in each generation that exhibit a combination of characters (uncapping behavior, hygienic pupal removal, grooming or biting, variations in their pheromones, social apoptosis, etc.) that in combination, confer the trait of “varroa resistance” upon the colony.
- That the combination of characters that confer that trait are heritable ― meaning that they can be passed to a new colony via the genetics (and epigenetics) of the queen and the sperm that she carries in her spermatheca.
- That one can identify which colonies exhibit the trait of resistance, by taking samples of a half cup of bees from a comb. Keep in mind that it’s not the queen that is resistant ― the trait is expressed at the colony level.
- That we can increase the prevalence of the trait of resistance in our breeding population by positive and/or negative selection of the queens of colonies expressing the trait of resistance (breed from those that head resistant colonies, eliminate those whose colonies show high mite levels).
Practical application: This article is mostly about my assessment of our progress, as determined by the increase in prevalence of the trait of varroa resistance in our breeding population
A Brief Description of our Methods
I am running this program as an experimental demonstration project (“walking the walk”) for the benefit of our commercial queen producers. I want to objectively see how much progress one can expect to make by using a highly-simplified “modified Bond” breeding program, in which no colonies need to be lost to varroa, and the only assessment used for the identification of breeders is mite washes [[5]].
We split all our hives into nucs when they return from almond pollination, requeening them with cells from breeders whose colonies exhibited full resistance the previous year. We apply an oxalic dribble at the brood break on Day 18 in order to start all the nucs with relatively low mite infestations.
Then in late June or July we take mite washes from every hive in our operation (which takes about 70 hours of labor per 1000 hives), identifying those that have mite counts of 0 or 1 as “potential breeders,” and treat the rest.
We then continue to sample those potential breeders over the course of the year (typically 4-5 times). Our most resistant colonies have records of 5 consecutive counts of zero (and to “make grade” must also be gentle and productive).
The next spring we then replace all of our queens with daughters grafted from larvae produced by queens that had headed colonies that kept mites to near zero for the entire year. Those virgins are then open mated with drones produced by the daughters of the previous year’s selected queens (whether their colonies exhibited resistance or not ― I’ll return to this shortcoming).
Each year I’ve published reports on our frustratingly slow progress to “fix” the trait of resistance in our breeding population.
Constraints of the Method
Our K.I.S.S. method intentionally avoids instrumental insemination, single-drone inseminations, marker-assisted selection, brood dissection, freeze-kill hygiene assessments, or tracking of bloodlines. Any of those methods could accelerate our rate of success, but again, this demonstration experiment is to determine what sort of progress we’ll make by “traditional breeding,” without those time-consuming or high-tech additions.
Another constraint is my intentional avoidance of excessive inbreeding, by not limiting our drone pool to only the drones produced by the current year’s breeder queens (which could not supply enough drones anyway), but rather by allowing the virgins to mate with drones produced by the daughters of the previous year’s queens ― all those daughters carrying the genetics of queens and drones from previous generations of selected breeders.
And we’re not just breeding for resistance ― we breed for stock that beekeepers would want to use. So we kick out any resistant colonies that are not gentle, strong, or productive.
The Low Heritability of the Trait of “Mite Resistance”
It’s relatively easy to selectively breed for color, hygienic behavior, or resistance to tracheal mite, but I have not found it so for the trait of “varroa resistance.” Mite resistance takes place at the colony level, and appears to have different alleles of multiple genes involved. A queen only supplies half the genetics of a colony, with the multiple drones that she mates with randomly supplying a mixture of different alleles.
Even after six years of intense selection, we haven’t yet seen a queen whose daughters mostly produced mite-resistant colonies. We’ve yet to “fix” the trait in any bloodline or our breeding population as a whole. This low degree of heritability [[6]] is disappointing, but that doesn’t mean that we’re not making progress.
Progress to Date
We started our program when I happened to notice “Queen Zero,” whose colony held a mite wash count of zero over the course of an entire year, without any treatments whatsoever. So at the start of our program, I’d identified 1 colony out of 1500 as exhibiting “full resistance” to varroa. So we started at 0.07% as our baseline prevalence of resistant colonies.
Since then we’ve requeened every one of our colonies each year with daughters grafted only from colonies exhibiting the ability to maintain mite wash counts at or near zero for an entire year, while surrounded with “normal” hives drifting mites into them (the potential breeders get no special treatment).
In recent years, we’ve averaged identifying around 70 “fully-resistant” colonies that have made the grade as breeders, grafting from the 30 with the best overall characteristics (this number in order to avoid bottlenecking our genetic diversity). Seventy out of 1500 works out to 4.5% — a 70x increase in the prevalence of identified fully-resistant colonies in our operation.
Have I Been Underestimating our Progress?
The key word is “identified.” In my update last year [[7]] I wrote: “I recently realized that we may have been inadvertently kicking a proportion of potentially-resistant colonies out of the program at our first mite wash assessment in June or July.” This is because I don’t know how many potentially-resistant colonies we fail to identify during the critical first round of mite washes, since we reject any colonies whose samples show more than a single mite (in a number of yards we even kick out any colonies with counts above zero). What I’ve observed is that a number of those rejected colonies are able to bring their counts back down to zero by themselves, but we wouldn’t know, since we treat them if they don’t make the cut on their first mite wash.
Not only that, but I haven’t been including the number of “moderately-resistant” colonies in my progress assessments — ones that might only need one or two treatments a year, as opposed to our normal regimen of four.
Practical application: “Mite resistance” is not a binary yes or no, but rather a range of degree. Many beekeepers would be happy with colonies that needed only a little help to fight the mite.
By luck, I had a chance this summer to investigate to what extent I’ve been underestimating our progress toward “resistant” stock ― including “overlooked resistant” and “moderately-resistant” colonies. I’ve now got revised numbers for the increase in prevalence of “resistant” colonies in our breeding population.
Tracking Rejected Colonies in their Second Year
Every February we take nearly all of our colonies to almond pollination, keeping only a few of our breeders behind (just in case something goes wrong). After return from almonds, we split all our colonies into nucs ― setting aside those queens that still made grade as potential breeders in splits (so that they don’t swarm). We requeen all our nucs with queen cells from the “best” breeders.
Later in the summer, I typically run a number of field trials to test the efficacy of various varroa treatments. For those trials, I need to use non-resistant colonies (for obvious reasons). So this spring I asked my sons, when they were splitting our hives, to save a few hundred second-year queens in nucs made with their own bees, giving them an oxalic dribble to reduce their starting infestation rates, and set them aside for me to evaluate later. They set up nine yards with those ostensibly non-resistant colonies (since they had never made the cut as potential breeders). In early June, the crew took mite wash counts to see to what degree varroa was building up in those yard (fearful that they’d be reaching dangerous levels). I sorted the results of their counts (number of mites per half cup of bees) in Table 1.
Table 1. These colonies were started in early March with splits containing the queens and bees of ostensibly non-resistant colonies that had been treated the previous season with some combination of formic, oxalic, or thymol treatments, and then given an oxalic dribble when we split them. They had by now mostly grown to 10-20 frames covered with bees (some up to 30), and their mites had been reproducing unchecked for 3 ½ months. I sorted their mite wash counts from lowest to highest, highlighting in red those colonies with dangerously high mite counts. Those high counts indicate how much the mite population could increase in non-resistant colonies over this period of time. Compare the high counts to those of the hives that still exhibited mite counts of zero to two, despite sitting in yards packed with those high-mite hives.
Interpretation

Row A (near the bottom of the table) is the count of hives in the yard.
Row B indicates the mean (arithmetical average) mite count for all the hives in that yard.
Row C indicates the median count (half the counts above, half below).
Row D shows the percentage of colonies in that yard that appear to demonstrate a substantial degree of mite resistance (exhibiting an infestation rate of less than 2%).
Bottom line: The above data really got me wondering whether I’ve been underestimating our degree of success in breeding for resistance!
Practical application: Aside from identifying potentially resistant colonies, as far as basing management decisions for treating for varroa upon monitoring via mite washes, is the difference between the mean and the median mite wash counts. From a management perspective, the median count is of more importance, since the mean mite count is skewed upward by a few non-resistant outlier hives with very high mite counts (typically around 10%, 13% red in the table above). We find it cheaper to take mite wash counts for every hive, than to waste money on unnecessary treatments. This one-time intense monitoring each season allows us to focus our attention on our “mite factory” hives.
The above data surprised me. At this point of time, out of the 496 hives above, it appeared that I’d have trouble coming up with the 250 demonstrably non-resistant colonies that I needed for my field trials! (Yes, we were laughing at ourselves for complaining about having too many hives with negligible mite counts!)
Practical application: It occurred to me that this data gave me an opportunity to estimate what the overall rate of “full to moderate” resistance was in our operation (since we sampled over 300 colonies from multiple mothers, in multiple yards).
So I allowed the mites to build up for another month. In mid-July we again took mite wash samples from four of the above yards to choose the 216 hives needed for one of my treatment trials, in order to record their starting infestation rates prior to applying the test treatments.
I arbitrarily decided to exclude any colonies exhibiting a mite wash count [[8]] that was still less than 5, since colonies with such a low rate of mite buildup after over four months of heavy brood rearing likely possessed some degree of resistance, despite not having been identified as potential breeders the year before.
Let’s first take a look at what an expected “normal” distribution (the “bell curve”) of mite counts for a yard if they evenly varied from the average. For Figure I used Excel to generate a normal distribution for 100 hives with an average count of 16 mites (similar to our actual averages per yard), and a standard deviation of 3 (to match our range of counts).

Fig. 1 This is a theoretical normal distribution — in histogram form, with each column representing the percentage of hives at each infestation level (all the columns in each graph add to 100%) — of the expected percentages of hives at each infestation level, for a yard with an average mite wash count of 16 (typical for our July counts). This shape of curve would be expected if all the colonies had the same degree of mite resistance, with random variation resulting in differences in measured mite counts [[9]].
So let’s compare the curve above to the actual distributions of mite counts for the four yards that we re-sampled in July.

Fig. 2 There was great variation in infestation rates in all the yards. Note how the curve for this yard is skewed to the left. Roughly 18% of the colonies had mite wash counts in the 0-5 range, suggesting relatively strong mite resistance, despite there being non-resistant colonies full of mites scattered within the yard.
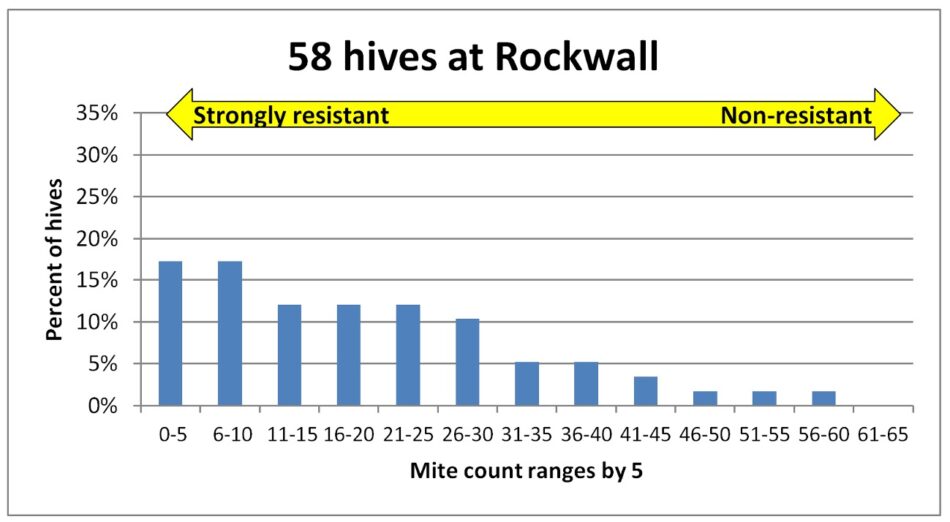
Fig. 3 The mite counts were similar in this yard, with some extreme outliers with very high infestation rates.

Fig. 4 In this larger yard, the distribution looked even more skewed toward resistance, with the majority of colonies exhibiting low counts, and around 28% suggesting strong mite resistance.
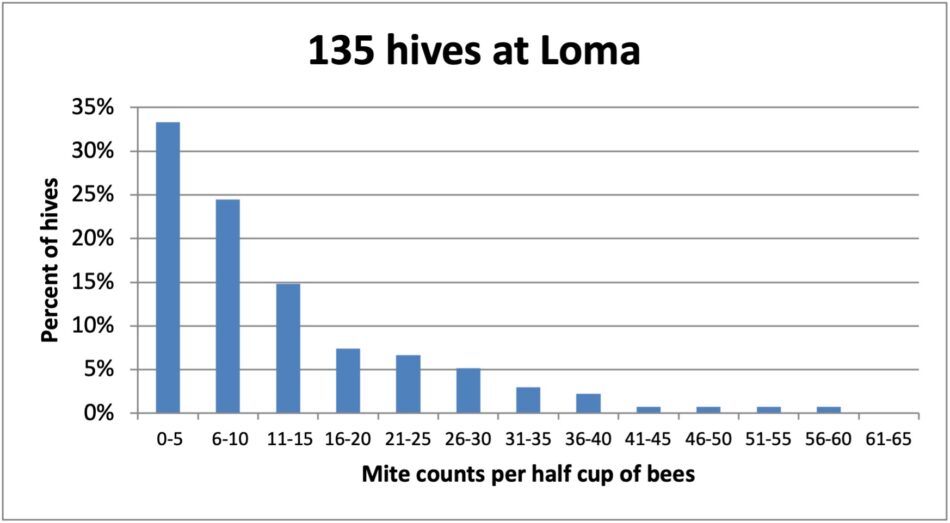
Fig. 5 Not only was this the yard with the most hives (staged from several locations). Over 30% of the colonies had mite wash counts of 5 or less, again with some clearly non-resistant “mite factories” scattered among them.
Practical application: Now add to the above figures the 4% of colonies that we’d already removed as breeders, suggesting that perhaps a third of the colonies in our operation (our breeding pool) are now exhibiting a goodly degree of mite resistance! This finding, to say the least, is very encouraging.
An Assessment of our Progress to Date
With only a third of our colonies exhibiting strong resistance, we’re not about to claim that we are yet producing mite-resistant stock — the heritability of the trait still remains low. But to chart our progress, we can compare the prevalence of resistant colonies to what we started with.
Going back to our starting baseline of perhaps a tenth of a percent of our colonies exhibiting resistance, the figures above suggest that after six years of selective breeding, we’ve increased the prevalence of mite-resistant colonies in our operation by 300x. So although slow, this is clearly progress!
Increasing the Selective Pressure
We’ve been practicing strong positive selection (breeding only from colonies able to maintain extremely low mite counts over the course of a year). We’re now going to increase negative selection ― replacing the queens of colonies that we identify with high mite counts to prevent them from adding their drones to next season’s drone pool when we mate out next year’s queens.
Wrap-up
Varroa is still our nemesis, but mite management is getting easier for us every year. Remember, we haven’t used synthetic miticides for 22 years now. My dream is that I’ll live to see the day when our bees “take care” of varroa by themselves (or with the occasional thymol or organic acid treatment).
Update
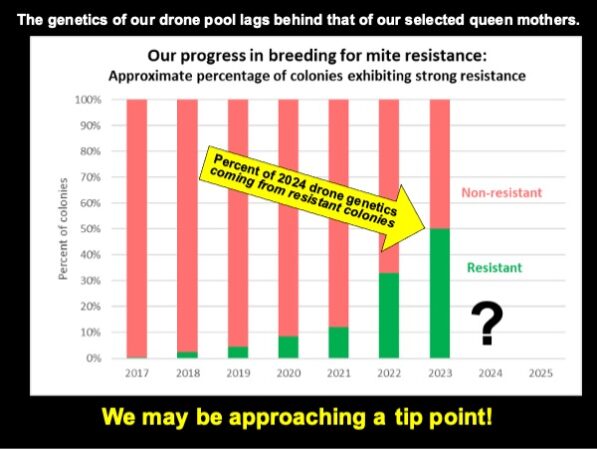
Since writing this article, we’ve taken our October mite wash counts. In some of our yards, it appears that we’re approaching 70% of the hives with mite wash counts not over 6 (less than a 2% infestation).
We may be finally reaching a tip point, where over 50% of our drone pool comes from resistant colonies.
We’ve been approached by our friend Ray Olivarez, who (after seeing the quality of our hives in almonds) asked whether he could sell production queens from our stock. He agreed to provide us a large isolated mating yard, which we could load with our own drone mother hives (allowing us for the first time to do so solely with resistant colonies). This is a win-win dream come true, since it may allow us to finally “fix” the genetics for mite resistance into our stock. Cross your fingers!
Disclaimer: I’m not running this program to promote our own stock, but rather to demonstrate to other commercial queen producers how to maintain a breeding program for resistant bees for their own or any other chosen stock, such as Harbo, VSH, POL line, Russian, Champlain Valley, locally-adapted ferals, or any other resistant stock (apologies if I didn’t include someone’s name). It’s time for our industry to shift to keeping mite-resistant bees!
Citations and Notes
[1] https://scientificbeekeeping.com/the-varroa-problem-part-6a/ (first published in American Bee Journal, March 2017)
[2] https://scientificbeekeeping.com/selective-breeding-for-mite-resistance-1000-hives-100-hours/#_edn1 (first published in American Bee Journal, March 2018)
[3] https://en.wikipedia.org/wiki/Selective_breeding
[4] Hill, WG (2001) https://www.sciencedirect.com/science/article/abs/pii/B0122270800011678
[5] https://scientificbeekeeping.com/the-varroa-problem-part-10/ (first published in American Bee Journal, September 2017)
[6] For a good read on the subject, see https://www.nature.com/scitable/topicpage/estimating-trait-heritability-46889/
[7] https://scientificbeekeeping.com/walking-the-walk-selective-breeding-for-mite-resistance-2022-update-part-1/#_Toc110874497 (first published in American Bee Journal, June 2022)
[8] Of a level half cup of bees, roughly 315 bees.
[9] I used a standard deviation of 3.
Contents
Testing Hypothesis #2. 2
Let’s Do the Calcs! 11
Conclusions 11
Why Would Mites Prefer Drones?. 12
Acknowledgements. 12
Citations and Notes 12
Drones and Varroa
Part 2
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ November 2023
In the May issue of this journal, Dr. Zac Lamas presented some findings of great interest, regarding the distribution of varroa mites on drones as compared to that on workers. Aha, I thought – this might help to explain why mite wash counts after the honey flow sometimes increase more strongly than expected. So I dove into some hives to determine to what extent the prevalence of drones in the hive could be involved.
If you haven’t already watched it, check out Dr. Lamas’ video [[1]], in which he goes into much greater depth than in his May article. He explains that the drones function as a “protective cushion” for the colony, due to the mites preferentially feeding upon them rather than the workers. Since drones are not involved in transferring food to other bees, nor in brood rearing, they are more expendable than the critical nurse bees, and their presence may help to reduce the impact of mites upon the nurse bees, as well as reduce varroa-mediated virus transmission within the hive, especially early in the season, when the relatively few mites present on the adult bees in a colony may be riding (and feeding) predominately on drones.
Practical question: The biotechnical method of using drone brood to trap and remove varroa can reduce the number of mites in a hive, but it would also remove the “protective cushion” that those drones would have provided had they been allowed to emerge. This tradeoff would be an interesting subject of research.
What especially intrigued me about Dr. Lamas’ presentation were two intriguing hypotheses:
- That for the purposes of varroa monitoring early in the season, sampling drones may better reflect a colony’s overall infestation rate than sampling bees shaken from the combs, and
- That the post-flow disappearance of drones from the hive would cause an appreciable increase the varroa infestation rate of the worker population.
I found both of the above hypotheses to be plausible and worth testing.
Regarding the first hypothesis, Dr. Lamas pointed out the problem with using a sampling methodology based on the assumption of a random distribution of the parasite on the bees, when the parasite actually occurs in an aggregated distribution – suggesting that hand-plucking of drones may be a better method than a wash of a half cup of workers. Last month I showed my own results, comparing the mite counts from worker samples to those of plucked samples of 25 drones from the same combs.
This brings us to the second hypothesis, which to me was of far greater interest, since it might help me to improve my varroa model [[2]]. Dr. Lamas’ proposed hypothesis was that when the drones “disappear” after the honey flow, the resultant shift of mites solely onto the workers might help to explain why mite wash counts suddenly increase, leading to viruses overwhelming the colony.
I found the above hypothesis to be plausible, and possibly explanatory for why my varroa model sometimes underestimates the observed rapid increase in mite wash counts in worker samples taken in late summer. So I decided that I’d better investigate.
Testing Hypothesis #2
First let me specify the question to answer:
Would the post-honey flow “disappearance” of drones from a colony result in an appreciable increase in the infestation rate of the adult workers?
It occurred to me that this would be a straightforward arithmetical calculation. So for our calculation, let’s imagine a hypothetical case in which all the drones in a colony were to suddenly fly off, leaving some of their mites behind.
To perform the calculation, we need to know:
- The total number of adult bees in the hive prior to drone fly off.
- The percentage of those bees that were drones.
- The mite infestation rate of the drones relative to that of the workers.
- The number of mites that the drones would be expected to carry out as they exited the hive.
We commonly hear that colonies reach populations of up to 60-80 thousand bees. But I was surprised by how difficult it was to find hard data supporting those numbers. So I decided that we should roll up our sleeves and count just how many workers and drones there were in some of my own hives in late June and early July (at the end of our honey flow).
Since I wanted to get counts for both the workers and the drones, I couldn’t just weigh the hive before and after shaking out the bees – I needed to first shake all the bees into a cage and weigh them (this part was relatively easy), then separate out the drones, and weigh them alone (that turned out to be a bit more difficult than I hoped) (Figures 1 & 2).

Fig. 1 I used a very handy battery-powered device from China to shake the bees from each comb. Just pull the trigger, and in two seconds all the bees are falling through the funnel (I used a standard bulk bee funnel). I built a special hive body “cage” to hold them for weighing.
By weighing the cage before and after shaking the bees into it, I could determine the net weight of the bees. I also took a sample (on ice) of at least 100 workers for later weighing. After the first hive, I first located and caged the queen prior to shaking.

Fig. 2 A view of the combs after shaking off the bees. The small amount of bees you see were returned foragers (which we then also shook into the cage).
It was relatively easy to determine the weight of the adult bee population. The tricky part was then to separate the drones from the workers. I had installed a queen excluder over the cage, so that after weighing, I could allow the workers to exit (Figure 3).

Fig. 3 Young workers avoid light; older workers fly towards it. I found that what worked best was to first replace the queen back into the empty hive (with its brood combs), then place the cage queen excluder side of the cage down, and allow the younger workers to climb through it onto the combs. Then I’d flip the cage over, and allow the older workers to exit up and out towards the light, leaving the drones still trapped inside.
Unfortunately, some workers stubbornly remain behind in the cage (there was a substantial hive to hive difference), preventing me from simply weighing a cage full of only drones. I tried transferring the remaining bees to a bucket to smoke the workers down through the excluder (Figure 4).

Fig. 4 Trying to smoke the workers down. Didn’t work.
So how about knocking them out? (Figure 5).

Fig. 5 I tried anesthetizing the drones and workers with CO2, but they recovered too quickly to separate them by hand.
What I unfortunately wound up having to do was to drench the drones (and any remaining recalcitrant workers) with detergent solution, and bag them for later drying, hand sorting, and weighing (Figures 6 & 7).

Fig. 6 We tediously hand sorted and weighed thousands of drones and workers.

Fig. 7 By counting out 100 workers or drones and accurately weighing them, we could determine their average weights, and then calculate how many of each were in the hive.
We shook the bees from five relatively strong double- and triple-deep hives. This was at the end of our honey flow, and we only shook out colonies in which I saw a lot of drones. I highlighted the description “relatively strong” since it was based upon my assessment of “cluster size” by looking down at the top bars – the colonies appeared to be “full of bees.” What was eye-opening for me were just how few bees were spread out over the combs of the colonies that we shook (Table 1).
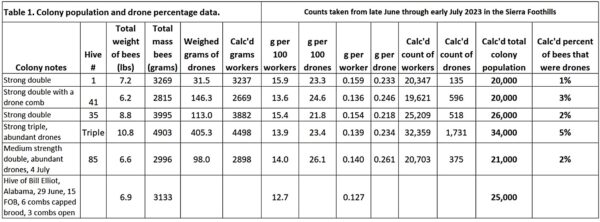
Practical note: Many years ago, Dr. Eric Mussen had commented to me that our colonies in California never reached the strength of those in Minnesota. So my results may not reflect what you’d see elsewhere.
I put out a request for other beekeepers to shake some of their hives. Bill Elliot from Alabama did so from a 1½-story hive around 20 days after his honey flow ended, and I included his finding. None of our shaken colonies were anywhere near the commonly stated 50-60,000 bee range. In the summer heat, the bees were not as packed on the combs as they are when it’s cool.
My bad: In my varroa model, I incorrectly assumed that there were as many bees on a comb during summer as there are when it’s cooler. That mistake leads to under calculating the expected mite wash counts during summer. I’ll address this in my next article.
Anyway, we’ve now got a figure for the total population of bees in post-flow hive. We now need a figure for the percentage of the population that were drones. Currie and Jay [[3]] stated that “Colonies with 24,000 – 27,000 workers contain approximately 1800 drones,” which works out to them constituting a bit more than 7% of the population. We didn’t find anywhere near that high a percentage, with some of the hives containing only 2-3% drones. Anyway, for our calculation, let’s use the highest drone percentage (5%) that I found in my hives.
The next figure that we need for the calculation would be the mite infestation rate of the drones relative to that of the workers – luckily something that I already had from the drone sampling that I described last month (Table 2).

I color coded the worker infestation rates in blue, and those of the drones in red. In black boldface below I calculated the “multiplier” – how many times higher the infestation rate of the drones was, relative to that of the workers.
In all the hives, the infestation rate of the drones was higher than that of the workers. For our calculation, let’s use the overall average multiplier of 5.5% (meaning that the average infestation rate of the drones was five and a half times higher than that of the workers).
The last figure that we’d need for our calculation would be how many mites the exiting drones would take with them. E.J. Steed collected flying (and thus presumably) older drones at drone congregation areas, and found that they were infested by mites at about two-thirds the rate of workers sampled from brood combs in nearby hives [[4]]. This indicates that drones that exit the hive take their mites with them. Dr. Lama’s data indicate that drones old enough to fly were roughly as attractive to mites as were workers of the same age, so for my calculations I’ll assume that when a drone exits a hive, its infestation rate would be the same as that of the worker average (as opposed to workers sampled from the brood combs).
Let’s Do the Calcs!
OK, we’ve now got all the information that we need to calculate to what degree a hypothetical fly-off of drones would increase the mite wash count in a sample of workers. So (as I’m prone to do) I took some time (actually a lot of time) to create a calculator [[5]] to see (Figure 8).
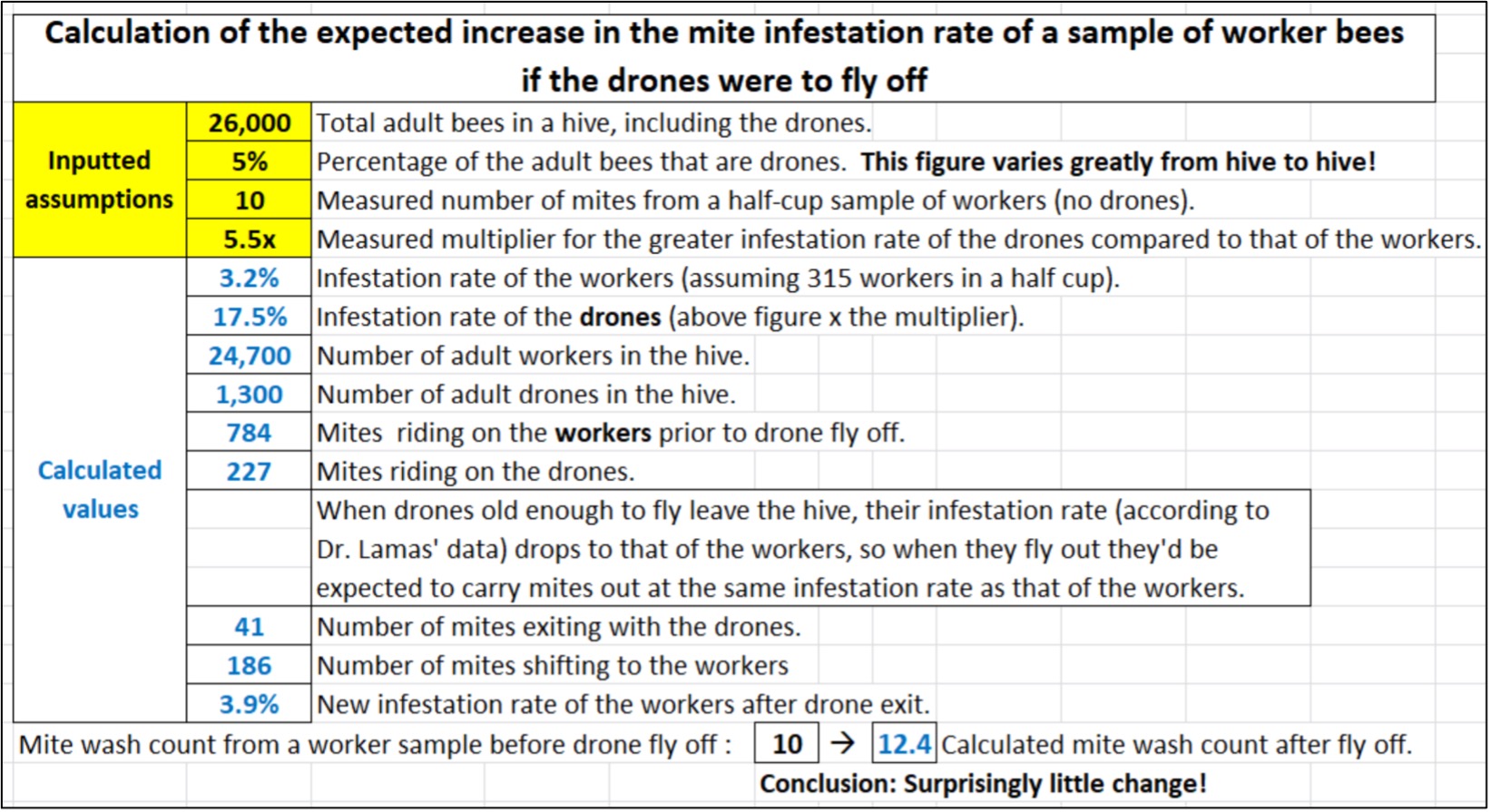
Fig. 8 In the above calculation run, I arbitrarily assumed that prior to drone fly off, the workers had reached an infestation rate of 10 mites per half-cup sample (a 3.2% infestation — a commonly accepted action threshold). The calculation indicates that even in the hypothetical case of the drones (infested at 5.5x that rate) suddenly leaving the hive, their exit would only result in a minimal increase in the infestation rate of the workers. Another tantalizing hypothesis bites the dust.
Conclusions
Although the hypothesis that post honey flow drone disappearance would result in a serious jump in the mite infestation rate of the workers was compelling, my data and calculations do not support it.
The cause of the rapid increase in the infestation appears to be mainly due to a combination of four factors:
- The exponential increase in the mite population, which doubles roughly each month [[6]].
- The reduction of the adult worker population, meaning more mites per bee [[7]].
- A decrease in the amount of sealed brood, which results in a greater proportion of mites on the adult bees.
- And possible immigration of mites due to worker drift or robbing.
Practical application: My measuring of the actual bee counts of my hives has opened my eyes to the fact that my varroa model needs adjustment. I’ll go into greater depth next month.
Why Would Mites Prefer Drones?
I wish to thank Dr. Lamas for his impressive body of research. His findings on the preference by mites for drones (which my data confirm) are of great interest, and I’m curious as to why, since their fat bodies (varroa’s target food) are not as developed as those of non-foraging workers. Haydak [[8]] found that the fat bodies in drones consisted of only “a few aggregations of cells, difficult to find,” and that “no fat-body was found in drones 3 days old or more.” However, the hemolymph of freshly emerged drones contains relatively more glycogen (an energy molecule) than in same-aged workers, and the protein and vitellogenin content of drone hemolymph “increases rapidly within the first days after emergence, reaching a peak at the 3rd day and decreasing after the first week” [[9]]. So what is it about drones that mites find so attractive?
We obviously still have a lot to learn about varroa and their affinity for drones! I look forward to reading Dr. Lamas’ studies when they become available.
Acknowledgements
Thanks for the field help from Corrine Jones, Kamon Reynolds, Victoria Knight, and Rose Pasetes.
Citations and notes
[1] Hidden in plain sight: Varroa aggregate on adult drones https://www.youtube.com/watch?v=mAsXFPakumU
[2] https://scientificbeekeeping.com/randys-varroa-model/
[3] Currie, R & S Jay (1991) The influence of a colony’s queen state on the drifting of drone honey bees (Apis mellifera L.). Apidologie 22: 183–195.
[4] Steed, EJ (2023) Parasites and pathogens at honey bee mating sites, and the implications for monitoring colony health. M.S. Thesis, The University of Waikato.
[5] I’d be happy to share a copy if you’re interested.
[6] See cell AG62 on the Current Version tab of my varroa model.
[7] https://scientificbeekeeping.com/ipm-3-strategy-understanding-varroa-population-dynamics/
[8] Haydak, MH (1957) Changes with age in the appearance of some internal organs of the honeybee. Bee World 38: 197–207.
[9] Hrassnigg, N & K Crailsheim (2005) Differences in drone and worker physiology in honeybees (Apis mellifera). Apidologie 36(2): 255-277.
Contents
The Proposed Hypotheses 2
Monitoring of the Mite Infestation. 3
How Best to Obtain a Representative and Consistent Sample?. 3
Testing Hypothesis #1. 4
Field Observations. 5
Results. 9
The Take Home. 15
Citations and Notes 16
Drones and Varroa
Part 1
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ October 2023
In the May issue of this journal, Dr. Zac Lamas asked “Why we aren’t we sampling drones” in order to monitor the mite infestation level of our colonies in the springtime? Since my sons and I perform a great number of mite washes, I found his findings and proposed hypotheses to be of great interest. So I pursued his suggestion that beekeepers compare the infestation rates of samples of hand-plucked drones to those of samples of bees shaken from the same or adjacent combs.
As a queen breeder, I’ve of course paid attention to the presence of drones (Figure 1).

Fig. 1 Drones are abundant in our hives from the time we return from almond pollination until at least early July, but I’d never thought about the possibility of using them for varroa monitoring.
So of course I was very curious about Dr. Lamas’ research, but his thesis is embargoed ‘til 2024. Fortunately, he presented some of his very interesting findings on YouTube [[1]]. His data indicates that the infestation rate of adult drones is much greater than that of similarly-aged adult workers for the first several days of their lives, after which the difference appears to largely disappear.
The Proposed Hypotheses
Dr. Lamas proposed two intriguing plausible hypotheses:
- That for the purposes of varroa monitoring early in the season, samples of drones may better reflect a colony’s infestation rate than samples of (mostly) worker bees shaken from the combs.
- That the summertime shift of mites from adult drones to adult workers would appreciably increase the varroa infestation rate of the worker population.
Since I’ve spent a great deal of time evaluating various methods for monitoring varroa [[2]] and perform thousands of mite washes each season, I found Dr. Lamas’ findings and proposed hypotheses to be of great interest. So I followed his suggestion for beekeepers to use his protocol to compare the infestation rates of samples of hand-plucked drones to those of samples of workers shaken from the same or adjacent combs. After that, I explored the hypothesis that the midsummer reduction in the amount of drones in a colony would result in an appreciable increase in the infestation rate of the adult workers.
Monitoring of the Mite Infestation
In Integrated Pest Management, one monitors the infestation rate of the pest in order to determine whether and when to take action against the pest, weighing the damage caused by the pest vs. the cost of control. There’s a good discussion on pest treatment thresholds at [[3]]. In short, there are two commonly confused terms. In the case of varroa management:
The Economic Injury Level: The infestation rate of mites that will cause yield losses (in pollination value or honey production) equal to the varroa management costs (the level at which it becomes cost effective to apply treatment).
The Economic Threshold (treatment action threshold): The varroa infestation rate (pest density) at which management action should be taken to proactively prevent the increasing mite population from reaching the economic injury level.
The key concept is to be proactive, since we can predict the rate that a varroa infestation will increase in a non-resistant colony (Figure 2).
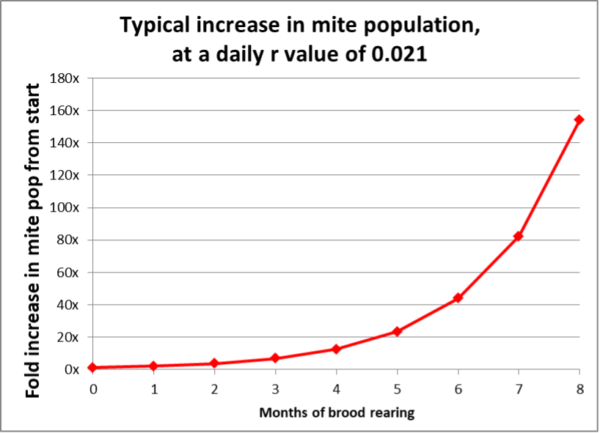
Fig. 2 The expected increase in a colony’s varroa population over time. I used the commonly observed intrinsic rate of population increase (r value) of 0.021 [[4]]. The thing to keep in mind is that with exponential increase, early intervention is critical.
Practical application: The most economically-successful beekeepers that I know attempt to maintain varroa levels well below the economic injury level at all times of the year — they don’t wait until they see high mite counts!
How best to obtain a representative and consistent sample?
The thing to keep in mind is that there’s only one way to precisely determine the mite population in a hive — that is to kill all the bees and brood, and then count every single mite (something that a number of researchers spent tedious hours doing in the early years of varroa research [[5]]). On the other hand, for monitoring purposes we attempt to take a representative sample of adult bees or pupae, and then extrapolate the average infestation rate of the bees in the hive.
Of equal importance, samples must be reliable and consistent — two or more samples taken from the same hive should come up with close to the same number.
Due to the inherent variation of the infestation rate from bee to bee, to extrapolate a colony’s average infestation rate from a sample, the sample must contain enough bees to overcome that variability, typically by using the Poisson distribution of probabilities to come up with the minimal required sample size [[6]]. For a mite wash, Dr. Katie Lee determined that a sample of 300 bees was adequate [[7]].
I’ve spent a great deal of time evaluating various methods for monitoring varroa and determining what would be the best representative sample [[8]], and have found that shaking bees from a frame adjacent to brood and allowing the older bees to fly off, would provide a sample representative of the colony as a whole. But I collected that data late in the season when there were few drones — I need to replicate the study in the springtime.
So even though a shook bee sample is representative of the mite infestation rate of the adult bees in the colony, it must be evaluated relative to the adult bee-to-brood ratio at the time it was collected. Take a look at Figure 3.
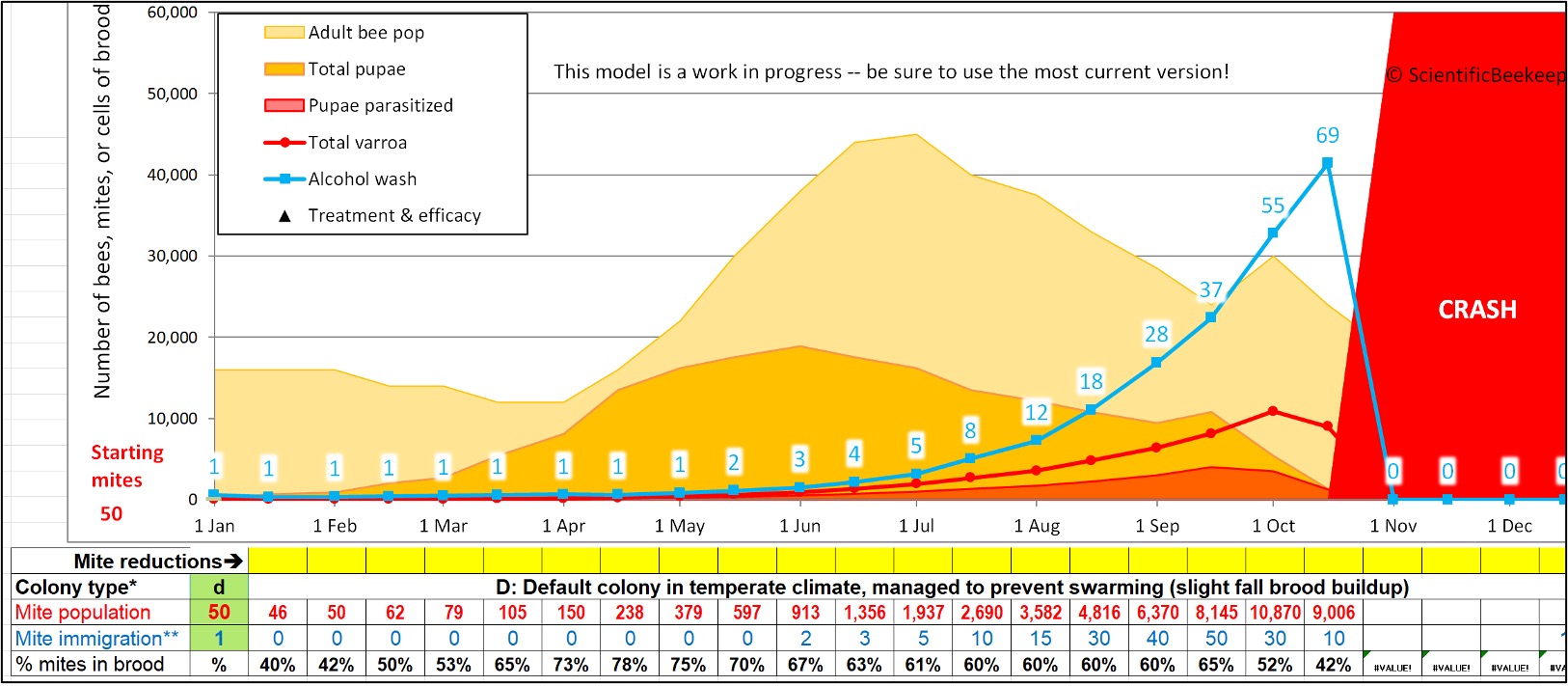
Fig. 3 My varroa model [[9]] calculates the estimated mite wash count based upon the inputted varroa population in the hive. And based upon the inputted amount of adult bees and brood (the vertical ratio of light orange to dark orange), it estimates the percentage of mites that are hidden in the brood (bottom row), as well as the expected mite wash count (blue figures). Note that during swarming season (when there are nearly as many pupae as there are adult bees), 4 out of 5 mites can be in the brood — and thus not on the adult bees (whether workers or drones). This means that one must interpret the mite wash count appropriately.
The thing to keep in mind is that any sampling of adult bees early in the season will underestimate the infestation rate of the colony as a whole. Note in the figure above how the infestation rate of the adult workers is low clear through the beginning of June (3 mites per half cup of bees), then appears to explode by the end of August (increasing over 9-fold to a count of 28).
Practical application: As illustrated in Figure 3, a mite wash count over 1 in April indicates that action should be taken before the honey flow, otherwise the colony’s infestation rate will have already allowed for DWV to go rampant by the time you pull your honey in mid-August.
Testing Hypothesis #1
Dr. Lamas questioned the efficacy of sampling worker bees to monitor the varroa infestation level of a colony early in the season. His proposed hypothesis is that taking a sample of 40 drones from a brood frame might provide a more representative assessment of the mite infestation rate of a colony as a whole than would using the standard method of taking a half cup sample of workers. So I roughly followed Dr. Lamas’ suggested protocol to compare the infestation rates of samples of hand-plucked drones to paired samples of workers taken from the same or adjacent combs. Helped by Jennifer Radke, Rose Pasetes, and Corrine Jones in late April and mid June, we dove into some high-mite colonies that I had moved home for experimentation, and took matching samples of drones and workers to compare their infestation rates (we used high-mite colonies so that we’d get enough mites in each sample to get good data to work with).
Field Observations
Most beekeepers notice that drones are more prevalent in frames to the sides of the broodnest, but when I actually started looking for drones to pluck off, it surprised me to see just how much they clumped together (Figure 4).

Fig. 4 A typical comb full of drones outside of the broodnest. It soon became apparent that it was often difficult to find the 40 drones on a brood frame as suggested by the protocol (since drones avoid this area), and that any sample of shook bees from an outer frame might contain a large percentage of drones.
I soon noticed that it was unusual to find a drone without at least one more drone nearby, so if I plucked one, I’d move the forceps right back to that area, and usually find at least a couple more. I found most of the drones on one or more of the outer frames, sometimes only on one side of the hive, as well as on the bottom board to the back of the hive (Figure 5).

Fig. 5 During the hot weather when we took the samples, drones were often clumped at the back of the bottom board.
A question: After observing how drones tend to clump together, it occurred to me that this appeared to be independent of temperature (I’m not discounting that they prefer cooler temperatures away from the brood nest). Since this clumping takes place in darkness, it is reasonable to assume that drones are attracted to the odor of other drones. Could this explain the common observation that some colonies (especially those without a laying queen) appear to be magnets for drifting drones?
I also noticed that there was often a large difference in the sizes of the drones in the same hive. I often notice this in colonies that have gone laying worker and produce stunted drones in extended worker cells, but this was in “normal” hives (Figure 6).
Fig. 6 Note the differences in sizes between the four drones on this comb. This amount of variation was normal.

It immediately became apparent to me that few beekeepers would have the patience to pluck 40 drones from a brood frame — firstly because there are often very few drones on a brood frame, and secondly because I found that it would take an inordinate amount of time to find, pluck, and successfully place in alcohol 40 drones.
Practical application: I quickly dismissed the practicality of collecting 40 drones for a sample, so arbitrarily took samples of 25 (which most beekeepers would still likely find to be too tedious). Even at a count of only 25, it was often difficult for me to collect more than a few drone samples from the twenty frames in each of the seven double-deep colonies from which we collected the paired samples. And if a drone managed to fly off when dropped into the collection cup, we sometimes had to recount — which would have been a real pain for a sample of 40!
I took as many paired samples of drones (Figure 7) and shook bees from the same or adjacent frames as we could spot drones on, taking the paired samples from combs throughout the hive (drones first, then shaking or brushing bees into a tub). The shook bee samples often contained a few drones (which would be typical for the half-cup of shook bee methodology), but we did not remove them.

Fig. 7 I plucked the drones off the combs with insect forceps and threw them into a Dawn detergent solution for washing (we double washed to confirm full mite recovery). I then shook or brushed bees (predominantly workers) from the same or facing comb into a tub, and then scooped up a level half cup of bees (roughly 315 workers) for the matching paired sample.
Since drones were relatively rare on brood combs, we checked all the frames in each hive for the presence of drones, in order to obtain as many paired samples from each hive as possible.
Results
Let’s take a look at the results of first hive from which we took paired samples (Figure 8):
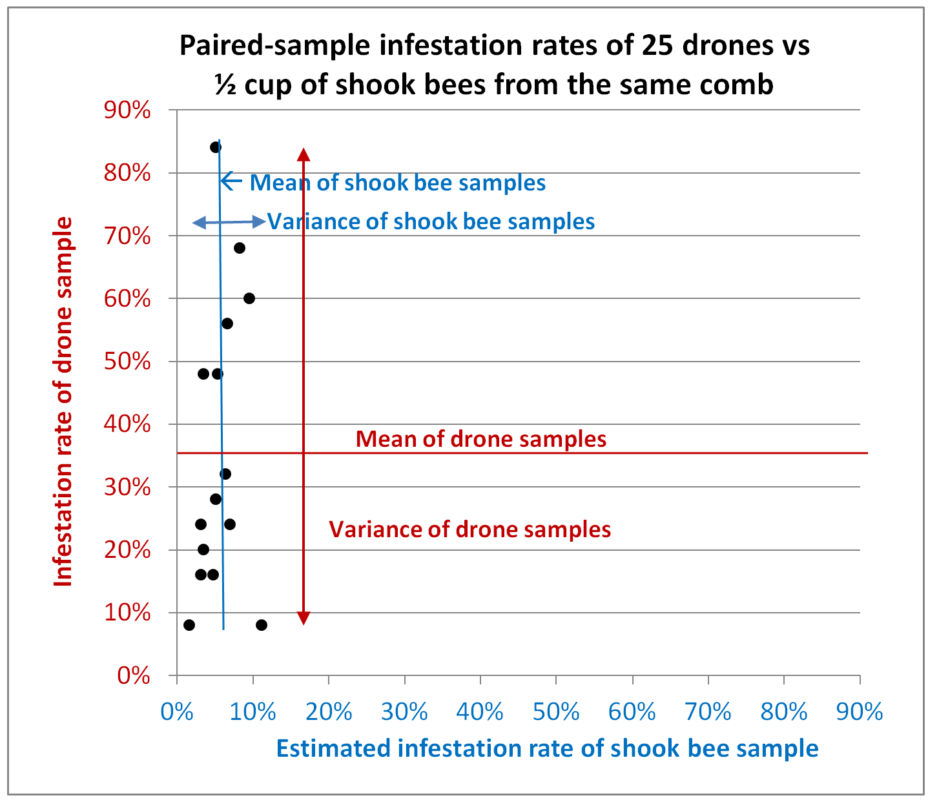
Fig. 8 Results from Hive A. All paired samples were taken from the same combs (or brushed from the interspace between adjacent combs). Each data point represents the infestation rates of two paired samples taken from a single colony, with the infestation rate of the shook bees on the blue x-axis, and that of the drones on the red y-axis. The average values for the two types of samples are indicated, and in this chart I’ve also indicated the variance for each type.
Interpretation: In this colony there was indeed a correlation between the infestation rates of the two types of samples (that of the drones being over 6x higher), but with a very low correlation coefficient (not shown) due to the far greater deviations from the average of the drone samples relative to those of the worker samples (compare the red variance arrow for the drone samples to the blue arrow for the shook bees).
We would expect variation in the worker infestation rates, since they are typically higher in samples taken from combs containing brood, but we’ll need to wait until Dr. Lamas publishes his results to see whether the same holds true for drones. The larger degree of variation in the drone samples is likely partially due to the smaller number of bees involved, but it is unrealistic to think that any beekeeper would hand pluck 315 drones for mite monitoring.
We continued sampling six more hives, some loaded with drones, some containing only a few (the differences in the amounts of drones in similar colonies sitting side by side surprised us). I collected as many paired samples as possible from each hive, sometimes with all of our eyes searching for enough drones to make up a sample of 25 (Figures 9-14).
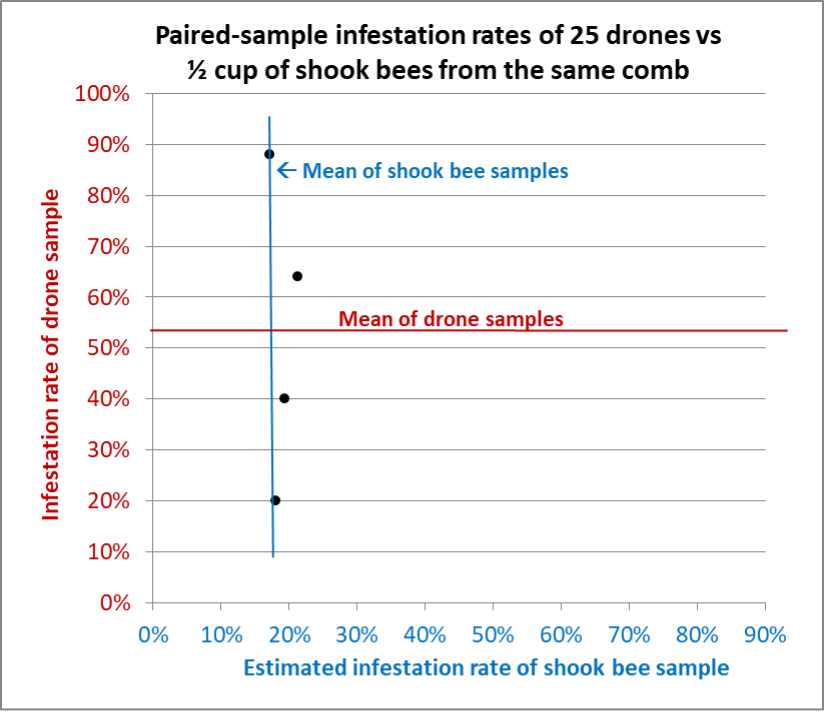
Fig. 9 Results from Hive B. Although we could only spot enough drones for four samples, similar to Hive A there was far greater variation in the drone samples than for the shook bee samples. Any one of the shook bee samples would have been representative of the whole-hive varroa infestation rate — something that could not be said for the drone samples.
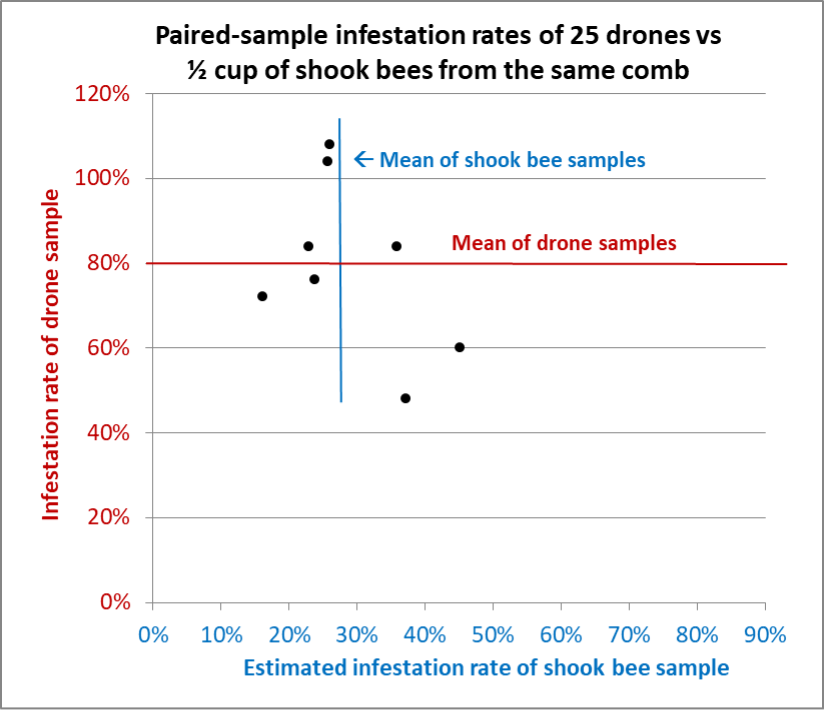
Fig. 10 Results from Hive C. This poor colony had an extremely high mite infestation rate, and a greater degree of variation in the shook bee samples than in other hives. Nevertheless, the standard deviation for the drone samples was over twice that of the shook bee samples. For those paying attention, the infestation rates of the drones that exceeded 100% mean that there were more mites than drones.
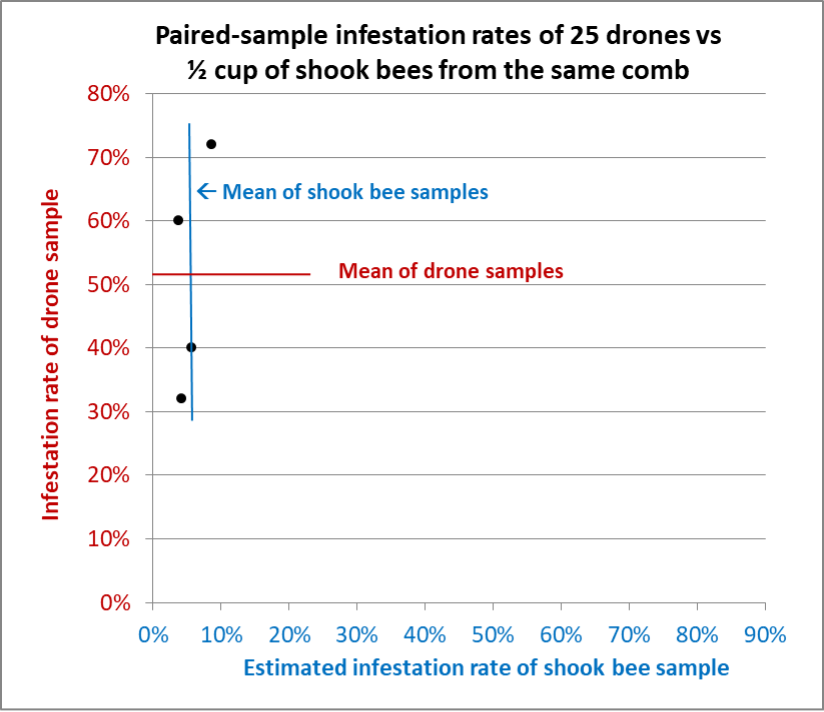
Fig. 11 Results from Hive D. Similar to the other hives, a single sample of 25 drones would not have been a reliable indicator of the colony’s overall infestation rate. On the other hand, the shook bee samples were remarkably consistent.
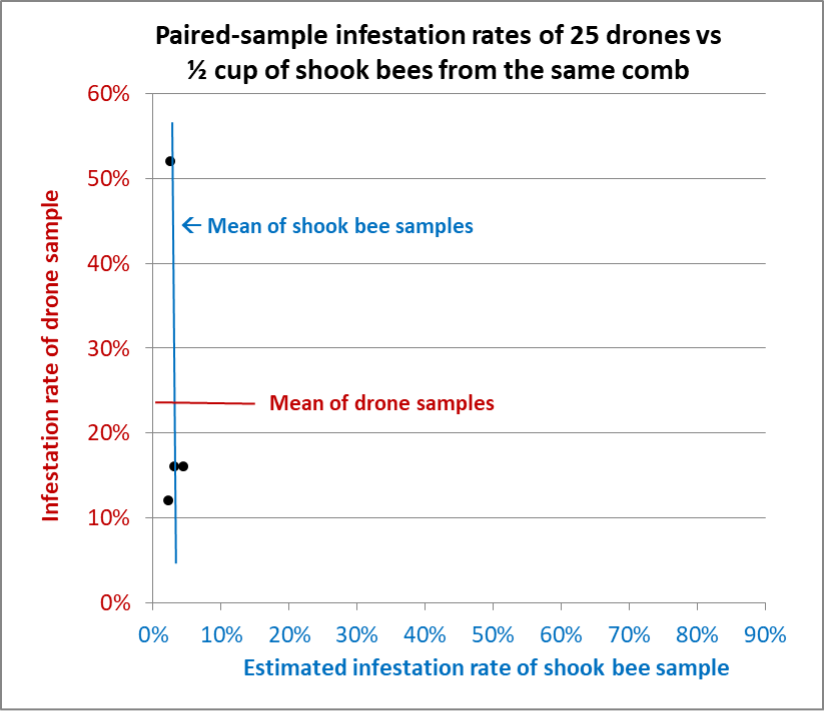
Fig. 12 Results from Hive E. Again, the shook bee samples all fell in the same range, compared to the drone samples, which varied widely.
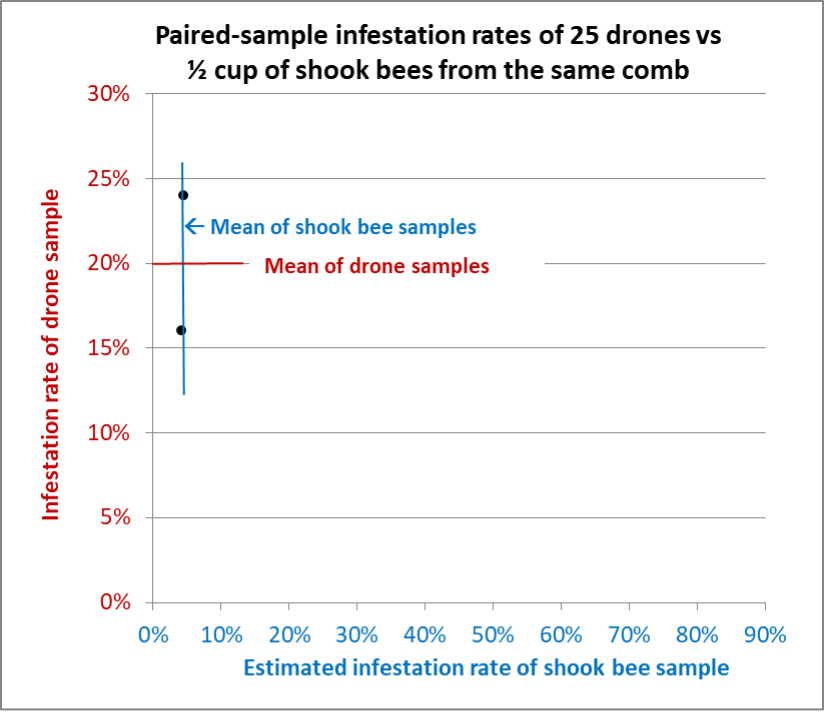
Fig. 13 Results from Hive F. We could only find enough drones for two paired samples. The infestation rates of the shook bee samples were nearly identical, compared to drone samples that differed by 20% to either side of the average.
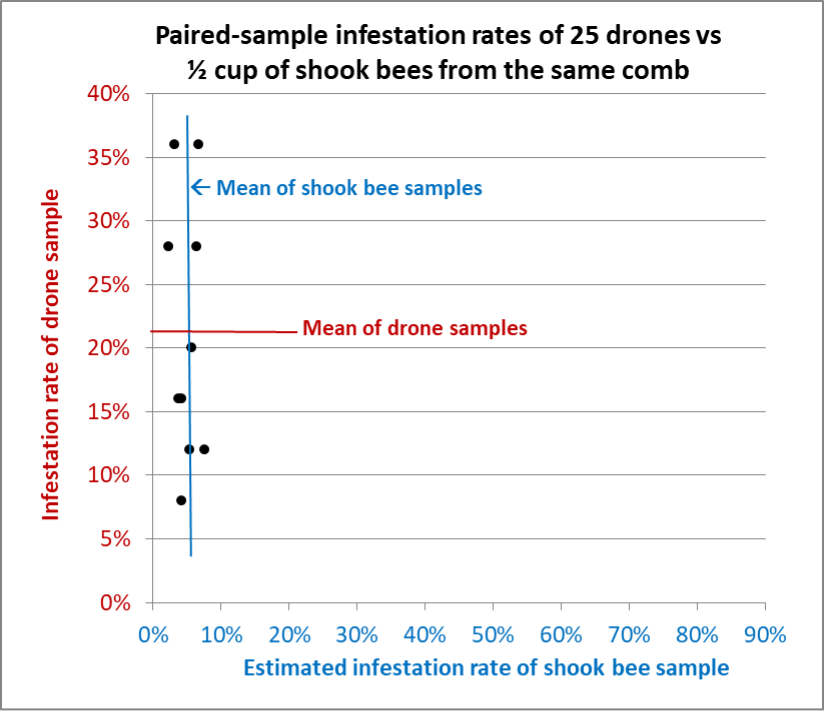
Fig. 14 Results from Hive G, from which we were able to take 10 paired samples (two exactly overlapped). Again, the shook bee samples varied only slightly, whereas the drone samples were all over the place.
The Take Home
Dr. Lamas’ findings about the distribution of mites on drones of different ages are of great interest, and may help us to better understand varroa and virus dynamics in the hive. But as far as using samples of drones that aren’t age marked in order to monitor a colony’s varroa infestation rate, we e found that taking a sample of even 25 drones was far more difficult and time-consuming than shaking a sample of a half cup of bees. Our findings from the seven hives from which we took paired samples in late April through mid-June indicated that a sample of 25 drones would not be a reliable indicator of the overall infestation rate of the adult bees in the hive, whereas a shook or brushed sample of a half cup of mostly worker bees generally would. In fact, inadvertently including a few young drones in such a sample might skew the mite count appreciably upward.
Practical application: For varroa monitoring purposes, we’re sticking with mite washes of a half cup of bees shaken from a comb adjacent to the broodnest.
The main thing to keep in mind is that early in the season most of the mites are in the brood, so it’s no surprise that the infestation rate of the workers will appreciably increase later in the season. So a mite wash of shook adult bees must be interpreted in that light — in our operation, we’d proactively treat if our colonies exhibited infestation rates of more than a single mite in a half cup of bees in April or May.
Next month I’ll detail how I tested Dr. Lamas’ second hypothesis, since it might help to explain the surprisingly sudden increase in infestation rates in August and September.
Citations and Notes
[1] SBGMI Presents: “Why don’t we sample drones?” https://www.youtube.com/watch?v=mAsXFPakumU
[2] Reconnaissance Mite Sampling Methods and Thresholds https://scientificbeekeeping.com/fighting-varroa-reconnaissance-mite-sampling/ (first published in March 2007 ABJ)
Re-Evaluating Varroa Monitoring:
Part 1- Methods https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-1/ (first published in March 2020 ABJ)
Part 2- Questions on Sampling Hives for Varroa https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-2/ (first published in April 2020 ABJ)
Part 4- What About Letting the Shook Bees Fly Off? https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-4/ (first published in June 2020 ABJ)
[3] https://cropwatch.unl.edu/economic-injury-level-and-economic-threshold-ipm
[4] The r value is slightly higher in the spring, and lower later in the season. See column AZ65 on the Current Version tab at https://scientificbeekeeping.com/randys-varroa-model/
[5] My hat is off to John Harbo, Jeff Harris, Lilia De Guzman, José Villa, and Bob Danka for their work!
[6] https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-2/ (first published in April 2020 ABJ)
[7] Parasite Monitoring https://scientificbeekeeping.com/sick-bees-part-11-mite-monitoring-methods/ (first published in August 2011 ABJ)
[8] Re-Evaluating Varroa Monitoring:
Part 1- Methods https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-1/ (first published in March 2020 ABJ)
Part 2- Questions on Sampling Hives for Varroa https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-2/ (first published in April 2020 ABJ)
Part 3- How Does Mite Distribution Vary Frame-to-Frame in a Hive? https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-3/ (first published in May 2020 ABJ)
Part 4- What About Letting the Shook Bees Fly Off? https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-4/ (first published in June 2020 ABJ)
[9] https://scientificbeekeeping.com/randys-varroa-model/
The Status of Our Industry Regarding Varroa Management
and
What Can We Do About It?
Part 2
First Published in ABJ August 2023
Randy Oliver
ScientificBeekeeping.com
CATCH UP
Last month I wrote about the options (legal or unapproved) that beekeepers are taking to deal with varroa as it evolves resistance to amitraz. In this installment, I’ll review proposed options for the beekeeping industry could take at the legislative level, specifically what we could lobby for in the upcoming Farm Bill. I’ve spent some time reviewing each proposal, and suggest that we don’t waste our time on those with little chance of success — such as the 2(ee) exemptions for extended-release oxalic acid passed by a few states, and then later denied by EPA.
THE SHIFT FROM XENOBIOTICS TO “NATURAL” BIOCHEMICALS
Some of the earliest pesticides used in agriculture, such as copper, arsenic, mercury, creosote, naphthalene and nicotine, were not in any way ecologically “friendly.” Since the Second World War, those nasty pesticides have largely been supplanted by synthetic manmade chemicals (xenobiotics not found in nature), such as organochlorides, organophosphates, carbamates, and neonicotinoids ― to which biological systems had never been exposed. Due to the persistence of some of these xenobiotics, their negative effects upon off-target species and the environment, and their unknown long-term effects upon our health, there is a push to instead use biochemicals naturally produced by plants or animals, which quickly biodegrade, and to which our bodily detoxification mechanisms have adapted over the course of evolution.
The EPA and USDA are very much in favor of this shift, making a special case for naturally-occurring “minimal risk” pesticides and “biopesticides” (naturally occurring substances that control pests by non-toxic mechanisms). Unfortunately, fine distinctions and arbitrary decisions have led to including varroa-toxic formic acid, menthol, and sucrose octanoate in EPA’s list of biopesticides [[1]], while excluding oxalic acid and thymol from both that list and the Minimal Risk list [[2]] (Table 1).
| Table 1. Pesticides with known or potential varroacidal action |
| Type of Pesticide |
Modes of Action |
Risks or Benefits |
Examples |
| Synthetic Xenobiotics |
Typically neurotoxins |
Persistent residues in beeswax, honey contamination, human health risks. |
Fluvalinate, bifenthrin, coumaphos, amitraz |
| Biopesticides: EPA’s definition is “naturally occurring substances that control pests by non-toxic mechanisms.”
But some of the active ingredients to the right are on EPA’s list of biopesticides, despite the fact that they exhibit strong toxic mechanisms against varroa! |
Caustic, cellular membrane disruptors, behavioral disruptors, other unknown actions. May exhibit neurotoxic, genotoxic or teratogenic effects. |
These would all be considered as natural treatments. Some are not only be toxic to varroa, but can also cause serious adverse effects to bees and brood. Fully biodegradable, some have tolerance exemptions in food, may be caustic or irritants to humans, but are of little overall concern about human health risk when applied to beehives. |
Acetic, phosphorous, and formic acids, menthol, sucrose octanoate, and the oils of balsam fir, canola, catnip, cedarwood, citronella, clove, eucalyptus, garlic, geranium, lavendin, lemongrass, menthol, mint, mustard, neem, orange, oregano, tea tree, thyme, and wintergreen |
| Natural or “Organic” Substances Not Listed as Biopesticides |
Similar to above |
Similar to above |
Oxalic and lactic acids, thymol, bergamot, melaleuca |
| Minimal Risk Pesticides (no registration required) |
Similar to above |
Determined to be of minimal risk |
Clove or thyme oils, citric acid |
| Home Use Food Products(can’t be sold as varroacides) |
Mechanical, behavioral disruptor, other action |
Of little concern |
Powdered sugar, Crisco, lemon juice, mustard |
| RNAi, Microbials, etc. |
Molecular or parasitic to varroa |
Generally of minor concern |
Products in development |
OUR PROBLEM WITH CURRENTLY-REGISTERED PRODUCTS: VARROA CONTROL WHILE COLONIES ARE PRODUCING HONEY
In many regions, due to climate or the timing of the nectar flows, there are no practical and efficacious registered treatments for controlling varroa while honey supers are on the hive. This is a problem, since during this period mites continue to reproduce at an exponentially-increasing rate — leading to serious, but preventable, colony losses later in the season.
There are three main issues involved:
- Due to the need to avoid contamination, some products are not allowed to be applied during the production of honey for human consumption.
- Label restrictions unreasonably limit application methods of registered products, even though they have tolerance exemptions in honey.
- Time-consuming labor or applicator safety issues are involved with the three natural treatments that are approved for use during the nectar flow (detailed below).
There is no need for beekeepers to fight the EPA, but rather we should ask EPA, and perhaps our legislators, to help us, as a minor industry, to be able to legally use expanded application methods for the safe and non-contaminating natural treatments, notably oxalic and formic acids, and thymol. Unfortunately, the few registered products on the market may be unreasonably expensive, and their labeling too restrictive for the needs of beekeepers in different climates and operations.
THE RISK TO APPLICATORS
The EPA must take into account the risk to the applicator involved with the application of any pesticide. But unlike a farmer who can spray a product onto their crop from the safety of the cab of an air-conditioned tractor, the beekeeper must apply varroa treatments — working in the full sun on hot days — to the brood chamber of each individual hive.
The application methods for the currently-registered miticides approved for application during the nectar flow are not only unreasonably time consuming, but also involve unacceptable risks to any beekeeper having more than a few hives. There are three main risks involved are:
- The physical exhaustion and chance of bodily injury involved in the removal and restacking of hundreds or thousands of heavy honey supers in order to apply the product to the brood chambers beneath them (especially when repeated applications are required).
- The heat stress involved with the above labor during the typical high temperatures that occur during the nectar flow, especially with the required Personal Protective Equipment (thick vinyl gloves, heavy clothing, or masks) called for by the label. The removal and restacking of honey supers is hard physical work, which in hot weather can easily lead to heat stroke.
- The exposure risk to the applicator involved with the unavoidable fumes of formic acid and heat-vaporized oxalic acid, especially when involved when treating thousands of individual hives during hot weather. The hard physical work and heat stress greatly increase the chance for accidents leading to acid vapor exposure.
Talking point for our legislators: What our industry needs are additional methods for controlling varroa while honey supers are on the hive, notably inexpensive natural treatments or application methods that pose less risk to the applicator.
UNDERSTANDING THE EPA
For us beekeepers to ask for any legislative action, we gotta understand the mandate that EPA works under.
The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) was signed into law by President Harry S. Truman in 1947 to regulate the distribution, sale, and use of pesticides. In 1972 power was transferred the Environmental Protection Agency (EPA) (which had been established by President Richard Nixon in 1970). The first iterations of the law focused on labeling and ensuring that chemicals were not adulterated, but did not focus on the environmental and public health effects of pesticide use; this did not come until 1972. Congress has passed amendments to FIFRA numerous times since then.
FIFRA now contains 284,623 words [[3]] and is enforced by the Office of Pesticide Programs (OPP). Unless specifically exempted, all pesticides distributed, sold, or used in the United States must be registered by the EPA and regulated by the Office of Pesticide Programs (OPP). In order to gain registration, a manufacturer must show that the pesticide will generally cause no unreasonable risk to man or the environment, while taking into consideration the economic, social, and environmental costs and benefits of use of the pesticide, dietary risk from residues, as well as not endangering agricultural workers during their application.
Practical consideration: It may be easier to get Congress to pass an amendment to FIFRA than to ask the OPP to approve a requested exemption.
USE, REGISTRATION, OR LEGISLATIVE OPTIONS ON THE TABLE
Below are the short versions of my assessments of each option (you can contact me for more detailed versions).
Potential Action Item #1: Applying for FIFRA Section 24(c) Special Local Need Registrations
Individual states are allowed to register a new end-use product or an additional use of a federally registered pesticide product to address an existing or imminent pest situation. The pest situation must be a special local need within the state that cannot be mitigated by a currently registered product [[4]].
We could argue that varroa’s development of resistance to amitraz is certainly an imminent problem, and that the very limited label directions for the currently-approved varroacides that can be applied during a nectar flow are too restrictive and unduly risky to the applicator.
Pro: Might work.
Cons: It would be a long shot. An SLN designation requires the consent of the Registrant of the active ingredient, which Chemicals Laif (Veto Pharma) is not interested in giving, so as far as any SLN registration of different application methods for oxalic acid, this option is Dead on Arrival.
My assessment: Since at best this would only apply to the use of Api-Bioxal, it’s doubtful that it would be worth the effort, especially since EPA may reject the applications. Chance of success is low.
Potential Action Item #2: Utilize USDA’s Office of Pest Management Policy (OPMP) to Help Us.
USDA, under §7653, with regard to the specific purpose of collaborating with the EPA and agricultural stakeholders in the availability, and use of economically and environmentally sound pest management tools, practices, and policies, established the Office of Pest Management Policy (OPMP), with the mandate to provide leadership to ensure coordination of interagency activities with the Environmental Protection Agency, the Food and Drug Administration, and other Federal and State agencies.
“The Office of Pest Management Policy shall consult with agricultural producers that may be affected by pest management or pesticide-related activities or actions of the Department or other agencies as necessary in carrying out the Office’s responsibilities under this section.” [[5]]
Limitation: The OPMP can only advocate, not regulate or approve varroacides.
My assessment: The OPMP is already helping us, but they don’t set actual pesticide policy.
Suggested action: Our industry needs to engage in consultation with OPMP and suggest how they might advocate on our behalf.
Potential Action Item #3: Ask IR-4 for Help
The IR-4 (Inter-Regional Research) Project (aka the Minor Crop Pest Management Program) develops data necessary for the registration of safe pesticides with EPA. IR-4’s mission is to facilitate registration of sustainable pest management technology for fruits, vegetables, ornamental plants, and other “minor” crops. IR-4 works in coordination with the Environmental Protection Agency to assist in the collection of residue and efficacy data in support of the registration or reregistration of minor use pesticides and the determination of tolerances for residues of minor use chemicals in or on raw agricultural commodities. Since its founding, IR-4 has facilitated the approval of thousands of pesticide registrations through EPA, many of which provide growers with innovative pesticide products (including varroacides) that minimize health and environmental risks.
There are synthetic miticides already registered for uses other than varroa control [[6]]. If a Registrant wanted to expand its use for beehives, it would require only a label amendment. IR-4 could help to collect required data, such as that required for a residue tolerance in honey. But our industry has already shown the chemical companies that if an already-registered plant-protection product (such as Mavrik or Taktic) is cheaper than a formulated product specifically for beehives (respectively Apistan or Apivar), that commercial beekeepers will likely buy it instead, so might not justify the cost involved in pursuing an additional registration.
However, as we discover other ingredients on the Biopesticide list, new modes of action against varroa (such as RNAi, odorants, or behavioral disruptors), IR-4 might help get them through the regulatory process.
Pro: IR-4 is willing to help us, although we would likely need to pay them for the necessary data collection.
Cons: IR-4 is mainly interested in the testing of novel active ingredients and formulations.
My assessment: IR-4 is mostly interested in helping to register biopesticides, but doesn’t set pesticide policy. The process of registering new products or application methods could take some time.
Potential Action Item #4: Shift regulation of Varroacides to the FDA
Currently, EPA and FDA determine regulatory oversight of pesticides and new animal drugs based on the rationale described in a Memorandum of Understanding (MOU) between the agencies signed in 1971 and revised in 1973. Parasite treatment products applied topically to animals (including pets) generally are regulated by EPA if they remain on the skin to control only external parasites, and by FDA if they are ingested and absorbed systemically into the bloodstream. Although this idea has been floated that varroacides might better be regulated by the FDA, either as over-the-counter products, or by veterinary prescription,
- The registration requirements by FDA for parasite treatments may be tougher than those by the EPA for pesticides.
- Vets have severe limitations for prescribing extra-label usage, and it’s doubtful that FDA would look more leniently upon alternate routes and dosages than the EPA.
- Since Api-Bioxal is already registered, FDA would not allow generic OA usage.
- FDA might revisit the residue concerns for honey.
Pros: Not clear.
Cons: Of questionable benefit to our industry. Most stakeholders are opposed.
My assessment: This option appears to be a non-starter.
Potential Action Item #5: Petition the EPA to Add Honey Bees to the Minor Use Crop List
Designation of a pesticide for minor use is intended to identify situations where registrants of potentially useful pesticide products (in our case, varroacides) may not need to apply for registration because the potential returns are low (This lack of economic incentive is a huge issue for us, greatly limiting the number of registered products available for our use). Crops (in our case beehives) qualify if there are fewer than 300,000 acres in production in the United States.
It takes 19,300 beehives to cover an acre. Thus the 3 million hives in the U.S. would cover 155 acres. So we should definitely qualify for Minor Use registration.
Minor uses also include pesticides applied for control of disease vectors such as mosquitoes, ticks, cockroaches, rodents and disease-causing organisms (varroa is the vector for DWV).
In 2018, the EPA released a Pesticide Registration Notice [[7]] to clarify and revise the EPA’s interpretation of “minor use” under FIFRA section 2(ll) as it applies to the registration of pesticides. Although there were several comments submitted to EPA, not one came from our industry representatives.
Suggestion: We beekeepers should make sure that our national organizations submit comments when EPA asks for input on decisions that may be of benefit to our industry!
Pro: If we were successful with this petition, it might decrease the costs for registering products intended for varroa control. So perhaps worth pursuing.
Cons: It’s unlikely that EPA would even consider granting exemptions for Minor Use Registrations of thymol, formic, or oxalic in generic form. EPA would likely only allow use of registered active ingredients from approved suppliers, new labels, etc.
My assessment: This may be worth a try, but we should check with EPA before pursuing. Again, the gears of EPA turn slowly, so this would not help us in the short term.
Potential Action Item #6: We Petition the EPA to Include Additional Active Ingredients to the “Minimal Risk” List
Specifically: Our industry could petition the EPA to add certain additional generic natural substances to their Minimal Risk list (limited specifically to application to beehives).
In 2021 EPA solicited public comments and suggestions about the petition process for exemptions regarding pesticides from registration and other requirements under FIFRA, where the pesticides are determined to be of a character unnecessary to be subject to regulation under FIFRA. The Agency was considering streamlining the petition process and revisions to how the Agency evaluates the potential minimum risk active and inert substances, factors used in classes of exemptions, state implementation of the minimum risk program and the need for any future exemptions or modifications to current exemptions.
Some 39 comments were submitted, but not one came from the beekeeping industry. We missed our chance to have thymol and formic and oxalic acids added to the Minimal Risk list.
Suggestion: We beekeepers should make sure that our national organizations submit comments when EPA asks for input on decisions that could be of benefit to our industry!
One public comment made the point that:
One of the processes that needs the most improvement is the cost and time burden on the implementation of decisions. Today, the current cost to introduce a new substance [can be] over $900,000 dollars … This is a burden that many businesses simply cannot afford. Second, the complexity of registering pesticidal products is too much for a small business to handle without outside guidance. Overall, [our] suggested action is to streamline the process … if the substance is widely used in the public.
Formic, oxalic, and thymol are all natural components of our food, and in “chemical form” are widely and safely used by the public (especially by beekeepers). Although the EPA “promotes the use of safer pesticides, including biopesticides, as components of IPM programs,” it hasn’t moved very quickly to add additional presumably safe ingredients to its Minimal Risk list; in fact they’ve added only one ingredient (chitosan) since publication of the original list in 1996 (for the mathematically disinclined, that was 27 years ago!).
Similar to how EPA responded to a petition to add chitosan to the Minimal Risk list, our industry could petition EPA to add more active ingredients already in their registry to the Minimal Risk list, including thymol, formic and oxalic acids, and a large number of aromatic plant oils which exhibit varroacidal action. Due to the relatively tiny amounts used by the beekeeping industry, the application of any of the above active ingredients to beehives would not cause any unreasonable risk to man or the environment. Beekeepers in New Zealand have set an example of how it works to allow them the freedom to apply these generic natural chemicals.
Pros: Since varroa is evolving more quickly than new miticidal products are being registered, it would be of great benefit to the United States beekeeping industry for EPA to expand their Minimum Risk Exemption to include formic, oxalic, and lactic acids, thymol, and additional plant oils that have shown promise.
If more ingredients were added to the Minimal Risk list, we could immediately begin experimenting with them, and likely quickly develop new varroa-control methods and formulations. And since beekeepers are already familiar with registered thymol and organic acid products, it would be a huge step forward to allow us to use the off-the-shelf generic ingredients in addition to registered formulations and products.
Pros: The addition of generic thymol, and oxalic and formic acids to the Minimal Risk list would help our industry immensely, since beekeepers could customize application methods for their specific conditions, and lead to the development and marketing of new formulated products.
Cons: It will take the submission of a petition and follow-up. We may need to file toxicology reports relevant to application to beehives. The current Registrants of formulated products may object to their active ingredients being given a “free pass” after spending large sums to get their products registered by EPA. Each state has its own statutes and regulations concerning pesticide registration and regulation, and the states are not required to permit the sale of an exempted product simply because it meets the 40 CFR 152.25(g) conditions for minimum risk exemption. But the biggest drawback would be that it would likely take a long time for EPA to go through the process.
My assessment: It’s a long shot, but perhaps EPA might add these generic substances to the List, perhaps limiting their use to varroa control in beehives. It’s worth formally petitioning them. But it would likely take a long time for them to go through the process, even if they decided to do so.
Potential Action Item #7: Ask our Legislators to Pass an Own Use Exemption Amendment to FIFRA
I’ve saved what I feel is our best option for last — it’s kinda a combination of Actions #5 & #6, but it would bypass the EPA by instead asking Congress to add a tiny amendment to FIFRA in the upcoming Farm Bill.
Here’s the problem: It is completely legal for a beekeeper to put generic oxalic or formic acid, thymol, or plant oils into their hives for the purpose of colony health, bee repellency, or the cleaning of frames or combs. Neither the EPA or FDA are concerned about risk to the environment nor to the honey consumer. But if in their mind the beekeeper is using them for pesticidal purposes, it would be against the law. I underlined the two key words —generic and use. This is not about the sale of pesticides, only with regard to use of off-the-shelf generic natural substances. Our predicament is that this creates a cloudy situation for beekeepers and those responsible for the enforcement of FIFRA.
New Zealand’s Ministry for Primary Industries, understanding this predicament, and realizing that (1) beekeepers needed help (and would likely help themselves anyway), and (2) that these generic natural substances applied to beehives posed no risk to man or the environment, wisely granted beekeepers an Own Use Exemption [[8]] for “compounding of substances for their own use.” To wit: “(1) The exemption applies to a substance or compound prepared by a person for use on animals or plants that they own, or on any land, place or water that they own or occupy. (2) In a beekeeping context, the ‘own use’ exemption is commonly used when a beekeeper prepares and applies preparations containing generic substances, such as oxalic acid or formic acid, to their own hives for control of Varroa mites.” The preparations can only be made by the beekeeper for their own use, and cannot be advertised or sold.
Pesticide regulation is different in the U.S. than in New Zealand, so (in my opinion) our best course of action is to combine the elements of the “Minor Use” and “Minimal Risk” components of FIFRA, and ask our legislators to place an amendment to FIFRA in the upcoming Farm Bill, granting an exemption to regulation for beekeepers applying generic thymol, oxalic, formic, or lactic acids, or food-grade aromatic plant oils to their own hives.
The logic for an exemption comes from the text of FIFRA itself:
7 U.S. Code § 136 – Registration of pesticides (a) Requirement of registration
“Except as provided by this subchapter, no person in any State may distribute or sell to any person any pesticide that is not registered under this subchapter. To the extent necessary to prevent unreasonable adverse effects on the environment, the Administrator may by regulation limit the distribution, sale, or use in any State of any pesticide that is not registered under this subchapter and that is not the subject of an experimental use permit under section 136c of this title or an emergency exemption under section 136p of this title” [highlighting mine].
I boldfaced the key words ― the Administrator may limit the use of a pesticide only if it causes unreasonable adverse effects. Since use of the generic forms of these natural generic substances within the confines of beehives would clearly not result in any unreasonable effects on the environment, it is not necessary for the Administrator to regulate their use (as opposed to registration or sale of formulated products), and such use should be exempt from regulation under FIFRA. We’d simply be asking for our legislators to codify that fact, not with an emergency exemption, but rather with a specific exemption amendment to section 136a.
Our fallback plan: If we can’t get Congress to pass an exemption, we could instead take the EPA to court to see whether we could convince a judge to agree that since the use of these substances by beekeepers poses no risk to the environment, that such use should be exempted by regulation under FIFRA.
As an example, groups supporting the development of plant biostimulants feel that the Farm Bill is “the most logical place for inclusion” of an amendment to FIFRA [[9]].
The advantages of this option are:
- This could be a minor amendment to FIFRA included in the Farm Bill, by simply adding an additional exemption.
- EPA would continue to regulate the sale of any products sold for their pesticidal effects against varroa.
- But beekeepers would be exempted from regulation for the use of (as opposed to the sale of) these specific natural substances.
- It would be limited solely to applications to beehives by the beekeepers themselves.
- Any preparations of the generic substances could only be made by the beekeeper for their own use.
- It would be up to the beekeeper to decide how to choose and use each substance or product (in rotation), following guidance by USDA’s Office of Pest Management Policy or by state agricultural extension.
- No prepared products could be advertised or sold for pesticidal purposes unless they were registered with the EPA.
From a legislative standpoint, this option is not theoretical ― it’s already being successfully used in New Zealand, and could simply be copied! The simple fact is that the use of these generic products in beehives should be exempt from regulation by FIFRA, since oxalic, formic, and thymol pose no unreasonable risk to man or the environment when limited to application to beehives, and all already have exemptions from tolerance in honey.And there is already a place for exemptions in FIFRA.
An Own Use Exemption would, similar to as in New Zealand, allow beekeeping suppliers to stock generic organic acids and thymol, but they could not make any pesticidal claims for them. The suppliers would continue to stock registered products for beekeepers wishing to use conveniently-packaged, tested formulations (such as the currently-registered plastic strips, formic pads, and thymol gel).
Pros: This would be the simplest and most straightforward solution, and allow beekeepers to avoid being pesticide scofflaws. This option would solve our predicament immediately, and allow us to use these generic natural substances without needing to wait for EPA to decide whether to add them to the Minimal Risk list. Adding a simple amendment to FIFRA to the farm bill should be apolitical, and strongly supported since we beekeepers are a very minor industry, but have some political clout due to our importance in crop pollination, plus the honey bee being a poster child for the environment.
Cons: Our legislators would likely ask EPA for their opinion, and if EPA objects strongly, they might be hesitant to pursue it. Thus beekeepers would need to request it even more strongly.
ACTIONS TO TAKE
At the state level
A number of beekeepers have asked me about actions to take at the state level. Unfortunately, states are allowed to impose pesticide regulations more restrictive than the EPA’s, but not less restrictive, so it may be a waste of time to pass pesticide exemptions at the state level, since (as with the case of the 2(ee) exemptions) EPA will likely overrule them.
However, in general, states (through their State Lead Agency) have primary authority for compliance monitoring and enforcement against illegal pesticide use. States can issue bulletins to their inspectors regarding guidance for discretion of enforcement.
At the federal level
Our best course of action would be at the federal level, but we’ll need state legislators to support an amendment to FIFRA in the Farm Bill. Should beekeepers in any state be able to catch the ear of a supportive legislator, they could ask them to contact the agricultural committee staffs of the chairpersons of the House and Senate Agriculture Committees.
A SUGGESTED AMENDMENT
I’ve posted suggested text for an amendment to FIFRA, plus suggested wording for pitching it to our legislators at [[10]].
CITATIONS AND NOTES
[1] https://www.epa.gov/ingredients-used-pesticide-products/biopesticide-active-ingredients
[2] https://www.epa.gov/sites/default/files/2018-01/documents/minrisk-active-ingredients-tolerances-jan-2018.pdf
[3] I haven’t yet read every word, but it would be something to do if you were ever spending a few years in prison due to an enforcement action.
[4] https://www.cdpr.ca.gov/docs/registration/manual/guidance.pdf
[5] https://uscode.house.gov/view.xhtml?req=granuleid:USC-2000-title7-section7653&num=0&edition=2000
[6] Bahreini, R, et al (2020). Evaluation of potential miticide toxicity to Varroa destructor and honey bees, Apis mellifera, under laboratory conditions. Scientific Reports 10(1) 21529.
[7] https://downloads.regulations.gov/EPA-HQ-OPP-2015-0814-0016/content.pdf
[8] https://www.mpi.govt.nz/dmsdocument/37901-Advertising-and-own-use-guidance-for-compounds-for-management-of-disease-in-beehives-Guidance
[9] Farm bill could include FIFRA exemption effort for plant biostimulants
The text of their bill is available at To amend the Federal Insecticide, Fungicide, and Rodenticide Act to provide for a consistent definition for plant biostimulants.
[10] https://scientificbeekeeping.com/suggested-wording-and-pitch-for-an-own-use-exemption/
A Study on Bee Drift and Mite Immigration
Part 6
Randy Oliver
ScientificBeekeeping.com
First published in ABJ July 2023
In our 2018 study in the California foothills, we confirmed that bees indeed drift from collapsing colonies to other hives, even to those at considerable distance. And that drifting can result in a substantial amount of mite immigration into those hives. But there’s surprisingly little hard data on how much mite immigration takes place in other settings.
It’s easy for beekeepers to blame rapidly-increasing mite wash counts in late summer or autumn on immigration of varroa due to drifting or robbing. But other than in the case of the robbing of a high-mite colony (which could result in mites catching a ride back on a robber), the normal hive-to-hive drifting of bees would be expected to have an overall effect of averaging the infestation levels of all colonies within flight range, rather than concentrating mites into only the well-managed hives. Although some colonies appear to be more attractive to drifting bees than others, the overall resulting mite drift would likely reflect the process of diffusion ― “the net movement of anything generally from a region of higher concentration to a region of lower concentration.”
The data from my home apiary that I showed in Part 4 of this series indicated an average immigration of only 245 mites per hive over a two-month period, despite having nine colonies collapsing from varroa in the same yard).
Practical application: That amount of immigration would barely be noticeable in a mite wash, since in a colony of 40,000 adult bees, assuming that half the immigrated mites wound up in the brood, those 245 mites, even if they had immigrated all at once, would have only increased the number of mites in a sample of a half cup of bees by a count of 1. So much for a “mite bombing” having taken place.
So although I’m suspecting that my sons and I can’t blame our August varroa spikes on bee drift, could it be plausible elsewhere? Something that really surprises me is the paucity of studies to quantify the actual amount of mite immigration typically taking place in different regions of the U.S. So in 2019 I put out a call for Citizen Science volunteers to collect mite immigration counts. I was disappointed that surprisingly few beekeepers responded. But those who did provided us with some valuable hard data ― thank you!
THE PROJECT
I provided the volunteers a protocol to follow [[1]], and my helper Brooke and I sent them packages of miticide strips and instructions, along with sample data sheets. As is usual with beekeepers unaccustomed to the amount of work involved in the setting up a field trial and collecting data, only a few were able to complete the necessary preparation and work. But I did receive good data from a range of localities.
VALIDATING THE METHODOLOGY
In order to quantify mite immigration, the protocol for this study involved proactively eliminating the reproducing varroa population from the hives — starting at least six weeks before data collection. This is done by simultaneously treating with Apivar and Apistan strips, which have two different modes of action (I’m currently also adding OAE sponges), and perhaps brood removal. As I pointed out in Part 4 of this series, one must then validate that the colonies to be monitored are actually free of mites. Such validation is not necessarily as easily confirmed as you might think!
The easiest and best way to confirm that a colony is free of mites is to monitor the sticky boards, but on days in which bees are flying, you can’t tell whether the dropped mites came from outside or inside of the hive. So for confirmation you need to check mite drop counts on rainy or cold days which prevent bee flight, thus eliminating the chance for mite immigration via drifting or robbing bees (as shown in Figure 8 in Part 4). But where I live, it doesn’t normally rain or get cold in the August-October time period during which we are concerned about mite immigration.
Another validation is that the mite counts drop to zero in November, which would certainly suggest that the hive is free of mites at that time, but doesn’t necessarily mean that the colony started free of mites at the beginning of data collection (since the extended-release miticides may cause a residual breeding population of mites in the colony at the start of monitoring to dwindle over time).
It occurred to me as I was writing this article that I should compare our mite drop counts to those of untreated Control hives in the same yard. Lacking those, I spent a couple of hours searching the internet for typical mite drop curves over the course of a season — I was surprised at how little data is actually published! (Please let me know if you know of any good data sets of natural mite falls in untreated hives over the course of a season).
One could perform sealed brood dissection, but that is tedious, and takes some practice, good lighting, and magnification.
So why not simple confirmation by a mite wash of a half cup of bees? To my surprise, a treated colony may exhibit mite wash counts of zero, but still be apparently producing substantial quantities of mites on its own (I’ll show an example next month). I highlighted “apparently” since out of a group of treated hives in the same apiary, one may continue to exhibit far higher daily (and consistent) mite drop counts than the rest — despite showing mite wash counts of zero day after day ― strongly suggesting that the colony is producing its own mites. One thing that I’ve noticed is that the location of the dropped mites on the sticky board may be an important clue (Figures 1 & 2).

Fig. 1 In this photo of a sticky board, I placed pieces of grass stem pointing to each mite on the board. What we observe is that in a mite-free colony, mites that have come in via immigration (and are then rapidly killed by the miticides) tend to fall outside of the area directly beneath the broodnest, which is indicated by the rows of uncapping debris that fell between the brood frames.

Fig. 2 On the other hand, on this sticky board the fallen mites are all directly beneath the brood area, leading me to suspect that mite reproduction was taking place in the hive.
I am greatly appreciative of the efforts put in by the volunteers involved with this study, and most of the data sent to me indicated that the monitored hives were indeed mite free (or nearly so). I censored the few mite count records that suggested that a colony was producing its own mites.
THE RESULTS
Let’s first take a look at the mite counts by Dr. Cahit Ozturk from the hot, dry, and sparse landscape of the Arizona State University Bee Lab (Figure 3).
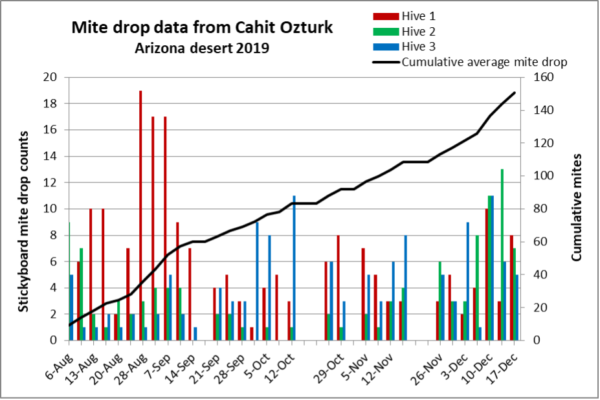
Fig. 3 Similar to my observations, there was large colony-to-colony variation in mite drops in Dr. Ozturk’s hives, as well as different curves for drop intensity. I checked the weather history for the time period, which indicated that temperatures were plenty warm for bee flight in November and December, but there was no precipitation. The average cumulative drop per hive was 151 mites — not a lot, but nearly continuous.
I asked Dr. Ozturk about the likely sources of the drifted mites; the university maintains around 100 European (and some captured feral) colonies under misted shade structures nearby. Now let’s move from dry Arizona to the moist coast of Maryland (Figure 4).
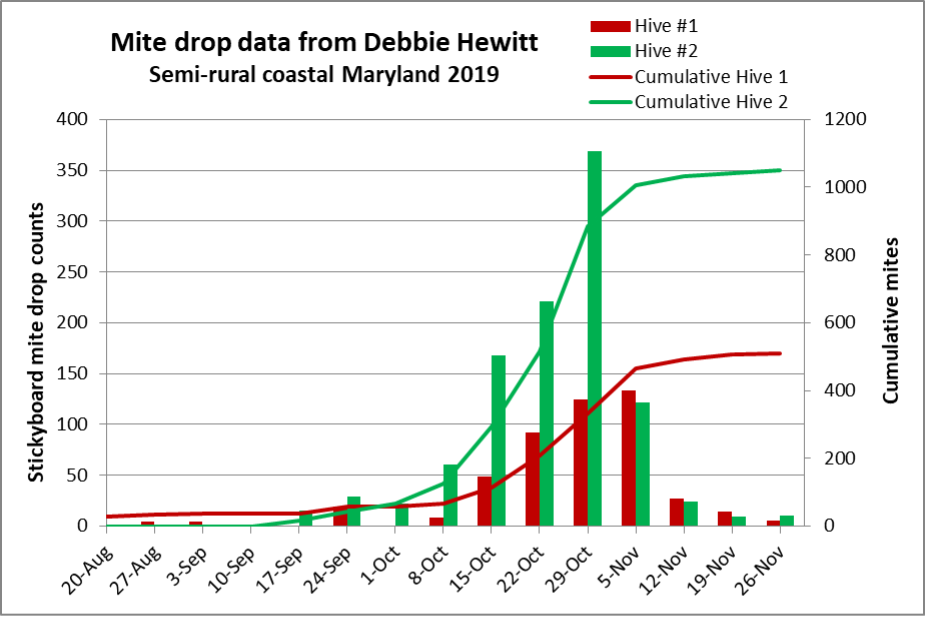
Fig. 4 Debbie Hewitt’s drop counts in moist, green coastal Maryland suggest that her hives were indeed mite-free prior to late-season immigration. One hive clearly took in more mites, so I broke down the cumulative count curves. Hive #1 got hammered with 1050 mites; Hive #2 with a bit over 500.
Practical application: In this case, Debbie could indeed say that her Hive #1 got mite bombed. A colony can generally tolerate a total mite population of 1000 going into winter. In Debbie’s location, it appears that even if her varroa management program involves a pre-winter treatment in early October, that a colony could get reinfested via mite immigration to a damaging level by the end of November. This is a perfect illustration of why ― once a colony goes broodless — the application of an early-winter treatment can be so important.
And now let’s move up to New York (Figure 5).

Fig. 5 Elwin Stillman monitored a single hive, starting with mite drop counts of zero until the end of June (great prep Elwin!). His mite immigration curve peaked earlier than did Debbie’s, as we would expect from his higher latitude. Although his hive clearly took in mites, it was not enough to make a big difference for varroa management.
There was another volunteer not far away in New York (Figure 6).

Fig. 6 Kyle Mulligan’s hives were not far from Elwin’s, and his counts also appear to reflect only immigration. Although the counts for the two hives did not peak at the same time, they both received nearly the same cumulative totals approaching 500 — maybe not enough to say that they were “bombed,” but clearly enough to indicate that he should include a “clean-up” treatment going into winter.
Let’s now return to California (Figures 7 & 8).
Fig. 7 I’ve visited Hal Liske’s well-managed apiary at his wonderful El Sol winery, and wouldn’t expect there to be much mite drift pressure.
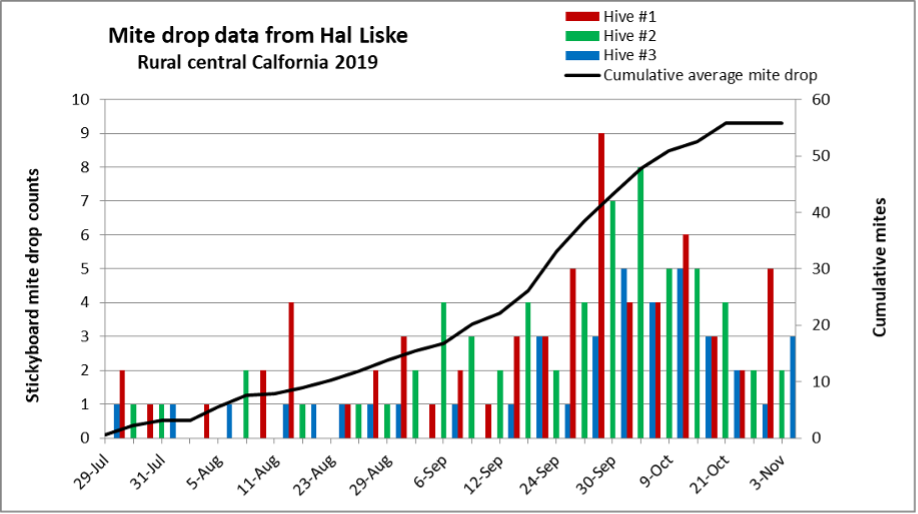
Fig. 8 Hal’s three monitored hives exhibited remarkably similar mite counts, and as expected, not much mite immigration.
Farther south of Hal is my longtime supporter Madeleine Mead (thanks Madeleine!), whose apiary is in a hot, arid, and sparse landscape with likely few surrounding colonies (Figure 9).
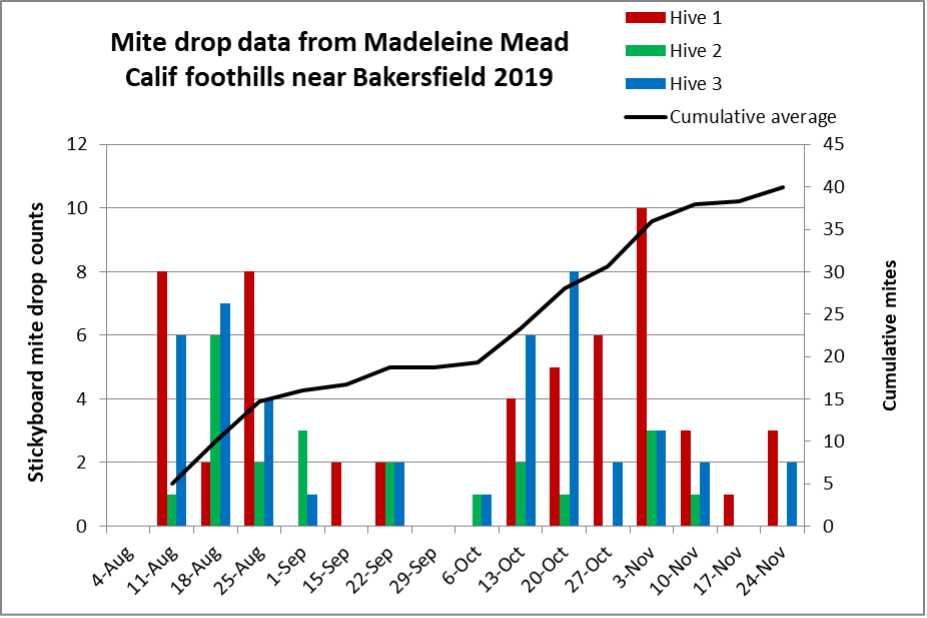
Fig. 9 Very few mites caught rides into Madeleine’s hives (lack of a color column indicates a zero count). I was curious about the near cessation of mite immigration in late September, so checked her weather history. The daily high dropped from 100°F to only 70, but there was no rain or wind, so I can’t explain. Mite immigration went back up during her warm October and immigration tapered off as the weather finally cooled at the end of November.
Practical application: A typical colony may emigrate far more mites every day on its own exiting workers who don’t return, than it receives from bees drifting in from other colonies.. Let’s do the math: From a colony in September, there are about 1000 bees a day flying off to die of old age. At a low mite infestation rate of even 1%, that would be 10 mites per day being flown out of the hive never to return. On only one day did any of Madeleine’s hives reach 10 incoming mites. That small amount of mite immigration would not be expected to tip the balance to any great extent.
DISCUSSION
Thank you volunteers ― the data that you collected was very informative!
The amount of mite immigration into the monitored hives in rural areas of the West was hardly enough to support the mite bomb hypothesis.
On the other hand, Debbie and Kyle’s apiaries on the East Coast, in areas in which I’d expect a lot of beekeepers and perhaps an abundance of feral colonies, not surprisingly exhibited far higher amounts of mite immigration, and they could legitimately say that they got bombed by mites (although it’s not clear as to whether any single colony was the bomb). Although I haven’t seen hard data, beekeepers in other urban areas where there are a bunch of beekeepers who don’t do a good job of managing varroa, tell me that mite immigration is a major issue for them late in the season.
Practical application: What with all the talk and blame among beekeepers about mite immigration into their hives, we could sure use more hard data such as that above for every region in the country. Let me know if you want to collect some!
Next month I’ll show the results of two experiments that we ran last year to test the effect of entrance guards on reducing mite immigration.
ACKNOWLEDGEMENTS
My great appreciation to my helper Brooke Molina, and volunteers Cahit Ozturk, Elwin Stillman, Hal Liske, Kyle Mulligan, Debbie Hewitt, Madeleine Mead, and Dave Munkvold.
CITATIONS AND NOTES
[1] https://scientificbeekeeping.com/mite-drift-quantification/
Tips for Using the XXX cup for Monitoring Varroa
TAKING THE SAMPLE
- Use an 18-quart dishwashing tub and a stainless-steel half cup (125 mL) measuring cup (for measuring live bees, a deep cup is more accurate than a shallow cup).
- Take the bee sample from a frame (or frames) adjacent to the broodnest, but not containing open brood — this will greatly reduce the chance of the queen being on the frame (check for her), and the sample will be most representative of the colony as a whole.
- Use a snap shake or brush to dislodge the bees from the frame into the tub (young bees hang on tighter than do old bees).
- Wait about 30 seconds for the older bees to fly off, shaking the tub, if necessary, to distribute the bees evenly. If more than a cup of bees remain in the tub, scoop out the excess so that you can more easily spot an inadvertently-shaken queen (the young bees will generally spread out evenly over the tub).
- Tilt and tap the tub firmly to shake the bees into a pile along one edge, and tip/scoop your half-cup sample of them into the cup. For the most accurate reading, use your finger to level them off before dumping them into the wash cup (young bees don’t sting).
PERFORMING THE WASH TO SEPARATE THE MITES
We don’t like to kill a bee any more than you do, but feel that any colony would gladly sacrifice a handful of workers in order to prevent facing an ugly death due to a varroa/virus overload. Our favorite agitation solution to use for separating the mites from the bees is Dawn Ultra detergent (2 tsp/quart water), but the XXX cup will also work with alcohol (use 90+% for highest mite recovery), or powdered sugar for dry shaking (this does not kill the bees, but produces inconsistent recovery).
For either Dawn or alcohol, first fill the cup to the line with solution, dump in the bee sample and immediately snap on the lid. Allow one minute for the mites to release from the bees, then gently swirl (don’t shake) the cup for a minute to precipitate the mites down through the bee bodies. Swirl just strongly enough so that all the bee bodies are in motion – vigorous agitation is counterproductive, since it keeps the mites stirred back up in the bees!
For a sugar shake, first place a tablespoon of powdered sugar into the cup, add the bees, snap on the lid, and then invert the cup back and forth until the bees are evenly coated. Wait a minute, then holding the cups upside down vigorously shake them up and down for a minute. Then pull off the outer cup and invert the inner cup over a white tub of water, and continue to shake it and the bees over the water until no more sugar (and mites) fall. The sugar will quickly dissolve into the water, leaving the mites temporarily floating on the surface for counting. The shaken bees can be returned to their hive.
DETERMINING THE MITE COUNT AFTER A WASH
Once you have separated the mites from the bees, remove the inner cup and discard the bees (compost them on the ground or save them for nosema microscopy). Then put on your reading glasses and lift the cup to view the bottom to count the mites. Better yet, hold the cup 4 inches above a 10x magnifying mirror to enlarge the mites for easy identification. This will be your “mite count” (you can divide the count by 3 to approximate the “percentage mite infestation rate if you wish).
TREATMENT THRESHOLDS FOR COLONY HEALTH AND SURVIVAL
So long as a colony’s mite count is below 2, varroa and its associated viruses have little impact upon colony health. Viruses begin to become a problem at counts above 6 mites, and colony performance declines noticeably once the count exceeds 15. A count above 40 will likely result in imminent colony collapse.
The key concept is to be proactive and stay ahead of the exponential rate of mite reproduction — the higher the mite count, the faster the rate of increase! When colonies are rearing brood, expect the mite count to double each month. Don’t be fooled by low counts in April – at that time most of the mites are hidden in the brood, and won’t show up in a mite wash!
To maintain optimal colony health, your treatment threshold counts would be:
January – April: zero-2 mites.
June-July: no more than 6 mites.
Mid-August: zero out the mites in preparation for their critical autumn brood rearing.
October – November: colonies may survive if they enter the winter in November with a count of up to 10 mites, but they’ll have a better chance if you get the count down to the zero-2 range (an oxalic acid or Hopguard treatment once they go broodless really helps).
Depending upon how many months the bees engage in brood rearing in your area, it will take one to three 95% reductions of the mite population (by whatever means) over the course of the season to maintain the above low levels of infestation. Your bees will thank you for helping them!
Updated 8 Jan 2023. I continually update this page, so please refer to the current version. For mite treatment options, search “Varroa Mite Management”
Beekeeping in a Nutshell
I’ve attempted to distill 50 year’s of beekeeping experience into a short set of instructions for starting out with bees in the Sierra Foothills. This page provides some quick step-by-step notes for your first year of beekeeping, written specifically for those starting with a nucleus hive or package bees purchased from me, but is generally applicable.
Bottom line: Once you get a colony started, other than adding space, the two most important things to pay attention to are varroa management and colony nutrition. If you do this, most other problems go away.
Below is a brief visual of hive management responsibilities in the Northern California Foothills. If you live somewhere else, the timing of the honey flow and length of winter may differ. I’ve written a step-by-step plan for colony management further down on this page.
Starting Out
First, educate yourself! A honey bee colony is a living animal that deserves to be cared for properly. You are beginning beekeeping at a time in which honey bees are struggling to stay alive–beekeeping is more difficult than it was prior to the parasitic varroa mite (which invaded around 1990). Although honey bees are essentially wild animals living in a box provided by their “keeper,” bees are in the midst of an evolutionary struggle due to the introduction of varroa (which completely changed colony stress and virus dynamics). In addition, the bee population now faces the additional novel stressors of persistent European foulbrood, loss of forage in many areas, the effects of climate change (shorter winters), increasing CO2 (which decreases the nutritional value of pollen), and perhaps pesticide exposure (this fear is greatly exaggerated as far as most areas are concerned, but may occur in areas with intensive commercial agriculture).
Other than varroa, however, basic beekeeping practices were well figured out long ago, and I highly recommend two classic USDA publications for best management practices. Be aware that both of these publications were for the North, and would need to be adjusted considerably for the West and southerly areas, but the main principles still apply. Farrar’s Productive Management of Honey Bee Colonies is a great read for understanding the winter cluster and swarm prevention; Beekeeping in the United States covers all regions, and has a great section by Moeller entitled Managing Colonies for High Honey Yields. I’ve linked these articles at Reading Materials.
It is more difficult to keep healthy bees than it is to care for most common pets (for which you generally need only to provide food). The more you understand the biology of colony health and dynamics, the more successful you can be at beekeeping. Bees see and respond to the world (environmental cues) very differently than do humans. In order to be a better beekeeper, I suggest that you try to learn to see the world through the eyes (and antennae) of the bee, and to “think” as does the honey bee superorganism.
What is your motivation?
Please allow me to be frank. If your motivation is to “save the bees,” please realize that the honey bee is in absolutely no danger of extinction. Caring for a hive of bees requires several hours of husbandry a year, involving opening and inspecting the frames inside, and getting stung. Keeping your bees alive and healthy these days requires management for the varroa mite–most beginners fail at this, and their colonies die an ugly death.
The number one feedback that I get from first-year beekeepers is that they did not realize how important it was to monitor and control varroa in their hive — most beginners lose their first hive to the mite.
So if your motivation is to help the bees, unless you’re willing to provide a hive with good husbandry, you’d do more service to bees, other pollinators, and the environment by planting flowers and flowering shrubs and trees, supporting small farmers, buying local produce, minimizing your carbon footprint, and telling your representatives to support the EPA.
On the other hand, the keeping of honey bees offers one a “tangible connection to the wild.” If you’re willing to make the effort, the honey bee can help to connect you to Nature, and to the joy of experiencing how this fascinating social insect manages to eke out a living.
“Treatment-Free” beekeeping
I kept bees “treatment-free” for two decades prior to the arrival of varroa, and my goal is to be able to do so again in my lifetime. Towards that end, I am a huge proponent for breeding bees for the traits involved in natural resistance to varroa, and am seriously involved in my own breeding program. That said, most lines of honey bees today will succumb to the varroa/virus complex within a year or two of starting the hive, unless realistic measures are taken by the beekeeper to reduce the mite population. Please read my article “The Varroa Problem Part 6b–Small-Scale Breeding,” in which I discuss some of the misconceptions often advocated by well-intentioned (but biologically misinformed) “treatment-free” promoters.
If you have an isolated apiary, or are fortunate enough to get a queen with the right genetics, your colony may survive without treatment. Sadly, this generally does not happen, and most beginning beekeepers lose their colonies to varroa. This is completely avoidable, and can be prevented by regular monitoring of your hive for its degree of varroa infestation with alcohol washes (my preferred method) or a well-done sugar shake. If thus indictated, there are a number of safe and effective (and organically-approved) products that can be used to reduce the mite population (detailed further down).
We love our bees, and hate to send our beautiful nucs off to certain death due to beekeeper negligence. Please, monitor your colonies for their varroa level from late June on, and help them to survive! Please be a responsible beekeeper–colonies that are allowed to die from varroa hurt the surrounding beekeeping community.
Books
There are several good beginners books.
First Lessons in Beekeeping, Dadant. Standard beginner reference.
The Beekeeper’s Handbook by Diana Sammataro & Alfonse Avitabile. Many recommend this one.
Storey’s Guide to Beekeeping. Easy reading style, straightforward information.
Beekeeping for Dummies. I haven’t yet read, but several beginners have recommended.
Honey Bee Hobbyist by Norm Gary—good overall understanding, rather than how-to.
Homegrown Honey Bees by Alethea Morrison. A fun journal of the experiences of a first-year beekeeper. The author ran it by me for accuracy prior to publication, so good info.
A Book of Bees, Sue Hubble–beautiful prose about being a beekeeper
And of course, ScientificBeekeeping.com. Although I have yet to summarize my many articles into a book, I suggest that you familiarize yourself with bees, starting with how the colony manages its labor pool, feeds itself, varroa management, The Rules of Beekeeping, and for a deeper understanding of the biology of the hive over the course of the year, my Colony Buildup and Decline series.
Find a Mentor
Beekeeping is difficult to learn from a book, and the proper handling of bees to avoid excess stinging even more difficult. The best thing to do is to get over the innate human fear of bees and of being stung. Bees sting. You want to keep bees. You’re gonna get stung, so get used to it! Experienced beekeepers generally feel that a regular dose of bee venom improves their health and well being.
HIVE INSPECTION
I strongly suggest that you find a mentor who can demonstrate handling technique. Find a mentor who does not normally wear gloves or much protective gear. You want to learn to approach your bees in a cooperative mindset, rather than an adversarial approach. If you learn while fully protected with gloves, you will get bad habits, as it prevents the bees from giving you “reminders” that your technique is sloppy or disrespectful to the hive.
Hive inspection on sunny days should involve minimal stingings, but I’d shoot for several dozen stings a season in order to avoid sensitizing yourself to bee venom (beekeepers who receive only a few stings a season set themselves up for true bee sting allergy late on). Learn to use smoke gently but appropriately. Move as though you are practicing Tai Chi–no sudden movements. The point is to avoid going past the bees’ threat response threshold. At that point they will start giving you “warning bumps” or stings. Learn to recognize those warnings.
Equipment
You’ll need a veil — defensive bees aim for the top of the intruder. The most painful places to be stung are all above your shoulders. Learn to work bees barehanded, but wear a veil until you no longer react to bee stings.

Image is important — the well-attired beekeeper looks sharp! Bow tie is optional.
Drawing from the book Beekeeping, by E.F. Phillips 1921.
I suggest a lightweight hooded jacket (we haven’t tested them all, but like the (widely-available) Eco-keeper hooded bee jacket (shown below). FWIW, I have no interested in promoting any products, but am happy to tell you which products we use in our own beekeeping business of 2000 hives.

A smoker — As far as smokers, I personally prefer dome-top rather than cone-top (funnel-shaped) smokers, in the 4″ x 7″ size (4 x 10 is too large and awkward). Dome top smokers better direct the smoke and don’t burn through the fuel as rapidly as do cone top). I like a stainless steel smoker, with a protective basket around it, with a square non-plastic bellows that is roughly level with the bottom of the smoker, so that it doesn’t tend to tip over (similar to that shown below).

Note: I do not recommend the stainless smokers with full inside insert, such as shown below–they do not work well! You want one with the perforated bottom plate with three legs. Check for what kind of insert is inside before ordering!

The hive tool — the smoker and hive tool are the only tools that I generally carry (plus a cigarette lighter to light the smoker). I prefer the curved-end hive tools as shown (rather than flat hook-end tools)–you can do everything with them.
 I greatly prefer tools with a 1/2″ offset rather than the more common 3/4″ curve, and I keep that edge sharp for scraping off propolis. I hold my hive tool to use the curved end for prying apart frames, since it gives you more leverage and control. I especially like the Jero brand 7-1/2″ hive tool for summer work when you don’t need a lot of leverage to break the propolis; I use a 10″ tool when it’s cooler. Caution–Jero’s come really sharp, so first rub the flat end against a rock; leave the curved end sharp.
I greatly prefer tools with a 1/2″ offset rather than the more common 3/4″ curve, and I keep that edge sharp for scraping off propolis. I hold my hive tool to use the curved end for prying apart frames, since it gives you more leverage and control. I especially like the Jero brand 7-1/2″ hive tool for summer work when you don’t need a lot of leverage to break the propolis; I use a 10″ tool when it’s cooler. Caution–Jero’s come really sharp, so first rub the flat end against a rock; leave the curved end sharp.
Gloves — best to learn without them, but I recommend vinyl (details below) or goatskin. Use Vaseline to keep leather gloves flexible–it’s better than any fancy treatment on the market, and doesn’t add odor.
You will be a far better beekeeper as soon as you learn to work without gloves, but I suggest that you learn to do so by working small colonies under perfect conditions. Under other circumstances try using 5 mil white nitrile, long-cuff gloves.

I’ve been happy with the two brands above. Each feels a bit different, but I can’t say that I prefer one over the other. These gloves prevent most stings, are long enough to tuck under the cuff of your jacket (I recommend a hooded jacket), and are remarkably durable (I’ve gotten 4 full days of hard work out of some pairs).

We really like these gloves when we are working bees in cold or rainy weather (we rarely wear gloves during warm weather). I put on a glove during warm weather for the photo above–yes, they get a bit sweaty, but your dexterity is so much better than with leather gloves. If you wish to wear leather gloves, get snug-fitting goatskin gloves.
You do not want to avoid stings–that will only predispose you to eventual bee sting allergy. Working with gloves allows you to get into bad habits–when gloveless, the bees will patiently remind you each time you make a mistake. If you’re getting stung, you’re doing something wrong (this does not apply so much with Africanized bees). It may be hard for beginners to believe, but once your immune system gets used to regular stinging, your body misses it when it doesn’t get its regular doses of bee venom.
Handling Tips:
Smoking the Hive
Forget about going “smoke free”–proper, light use of smoke is the beekeeper’s best tool. You only need to use a little, but apply that little bit immediately upon opening any part of the hive–timing is everything!
You only need to smoke the guard bees–bees in the middle of the cluster rarely sting–apply gentle smoke to those on the periphery. Look at their “faces”–if they are looking at you, then they are aware of you! A little bit of smoke goes a long way — don’t over smoke your colony — use only enough smoke to get the bees off the top bars, and so that none are looking at you.

Never reach for a frame if there are bees looking at you. Notice the bees in the photo above that are looking at you. They are guard bees, and since they’re looking at you, they are aware of you. Give them a gentle puff of smoke to turn them away from you. Only when you don’t see any faces looking at you should you reach towards a frame. Keep an eye on the bees, and when they start to look at you again, give another gentle puff of smoke.
Make sure that your smoke comes out cool, WHITE, and DENSE. Bees do not respond to weak or grey smoke like they do to dense white smoke. Many beekeepers use burlap, but I dislike the smell. Many natural materials work well–ask a local beekeeper. My favorites are pine needles fluffed by car tires on an asphalt road after the first autumn rainstorm, punky oak wood, eucalyptus bark, oak leaves, or wood pellets (harder to light, but burn for a long time).
Working the hive
Beginners should always wear a veil! (Thanks to beekeeper Trish Harness for this suggestion). The most painful places to be stung are all on your head, and that’s exactly where defending bees aim! Until you’ve build up “immunity” to stings, always wear a veil.
Approach bees with respect, but get over your fear of them as quickly as possible. Stings are the bees’ way of telling you that you have made them think that they need to defend their colony–once you learn not to give them reason, you can make it look like magic.
In my beginners demonstrations, I get people over their initial fear by my example of wearing only shorts and a tee shirt, opening a hive,, and then shaking a tub full of bees from the comb, then ladling scoops of bees with my bare hand into their bare hands–no one ever gets stung. After this demonstration, everyone is far more relaxed around the bees. But I know exactly what I’m doing.
And I’ve been stung so many times that I no longer swell up or itch after a sting (OK, slight temporary swelling when stung on the lip or eyelid). One gets used to stings to the hands and elsewhere, but the ones to the face still hurt like hell for a few minutes. So although you will see many experienced beekeepers working without veils (because it’s so much easier to see the queen, eggs, etc.), I strongly suggest that beginners always wear a veil until they no longer swell in response to stings. And make sure that any visitors wear a veil. Practical tip: put on your veil before you enter the apiary, and don’t remove it until you are out of sight of the hives (this is when many get stung to the face). We really like the hooded jackets that unzip from the front and allow us to flip the hood forward or backward.

Typical bee working for my sons and I (we’re in an almond orchard, performing alcohol washes for varroa). Note that when it’s warm, my sons typically throw a hooded veil over their heads, unzipped from the jacket.
Move smoothly–like you’re doing Tai Chi. Bees only sting when they feel that you are threatening their hive. So don’t do anything threatening! Fast or jerky movements appear threatening. Always use smoke, but use it sparingly. The only bees that will generally sting are the guard bees on the periphery of the cluster–especially at the entrance and at the top bars. Bees that are not looking at you aren’t interested in you. If you see bees looking at you, give them a little puff of smoke and wait until they turn away from you. It is only safe to pick up a frame if there are no bees looking at you.
Bees will often fly at your hands or face and give “warning bumps” prior to actual stinging. Pay attention to what they are telling you–BACK OFF! They either don’t want to be disturbed at that moment, or you have not used enough smoke, or you’ve been too rough.
If there are bees looking at you, warning bumps, stinging, or the smell of alarm pheromone, STOP WHAT YOU ARE DOING! Do not keep going, or things will quickly get worse–you don’t want to go there! Give the bees a chance to calm down, and for alarm pheromone to dissipate. Give the top bars a light puff or two of smoke until there are no bees facing you. If you can’t get them to calm down, then just close the hive up for the day.
The best way to learn how to work gently with bees is to carefully watch an experienced beekeeper who doesn’t usually wear gloves (or veil). Such a beekeeper has learned how to work bees with care and respect. Those who always wear gloves and full gear often have very bad habits. I strongly suggest that you learn to work bees barehanded in good weather–thin latex or nitrile gloves are a good way to eliminate most stings, but still get a “feel” for the bees.
The Rules
Q: What “should I do?”
A: There are no “shoulds” in beekeeping other than practicing good animal husbandry. Bees need only a dry cavity, food (nectar, pollen, or syrup and pollen supplement if lacking from natural sources), and parasite management. My basic rule is to not do anything unless you completely understand the reason that you are doing it!
You will find on the internet a plethora of opinions (often strongly expressed) on the “right way” to keep bees. In general, the more emphatic the author, the less I’d trust the information. For a review of the rules according to the bees, see The Rules and The Rules Redux.

My advice–concentrate on (1) varroa management and (2) nutrition (especially in the late summer), and do so proactively–before mites or poor nutrition have seriously stressed the colony.
Getting Started
I strongly suggest that you start with two deep Langstroth brood chambers (to establish the brood chamber), and later “medium” honey supers over a queen excluder (lots of reasons). Such equipment has truly stood the test of time (since the mid 1800’s) and is by far the easiest way to learn to keep bees. You can try other systems (top bar, Warre, etc.) later, after you’ve experienced success with “standard” equipment. If weight (of the boxes) is an issue for you, I suggest using 8-frame Langstroth equipment (same as above, but a narrower, lighter box), rather than using medium supers for the brood chamber.
Location
Place your hives in a sunny location with morning sun, so that they can get an early start at foraging in the morning. If you live in an especially hot area, they will benefit from afternoon shade. Hives will do OK in the shade, but are much more “pissy” to work. Place the hives in a dry area with good air drainage. The best hive stands are two 8″x8″x16″ cinder blocks, placed flat sides down, with the hive tipped slightly forward for drainage. Do not place more than one hive on a stand, as vibrations from working will transmit via the stand to the other hives. If you have neighbors, place a fish pond with aquatic plants and floating wood as a water source for your bees (so that the bees won’t cause problems with your neighbors).
If you live in an area with bears, you MUST protect the hive. Plans for an inexpensive bear fence are here.
The Plan — Step by step
A nuc or package comes with a vigorous young queen and a cluster of bees. A nuc, due to already having brood, will grow much more quickly. If you are a first-time beekeeper, I suggest that you request a weak nuc, so that you have more time to learn before it grows into a large and more defensive colony.
Below is an outline of your management plan. Further down are more details.
First-year steps:
Establishment
- Install the nuc/package into a brood chamber. Add drawn comb if you have it, otherwise frames of foundation (wax-coated plastic foundation is by far the easiest to use). Foundation is not necessary, but it makes beekeeping MUCH easier. Place 10 frames total in the hive body, all always squeezed tightly together.
- Unless there is a strong nectar flow on, feed the colony as much 1:1 sugar syrup as they will take (at least a half gallon a day if on foundation). This will allow the colony to produce the beeswax necessary to draw comb. A strong nuc lacking honey stores can starve in a day — make sure that they have a comb of honey when installed, or feed at least a gallon of sugar syrup immediately.
- Continue feeding until all 10 combs are drawn, and the outer two combs are filled with “honey.” Only then will you add the second brood chamber (by this time all 10 frames will typically also be covered with bees).
Rate of colony growth: A strong nuc full of sealed brood can grow explosively! It can fill 1o frames in less than a week. That’s why I suggest that first-timers ask for a weak nuc — so that they can enjoy opening it up a few times a week and watching it grow.
One problem for beginners is that a new hive is much easier to manage on already “drawn” comb than if you are starting with foundation. A strong nuc, if not fed enough syrup to draw comb, may feel “crowded” and swarm! Feed the colony enough so that they are producing “white wax” and drawing comb continuously.
Note: it is generally not necessary to feed colonies sugar syrup. On the other hand, bees LOVE sugar syrup, and it is one of our best management tools. If there is no natural nectar flow occurring, the feeding of syrup will allow a colony to continue growing and drawing foundation, or to put on winter stores. The more hives in the neighborhood, the more likely that there will be nectar dearths, since small growing colonies cannot compete against large established colonies.
Adding the second brood chamber
- Add the second brood chamber — with 10 frames of foundation or drawn comb, all squeezed together. In general, you will get straighter combs if you don’t mix frames of foundation with frames of drawn comb.
- At this point you can decide whether to continue feeding syrup, or if the honey flow has begun, to allow the bees to store pure honey. If you choose the latter, you can later extract some of the honey, and then feed syrup to replace it for winter stores.
Supering for honey
- In general in the West, a nuc or package won’t grow larger than two deeps high in the first year. But if it does, you can place a queen excluder over it (I like queen excluders), and then place a medium super on top for honey collection. Tip: bees are loathe to draw foundation above a queen excluder if there is a band of honey in the combs immediately below the excluder. If this is the case, rotate some brood combs from the lower brood chamber into the middle of the upper brood chamber, so that brood is right up against the excluder. This will encourage the bees to pass through the excluder and draw comb. Important note: bees will not draw foundation until every drawn cell in the boxes below is filled with nectar! There are no tricks to induce them to draw foundation otherwise.
June/July management
- Starting in June, perform alcohol wash or sugar roll to monitor the mite level. Treat if more than 6 mites per half cup of bees.
- Your first-year goal is to build the colony to a double-deep brood chamber, with the upper box mostly filled with honey to provide winter stores.
August management
- Control varroa! The honey flow is typically over, unless you are surrounded by star thistle, or incense cedar.
September/October management
- While the weather is still warm, make sure the colony has enough honey stores for winter. The upper brood chamber should be largely full of honey, with the broodnest all in the lower brood chamber. If not, start feeding heavy syrup. Unless there is plenty of pollen coming in, we also will feed high-quality pollen sub (2.5 pounds every 10 days) to allow the bees to brood up heavily. It is the bees that emerge from brood reared in October that will form the winter cluster.
Note: if you live in the Bay Area, you may get a large eucalypt honey flow in November. Talk to local beekeepers for advice.
December management:
- Varroa: once most of the brood has emerged, apply an oxalic acid dribble or vaporization so that the colony goes into winter with a minimal mite load.
More detailed management tips
Installing the bees
Congratulations, you’ve just purchased either a quality nucleus colony (“nuc”) or a shook swarm (“package bees”) with a newly-mated queen from selected stock!
Warning: A strong nuc is full of brood and ready to grow explosively! You may need to transfer your nuc into a larger hive within a day or so, or they may swarm. And unless they have at least half a frame of honey stored, they may starve if not given some sugar syrup.
Some nucs (and all packages) have the queen caged. If so, remove the candy cap when you get home to allow the bees to chew through the candy and release the queen. But do NOT remove the candy cap if the bees are tightly balled over the cage, indicating that they have not yet accepted the queen–wait another day until they are walking lightly over the cage.
Install the nuc in the center of a brood box with additional frames to fill the box. Keep the frames of the nuc in their original order. Be very careful not to crush the queen–a substantial percentage of queens are accidentally killed by new beekeepers during the initial transfer process.
For a package, there are a zillion recommendations for specifics as to how to install the bees. It makes little difference if you are installing only a single package if there are no other hives immediately nearby (if there are, install late near dusk to avoid drift of bees to the established hive). In general, lift out the feeder can to remove the queen cage, and hang the cage (use a grocery store twist tie or toothpick) from the top of a center frame. Then shake the bees out of the cage into the hive (you can temporarily remove frames). Use no smoke during the installation. Then replace the hive cover (you can place the package with the hole next to the entrance to allow the rest of the bees to find their way into the hive.
Feeding your bees
Your nuc may arrive hungry–check to make sure that at least one of the outer combs contains stored honey. If there is a good nectar flow on when your nuc is installed, there is no need to feed, but the feeding of sugar syrup to the small colony frees the bees from the need to forage for nectar, and they can use their efforts instead to collect pollen, rear brood, produce beeswax, and draw out comb. The production of beeswax in order to “draw out” the frames of foundation into “drawn combs” requires a great deal of sugar (whether from nectar or syrup). Therefore, it is generally a good idea to consistently feed sugar syrup to the new colony until all the frames in the lower box are fully drawn.
A growing colony needing to draw foundation can utilize more sugar syrup than you can imagine (gallons)–feed it as much as it will consume until all 10 frames in the lower brood chamber are drawn out.
Here’s the art: the bees can only build comb on warmed frames, so unless the weather is quite warm, they won’t be able to draw comb outside of the cluster. If you overfeed, the bees will fill the broodnest full of nectar, thus preventing the queen from laying more eggs. So what you want to do is to observe the broodnest, and feed enough syrup for the bees to be creating “white wax” at the edges of the cluster (meaning that the amount of incoming nectar and syrup, relative to the amount of free open cells, is stimulating them to activate their wax glands). Once the sealed brood of the nuc starts emerging, the colony (cluster size) will start expanding rapidly, and you can then feed heavily to allow them to draw comb.
So at first, the colony may need perhaps a cup of syrup per day. Later on, it may take a gallon a day. Once all the frames in the first brood chamber are drawn, then it is up to you whether you wish to continue to feed. Typically, in the Sierra, the main honey flow may be on by that time, and you may wish to collect some honey. You can always return to feeding later in the season (July), if necessary, to help them to draw out all the combs in the upper brood chamber, and to put on honey stores (in Sept and Oct) for winter.
I recommend making a dedicated feeder lid—make it from a 16¼” x 20” piece of plywood, with an approx. 1½” hole in the center. Use this lid temporarily in place of your regular lid(s). Make a feeder bottle from any wide-mouthed quart jar, with about three small holes punched closely together in the center of the lid. To use the feeder, fill the jar 2/3rds full of white granulated sugar (feed no other sugars*), then add hot tap water until nearly full. Place your finger over the holes, and shake ‘til the sugar’s dissolved. Invert the jar, centered over the hole in the feeder lid. Generally, feed as much syrup as the bees will take (at least a quart per day, often more).
*Nectar contains sucrose, fructose, and glucose–all of which bees can easily digest and utilize. Bees cannot digest some of the complex sugars or minerals in raw or brown sugar, some fruit sugars, and food waste sugars–feeding any of these may cause yeast infections in the bee hindgut and dysentery–additional info here. Old honey may contain toxic HMF or AFB spores, so is not recommended. Each type of sugar syrup has advantages and disadvantages as far as granulation issues, etc. It’s generally easiest for the hobbyist to feed sucrose syrup, using plain old “white” granulated cane or beet sugar (ignore the internet chatter about purported GMO issues).
Use “light” syrup for stimulation, “heavy” syrup for winter stores. Light syrup is 1 part sugar: 1 part water (by either weight or volume)–this is similar to the most sugar-rich natural nectars; heavy syrup is 2 parts sugar:1 part very hot water, and is quickly converted by the bees to “honey” for winter consumption. Bees also do very well on a commercial sucrose:HFCS mix, such as Mann Lake’s Prosweet, commonly used by professional beekeepers (other manufacturers carry different sugar products at different branches, so I can’t make blanket recommendations).
C&H Baker’s Drivert (a dry flaky product from bakeries) makes for a great dry feed for stimulation–useful in nucs. Baker’s fondant, hard “candy boards,” or even granulated sugar over newspaper can be used for winter feed (although simply leaving the bees enough natural honey is usually best, unless they have stored honeydew, which can cause dysentery).
I’ve seen no evidence of benefit from adding acid, essential oils, or purported stimulants or health supplements to the syrup. I do add a half tsp of unscented household bleach per gallon to light syrup fed during hot weather, in order to prevent harmful microbial growth in the feeders. The bees appear to love chlorine!
It may never be necessary to feed sugar to your colonies–I kept bees for 25 years without ever feeding syrup. But then I learned that syrup can be of great help to the bees when there is no nectar flow, and can greatly assist colonies in “getting going” and drawing comb.
No matter what you feed, be sure to keep your honey pure by never feeding syrup in any way that it could possibly get into honey to be extracted!

An example of a simple feeder jar and temporary hive cover for feeding. By making the hole smaller than the size of the lid, plenty of beespace is left above the top bars. Use a wide-mouth jar for stability.
Management summary for the foothills
Hive Inspection
For best queen survival, you should not disturb the new colony for a few days (other than feeding). Inspect the colony after a week. Use little smoke and minimal disturbance. If all’s well, the bees should have started drawing out fresh comb, there should be brood of all ages, including white larvae and eggs. Note that eggs are very difficult for the beginner to see, especially against new comb. The presence of any eggs, or young larvae in royal jelly, means that you have a queen, and all’s well—you need not actually see the queen!
Q: How often “should” I inspect my colony?
A: There are no “shoulds” in beekeeping. You are learning about beekeeping. You can learn some things from books, but there is nothing like the hands-on experience that you get from closely observing what is going on inside the box. So open your hive as often as you can (every other day is OK) so long as you use only enough smoke to keep the bees from looking at you, and handle the frames carefully and gently. Sure, such frequent opening is disruptive to the bees and may result in some degree of queen losses, but it is the only way you will ever become a good beekeeper. It is far easier to observe the growth of a small nucleus colony than it is to tear into a hive when it is huge. So get in there and watch your colony grow! Learn to recognize the ages of the brood, fresh eggs, the expansion of the broodnest, pollen stores, comb building, etc. It is not necessary to ever actually see the queen, so long as you see eggs or young brood.
Colony Buildup
The primary requirement for colonies to grow is plenty of nutritious pollen (some pollens are more nutritious than others). Colonies grow only when pollen (and generally at least some nectar) is coming in. For my local area in the Sierra Foothills, pollen income and colony growth are as below:
Note the typical amounts of time required for an overwintered colony to build up to swarming, and then to its maximum population. And note how buildup follows pollen availability.
Management in a Nutshell

Reading the Combs
There is little need to ask others for advice–if you learn to read the bees and the combs, the colony will tell you its exact condition, and whatever it needs. I’ve labeled the components below.
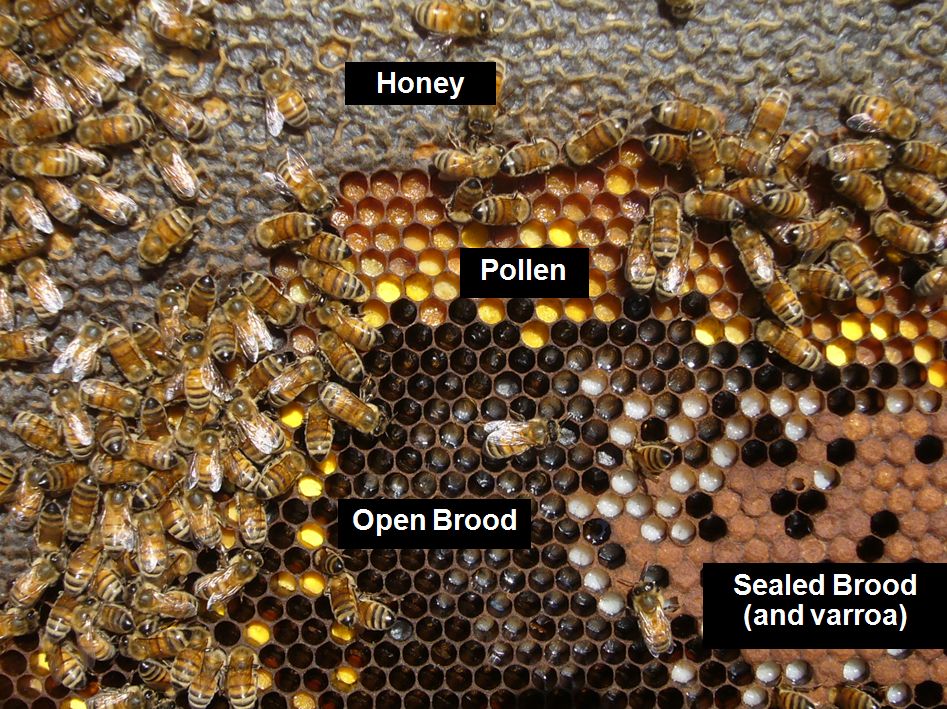
To determine colony condition, work your way to a center brood comb, and pay most attention to the interface between the honey above, and the brood below.

In this dynamic interface, you will be able to tell whether the colony is hungry or storing honey, and how good their protein reserves are.
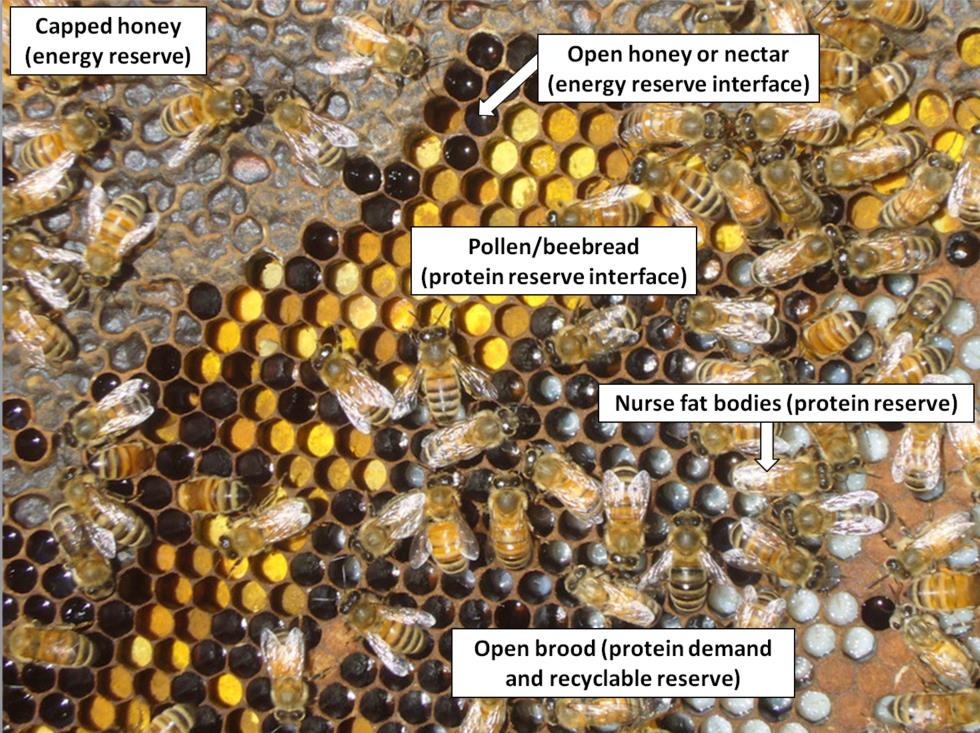
You always want to see a nice band of beebread around the brood. Should this band disappear, suspect that the colony is suffering from nutritional stress (more later). There is no benefit from feeding pollen sub patties to colonies if they are already gathering plenty of natural pollen (in the Foothills, typically from February through the end of June, unless there is prolonged poor weather).
I have written a lengthy “Understanding Colony Buildup and Decline” series–all posted to ScientificBeekeeping.com. Reading this series from beginning to end will give you a deep understanding of the biology of this fascinating creature, and allow you to make better management decisions on your own.
Initial Varroa Management
Unless I tell you otherwise, any nuc that I sell has already been treated to reduce varroa levels (we use a dribble of oxalic acid syrup during a critical window of time; see Oxalic Treatment of Nucs). For packages, I suggest that you give any package a similar oxalic dribble treatment prior to Day 8 after installation (at which time varroa can begin to “hide” in the sealed brood). If the nuc has not been treated, I suggest the application of either a Hopguard II strip (considered an “organic” treatment, but less effective), or an Apivar strip (synthetic, but highly effective, since our mites have not previously been exposed to amitraz).
Helping the Colony to Grow
As the population grows, and the colony can cover more frames, the bees will draw combs of foundation from the center out, and the queen will start laying in those combs. This is your chance to watch a colony grow! (The population of bees in a nuc will expand quickly; that of a package, not until 3 weeks after installation).
Growth of your colony will be dependent upon the local nectar and pollen flows.
The colony can’t raise more bees without pollen–pollen is the main limiting factor for colony growth and well being. Colonies also need energy-rich nectar to heat and fly, and for producing beeswax.
In general, pollen flows are adequate in most areas in spring, but if rainy weather confines the foragers, the nuc will benefit from being given a pollen sub patty or two (no benefit if there is natural pollen coming in). And unless there is a very heavy natural nectar flow, the colony will greatly benefit from being fed all the 1:1 sugar syrup that they will consume (a half gallon a day if they will take it). Feed syrup until you see white wax being produced–they will not draw comb unless they are producing white wax!
Making sugar syrup: use only white granulated sugar (cane or beet). Sugars with any color may contain undigestible carbohydrates or minerals that may cause gut dysbiosis. Feed “light syrup” for buildup (one part sugar to one part water, by weight or volume); feed “heavy syrup” for putting on winter stores in fall (2 parts sugar to 1 part boiling water).

Learn to recognize “white wax.” It is only when bees are producing white wax that they will build comb and draw foundation. It is a sign to the beekeeper that the bees have filled every available cell with nectar, and are crying for more room. Thus, it is an indicator of a strong nectar flow, and a signal to add honey supers immediately.
Frequency of Inspection and arrangement of combs
You may check the colony as often as you like, but be aware that clumsy handling by beginners often results in queen loss–so handle the combs carefully.
In general, place the combs back into the same arrangement that the bees had. Always keep brood (and frames of eggs) together, honey to the outside, and pollen at the edge of the brood nest.
Exception: It’s hard for the bees to draw out the outermost combs of foundation. Once the bees draw out the inner side of the second combs in, reverse those combs, and move them to the outside (only if they contain no eggs–see illustration).
.
Once the combs have been drawn and filled, their positions can in general be exchanged, just be sure to keep brood combs together, and in the middle of the hive.
Handling tip: always run 10 frames in your brood chambers, squeezing them back tightly together after each inspection, and then centering them in the box.

Prevent propolis from building up between the end bars–this will maintain proper bee space between the combs. Always squeeze center the frames tightly together, and then center them in the box–you can pry them all sideways to leave 3/8″ inch clearance between the end bars of the outer combs and the sides of the box. If you always do this, you will generally get straight combs, and will always have working room between the combs.
Feed the colony sugar syrup continuously, but not to the extent that the queen is unable to expand the brood nest due to excess stored syrup and nectar. If the bees store syrup in the center of the brood area, cut back on feeding.
Note that there is often a large colony-to-colony variation in buildup and honey production. Unless you have other colonies to compare to, it’s difficult to know how well your colony is doing, compared to the “norm.”
Make sure you have a queen—indicated by eggs and brood of all ages. Note that it is common for beginners to inadvertently kill the queen by inexpert handling of the frames!
Queen Issues
Check to see that the queen is laying a regular pattern of brood in concentric rings by age. If brood pattern is spotty or uneven, the queen may have a problem, or the colony may be suffering from a brood disease.

Above, a colony with well-fed, healthy brood. Note the even stepwise progression of larval ages,their high rate of survival, and the copious jelly being fed to the young larvae–all signs of a good queen in a colony in good health, enjoying good forage conditions.
It’s well known that in humans, the females improve with age. Not so with honey bee queens. Once a queen has mated, her supply of stored spermatozoa is finite, and typically begins to run short after two seasons of broodrearing in a large colony (she’ll last longer where seasons are short, or in a small colony). This depletion typically occurs in the late summer of their second season. At this point, some of the eggs that she lays in worker cells may not get fertilized, and the queen becomes a “drone layer” (photo below). In nature, the bees typically replace their queen when this occurs via a process called supersedure.

(Above) signs of a failing queen. Unfertilized eggs laid in worker cells (resulting in scattered drone brood), and a supersedure queen cup near the top of the photo. Time to replace the queen!

Colonies replace their queen by producing one or more supersedure cells, typically on a comb face. Allow them to do so. The mother and her daughter may coexist happily together in the hive for some time.
If there are multiple scattered eggs in a cell, or you see bullet-shaped cappings in worker brood cells, the colony may be “hopelessly queenless” with “laying workers.” Such colonies typically exhibit a “queenless roar” when smoked, their wings are all jittery when disturbed, and the workers get pissy. Laying worker colonies are difficult to requeen–it’s easiest to combine them with a queenright colony–just set them right on top.

Queen cells
If there are queen cells being formed, the colony may have lost their queen (you’ll see several small, scattered emergency queen cells), may be superseding the queen (one or two very large queen cells on the side of a frame; allow the process to continue), or be preparing to swarm (large queen cells often at the bottom of the frames–colony needs more room).

If you see emergency queen cells with a larva and jelly inside, you have likely lost the queen–either requeen or allow the colony 25 days to requeen itself. Smaller “queen cups” without eggs or jelly inside or normal.

In the springtime, a crowded colony (not given enough space) will build swarm cells at the bottoms of the combs. In the above photo, there is one queen cup, two starting swarm cells, one nearly complete cell, and one sealed swarm cell. Queen cells are always vertically oriented– the other large horizontal cells are normal drone cells.
Requeening
There are a few ways to requeen your colony. You can allow the bees to do it themselves via supersedure, but this is risky in the foothills due to the limited supply of drones in late summer, perhaps resulting in a poorly-mated new queen going into winter.
You can rear your own daughters from your favorite queen by simply creating a few nucs in springtime, and allowing each to rear emergency queen cells. It will take a minimum of 24 days before the new queen begins laying eggs.
You can speed up the process by purchasing or grafting queen cells–see Queens for Pennies.
Despite claims, attempts to requeen a queenright colony by placing a queen cell into it are generally unsuccessful–you need to remove the old queen first. I generally prefer to introduce my queen cells into nucs for mating out, and then after seeing the new queen’s brood pattern, then introduce the nuc into a colony that has sat for a day after removing its queen. Gently place the nuc into a full-sized brood chamber, remove the cover from the colony to be requeened (no queen excluder), lay a strip of newspaper at least as wide as the nuc lengthwise over the top bars (this prevents the new queen from running downhill into foreign bees), and place the cover over the top. After a few days, you can rearrange the combs.
If instead you purchase a caged queen, if she’s been banked, she may need to be fed by the bees for a day to get her into laying condition and to pick up the colony odor for better acceptance. A good way to ensure that your caged queen will be accepted is to do a two-step process:
- First, queen acceptance is best when there is a nectar flow on and no robbing–it may help to feed the colony syrup. Also, queens are more readily accepted by young bees with brood–if your colony has been queenless for some time, it may help to add a frame of young brood with the adhering nurse bees. And in any case, you’ll generally get best acceptance into a newly-made nuc that has sat queenless for a day or two.
- Press the queen in her cage, in the brood area, with the screen of the cage fully exposed to the bees, into the comb (avoid comb with honey, as you can drown the queen). Keep the candy covered with the cork or cap, or vinyl tape. Wait 24 hours. After 24 hours gently open the hive with minimal smoke and inspect the behavior of the bees on the cage. If they are walking lightly over the screen as below, and you can see them offering the queen food, and the bees can be moved with a gentle touch of your finger, they’ve accepted the queen. You can then expose the candy, replace the cage, and allow the bees to chew through the candy to release her after you’ve put the hive back together.
 In the photo above, the bees have accepted the queen. In the photo below, they have not, and are attempting to “ball” the queen and cook her. The bees below are behaving aggressively towards the queen, and will not move when you touch them.
In the photo above, the bees have accepted the queen. In the photo below, they have not, and are attempting to “ball” the queen and cook her. The bees below are behaving aggressively towards the queen, and will not move when you touch them.
 In the case above, you most likely have a loose queen remaining in the hive (or perhaps she needs another day)–no sense to release the new queen, as she’ll be killed. You need to either find the other queen, or introduce the queen into a queenless nuc.
In the case above, you most likely have a loose queen remaining in the hive (or perhaps she needs another day)–no sense to release the new queen, as she’ll be killed. You need to either find the other queen, or introduce the queen into a queenless nuc.
Another method that works well is to make a “press in cage” of 1/8″ hardware cloth (washed to remove any oil). Place the cage over some emerging brood, tip it up and carefully release the caged queen into the screen cage, and gently press the screen fairly deep into the comb. Then open the hive after a couple of days and if all looks well, gently release the queen–watching to make sure that the bees don’t attack her.
 A tip: new beekeepers often manage to allow a caged queen to fly off. If you do so, freeze, and she will often return to the cage in a few minutes. Prevent this from occurring by handling caged queens in a closed room, your car, or in your removed veil.
A tip: new beekeepers often manage to allow a caged queen to fly off. If you do so, freeze, and she will often return to the cage in a few minutes. Prevent this from occurring by handling caged queens in a closed room, your car, or in your removed veil.
Problems and Diseases
Hive Invaders
Bears
If you live where there are bears, you’ll need to protect your hives from them. Bears are surprisingly strong, but can be deterred by a well-constructed electric fence. For the fence design that we use successfully in the Sierra Foothills, see Bear Fence Instructions.
Skunks
Skunks may scratch at the hive entrances at night. This can be prevented by nailing carpet tack strips across the entrance, or if the hive is on the ground, simply sprinkle a teaspoon of lye crystals onto the scratched ground, the skunks don’t like the taste, and can be easily trained to stop bothering the hive. Racoons don’t bother hives with live bees in them.
 Signs of skunk scratching. We don’t want to harm our skunks, so instead train them with an sprinkling of lye crystals on the scratched ground.
Signs of skunk scratching. We don’t want to harm our skunks, so instead train them with an sprinkling of lye crystals on the scratched ground.
Ant Control
Ants may be problematic in some areas. It’s common to find ants nesting between the outer and inner cover, or around the hive. But there are only a few ant species that will actually invade a hive. In California, Argentine ants can be a serious problem. I have no experience with ant species found in other states.
It’s best not to spray or spread insecticides near the hive, since ants are closely related to bees, and are susceptible to the same insecticides!
You have two options:
1. Poison the ants. For actual long-term success, you must use a bait that the ants will carry back to their colony and kill the queen. For that purpose, borax or fipronil work best.
Since most invasive ants are seeking sugar, a sugar bait such as Terro, or homemade bait of 5% borax (sodium tetraborate) in honey or sugar syrup, offered in a Mason jar with 1/8″ screen in the lid works well. Fipronil-based ant bait stations are also very effective at killing an ant colony, but be sure that your bees can’t gain access to the bait.
2. Protect the hive by placing it on a stand which ants are unable to traverse.
I recently noticed some pretty cool ant stands being sold by a small California company: https://defyantstands.com/
Note, combs and boxes from diseased or “deadout” hives can be reused without sterilization, except for AFB.
Chilled Brood
An unexpected cold night in the spring can result in some chilling of the brood at the periphery of the cluster. You may see pulled out pupae on the landing board the next morning. You do not need to take any action.

If the brood is “spotty,” look for signs of brood disease—chalkbrood (white & tan “mummies”), EFB (twisted white & yellow larvae–often hard to diagnose), or AFB (sunken perforated cappings, melted brown larvae that “rope”).
EFB
EFB is a common bacterial disease, especially in stressed colonies in the spring.

European Foulbrood (EFB) above. EFB used to just “go away” on its own; our current strain doesn’t. Treat with oxytetracycline — a natural antibiotic. Oxytet now requires a veterinary prescription, but any professional beekeeper will likely have some on hand.
Chalkbrood
Another brood disease is Chalkbrood (a fungal disease):
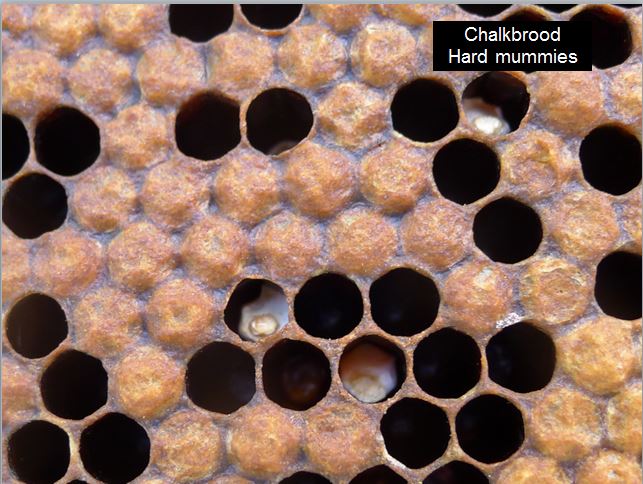
Chalkbrood produces distinctive, two-toned hard “mummies” in the combs.

There is no treatment for chalkbrood. If it doesn’t spontaneously disappear, the best advice is to requeen the hive.
AFB
The most serious disease (other than varroa-induced virus collapse) is American Foulbrood (AFB)–a highly contagious bacterium that can spread to your neighbors’ hives. Although AFB is now uncommon in professional apiaries, it is currently resurging in some urban areas that have a lot of hobby beekeepers who don’t recognize this serious problem.
AFB is nothing to mess with–ask for help if you suspect that you see it, and then scrupulously eliminate it before it spread to your neighbors!

AFB produces a distinctive, “salt and pepper” brood pattern, with some cells having sunken, perforated cappings. It generally has a strong distinctive unpleasant odor (a cross between gym socks and dead rotting bees).

The caramel-colored melted pupae (above) are distinctive for AFB. Stir a dry stick into the melted brood cell and then slowly withdraw it. If the slime ropes out for 3/4″, that is diagnostic of AFB. We burn colonies if we see even one cell! Do not ignore AFB!!!!

Colonies that have died from AFB will have black “scales” tightly adhered to the bottom of the brood cells. If buying or reusing combs, check them carefully for AFB scales, as this is a common way to spread the disease.
AFB combs must be burned or safely disposed of in a landfill. The boxes can be sterilized and reused.
The Clean Up Crew
Once a colony weakens or dies, if the weather is warm, the Wax Moth will move in and lay eggs. Its caterpillars will rapidly consume any pollen stores, boring through the combs, and building silken tubes and webbing, and chewing into the wood to pupate. You can remove the silk (especially the white cocoons), and reuse the frames with no additional cleaning.

Wax moth is generally only a problem when temperatures are above about 70°F. Store boxes of dark combs with light and air exposure (never wrapped up or stacked) to avoid wax moth damage. BTW, wax worms make great pet food, and are tasty to eat, raw or cooked.
OK, most of you won’t have any of the problems above, and your nuc or package will rapidly outgrow their first brood chamber.
Adding the Second Brood Chamber
Once the bees of your colony cover all 10 combs in the first brood chamber, it’s time to add a second brood chamber of foundation above it. At this point of time, you should assess the varroa infestation level in your colony. I’ll return to adding your second brood chamber after I discuss varroa management.
Varroa Mite Management
Varroa is the Leading Cause of Colony Morbidity and Mortality
The leading cause of colony failure for beginners is from not managing varroa mite levels, which then allows viruses and nosema to kill the colony.

The varroa mite transmits viruses (mainly Deformed Wing Virus) within the hive, resulting in dying brood. This is called Parasitic Mite Syndrome (PMS). Once a colony reaches this stage (above), it is difficult to save.

Note the two bees with deformed wings in the photo above–they are suffering from varroa-transmitted DWV. Also note the dying brood, and bees unable to emerge.
If your colony was strong in summer, and then the bees suddenly disappear in fall, leaving behind plenty of honey above, and scattered sealed brood in the lower box, varroa is generally the culprit. You can diagnose by holding a brood frame horizontal with the top bar away from you. With the sun coming over your shoulder to illuminate the “ceilings” of the cells, look for the telltale white guanine deposits left by mites. If you see them, it was most likely varroa that did your colony in. Here are some photos of typical varroa deadouts:
 In this photo I have the top bar toward me (sun to my back), looking at the “floors” of the cells for AFB scale. To look for guanine deposits from varroa, turn the top bar away from you.
In this photo I have the top bar toward me (sun to my back), looking at the “floors” of the cells for AFB scale. To look for guanine deposits from varroa, turn the top bar away from you.
Tip: when inspecting combs, look for the shadow of your head on the ground, and then face it. This will put the sun directly behind your back (without blinding yourself by looking directly at the sun).
 A colony dying from varroa/DWV. Note the deformed wings on the bee, and the fully-formed adults in the cells unable to emerge–a typical sign. Note the white guanine deposits left by the mites on the cell “ceilings.”
A colony dying from varroa/DWV. Note the deformed wings on the bee, and the fully-formed adults in the cells unable to emerge–a typical sign. Note the white guanine deposits left by the mites on the cell “ceilings.”
 Another shot of a colony dying from varroa/DWV. Again, note the white deposits.
Another shot of a colony dying from varroa/DWV. Again, note the white deposits.
 Close up of some guanine deposits, some adult brood unable to emerge, and some cells with the cappings partially chewed open. All typical of a varroa kill.
Close up of some guanine deposits, some adult brood unable to emerge, and some cells with the cappings partially chewed open. All typical of a varroa kill.
“Treatment Free” Beekeeping
Although my goal is to develop a stock of bees that require no treatment for varroa, I adamantly do not recommend “treatment-free” beekeeping for beginners. It is biologically and ethically unjustified, inexcusable as far as practicing good animal husbandry, and spreads mites and viruses to your neighboring beekeepers and any struggling populations of feral honey bees, and viruses to native pollinators.
Control varroa, or colonies will generally die an ugly and unnecessary death from the varroa/virus complex. Not only that, but when that colony crashes, it floods the surrounding colonies with varroa mites, making you a nuisance to both surrounding beekeepers, and the feral population of bees that is slowly evolving resistance to the mite. PLEASE MANAGE MITE LEVELS IN YOUR HIVES AND DO NOT ALLOW COLONIES TO COLLAPSE FROM VARROA.
Once you’ve mastered keeping colonies alive and healthy for at least three years, then you can experiment with going without mite treatments (don’t expect most commercial bee stocks to survive without treatment–you will likely have better luck with VSH, Russian, or feral survivors). There are several easy-to-use and effective “natural” mite treatments on the market that will not contaminate your combs or honey, as well as biotechnical methods such as drone brood trapping and queen caging.
Monitoring Varroa Infestation Levels
Every bee colony contains varroa mites, and more drift in from other hives. And although we breed for resistant stock, the vast majority of our hives require mite treatments to survive. Mite buildup follows a predictable growth curve. My mite model demonstrates the expected mite population and alcohol wash counts for a nuc, treated before purchase:
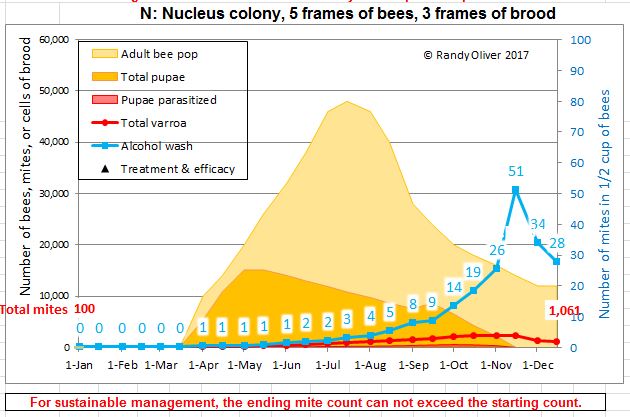
The graph above is from Randy’s Varroa Model — a free download that runs on Excel https://scientificbeekeeping.com/randys-varroa-model/
In the above chart, the nuc started with a reasonable 100 mites in April. Note the progression of alcohol wash counts. This nuc had a chance to survive the winter, but would start the next season with far too many mites to survive a second year. Note: the alcohol wash mite counts rise substantially more slowly than the actual mite population–realize that the mite wash count will rise rapidly in late summer as the colony bee
population begins to decline. You want to be proactive rather than reactive with mite management–intervene in early July if mite counts exceed 4 mites!
Compare to the chart below, with a somewhat 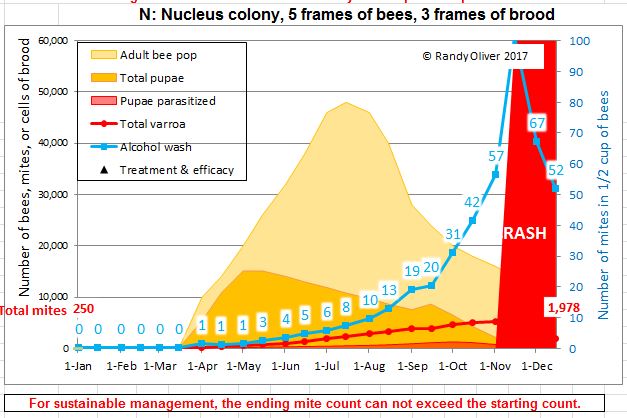
In the above chart, I started with 250 mites in April. Note that the starting alcohol was count was still low, but the colony crashed in November. Had the hive been in an area where there were other high-mite hives, it would have crashed even earlier, due to mite immigration from your neighbors.
Practical suggestion:
For Joe Smallscale, use my varroa model to put together a seasonal mite management schedule of treatments for your particular operation and location.
Unless you’re a breeder, there’s little reason to wait until monitoring suggests that you’ve reached an action threshold.
Instead, use spot-monitoring of 300-bee samples (by detergent wash) to confirm that you’re maintaining adequate mite suppression in your operation.
If your counts are always zeroes, you can likely back off a bit on your treatment schedule.
If instead, counts start to exceed 6 mites, adjust your treatment schedule.
This is especially important in late summer if there is much mite drift in your area.
Expect mite counts to jump a bit when the colonies wind down brood rearing in fall (or during dearth) — confirm by monitoring that your treatment schedule takes advantage of hitting the mites when they are all out of the brood (we prefer OA dribble or extended-release).
Here in the California foothills, with 11 months of broodrearing, most colonies will require three or four 95% reductions in their mite levels during the season.
Starting in July after installing a new nuc or package, you should test it for mite level, using a sticky board or other method (I strongly recommend the “alcohol wash” or “sugar shake”). Assume that all nucs have varroa mite. You must monitor mite level, and treat if the mite level exceeds the seasonal “treatment threshold” (3 mites/half cup of bees prior to July 1, 6 mites from July 1 through late fall). We are having great success with mite-tolerant queens, biotechnical methods, and “natural” treatments.
I recommend mite testing in early spring, before supering, again in early August, and in late September (Northern Calif).
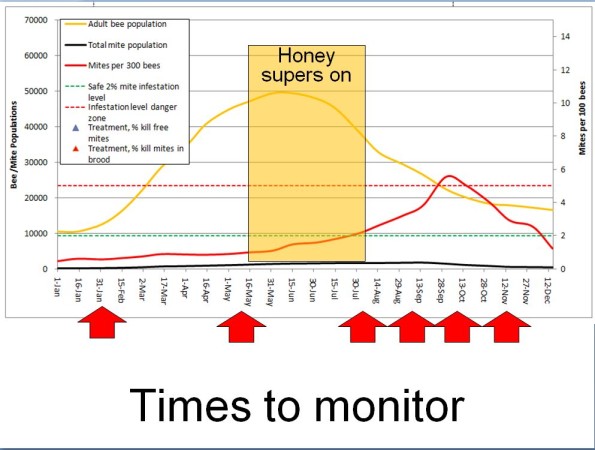
Due to the huge variability of stickyboard counts, any single stickyboard count is nearly meaningless. However, they can be of use if done regularly.
The best test is an alcohol or detergent wash of a level half cup of bees (~320 bees), or the “sugar shake” (see https://scientificbeekeeping.com/sick-bees-part-11-mite-monitoring-methods/)–I recommend the alcohol wash over the sugar shake. Learning to do an alcohol wash is so important, that I’m going to go into detail as to how to do it!
Update August 2020: We have switched from using alcohol to Dawn Ultra dishwashing liquid, diluted to 2 Tablespoons per gallon. Allow the bees to soak in the detergent for 1 minute prior to a swirl agitation. Due to the foam, then count the mites looking up from the bottom of the cup — a 10X makeup magnifying mirror helps.

First, build a mite wash cup set. For details, see these links: An Improved mite washer and Still improving.
The simplest is made from two clear 16-oz plastic cups with a snap-on lid, plus a piece of mesh fabric. Use a heated knife blade to cut roughly 1/2-3/4″ off the bottom of one cup, leaving a smooth edge:

Then cut a 12″ square of “mesh” or “Tulle” fabric–not the hole size, which must be large enough for the pinhead-sized mites to pass through.

The cut cup goes into the uncut cup, with the mesh between them:

We hate to see the bees that we sell die from neglect, and are happy to give you a prepared mite wash cup as above any time–just ask when you come by.
Fill the cup about 3/4ths full of 91% rubbing alcohol: Update: We’ve found that Dawn Ultra dishwashing liquid, diluted to 1 Tbl per half gallon of water, works even better than alcohol.

Now you need some nurse bees to sample. Yes, you will unfortunately need to sacrifice ~300 bees, but realize that in a typical hive, roughly 1000 bees die of old age each day. The 300 bees are a small sacrifice in order to save the life of the entire colony!
Nurse bees are scattered throughout the hive, but mostly on frames with brood or beebread. In order to avoid harming the queen or disturbing the broodnest, simply take your bee sample from a frame from the upper brood chamber, to the side of the broodnest:

The above frame is filled with beebread, upon which only nurse bees feed. This is a perfect frame from which to take your sample.

Shake the bees off the frame into the tub–one frame’s worth of bees is usually plenty.
Now say the magic words: “Old bees fly off!” The older bees will immediately respond to your command and fly home, leaving only the nurse bees, which will briefly spread into a single layer, in which it is easy to spot the queen, should you have accidentally collected her. Although noisy, the bees flying off are not in any way inclined to sting–we do dozens of these shakes a day, and never have a bee attempt to sting us.

The young bees remaining in the tub are completely non-defensive, and if you wish, can be gently scooped up into your hand, and poured gently from hand to hand (I often do this at field demonstrations to show that young bees rarely sting, unless you squeeze them):

A mite wash does require the sacrifice of ~300 workers. As much as any of us hate to kill a single bee, the reality is, that from midsummer on, a typical colony of bees is losing more than 1000 workers a day to natural attrition, so a 300-bee sacrifice is a small price for a colony to pay in order to be saved from varroa. Keep in mind that any worker in the colony would unhesitatingly sacrifice itself to benefit the colony as a whole.
Tip the tub on edge and tap the bees down into a pile, then turn the tub and use the measuring cup to scoop upward to fill it overflowing with bees.

You can then use your finger to level the bees off for an exact half cup sample. I do this hundreds of times a season, and have never been stung in the finger:

Quickly dump the bees into the alcohol before they fly out of the cup:

It helps to immediately cover the bees floating on the alcohol with the bottom of the cup until they’ve submerged. They all quickly and humanely die. After the wash, dump the dead bees on the ground and the night critters will eagerly consume them. Or you can use them to check for nosema under the microscope.
Add alcohol if necessary to have 1/4″ of alcohol above the bees, in order to allow them to tumble as you swirl the cup. Snap the lid onto the upper cup, and swirl the cup with a small, easy circular motion (fingers pointing down) such that you can see the bees at the bottom tumbling over one another. Mites will begin to drop immediately, and most will drop by 30 seconds.


With a good swirl, you’ll get 100% mite recovery by 45 seconds. At that point, lift the upper cup, along with the mesh, free of the lower cup. You can then view the lower cup from below to count the mites. They are the size of a pin head, oval, and dark reddish-brown. There are two mites in the photo below:

We want to see no more than 1 mite early in the spring, not more than 3 or 4, in June, and intervene with some sort of treatment if the count goes above 6 (at most 8 mites/wash in September). Update, with detergent rather than alcohol, you must count from the bottom, due to the foam.
At less than 2 mites per 100 bees (6 in an alcohol wash of 1/2 cup of bees), virus transmission by mites is not a major issue. At 5 per 100 (15 in a wash), some viruses begin to go epidemic. At 15/100 (45 in a wash), colonies generally start to go into a death spiral. There are roughly 82 mites in the photo below. This is not at all unusual for me to see in hobby beekeeper hives in late summer. This colony looked fine, but will collapse before midwinter. With this many mites, it already is suffering from a raging virus epidemic, and is nearly impossible to save.

The most important times to sample are early spring, and then regularly between August 15 and the onset of winter.
Treatments for varroa control
Suggestion #1: Be proactive rather than reactive! It is far easier to keep the varroa level down from the start, than to later attempt to bring it down once the mites have overrun the colony.
In California, with our long broodrearing season, depending upon how resistant your bee stock is to varroa, it will generally require 2-3 90% kills of the mite per season to keep the mites under control. I suggest that you ignore much of the information on the internet, and use the well-proven effective treatments detailed below (most are approved for organic production). FWIW, although the essential oils sound alluring, bees as a rule do not like them, and they are stressful to the colony, as well as (other than thymol) exhibiting poor mite control.
We have very good success with Apiguard gel (thymol) or Formic Pro (formic acid). I also recommend a 3.5% oxalic acid dribble (applied accurately) before Christmas (Northern Calif). I’ve detailed your seasonal choices for mite treatments below.
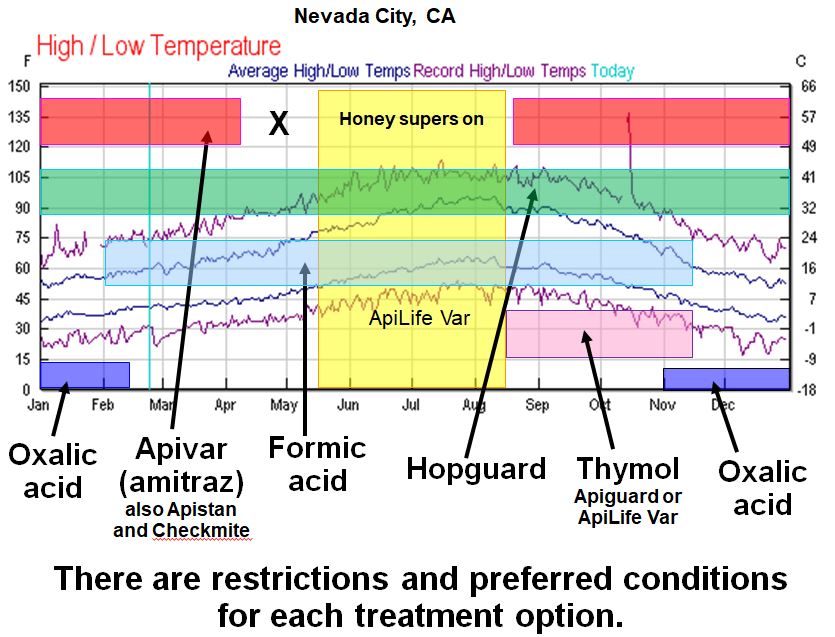
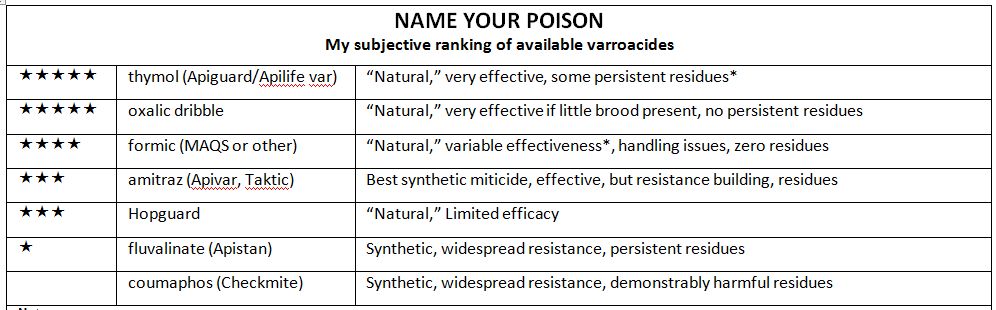
Expected mite reductions by treatment option: Use the figures below with Randy’s Varroa Model to plan your mite management strategy.
95%: High-efficacy synthetic miticide, such as amitraz applied over several weeks. Not quick acting, so best applied proactively, early in the season.
90%: Two 50-g Apiguard treatments at 10 days, in a rim. Best used in August, as it suppresses broodrearing.70-90% (sometimes less): 2 MAQS or Formic Pro pads, or strong formic acid treatment (time release or hard flash). Best treatment for quick mite knockdown in warm weather. Can be used during honey flow.
50% (approximate): 1 MAQS or Formic Pro pad, or single weak formic acid “knockback” treatment. Quick knockback for buying time, easier on the queen.
80-90%: Hopguard 3 if colony is broodless; would require repeated applications if brood present. Can be used during honey flow.
25%: A single powdered sugar dusting, assuming 50% drop of phoretic mites. Requires multiple and continued applications.
15-20%: Complete removal of drone brood, if done early in the season, each removal. Provides some reduction, but labor intensive.
40-60%: The issuance of a prime spring swarm.
80-95%: Oxalic dribble or vaporization when broodless. Excellent reduction when colony is broodless, due to natural or induced brood break.
15-45%: One Oxalic dribble or vaporization in hive containing brood, proportional to the % mites of phoretic. Limited reduction when colonies contain brood.
85%, 3x: Extended-release OA/glycerin towels or sponges; for end result at 45 days, enter 85% for three consecutive time periods, starting at time of application. Not yet approved.
Seasonally-appropriate choice of treatment options
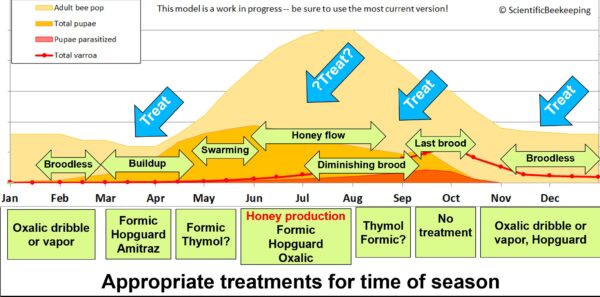
Each treatment option has advantages and limitations, which can be taken into account when formulating an annual mite management plan.
Amitraz applied as Apivar strips can hit the mites emerging from brood, but takes 6-8 weeks to obtain full efficacy, provided that your mites don’t exhibit resistance to the product. Thus, the best treatment window for Apivar is either in the springtime, at least 8 weeks prior to the honey flow, or immediately after pulling honey no later than mid August. Application later in the season may be too late.
Formic acid is the treatment of choice for immediate mite reduction, and strong applications can kill the mites within the brood cells. Formic treatment does cause about a 5-day brood break, but then the new brood looks great. There is also the chance of queen loss (especially during hot weather), which can normally be prevented. Formic is appropriate in springtime, when honey is on the hive, or for serious “clean up” of high-mite hives.
Thymol is disruptive to the colony, and will cause reduction in brood rearing, and is not approved for use when honey supers are on the hive. It thus may not be appropriate in springtime or fall (when you want to encourage brood rearing). We find it to be efficacious in August.
Oxalic acid is very efficacious applied by dribble or vapor only when a colony is without brood. It can be used when splitting colonies in springtime to eliminate most of the mites, in early winter, or with an induced brood break. Extended-release OA (with glycerin) is currently being registered, and can be efficacious while a colony has brood present.
The beekeeper can use Randy’s Varroa Model to figure out how many mite reductions per year are necessary in their environment, and the most appropriate treatment type to use for each reduction.
Top Bar Beekeepers
The varroa mite does not care that you’re keeping your bees in a top bar hive–colonies in top bar hives are just as susceptible to the mite. Unless you are running a truly mite-proof bee stock, you can expect the same sort of buildup of mite populations as in Langstroth hives in late summer, and I have seen plenty of top bar deadouts with clear signs of mite overload.
Top bar hives make mite management a bit more difficult, but can be done. For some tips, see: Mite management in top bar hives
Notes:
All miticides are poisons, but some are safer than others to both the bees and to humans. The “natural” treatments are all part of our normal diet, and most are naturally found in the hive at low concentrations. I do not hesitate to use them, since the benefit of controlling varroa far outweighs any health concerns to the bees or their keeper. That said, the natural treatments are all stressful to the colony, so application instructions must be carefully followed. Proactive treatment allows you to use lower (and less stressful) doses.
The “natural” miticides
These organic acids and essential oils are approved for organic certification. Although more difficult to use than the synthetic miticides, they leave little or no residues in the combs. These are all that we have used in our operation since the year 2000. That said, Apivar strips (scroll down) are very effective for those wishing the easiest mite management.
Thymol: Natural and safe (25 g of Apiguard gel contains the same amount of thymol as does 1 lb of fresh thyme herb). Thymol is the only consistently effective essential oil (actually a crystal at room temperature) found to date. I suggest wearing disposable gloves (to remind you not to rub your eyes) . We’ve used Apiguard successfully from 55°F to 95°F, applying 25 g (in a lump, not spread out) on an index card laid across the center of the top bars between the brood chambers (application without the card can result in excessive colony disturbance) https://scientificbeekeeping.com/the-learning-curve-part-3-the-natural-miticides/. We adjust the dose according to cluster size and temperature—the proper dose will cause the removal of a handful of pupae onto the bottom board by the next morning (the colony will remove them from the hive soon afterward). I do not recommend applying under the lid, especially in hot weather. Repeat in a week to 10 days if mite count is not reduced adequately. Do not apply if honey supers are on.

Above is 50g of Apiguard thymol gel being applied in a 1-1/2″ rim. Hobbyists can purchase it in 50-gram aluminum tins. This is a very effective late-summer treatment in the foothills, once you’ve pulled the honey.
Update 2016: recent tests of mine are showing better mite control by applying two 50-g doses of Apiguard, 10 days apart, on top of the hive (as per mfr’s instructions) in a 1.5″ wooden rim (to provide better air circulation around the thymol). This works very well at daily high temps around 85F, and can be used up to 105F (but the colony will be somewhat stressed during treatment). We do not taste thymol residues in the honey after treatment, but the label calls for it not to be applied with honey supers on. We get high efficacy with two 50-gram doses applied 10 days apart. We get great results with Apiguard in August or September.
Thymol treatment causes the bees to move the broodnest and reduce broodrearing, but after the 20-day treatment, the colony immediately recovers.
Formic acid: Natural and pretty safe, depending upon how you handle it (definitely wear nitrile gloves; the respirator is generally unnecessary). The three main advantages of formic are that it effectively kills mites, is already a natural component of honey, and that it leaves zero residues in the hive. The problem is that the liquid form is somewhat dangerous to handle, and getting an appropriate release of vapors is tricky. The product Mite Away Quick Strips (MAQS) is the most user friendly https://scientificbeekeeping.com/an-early-summer-test-of-mite-away-quick-strips/, https://scientificbeekeeping.com/miticides-2011/. Works best in doubles without supers. A single-strip treatment gives decent mite “knock back.” A half strip works for singles with at least 5 frames of bees. Applied in doubles at full dose (two strips) in summer, we lose about 1 queen out of 25. Mite kill may not be consistent, so check back. MAQS may cause the loss of poorly-performing queens when applied at daily high temps above 85F.

Applying a single MAQS. We use this for mite knock back while honey supers are still on. I prefer the new Formic Pro strips over MAQS.
Tip for hobby beekeepers: to avoid queen loss, temporarily remove the queen from the hive during treatment– to a cage indoors, or to a two-frame nuc. Then you can blast the hive with formic for an effective mite kill, and return the queen after treatment (after 24 hrs with formic flash, after 5 days with MAQS).
Oxalic dribble: Natural and safe (the amount used is equivalent to that in a 3½-oz serving of spinach). This product is available at any hardware store as Wood Bleach. Follow the mixing directions at https://scientificbeekeeping.com/oxalic-acid-treatment-table/ exactly, as there is only a small margin of safety to the bees. The oxalic syrup is very safe to handle, but care should be taken not to splash it into your eyes. The treatment is by far most effective if the colony is broodless. Best use is for a single application in November, or earlier if the colony has shut down broodrearing. You can also apply in summer—3 applications, a week apart. Excellent for “cleaning up” nucs in the spring https://scientificbeekeeping.com/simple-early-treatment-of-nucs-against-varroa/.

Applying oxalic acid dribble, an easy way to control varroa, provided that there is very little sealed brood in the hive.
Others are using oxalic vaporization, which can also give very good results, but has operator safety issues–be sure not to breathe the vapor!
Hobby beekeepers can create an induced brood break by caging the queen for 12 days, then releasing her, and treating the hive with oxalic dribble or vapor 6-7 days later (day 18-19 after first caging). This is a very inexpensive and effective way to control varroa. Even better is to use queen excluder division boards to confine the queen for 14 days to a single “mite trap” frame.

A home-made queen excluder division board. Use two, plus a full queen excluder, to confine the queen to a single frame.
MITE CONTROL USING A 14-DAY BROOD BREAK WITH OXALIC DRIBBLE
- Day 1: Confine the queen to one “varroa trap” frame, using queen excluder dividers.
- Day 14: Release the queen.
- Day 21: Remove the varroa trap frame and dribble the colony with oxalic acid.
See also “How to kill three birds with one stone” below.
Update May 2017: I’m currently testing OA/glycerin extendend release towels, which appear very promising See Oxalic Shop Towel Updates
Hopguard: this recent addition to the arsenal is completely natural, not surprisingly tasting like bitter, concentrated hops https://scientificbeekeeping.com/miticides-2011/. You’ll want to handle it with disposable gloves due to its messiness. Its utility and effectiveness are similar to oxalic acid, but it is more costly. The new Hopguard II strips are effective in nucs, packages, or broodless colonies; just be sure not to directly hit the queen with the strip when you insert them.

The new Hopguard II strips hold much more active ingredient, and release it over a longer period of time. These are handy for nucs (for which thymol and formic may be too rough). You may need to repeat treatment.
The synthetic miticides
Amitraz: Commercial U.S. beekeepers have used the agricultural product Taktic, generally mixed with oil or shortening, for a number of years with consistent success (although this practice is illegal). Apivar strips are an improvement, being legal, as well as delivering a reduced and safer dose over 42 days, resulting in high efficacy of kill, and fewer residues in the hive. The strips, however, do not cause a quick kill, so may be most effective in either spring (applied at least 42 days before the honey flow), or in fall if mite levels are not too high, and there is enough time before cold weather sets in. I gave Apivar only three stars since it does leave a residue, which recent studies suggest may affect sperm viability in the queen and have other sublethal effects on the bees, as well as the EPA’s legitimate concerns about its safety to humans. There is also evidence that some mite populations have developed resistance to the active ingredient.

Apivar is applied at the rate of one strip per 5 frames of bees. It is slow-release, and requires 8 weeks for full efficacy, so is best used in springtime. This synthetic miticide is also used in tick collars for dogs. It is effective, especially when applied in the spring (a single strip is great for nucs or packages, and exhibits few adverse effects upon the colony.
Fluvalinate and Coumaphos: These first-generation synthetic miticides (along with the off-label use of agricultural products containing the active ingredients) quickly bred for resistant mites, and contaminated the entire U.S. beeswax supply. I cannot recommend their use.
Our typical mite management regime in Grass Valley is as below:
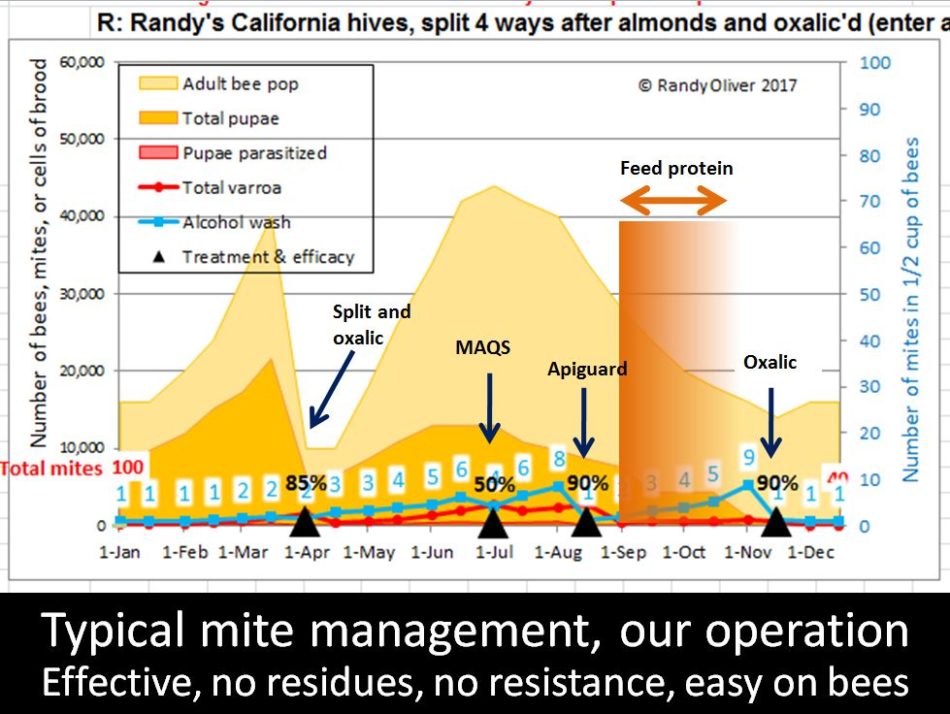
Update November 2022
Treatment Suggestions by Season
Each sort of mite treatment product is most suitable for different times of the year. Below are what we’ve found to work well in the Sierra Foothills–where we have a short brood break during cold winter, early spring buildup, hot, dry summer, little pollen after mid July.
Midwinter--generally leave colonies alone
Early spring–oxalic dribble* before there is much sealed brood; formic acid, or Apivar (amitraz strips–the easiest and most effective, but must be removed prior to collecting honey). Feed pollen sub if poor foraging weather.
Mid spring–drone brood removal, splitting with induced brood break and requeening, coupled with oxalic dribble*. Formic Pro–half or full dose. Likely too late for Apivar.
Late spring–apply extended-release OA/glycerin treatment on cellulose towel (if legal in your country). Formic Pro–half or full dose.
Late June–check mite levels while honey supers are still on; treat with formic acid if indicated Formic Pro–half or full dose.
Mid August (there is no advantage to us for broodrearing at this time)–50 g Apiguard thymol gel, 10 days to 2 weeks apart, in a 1.5-inch rim above the brood nest (or two 25-g applications between the brood chambers). Hard formic blast to hives with excessively high mite counts. Apivar strips (this is the latest application date for adequate mite control before autumn).
Early September through October–Mite control should have been completed by now; if not, use Formic Pro for a quick treatment. Feed pollen sub (and syrup if necessary) to stimulate the rearing of healthy brood that will form the winter cluster. Due to possible mite immigration from other hives, monitor mite levels, and treat with OA* or Formic Pro–half or full dose– if necessary.
Late November/December (as the last sealed brood emerges)–dribble with OA*. OA vaporization also an option.
*We prefer the oxalic dribble in 40% glycerin (Google my “Oxalic Acid Treatment Table”), but OA vaporization will give similar results, with slow-release vaporizers possibly being more effective.
I’m also very impressed by extended-release oxalic acid in glycerin–not the Argentine formula, but the formula at the end of
this article. However, this treatment must be proactive–before mite levels are high, since it takes 50 days for full efficacy. This treatment is not yet legal in the U.S., so I cannot yet recommend it.
Fomic acid details
In late summer, when colonies are full of brood, and mite levels get high, we use formic acid. I’m experimenting with different application methods, trying to find ones that are safe for the applicator, and that don’t cause queen loss in hot weather.
The beauty of formic acid is that it penetrates the cappings if applied at a high enough concentration, leaves no residues in the hive or honey, and gives a colony a new lease on life (the following brood patterns are typically beautiful).
Here’s what we’ve found to work for our double-deep Langstroth hives (may be different if your beekeepers have different hive configurations):
- A single MiteAway Quick Strip (MAQS) between the brood chambers. About 50% mite reduction, but no queen loss.
- Two MAQS between the brood chambers, but with the upper chamber shifted front or back to leave a 2 cm space for ventilation. There may be some loss of already-failing queens.
- Top formic flash, 65% formic applied by a fume board. 30 mL will give a mite knock back, 90 mL will give near complete mite kill, but will also kill some queens in hot weather. The beekeeper can always remove the queen to a cage or a nuc overnight while doing the flash.
- The Nassenheider formic applicator also works, but is more complicated.
- For really high-mite hives, we split down to single boxes, and apply 300 mL of 65% formic absorbed into some cellulose, placed in a plastic bag with around 50 paper-punch holes. This will kill all the mites in the box, but not kill the sealed brood or adult bees (expect to see about 50-100 dead adults the next day). It will kill a large percentage of queens if they are not removed. The treatment takes about a week, but if you add a new queen back, the colony will quickly rebound like new.
- For nucs, you must limit the release rate of the formic. We use one-half of a MAQS, set on the top bars, with the top of the strip tightly covered by either the hive cover or plastic. This limits formic release greatly–it takes well over a week for the strip to go dry.
For further information, I suggest the following articles:
Varroa Strategy
Mite Treatments 1
Mite Treatments 2
Formic acid strips
Oxalic Acid
https://scientificbeekeeping.com/sick-bees-part-12-varroa-management-getting-down-to-brass-tacks/
CCD
Back to the hive: adding another brood chamber
Once the bees have drawn out all the combs in the first brood box, you can add the second brood box (you may wish to add a drone frame for mite trapping).
Do not mix drawn combs with the foundation; just add 10 frames of foundation-this will produce the straightest combs.
We have our best luck at drawing foundation by adding full boxes of 10 frames directly over the brood of strong (at least 10 frames covered with bees) growing colonies during a strong nectar flow.
Keep feeding the colony until all the combs are drawn out. At this point, the brood chamber is complete (assuming a double deep brood chamber), and you should discontinue feeding.

A healthy colony will often create drone comb between the two brood chambers in spring. Always check such exposed brood immediately for the presence of varroa, as it is a decent indicator of brewing problems.
If the colony fails to move up onto foundation in an added box, it is generally due to a lack of nectar flow. In that case, keep feeding sugar syrup. Bees will not draw foundation unless they are “whitening wax” due to intense feeding or an intense nectar flow. It takes a minimum of 2 gallons of heavy syrup to draw the wax on 10 deep combs, and another 5 gallons to fill them with sugar “honey” for winter stores.
Adding Honey Supers
Once the colony has filled two brood chambers (this may not occur in your first year), you can then add a queen excluder (I highly recommend) and honey supers.
Don’t bother adding foundation unless you see white wax. But always add more combs if you do!
Queen Excluders
Bees do not like to draw foundation placed above an excluder (not a problem if you are adding drawn comb, which you will unfortunately not have available your first season). To encourage bees to work through the excluder, I suggest “reversing” the brood chambers if there is a band of honey across the top of the upper combs. This maneuver will place brood directly below the excluder, which encourages the bees to work upward.
Keep in mind that bees will not draw foundation unless they are producing white wax. This rule applies even more emphatically if you are trying to get them to work through an excluder onto foundation.
There is a major advantage of keeping your honey supers free of brood cocoons–wax moth cannot grow on stored “white” combs free of brood cocoons or beebread (not enough protein). By using queen excluders, you can keep your honey combs free of brood cocoons, and thus eliminate the storage problem from wax moth.
Getting a Taste of Honey
OK, you may not get a full honey crop your first season, but you can at least steal a little from the bees so long as you replace it by feeding them heavy (2:1 sugar:water) syrup before cool weather sets in.
Let’s say that the first brood box is full, and now the honeyflow is starting, and you’d really like to get a little honey the first year. Just put on the second brood box, and stop feeding syrup. Let the bees work up and fill the box with brood and honey. Once the honeyflow’s over (usually once blackberry stops blooming at the end of June), you can pull out frames of honey and do a “scrape extraction.” Use a tablespoon to scrape the honey off the foundation into a nylon stocking hung in a gallon jar, then return the scraped frame to the hive, and resume feeding until the bees draw it out again. They will winter just fine on honey made from sugar syrup.
Supering your Hive for Honey Production
I love queen excluders–they allow you to keep the queen out of your honey supers. The world’s largest crops of honey (in Australia) are all produced through queen excluders.
One of the most common complaints is the “my bees wouldn’t go through the queen excluder to draw foundation.” Bees won’t do so until every single cell of drawn comb below the excluder is full of nectar–only then will they work foundation, and as I mentioned above, they won’t draw foundation unless there is a very heavy nectar flow on. Bees also are adverse to crossing a solid “honey band” below the excluder. A Tip: you can encourage them to move through the excluder by rearranging your brood boxes or frames so that there are frames of brood right up to the queen excluder.
Late-Summer Management and Varroa Control
Your colony will generally reach its maximum size around July 1, and get somewhat smaller during the late summer and fall. In the foothills, there is typically a pollen dearth from early July through October, and the colony will minimize its broodrearing. This is good, since it reduces varroa reproduction. Use this window of opportunity to control varroa in August (Apiguard thymol gel works very well).
After you’ve got varroa under control, you may then wish to provide the hive with high-quality pollen sub as below:
Winter Preparation
Colonies winter best (in the foothills) in two deep brood chambers or equivalent, with a total weight (mainly due to honey) of about 130 lbs (you need at least two fingers to tip the hive). If the hive is not heavy by the end of September, feed heavy sugar syrup until it is.
If your apiary is located in a dry area of the foothills, there may not be enough pollen to sustain late summer broodrearing–colonies in these areas greatly benefit from late summer feeding with pollen supplement. Any of the top-tier pollen supplements work–if you don’t see pollen beebread in an arc above the brood, the colony is likely hurting for protein. We typically feed each colony several pounds of pollen supplement during August through October. This may not be necessary if you live in town or in an irrigated area.
The best indicator of protein status is how much jelly the nurse bees are feeding to the young larvae. Larvae fed generously with jelly are called “wet brood.”

When a colony suffers from protein deficiency, the nurses will cut back on the amount of jelly that they feed to larvae. See the photo below for “dry” brood.
 There are eggs in the center cells; larvae being given minimal jelly in the cells below. When we see such “dry” larvae, we immediately feed patties of pollen substitute (see Comparative Test of Pollen Subs).
There are eggs in the center cells; larvae being given minimal jelly in the cells below. When we see such “dry” larvae, we immediately feed patties of pollen substitute (see Comparative Test of Pollen Subs).
There is a trade off regarding the fall feeding of your hives. Colonies that are strong and well fed in fall winter well, but will then also be strong in spring. They will then want to swarm. Escaped swarms later become “mite bombs” that will come back to haunt you. So if you build your colonies up by feeding, please then manage them to prevent swarming the next spring–this will help the beekeeping community overall.
Rust fungus spores
Be aware that in the Sierra foothills, in late summer the bees may be tricked by a fungus into gathering their spores instead of nutritious pollen. The blackberry rust fungus produces sweetened fluorescent-orange spores that the protein-desperate bees will gather and store as beebread–below:

This bright orange beebread consists nearly entirely of rust fungus spores. Colonies quickly go downhill on this diet–note the spotty brood pattern. Don’t let this beebread fool you! If we see this occurring, we give the hives two rounds of 2-3 lbs of high quality pollen sub:

If you live in town, there will likely be enough pollen to keep your colonies healthy during late summer. On the contrary, in dry rural areas, colonies can go downhill due to lack of pollen (and especially if they are gathering rust fungus spores). Feeding pollen sub (above) can rejuvenate those colonies. But be aware that its a trade off–increasing broodrearing also increases varroa reproduction.
It takes a fair amount of pollen sub to provide enough protein–about one 1-lb patty per week (you can feed several pounds at one time if you don’t have Small Hive Beetle in your area).
Winter Prep
Winter prep consists of having the hives off the ground and in a well-drained sunny location. It helps to reduce the entrance. A well-prepared colony (well fed, and low mite level) should need no care during the winter, and will begin to rebuild its population in January. An insulated cover (such as with 1″ of Styrofoam) will help the colony.
Next Spring and Splitting
Most strong colonies will attempt to reproduce in spring by swarming.

If you lift the upper box of the broodnest of a colony preparing to swarm, you’ll typically see swarm cells hanging from the bottom bars as shown above (but there may well be swarm cells elsewhere in the hive). The old queen and a swarm may leave any time once the first cells are sealed.
There are management techniques than can be used to minimize the cues that initiate the swarm impulse (see Minimizing Swarming ). One of the most effective techniques is to simply split the colony. You can split a strong colony in half, or make up to 5 nucleus hives from it. However, keep in mind that there may be a tradeoff between later honey production and “making increase.” Ideally, you’d remove only enough bees to avert swarming, but leave enough for the colony to recover its maximum population just in time for the start of the main honeyflow in your area.
There is also the issue of how to provide queens to the splits–some methods result in a very long break during which the colony will have no emerging adult bees–see the table below:

In general, the more brood that you can put into a split, the better (the amount of brood is more important than the amount of adult workers). Typically, make your splits with at least 5 frames of bees, a ripe queen cell, and a minimum of 2 full frames of brood. But such a split may not make a honey crop in the first year. The same split made with 4 full frames of brood and a laying queen, on the other hand, can well grow quickly and make a honey crop, if immediately given plenty of drawn comb, and fed some sugar syrup.
How to kill three birds with one stone
You can kill three birds with one stone each spring–preventing swarming, requeening, and mite control. See the graphic below:
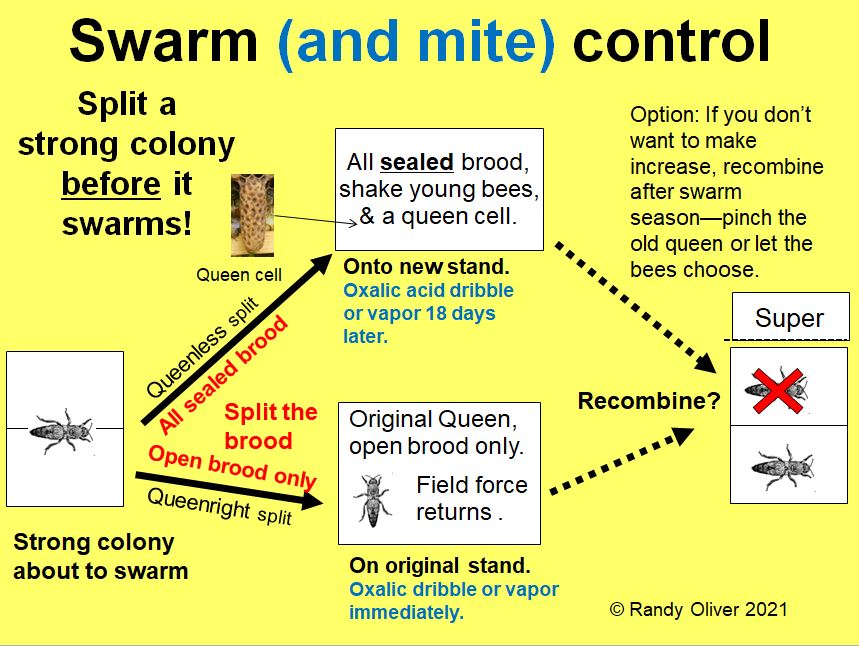
By splitting your colony when it is thinking of swarming (by using either a natural swarm cell or a purchased one), you can use oxalic acid dribbles (at different times) to nearly eliminate varroa from the two splits (by creating induced brood breaks). The small-scale beekeeper can easily manage varroa by using the above or similar creative methods of creating brood breaks.
Miscellaneous
Read all about beekeeping, but remember that there is no substitute for hands-on experience in the beeyard with an seasoned beekeeper. Find a mentor in your area. The easiest test for choosing a mentor is to find someone who consistently has extra bees for sale each spring (not necessarily the one who is most strongly opinionated).
Getting a colony of bees is not like getting a puppy–you expect the puppy to live to old age; this may not be the case with bees. In nature, a large percentage of colonies die each year. Even the best beekeepers may have a third of their colonies die in some years. It helps to keep more than one colony, so that you can split it in spring to make up for losses.
Good luck with your new colony—I hope you have great success this year! If you have suggestions as to how I can improve these instructions, please let me know!

Additional resources:
For a good, standard, no nonsense summary of good beekeeping practices, see
http://www.beesource.com/resources/usda/productive-management-of-honey-bee-colonies/
Contents
Introduction: A Reality Check on the American Beekeeping Industry’s Varroa Control Predicament 2
The Lead Up. 2
The Elephant in the Room.. 2
Our Conundrum.. 3
Our Predicament 3
A Personal Note. 4
Rationalization and Lack of Unreasonable Risk. 4
How the EPA Can Help Us 4
Our Current Arsenal: The Registered Varroacides 5
The Case for Approval of Extended-Release Oxalic Acid (OAE) 5
A Reality Check on Current Options for Mite Control with Natural Miticides 5
Fully Legal Option #1: Use Currently Registered Products 6
Fully Legal Option #2: Personal Use or Sale of Minimal-Risk Pesticides 6
Question #1: “Can I Use Less-Expensive Wood Bleach or Off-the-Shelf Oxalic Acid?” 7
Question #2: “Can I Up the Dose by Using Repeated Oxalic Applications?” 7
Question #3: “Can I Qualify for Experimental Use of Oxalic Acid?”. 7
Question #4: “What if I Say That I’m Using These Treatments for Something Else?”. 7
Question #5: “Can I Feed Aromatic Food Ingredients to Balance my Colony’s Ethereal Energy?”. 8
Question #6: “Am I Going to Get Busted if I Treat my Hives with Generic Oxalic Acid (wood bleach) by the Extended-Release Method?” 8
Coming Next Month. 9
Citations and Notes 9
The Status of Our Industry Regarding Varroa Management
and
What can we do about it?
Part 1
Randy Oliver
ScientificBeekeeping.com
First published in ABJ July 2023
Introduction: A Reality Check on the American Beekeeping Industry’s Varroa Control Predicament
The introduction of the parasitic varroa mite completely changed our industry, leading to a huge increase in rental rates for almond pollination, and a shift for most commercial beekeepers from managing for honey production to focusing upon having enough strong hives in February to fill pollination contracts. Our commercial industry was only saved by its unapproved uses of agricultural miticides. But commercial beekeepers have long known that that would only be a temporary fix. Evolution is catching up with us as varroa evolves resistance to the mainstay of our industry. The times they are a-changin’, and beekeepers are asking, “What are we gonna do?” One problem is that it’s not up to just us to figure out ― we gotta work it out with the EPA.
The Lead Up
The introduction or evolution of a pest, parasite, or pathogen can wreak havoc in agriculture, and the varroa mite did just that for the beekeeping industry. As researchers and beekeepers worldwide desperately scrambled to find efficacious and affordable mite treatments, the frustratingly-slow lag time between discovery and getting a formulated product through EPA’s registration process sometimes left us in the lurch. This occurred first with fluvalinate, granted a Section 18 emergency registration in 1987 to use the neurotoxic miticide for varroa detection by soaking it into wooden strips. Although EPA granted registration of Apistan strips (as a treatment) the following year, the cat was already out of the bag, and commercial beekeepers were soon making up their own inexpensive off-label fluvalinate treatments using the cattle dip Mavrik, which not only became an industry “standard,” but resulted in comb contamination and likely accelerated the evolution of resistant mites.
The situation didn’t improve when, after only about six years, mites started to exhibit resistance to fluvalinate. In response the neurotoxic organophosphate coumaphos got registered as Checkmite strips. Many beekeepers, accustomed to making up treatments at home, very unwisely and unsafely used a cattle dip powder with the same active ingredient (again leading to serious comb contamination).
And when the danged mite very rapidly developed resistance to coumaphos, beekeepers again faced a paucity of registered efficacious treatments, partially triggered by a lawsuit against Miticur (the first amitraz product labeled for honey bees), leading them to start making treatments using amitraz sourced from registered cattle dip products. Off-label use of amitraz soon again became the industry standard, indeed being the sole miticide used for the vast majority of beehives in the U.S. for the last twenty years, despite the registration of Apivar strips in 2013 (in exchange for the concurrent withdrawal of Taktic in 2014). Surprisingly, it’s taken an unexpectedly long time for varroa to show signs of resistance to amitraz.
The Elephant in the Room
Amitraz isn’t the most innocuous thing to be putting into our bee hives. It not only causes adverse effects upon the colony, but also endocrine disruption, neuro-, immuno-, reproductive, and developmental toxicity in mammals such as ourselves [[1]]. EPA was concerned about the total “risk cup” of human exposure to amitraz, and required the registrant to remove its use as a cattle dip in the U.S. in order to register it for use in beehives.
Appallingly, our honey and combs are now contaminated with its degradation products. The elephant in the room is the fact that since 2014 our commercial beekeeping industry has been almost entirely dependent upon the use of amitraz products illegally imported into the U.S.
EPA is aware of this, and issues Stop Sale, Use, or Removal Orders to advertisers on sites such as eBay and Amazon, and has recently taken action (involving fines and prison terms) against some smugglers. Yet, despite the FIFRA Enforcement Response Policy allowing for the issuance of Notices of Warning and penalties of $500 for a first offense, to date there has been little enforcement action taken on the illegal use of pesticides by beekeepers [[2]].
But this may be about to change. The State FIFRA Issues Research and Evaluation Group (SFIREG) has told the EPA, “Working to control and regulate pesticide misuse in the pollinator industry is very urgent” [[3]], and has formally requested “EPA to organize and respond to this important issue topic and to work quickly with SLAs to stop pesticide misuse [by beekeepers].”
The irony of this is that while beekeepers loudly complain about pesticides improperly applied by farmers, they themselves not only are the main source of pesticide exposure to their colonies, but often act as “pesticide scofflaws” themselves ― an irony not lost on the EPA.
But don’t get me wrong — in my opinion, the EPA has been unreasonably restrictive in registering products for us to use for varroa control. An important point not adequately taken into concern by the EPA is that, as opposed to the slaughter of millions of harmless insects, other invertebrates, birds, and aquatic species when farmers treat vast acreages with insecticides, the miticides that beekeepers apply within their hives have virtually no impact on the environment outside those hives. So it’s a valid question as to whether the same rules should apply to miticides that we use for varroa control (other than regulation to minimize any harmful contamination of honey).
A huge frustration to beekeepers is that research has demonstrated that the safe and eco-friendly extended-release application method of oxalic acid could greatly reduce our industry’s dependence upon amitraz, but there’s little sign that an inexpensive and registered formulated product will soon be on the market, leading many beekeepers to make up their own treatments using generic wood bleach (being openly sold by beekeeping supply houses). A few states tried to solve the problem by filing for Section 2(ee) exemptions for extended-release oxalic acid, but those exemptions were predictably rejected by the EPA. Beekeepers are now upset that they lost what they hoped would be a solution to “our varroa problem.”
Our Conundrum
As varroa begins to exhibit resistance to amitraz, our industry is now going into crisis mode, since there are an inadequate number of registered alternative efficacious varroacides on the market to fulfill the widely-varying needs of both recreational and commercial beekeepers ― notably when honey is being produced. Their current label restrictions require excessive time and labor, are physically demanding, and involve unnecessary risk to the applicators when applied to large numbers of colonies during hot weather.
Beekeepers would prefer to be able to control varroa with cost-effective approved miticides. Ideally, we would avoid the synthetic miticides (which contaminate our beeswax and honey with residues), and instead use the more consumer-friendly “natural” treatments. But since there is such a small margin of profit in beekeeping, the often high cost and narrow label application restrictions of registered miticides tempt beekeepers to mix up their own off-the-shelf treatments.
Our Predicament
- Since beekeepers are such a minor industry, there is little financial incentive for Registrants to update their labels, or for companies to develop and register new or improved formulated products for varroa control.
- Beekeepers are partly to blame for this, since we’ve shown the pesticide companies that it may not be worth their effort to reformulate or re-label an already-registered agricultural miticide into an expensive product for application to beehives, when they know that beekeepers will likely just prepare their own treatments from the less-expensive agricultural product, or use off-the-shelf chemicals already on the market.
- So to our dismay, there appear to be few novel varroa control products in the registration pipeline.
- The commercial beekeepers who manage the vast majority of colonies thus feel forced to use active ingredients out of compliance with the law.
- Our state lead agencies and bee inspectors are stuck in the conundrum of knowing that our entire industry is dependent upon such unapproved applications, yet do not want to take enforcement action if the beekeepers have no good alternatives. This puts them in a difficult place.
- Thus beekeepers are highly tempted to use readily available off-the-shelf natural treatments (formic, oxalic, thymol and other plant oils) that present no unreasonable risk to man or the environment and would be of little concern to inspectors, but due to EPA’s stringent requirement to rigidly follow their FIFRA mandate, are not legally allowed.
A Personal Note
As an independent honey bee researcher, supported financially by donations from beekeepers themselves, much of my research (under Pesticide Research Authorizations) involves the development and improvement of “natural” treatments for varroa control. Since I am also a professional beekeeper who successfully shifted from synthetic miticides to natural treatments over twenty years ago (using only oxalic, formic, and thymol in rotation), I’ve been frustrated by some of the shortcomings of the currently-registered treatments and their label restrictions, and publish my research findings on alternative application methods (sometimes collaborating with their Registrants).
But it takes an excruciatingly long time (and sometimes a lot of money) for a Registrant to get a revised application method, or a new formulated product, through the EPA registration process. This frustrating situation precipitated my writing of this “white paper“ as an objective, reality-based status report and assessment of the current predicament that our industry and its regulatory agencies are involved in, the various options that we beekeepers have at our disposal (or are currently using), and potential action items that our industry can take (initially with regard to registration of the extended-release method for application of oxalic acid, but then expanded to see how we might increase the overall availability of additional “natural” treatments for the control of varroa).
Rationalization and Lack of Unreasonable Risk
What our regulators should keep in mind is that people only respect laws that they feel are reasonable. Unreasonable laws tend to be ignored and not enforced.
Humans can rationalize most any behavior, and when a law seems unreasonable, it’s very easy to rationalize “breaking it.” We now have an entire industry that has rationalized using unapproved methods to control varroa.
FIFRA’s mandate is that the application of any pesticide should result in “no unreasonable risk to man or the environment.” It’s obvious to any beekeeper that using oxalic acid, formic acid, or thymol in their hive would in no way create either of those risks. Our regulators need to understand that for us to respect them, EPA’s regulations must make “common sense.”
The EPA’s strict adherence to the letter of the law regarding FIFRA, and to protect themselves from liability or lawsuits, may put them at odds with common sense and beekeeper practical experience. The active ingredients of the organic acids oxalic or formic, or the plant extract thymol, due to their rapid biodegradation, when applied to beehives pose absolutely no unreasonable risk to the environment, and since they all have tolerance exemptions in honey, no risk to the consumer, and with reasonable precaution, no more risk to the applicator than do commonly-used oven or toilet cleaners, bleaches, swimming pool treatments, etc. (Feel free to use this paragraph if you speak to your legislators).
How the EPA Can Help Us
As it is, the beekeeping industry is crying out for help. We simply do not have an adequate number of legal and practical options available to control the mite (notably during the honey flow), especially for the commercial operations that manage the vast majority of honey bee colonies and provide pollination services to the agricultural sector.
Our current situation opens a window of opportunity for the EPA or our legislators to step in to help the beekeeping industry shift from dependence upon a single synthetic miticide, to a range of natural treatments (to better allow us to practice integrated pest management, and to avoid the development of pesticide resistance). It also offers EPA the opportunity to help bring the beekeeping industry into compliance.
I fully support the EPA in its mandate to ensure that the miticides that we are allowed to use present no unreasonable risk to man or the environment, and do not wish for my research findings to encourage unapproved use. That said, potential registrants with whom I’ve consulted find that the procedures and requirements of the EPA make it frustratingly difficult and expensive, not to mention the years of effort involved in the process, to bring to market a registered varroa control product.
Our Current Arsenal: The Registered Varroacides
There are currently only a few registered varroacides on the market (Checkmite + and Sucrocide appear to be unavailable) ― only three of which are approved for application while colonies are producing honey (Table 1) [[4]].
| Table 1. Currently-registered varroacides (“natural” pesticides highlighted in green) |
| Formulated
Product |
Synthetic or
“natural” |
Active Ingredient(s) |
Demonstrated
resistance? |
Comb
contamination? |
Can be used during honey flow? |
| Apistan Strips |
synthetic |
Fluvalinate |
Yes |
Yes |
No |
| Checkmite+ |
synthetic |
Coumaphos |
Yes |
Yes |
Yes |
| Apivar Strips |
synthetic |
Amitraz |
Yes |
Yes |
No |
| Amiflex |
synthetic |
Amitraz |
Yes |
Yes |
No |
| Api Life Var |
“natural” |
Thymol, eucalyptus, menthol |
No |
Slight |
No |
| Apiguard |
“natural” |
Thymol |
No |
Slight |
No |
| MAQS, Formic Pro |
“natural” |
Formic acid |
No |
No |
Yes |
| Hopguard III |
“natural” |
Hop beta acids |
No |
No |
Yes |
| Api-Bioxal |
“natural” |
Oxalic acid |
No |
No |
Yes |
The three registered xenobiotic synthetic chemicals are not only of concern to consumers who consider honey to be a “natural” product, but their persistent nature has caused serious contamination of beeswax and honey, and due to the evolution of pesticide resistance are all now considered to be ineffective or unreliable. Thus our industry is greatly concerned as to how we are going to manage this destructive parasite, and is showing greater interest in the natural treatments, which although trickier to use, have fewer issues with comb or honey contamination.
The registered natural varroacides highlighted in green above, although great products, have label restrictions that limit their utility, or present unreasonable risks, to many of the diverse range of beekeeping operations.
The Case for Approval of Extended-Release Oxalic Acid (OAE)
There is an application method of oxalic acid that is registered by Aluen CAP in a number of countries, but not yet in the U.S. This application method is highly efficacious when brood is present in the hive, can be used during honey production, eliminates the need to remove and restack honey supers (since it can be applied before they are added), and presents less exposure risk to the applicator than the currently-approved application methods. For more details, please refer to Supplemental Information for OAE [[5]].
A Reality Check on Current Options for Mite Control with Natural Miticides
I initially wrote this document for the benefit of our industry leaders, to be used to inform our regulators and legislators. I am by no means a lawyer, but have interpreted Title 7 and FIFRA as best I can (with some guidance from those with experience in various agencies). Allow me to objectively present the current legal options available individual to beekeepers, as well as answers to some questions that beekeepers have asked me about.
Fully Legal Option #1: Use Currently Registered Products
This is the legal default ― to use only registered products per the label instructions. The recent registration of Amiflex paste takes away the excuse by commercial beekeepers that Apivar strips don’t work fast enough. We now have every reason to expect our state lead agencies to start cracking down on the illegal importation and use of amitraz cattle dips. Until there is inforcement action taken against beekeepers who use inexpensive smuggled amitraz, it creates an unlevel playing field ― conferring an unfair economic advantage to those who use unregistered products.
For most recreational beekeepers this is doable, as evidenced by my success in our own operation. But it may not be realistic for larger commercial operations, or for beekeepers with short seasons, or other issues.
The problem with the wait: Registrants are reluctant to go through the process of getting changes approved for their labels. Some have asked Chemicals Laif (the registrant of Api-Bioxal) to submit a change in label request to EPA to add the extended-release application method, or to allow a state to apply for a Section 24(c) Special Local Need Registration. The company does not appear to be interested. The reality is that even if it (or another company) eventually gets a label change or formulated product (such as Aluen CAP) approved, beekeepers across the country will already be using this method (using generic oxalic acid), and may be resistant to purchase a far more expensive registered product. Thus there is little incentive for companies to invest in new registrations.
Fully Legal Option #2: Personal Use or Sale of Minimal-Risk Pesticides
EPA offers individuals and companies the option of using pesticides on the Minimal Risk list without need for registration: “Generally, we do not review products that claim to meet the criteria set by 40 CFR 152.25(f) for exemption from pesticide regulation for companies planning to market such a product. We also do not provide a label review of such products. The producer is responsible to carefully read the criteria and make an evaluation of how the product meets (or does not meet) the criteria.” [[6]]
It is up to beekeepers, researchers, and private companies to develop some Minimal Risk ingredients into efficacious and safe varroa treatments.
This option would not immediately solve the urgent during-the-honey-flow problem, but allows beekeepers, researchers, and companies to experiment with and perhaps quickly develop new minimum-risk application methods or formulated products for varroa control.
Exempted products. Products containing the following active ingredients, alone or in combination with some other substances or inerts, are exempt from the requirements of FIFRA provided that all of the criteria of Section 40 CFR 152.25(f) are met. Refer to [[7]].
| Minimum-risk Pesticides with potential for varroa control |
| Thyme oil |
Citronella oil |
| Clove oil (eugenol) |
Geranium oil |
| Peppermint oil |
Rosemary oil |
| Lemongrass oil |
Cedarwood oil |
| Cinnamon oil |
Citric acid |
All the above oils are readily available and not too expensive. Unfortunately, most of them also exhibit adverse effects on the bees at dosages (typically ~10 mL) adequate to kill varroa [[8]]. One can find plenty of studies on Google Scholar, and some appear to show promise. Our industry should request the USDA and funding agencies to support further research into this list of products for varroa control!
Question #1: “Can I Use Less-Expensive Wood Bleach or Off-the-Shelf Oxalic Acid?”
The only oxalic acid source currently approved for varroa control is that registered and sold by Api-Bioxal, which unfortunately is priced far more expensively than off-the-shelf 99.6% pure oxalic acid. Beekeepers may do dumb things, but they ain’t stupid. To save money, some purchase a bag of expensive Api-Bioxol to keep on hand in case an inspector asks, and then treat their colonies with generic oxalic acid. This is not in any way legal. I’m in no position to guess how likely you are to get busted.
Question #2: “Can I Up the Dose by Using Repeated Oxalic Applications?”
The label for Api-Bioxal states: “Consult state guidelines and local extension experts about best application practices when applying Oxalic Acid Dihydrate when capped brood is present because multiple treatments several days apart will be needed to reduce successive cohorts of adult mites.” But those entities are not allowed to suggest any deviation to the application directions on the label!
There are problems with repeated dribble application, due not only to the labor involved in removing and replacing the honey supers above the brood chambers, but also the known adverse effects that repeated dribbles have upon the colony. This leaves the vaporization method as the only summer option. But vaporization (OAV) with the approved 1-gram dose per brood chamber exhibits very poor efficacy when brood is present, and requires 7-10 applications [[9]]. Recent research has shown that it takes about 3x the approved dosage to attain good efficacy [[10]].
Legality: Since there are no label restrictions for re-application intervals for oxalic vapor, to obtain efficacy when their colonies contain brood, some beekeepers are simply repeating applications every few days (or even during the same day to legally apply a larger dose). Technically, this would be legal. But using off-the-shelf wood bleach instead of Api-Bioxal would be illegal.
Question #3: “Can I Qualify for Experimental Use of Oxalic Acid?”
Until recently, the application of oxalic acid to beehives for pesticidal use remained illegal in California. So to do my research, I obtain a Pesticide Research Authorization from my state lead agency (SLA) each year. But in some states, their SLA defers to the EPA as far as experimental use of pesticides.
The EPA has informed me in writing that the Agency does not require — at the federal level — a beekeeper to obtain an Experimental Use Permit for experimental use of oxalic acid on their own hives (the use must be for experimental purposes only). However, your state may have more stringent requirements. You’d need to check with your state lead agency. It’s not clear to me whether experimental use would apply to generic oxalic acid, or only the use of Api-Bioxal. The beekeeper could not sell any honey produced during the experiments.
Question #4: “What if I Say that I’m Using These Treatments for Something Else?”
When laws appear to be unreasonable, some people skirt the intent of the statute by making disingenuous claims or performing quasi-legal workarounds. I by no means suggest that beekeepers engage in such quasi-legal workarounds, but some beekeepers who feel that they just can’t wait for EPA to approve new application methods for thymol or oxalic acid are covering their backsides by citing Title 40.I.E.152.8, Products that are not pesticides because they are not for use against pests [[11]].
Since Section (b) states that “A product intended to force bees from hives for the collection of honey crops is exempted,” some beekeepers are making the disingenuous claim that they are using thymol as a bee repellent. But if their thought bubble includes killing varroa, they’d be breaking the law.
Others cite Section (b): “The following types of products or articles are not considered to be pesticides unless a pesticidal claim is made on their labeling or in connection with their sale and distribution: (a) Deodorizers, bleaches, and cleaning agents.”
I’ve heard plenty of beekeepers claim that they are “bleaching their top bars,” using oxalic acid dissolved in glycerin as a wood bleach — leaving the pads in the hive between scrubbings. So long as their intent is solely for bleaching, and not for pesticidal use, this practice is technically legal. Again, if your thought bubble contains the word varroa, you’d be breaking the law.
Such clearly-intended workarounds place our state enforcement agents in a tough situation, since they can either wink and turn a blind eye, or pursue a difficult-to-win prosecution (from what I hear, most inspectors are not concerned about either of these ingredients, and are accustomed to seeing various pads in beehives).
Question #5: “Can I Feed Aromatic Food Ingredients to Balance my Colony’s Ethereal Energy?”
The EPA regulates pesticides; the FDA regulates the ingredients allowed to be used in animal feeds for sale. No agency cares much about what food products (or over-the-counter supplements) you feed to your own pets or livestock. Beekeepers are well known for feeding all sorts of things to their colonies to “improve colony health,” and this gets us into a murky area of the law, so long as the feeding is done without the intent of it functioning as a pesticide.
Not surprisingly, any number of plant products that may be found in your kitchen (and that you might even feed to your kids), when introduced into beehives may also inadvertently kill some mites, which would indeed likely improve colony health. Some that come to mind are anise, basil, cinnamon, coconut oil, fennel, garlic, geranium, lemon juice, mustard condiment, oregano, powdered sugar, turmeric, and wintergreen (and this is hardly a complete list). It would technically be illegal to use (or sell) them as varroa control agents, since that would be a pesticidal use, but similar to Honey B Healthy (which contains active ingredients known to exhibit varroacidal properties), it would be legal to feed any of the above food products to one’s colony with the sole intent of “improving colony health,” “stimulating buildup,” or “balancing their ethereal energy.” We clearly need more research on the effects of feeding such food products to our colonies!
Question #6: “Am I Going to Get Busted if I Treat my Hives with Generic Oxalic Acid (Wood Bleach) by the Extended-Release Method?”
Disclaimer: Randy Oliver does not encourage or condone any off-label use of any pesticide.
The sad fact is that beekeepers have unfortunately become accustomed to applying off-label treatments to their hives. Apiary inspectors, understanding our predicament, have long been turning a blind eye toward off-label varroa treatments (although a number are asking EPA for a crackdown on illegal use of smuggled amitraz).
We beekeepers are in the unfortunate situation that the only registered source of oxalic acid costs up to 50 times as much per gram as does readily-available 99.6% pure generic product. And the registered product’s label does not allow for application of it by the extended-release method. One can understand why beekeepers are tempted to skirt the law.
Desperate for an efficacious mite treatment that can be used during the honey flow, beekeepers nationwide are already making up their own extended-release oxalic acid pads (using generic oxalic acid), and will likely continue to do so even if an inexpensive formulated product comes to market. That said, they do face possible enforcement action (most likely a warning) for unauthorized use of generic oxalic acid as a pesticide.
Reality check: It takes no stretch of the imagination to expect that this application method of generic oxalic acid will likely become a “standard” for our industry. This is a bummer, since we would still be pesticide scofflaws, even though it’s obvious that putting some wood bleach into our hives poses “no unreasonable risk to man or the environment.”
Coming Next Month
Our industry leaders have been weighing options for proposed actions that we might take to help with “our varroa problem” and get us into compliance with the law. But similar to the 2(ee) exemptions for OAE requested by a number of states [[12]], we should do our homework before wasting our time (it was obvious that there was no way that the EPA could grant that exemption, since it included an increase in the dosage).
So I took it upon myself to do the homework for our leaders, going over the pros and cons of the various action items proposed for our industry to petition our regulators and legislators for. I’ve been in contact with some leaders as well as our industry lobbyist (and am already sharing information with them). I feel that all of us beekeepers should be fully informed (so that we can express our opinions to our representatives), so will lay out a summary of my assessment of the options for you next month.
Citations and Notes
[1] Del Pino, J, et al (2015). Molecular mechanisms of amitraz mammalian toxicity: a comprehensive review of existing data. Chemical research in toxicology 28(6): 1073-1094.
[2] The Adees did get fined in 2007 for applying fluvalinate and oxalic acid on blue shop towels.
[3] https://aapco.org/wp-content/uploads/2021/08/SFIREG-Letter-to-EPA-for-Managed-Pollinator-Issue-Paper-August-4-2021.pdf
[4] https://www.epa.gov/pollinator-protection/epa-registered-pesticide-products-approved-use-against-varroa-mites-bee-hives
[5] https://scientificbeekeeping.com/scibeeimages/Supplemental-Information-for-OAE.pdf
[6] https://www.epa.gov/minimum-risk-pesticides/minimum-risk-pesticide-definition-and-product-confirmation
[7] https://www.cdpr.ca.gov/docs/registration/sec25/minimum_risk_flowchart.pdf
[8] Imdorf, A, et al (1999) Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie 30(2-3): 209-228.
[9] Oliver, Randy, data sets in prep.
[10] Jack, C. J., van Santen, E., & Ellis, J. D. (2020) Evaluating the efficacy of oxalic acid vaporization and brood interruption in controlling the honey bee pest Varroa destructor (Acari: Varroidae). Journal of economic entomology, 113(2), 582-588.
[11] [66 FR 64763, Dec. 14, 2001, as amended at 73 FR 75594, Dec. 12, 2008]
- 152.8 Products that are not pesticides because they are not for use against pests.
A substance or article is not a pesticide, because it is not intended for use against “pests” as defined in § 152.5, if it is:
(a) A fertilizer product not containing a pesticide.
(b) A product intended to force bees from hives for the collection of honey crops.
[53 FR 15975, May 4, 1988, as amended at 66 FR 64764, Dec. 14, 2001]
- 152.10 Products that are not pesticides because they are not intended for a pesticidal purpose.
A product that is not intended to prevent, destroy, repel, or mitigate a pest, or to defoliate, desiccate or regulate the growth of plants, is not considered to be a pesticide. The following types of products or articles are not considered to be pesticides unless a pesticidal claim is made on their labeling or in connection with their sale and distribution:
(a) Deodorizers, bleaches, and cleaning agents;
[12] https://agriculture.vermont.gov/sites/agriculture/files/Oxalic%202ee.pdf
There is a great deal of argument about how “naturally” we beekeepers should manage our colonies. But the mere act of “keeping” bees implies that it is unnatural. Truly natural “beekeeping” would be to set out empty cavities (hives or other hollows) for swarms to move into (but not more cavities than would “naturally” exist in the landscape − and even that is questionable if the honey bee is an introduced species in your area). Then if swarms happen to move in, the colonies could naturally exist in balance with nature and competition with each other. You could observe them, but any intervention on your part would be unnatural. And when each of them eventually die, you’d wait for a new swarm to move in.
But most of us want to help our colonies to thrive. For my first 20 years of beekeeping, I eschewed feeding any sugar syrup, since it would be “unnatural.” Then one day when I was talking with a commercial beekeeper friend, he said “I don’t know whether feeding syrup is natural or not, but I can tell you that a colony certainly responds positively when fed a gallon of syrup.” That sentence made me reevaluate what aspects of my management were based upon what was good for my bees, as opposed to some idealistic dogma.
For example, it is entirely unnatural to place empty combs above the broodnest (since a natural colony always builds and fills from the top down), but placing empty combs there during a nectar flow sure helps the colony to draw comb and store honey.
Honey bees also evolved to exploit the vertical cavities in hollow trees, and although they can adapt to horizontal cavities, horizontal hives would be unnatural and more difficult for a colony to overwinter in.
As far as being “treatment free” (against varroa), every ethical standard for animal husbandry (including Certified Organic) calls for intervention to help one’s livestock to deal with parasites. There is no excuse for allowing a colony to suffer an ugly death from varroa and deformed wing virus. Selective breeding for mite resistance does not require any colony deaths — the Modified Bond Method (which I personally use) allows one to select for resistance without causing colonies to needlessly suffer.
Anyway, I felt that the following article in ABJ covered the subject of the ethical responsibilities involved in being a bee-keeper, and asked the author to allow me to post it.
Natural Beekeeping: A Reckoning
Fred Jones
First published in ABJ March 2023
“Nature is not benevolent; with ruthless indifference she makes all things serve their purposes.” -Lao Tzu
Natural Beekeeping: A Reckoning “Nature is not benevolent; with ruthless indifference she makes all things serve their purposes.” -Lao Tzu
Less than 50 years ago a young man aspired to a career working with animals. At the beginning of his third year of veterinary school he was assigned a “surgery” dog. Through the course of the semester the dog was subjected to multiple unneeded procedures. After the final surgery the dog was euthanized. Pain medications were not part of post-surgical protocols.
How could a person reconcile a career helping animals with committing such cruel acts on a helpless, abandoned dog? Was it enough to justify these events by claiming “good intentions”? Was it OK to pass the buck and blame the experts, his mentors?
Similar ethical dilemmas are now less common. Guidelines for animal welfare were first developed in 1965 when The Farm Animal Welfare Council laid down a set of conditions meant to apply to all animals under human care. Those conditions, known as the Five Freedoms, have been subsequently adopted globally by research labs, zoos, aquariums, farms, animal shelters, and yes, veterinary schools.
Our changing landscape, the introduction of new pests and pathogens, development of deadly pesticides, and climate change have all played a role in massive colony deaths every year since the 90s. Some claim conventional beekeeping techniques are also to blame. In response, natural beekeeping has gained a broad audience particularly among new beekeepers wanting to save the bees.
An online search for natural beekeeping will result in a multitude of definitions and approaches. Most look to how bees live in the wild as a guide for successful beekeeping. Some reason that human interaction with honey bees is unique in that Apis mellifera has never been fully domesticated. But bees are not alone in the ability to tread the line between life under our care and life in the wild. In the US, populations of donkeys, horses, dogs, chickens, cats and pigs exist and, in some cases, even flourish in nature. Does the ability for a species to survive in the wild imply that copying nature will yield a stress-free existence and better health? Is it valid to simply replicate conditions in the wild as a guide for animal care? Why is there so little promotion of natural husbandry for other species yet is continues to gain traction for honey bees? It has been said that natural beekeeping puts the needs of bees before those of the beekeeper. This article will explore just that. Let’s look at how conventional and natural beekeeping compare under the microscope of the Five Freedoms.
- Freedom from Hunger and Thirst
Honey has been central to man’s relationship with bees throughout history. Evidence for human interaction with honey bees extends back almost 9000 years in the form of cave paintings depicting honey hunters on cliffs. Demand for honey continues to this day with US consumption of honey in 2021 exceeding 600 million pounds. Domestic production, however, provided less than a quarter of that amount and fraudulent importation of adulterated honey is well documented. Yet accusations that harvesting honey from colonies amounts to exploitation is one of the justifications for natural beekeeping.
It has long been recognized that under ideal conditions strong honey bee colonies will produce quantities of honey that exceed colony needs. Knowing how much honey a colony requires for survival is critical to knowing how much surplus can be harvested and is part of the art of beekeeping. That determination is dependent on such variables as when the harvest occurs, the likelihood of a summer dearth, the possibility of a fall nectar flow, the length and severity of winter, and the population of the hive as it enters winter. One strategy is to simply leave the honey on the colony over the course of the winter and harvest the remainder in the spring. Doing so risks the possibility that the honey will crystalize in the comb and extracting crystalized honey can turn an already tough job into a nightmare. Conventional beekeepers weigh these factors and harvest honey with the goal to leave enough for colony needs and allow some extra as a buffer. I once salvaged a colony from a tree cut by a logging crew. It was mid-February and this large colony had only about two pounds of honey remaining with at least a month before a reliable nectar flow. Starvation was inevitable. Sometimes even nature gets it wrong.
Beekeepers, both conventional and natural, are probably in agreement that honey and pollen comprise the best diet for bees. They differ in that natural beekeepers eschew the feeding of sugar and syrup. Conventional beekeepers believe that when conditions merit, the augmentation of a colony’s food stores with pollen patties, fondant, sugar patties or granular sugar can make the difference between survival and starvation.
- Freedom from Discomfort by Providing an Appropriate Environment
Langstroth hives have been the industry standard in this country for 170 years, long before the advent of the massive colony losses that began in the 1980s and continue to this day. Their success is undeniable and claims that this format contributes to recent colony losses is nonsense. Honey bees are remarkably adaptable to a wide variety of cavities. Claims that one type of hive yields a healthier environment for a colony remain unsubstantiated. Research has demonstrated that, given the option, bees in the wild prefer a smaller cavity than what is used by conventional beekeepers. Natural beekeepers often choose a restricted hive size knowing it will result in a greater likelihood of swarming. As will be described later, swarming can have a detrimental impact on colonies.
- Freedom from Pain, Injury, and Disease
Varroa mites and the viruses they transmit are likely the single greatest management problem for beekeepers world-wide. Tracheal mites were at one time of similar concern, however, honey bees have apparently developed a significant level genetic of resistance or tolerance. Will honey bees follow the same path to genetic control of Varroa mites and, if so, how long will it take?
Fortunately, there has been some progress in finding a genetic defense against Varroa. But for it to be effective it must be sustainable and therein lies the problem. Natural beekeepers advocate allowing natural selection to occur. No colonies are treated, thus, colonies susceptible to Varroa infestation perish which will bring an end to their gene pool. Survivor colonies will prevail and carry forth their superior family lines. Complicating this strategy is the polyandry of honey bees. Virgin queens fly to drone congregation areas (DCAs) to mate with multiple drones. DCAs are populated with drones from a variety of sources in the region. Meaningful natural selection would require either isolation as has apparently occurred in some remote areas like the Arnot forest of New York. Without isolation, hybridization with other genetic lines can dilute the desired trait.
Conventional beekeeping approaches disease in the same manner as veterinary or human medicine. Prevention and treatment are the rule and the only compassionate option considered. Imagine if veterinarians proclaimed dogs would no longer receive heartworm preventative in order to allow natural selection to occur. What if physicians no longer treated children for scarlet fever for the same reason? It is simply unthinkable. Instead, many conventional beekeepers utilize queens with known Varroa resistance. When possible they set up drone out yards with bees exhibiting desirable traits. Monitoring mite levels determines which colonies are in need of treatment and which colonies are showing resistance. Until highly heritable Varroa resistance is discovered, monitoring and treatment appear to be the only compassionate way forward.
- Freedom to Express Normal Behavior
Swarming is accepted and encouraged by many natural beekeepers. It is reproduction at the colony level and happens in most wild colonies one or more times annually. Swarming provides a brood break for both the swarm and the parent colony. Since Varroa replicates in brood, these broodless periods can significantly lower mite numbers and transmission of Deformed Wing Virus (DWV). Yet a study done in the UK showed Deformed Wing Virus levels were 2.4 times higher in feral colonies than in managed colonies. Swarming is hardly sufficient to manage mite populations. Research shows that survival rates of swarms in the wild are at best 25% after one year. Even the founding (parent) colony is at risk since their fate is dependent on the successful mating flight of the new virgin queen. All told, swarms result in a lot of dead bees!
While swarming might be considered by some to be natural, it is actually a response to specific hive conditions. During the course of a year most bees raised in an established feral colony will not participate in a swarming event. Swarming is hardly normal day to day behavior. Conventional beekeepers try to prevent the conditions that lead to swarming or they simulate that behavior by splitting crowded colonies in a controlled manner. In so doing they prevent the likely death of over 50% of the colony population. Likewise, reproductive control in other species is sound management practice and well accepted.
- Freedom from Fear and Distress
Champions of natural beekeeping subscribe to minimal colony manipulations claiming these place stresses on bees leaving them less healthy. Indeed, if a beekeeper does not monitor or treat for disease, chooses to use small hives, allows colonies to swarm at will, and does not monitor honey stores or feed if indicated then what need is there for inspections? Another word for this is neglect.
For conventional beekeepers monitoring and working colonies is the heart and soul of our craft. How else are we to know if a queen is failing, a disease is present, how much honey or pollen is present, if a colony is getting crowded, or how well the colony is prepared for winter? What better way could there be for a new beekeeper to learn how to read frames, to gain expertise in recognizing problems and to witness the seasonal rhythms of a colony than to pull frames and experience the sights, the smells and the sounds of the bees? Certainly, it can set back a colony to some extent. But it is a small price to pay for the knowledge gained which leads to a better state of health for the apiary.
The attraction of natural beekeeping is understandable. We are conditioned to hold nature in awe. National Parks and Wilderness areas provide a strong sense of pride for Americans. Advertisers embrace nature; for example large numbers of car and truck commercials are set in the back country. Even our diet is tied to nature; think free range chickens, grass fed beef, nonGMO organic vegetables, and wild-caught salmon. They are all seen as preferable to what is produced from conventional factory farms. Media attention to the plight of honey bees has created a lot of concern. Sadly, it is well meaning people who are drawn into the movement. They are convinced that something is wrong, and that the solution involves a radical change. Natural beekeeping is an easy sell. But making it work is another story altogether.
The reality of natural beekeeping is that it takes a toll not only on honey bees, but also on prospective beekeepers. My evidence is anecdotal, but it is reinforced by conversations with others in local beekeeping associations. Bereft beginners seldom endure more than two years of repeated colony losses. In this regard, natural beekeeping appears to fail in meeting the needs of both the bees and the beekeeper. Will beekeeping follow trends similar to other segments of agriculture with stewardship falling into the hands of a shrinking number of people?
The future of beekeeping is filled with challenges. How do we best deal with invasive pests and pathogens in our bees? How do we select for genetic solutions to disease yet maintain the genetic diversity crucial to the success of honey bees as a species? Studies of feral bee populations can yield important information for improving our knowledge of honey bees, and help to meet these and other threats. The lives of feral colonies should not be a guidebook for beekeepers. The aura of idealism surrounding nature does not absolve us of the duty to think critically about our actions when it comes to keeping bees.
My surgery experience as a vet student led to a lifetime of guilt. Good intentions can have unintended consequences. I now know that even the most charismatic, impassioned experts are fallible. A categorical defense of natural beekeeping may stand on a philosophical, ecological, biodynamic, or Darwinian basis. But when it comes to animal welfare it fails miserably. Wild bees will continue to exist without our help. Colonies under our watch are deserving of nurture and care.
Contents
A Deeper Look into our Data. 1
Was Robbing Involved with Mite Immigration?. 6
Discussion on Robbing. 8
Study Wrap Up. 9
Review.. 10
Next Month. 10
Acknowledgements. 11
Citations and Notes 11
A Study on Bee Drift and Mite Immigration
Part 5
Randy Oliver
ScientificBeekeeping.com
First published in ABJ June 2023
There was clearly drifting of bees from the collapsing colonies, with them presumably carrying hitchhiking mites. But averages and statistics are not nearly as informative as looking at the data from each individual hive.
A Deeper Look into Our Data
Question: Is mite immigration steady or episodic?
Let’s look at the semi-weekly mite drop counts over time for the Receiver hives, again broken down by distance from the Donors (Figure 1).
Fig. 1. Mite immigration followed a remarkably smooth and consistent curve, peaking in October (coinciding not only with Donors collapsing, but also when their mite levels peaked). The colored columns indicate the average semi-weekly per-hive mite drop for each distance group of Receiver hives; the black columns are the total number of drifted bee tags recovered on each date. The low initial rate of tag recovery is likely an artifact of us not beginning our tagging of young bees until 17 September, from which we wouldn’t expect any flight or drift until at least several days later.
The tall red columns indicate higher mite drops in the single Receiver at 15 feet, but surprisingly, there wasn’t much difference in average mite drops between the 60- and 500-foot groups of Receivers.
Also note the substantial decrease in mite immigration beginning on 25 October. It didn’t appear to be any change in the weather (Figure 2).
Fig. 2. Days were warm and sunny throughout the duration of this study.
One thing that had changed was that the high-mite Donors had largely collapsed by that time, so the amount of apparent mite immigration did appear to coincide with the two episodes of Donor colonies collapsing.
Question: How closely did mite immigration correlate with the drifting of tagged bees?
The black columns in Figure 1 indicate that there was often a correlation between the drifting of tagged bees and mite immigration. But since we were intentionally tagging bees during the process of each Donor colony collapsing, it’s not surprising that there would be a correlation. So let’s break the data down by hive (Figure 3). (Reminder on hive numbering: D1 means Donor #1; R1 means Receiver #1).
Fig. 3. For some Receivers, the correlation between mite drop and incoming drifted tagged bees was strong; for others it was weak. So it’s difficult to draw conclusions (especially since the chance of a drifted bee carrying one or more mites is likely random). Again, since we tagged such a low percentage of bees per Donor, it’s not clear whether the immigrated mites came mainly from the Donors, or from unknown colonies (managed or feral) in the neighborhood.
An important qualifier as far as considering this as a longitudinal study: This study was set up to determine whether the relatively-sudden increases in mite infestation rates in our colonies was due to mite immigration, and from colonies at what distance. For that reason, we intentionally tagged bees in the Donor hives when we observed that their mite levels were peaking and they were undergoing the process of collapse. Thus the overall correlation between tagged bee recovery and mite immigration is likely at least partially an artifact of the timing of when we tagged bees. So although the mite drop counts qualify as a longitudinal study, our tag recovery data are skewed by our uneven tagging of bees week by week. Thus the emigration of our tagged bees may not reflect the actual timing and rates of emigration of the number of untagged mite-carrying bees that drifted to the Receivers (and their associated transfer of mites).
Interpretation: It’s not clear whether the drifting of tagged bees (and mites) was the result of simple diffusion of bees drifting in both directions (Donor-to-Receiver and Receiver-to-Donor), or rather due to there being an increased exodus of bees from colonies as they collapsed. Nonetheless, despite the wide hive-to-hive variations in the total drifted tag recoveries and total apparent mite immigration over the course of the entire study, it may help to break that data down further.
Another way of looking at our data is to use a scattergram (starting on 1 October when we started to recover tags). This allows us to determine, for each hive check, the degree of correlation between the number of drifted tags, relative to the corresponding mite drop count for that specific time period, independent of calendar date (Figure 4).
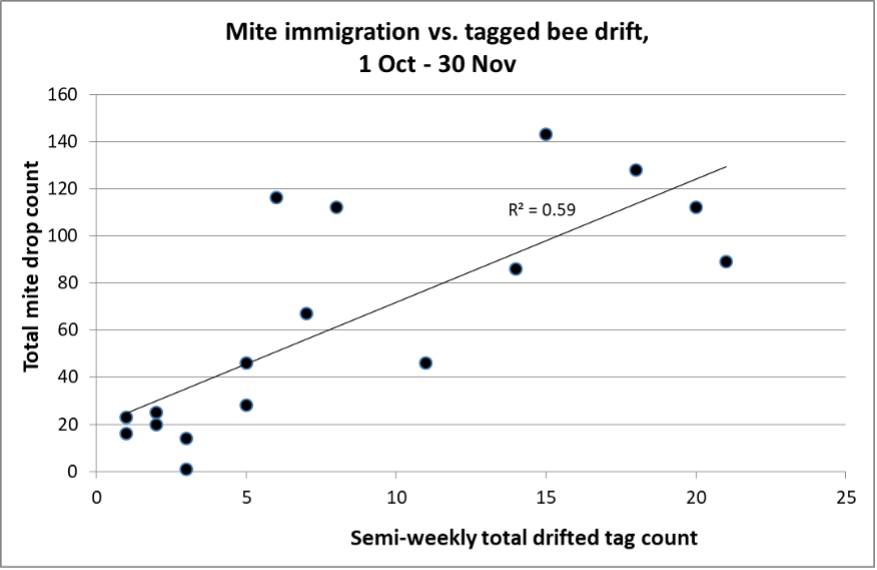
Fig. 4. Viewed this way, there was a remarkably strong correlation between the number of drifted tags from the Donor hives, relative to the number of apparently immigrated mites on the stickyboards (the counts are not ordered by date).
Since we don’t know for sure the source colonies for all the immigrated mites (and since the tagged bees represented such a small proportion of the Donor hive workers), it’s impossible to draw firm conclusions from this chart ― the correlation may well have been even higher if there had been no other colonies within flight range, or if we had tagged a larger proportion of the workers in the Donor hives.
Interpretation: Although tagged bee drift and mite immigration both coincided with the collapses of the Donor colonies, we didn’t begin tagging bees early enough, or consistently enough, to tell whether the process of collapse caused an exodus of drifting mite-ridden bees (Mite Bombs), or whether it was simply due to normal drifting. One would need to repeat the study ― tagging the same number of bees each week — to find out. In any case, it certainly appears that there was a strong correlation between the drifting of tagged bees and mite immigration. That fact suggests that the immigrated mites did at least largely come from the Donors, rather than from unknown hives, since those unknown colonies would have needed to have been shedding mites on the same days that our Donor hives were dispersing drifting tagged bees.
We can take even a closer look at our data, which I’ve broken it down in two charts — one for the close Receivers, the other for those in the 500-foot group (Figures 5 & 6). Remember that some Donors collapsed in mid-to-late September, the rest in mid-to-late October, which is reflected in the stickyboard counts (in this case ordered by date).
Fig. 5. For the Receivers located near the Donors, it appears that some were “mite magnets” (red and purple), whereas others suffered from little mite immigration.
Fig. 6. Similar to the close Receivers, some of these more-distant Receivers consistently dropped more mites than others, independent of their distance from the Donors.
Interpretation: What surprises me is the consistency of the apparent mite immigration into some of the hives, whereas in others it would go up and down (as I would expect from random drift). And as far as my nagging question about whether the high-count receivers were generating their own mites, take a look at the sky-high stickyboard counts of R5 and R7 (red columns), which I inspected on 17 October, and confirmed that they were essentially mite free.
Note that by early November, the stickyboard counts dropped to very low numbers, despite there still being plenty of bee flight due to the weather remaining warm.
We have only one last question to go:
Was Robbing Involved with Mite Immigration?
Question: Did mite immigration Correlate with Robbing at My Location?
Earlier in this series I pointed out that although healthy colonies may rob mite-infested colonies if they contain ripening nectar at the time of their collapse, that didn’t happen here. We had placed scales under both our Donor and Receiver hives in order to see whether the Donors lost weight during collapse (which would indicate that they were being robbed), or whether the Receivers gained weight during our dearth (indicating that they had robbed a collapsing colony) (Figures 7-9).
Fig. 7. An example of what I looked for ― a Donor losing weight at the same time that a Receiver was gaining it. I’ve aligned these two snips by date, to show D8 losing weight while R4 gained it. But looking back to Figure 5, one can see that R4 exhibited minimal mite immigration during that time period. So even if it was robbing, it wasn’t bringing back mites.
Fig. 8. A screenshot of scale data for the Donors [[1]].The only sudden weight losses that we observed were for Donor D6 and D8 (not shown in this chart) ― the rest of the Donors showed no indication of getting robbed during their collapse. But the bulk of those weight reductions may well have simply represented the body mass of the workers that abandoned the hive (the bees covering 8 combs weigh roughly 4 pounds).
Fig. 9. A screenshot of typical scale data for the Receivers, with weight gains by R4 and R7.
Discussion on Robbing
In my arid climate ― in which there is no appreciable nectar flow in late summer — we saw no indication of weight loss due to robbing taking place as the Donors collapsed, nor any appreciable weight gains to suggest that the Receivers were robbing other colonies.
This is not to say that mite immigration can’t also be associated with robbing in areas in which robbing does take place as colonies collapse, such as where nectar is ripening in the hive at time of collapse.
Even in those areas, actual field data indicating that robbing is more of a factor than is drifting, is (to date) largely circumstantial. Although it “makes sense” that mites would exhibit a behavior to hop on robbing bees for a ride to a new host colony, one thing that I’ve found out over the years is that just because something “makes sense” to the human mind, that doesn’t necessarily mean that it actually takes place with honey bees. So let’s take a look at the evidence.
Ever since Del Piccolo [[2]] reported that mites “preferred” nurse bees over pollen foragers, apparently due to differences in their cuticular hydrocarbons, it’s been pretty well established that there is benefit for a mite to find a nurse bee to feed upon (reviewed by Xie [[3]]). My own testing [[4]] confirmed that non-flying (presumably young) workers typically exhibit higher infestation rates than do flying workers sampled from the same comb.
But Cervo [[5]], discovered (via GC-MS) that as the varroa infestation rate of a colony increased, the difference in odor between nurse bees and foragers disappeared, as did the preference of mites for nurses over foragers (as determined by host-preference tests in petri dishes). That finding would suggest that the mite infestation rate of foragers, relative to that of the nurse bees, would increase as the infestation rate grew — thus resulting in a larger number of mites on the older workers drifting from highly-infested colonies.
But what happens in the case of foragers from low-mite colonies robbing out high-mite colonies? Peck and Seeley [[6]] observed a circumstantial connection, leading us to wonder whether the mites in dying colonies recognize the robbers as being “foreign,” and then hop onto them in order to catch a ride out of their sinking ship (this would make “total sense” since it would aid in horizontal transmission). But alas, Cervo’s findings do not support that hypothesis — they found that mites “did not show any preference between homocolonial and heterocolonial foragers at any mite abundance of the colony.”
And in perhaps the only study in which mite levels on returning foragers were quantified, DeGrandi-Hoffman [[7]] found that:
We did not detect high proportions of [foragers with mites] during any sampling period as would be expected if foragers were robbing heavily infested collapsing colonies. Though increases in mite numbers due to robbing probably occur, our data suggest other possible explanations for unexpected growth in mite populations. [Foragers with mites] comprised at most 2–3 % of all the foragers we captured in a sampling interval.
DeGrandi-Hoffman’s study took place in Tucson, Arizona, where there are late-season nectar flows [[8]], so it’s not clear whether robbing was taking place. I encourage further research to explore the actual amount of mite transfer that takes place due to robbing!
Field Study Wrap Up
The weather finally turned cold in late November (Figure 10).
Fig. 10. Ambient temperatures remained high well into November
This gave me a chance to confirm that our Receivers were still mite free. We took our last two stickyboard counts on 26 and 30 November, during the interim in which it finally got cold (and rainy) enough to prevent bee flight (and thus mite immigration due to robbing or drifting bees). The stickyboard counts on the 26th were 9,,6,1,2,8,5,2,4,14,7,10,2. By the 30th they had dropped to 0,1,0,0,3,0,0,0,0,0,1,0. Again, strong evidence that the Receivers were not producing their own mites.
I also checked the Donors—all were devoid of bees, other than D4, D5, D7, and D8, which had tiny clusters just under the lid. And I only observed a single remaining tagged bee (in D8). With that, I wrapped up the study.
Review
So what are the take-homes from our study?
- I set up this trial to determine whether the late-season increase in mite infestation rates in our hives could be due from the drifting of bees from infested colonies outside our apiary. We found that there can be substantial bee drift into hives a ½ mile distant, and some drift to colonies a full mile away. Thus we now have hard evidence to support just how important it is to encourage your neighbors to manage varroa in their colonies!
- We counted nearly 3000 apparently-immigrated mites from our Receiver hives ― an average of 245 mites per hive. Those immigrated mites were not evenly distributed, but even in the hives receiving the most mites, those immigrated mites would only have increased the infestation rates of those colonies by about 1% (3 mites per half cup of bees). So even with 9 colonies collapsing in this apiary, we could hardly blame any large increases in mite wash counts in the surrounding colonies solely upon mite immigration from other hives.
- Other research has shown that a substantial percentage of workers may drift between hives in closely-spaced apiaries. In this trial we know from recovered tags that at least 4% of the bees we tagged drifted into the 21 hives that we monitored, but since there were also unmonitored hives in the area, that 4% figure is clearly an underestimate of the total amount of out-drifting that actually took place. Based upon the strong correlation between tag recovery and mite drop, there is reason to suspect that our sporadic tagging of bees (in an attempt to coincide with collapse), likely underestimated the true degree of drifting of mite-infested bees that actually occurred.
- There was far more drift to distant colonies than would be expected by chance. This finding is completely contrary to “common sense,” and shows how little we understand how and why bees drift. It also brings into question recommendations regarding the spacing of hives in an apiary to reduce drifting.
- There was a consistently large variation in mite immigration between hives that were side-by-side at various distances from the Donors, indicating that some colonies appear to be more (or less) attractive to (or willing to accept) drifting bees than others (although the amount of mite immigration did not necessarily correlate with the amount of entered drifted tagged bees).
- We observed that the most intense mite immigration into the Receivers took place when the Donor colonies were at their highest infestation rates (and also collapsing). It’s not clear whether mite immigration was mainly the result of infested workers abandoning colonies and seeking out other hives as they collapse (the “Mite Bomb” hypothesis), or simply due to the continual mite diffusion associated with “normal” bidirectional bee drifting.
- In our environment, without a substantial nectar flow occurring during the study, the collapsing colonies did not get immediately robbed, and there was no correlation between weight gain (indicating robbing) and mite immigration.
Update August 2023: I brought a number of colonies with very high mite counts (30-70 mites per half cup of bees) to my home yard in order to harvest live mites from them for my studies. To my dismay, a number of them got robbed out as their populations were collapsing. Since I was inspecting the colonies on a regular basis, I was able to closely watch the process of collapse and then robbing.
Once a colony reaches the DWV “tip point” (mite counts around 50 or above), its amount of brood starts to decrease, and the worker population begins to collapse rapidly (by up to two frames of bees per day). And once the cluster can barely cover three frames, in a crowded yard, robbing pressure can become too much (isolated colonies with only a handful of workers are able to defend themselves in my area). Once robbing starts, it explodes! No noticeable robbing in the morning, but within an hour of when it begins, the hive is mobbed by robbers. Although the colony’s workers valiantly attempt to repel the robbers, they get overwhelmed, and a double-deep hive is typically “cleaned out” of honey within a day or two.
The Aftermath
When I inspect the hive immediately after robbing ceases, the combs are devoid of honey, and much of the brood has disappeared. There are dead bees on the bottom board, but I do not know whether they were defenders or robbers. But there is often a remaining reformed cluster of live, hungry bees – they do not necessarily die or drift away. That cluster may represent a substantial proportion of the workers present prior to the initiation of robbing.
I was curious this morning when I inspected a colony that had been robbed out over the past two days, whether the remaining bees had given up their mites to the robbers. As much as I hated to sacrifice those poor survivors, I took a sample of a half cup of them and washed the mites off them.
There were too many to count in a pile, so I divided them up and counted them twice. There were 117 mites on those approximately 315 bees! It appears that their mites had not hopped off on the robbers to any extent, since my mite wash count from the same colony a few days earlier had been only 44.
Next Month
My purpose for undertaking this study was to answer my question “Are the seemingly-rapid spikes in our mite wash counts in late summer mainly due to the immigration of mites from other hives?” That explanatory hypothesis is extremely attractive, since it puts the blame on someone else, rather than on one’s own inadequate varroa management. But I’m not sure that it holds water. Next month we’ll look at additional hard data, and run some simulations.
Acknowledgements
Thanks again to my helpers Brion and Alice Dunbar, Sandy Honigsberg, Anna Mudd, and Brooke Molina. And to Dr. David Peck for reviewing my data.
Citations and Notes
[1] Due to unfamiliarity with data preservation for the scale hives, we lost all our raw data when we removed the batteries from the scales at the end of the trial. Luckily, I had been following the weight data during the course of the trial, and had taken a few screenshots. I fully expected to see evidence of robbing correlating with mite immigration, but did not.
[2] Del Piccolo, F, et al (2010) Selection of Apis mellifera workers by the parasitic mite Varroa destructor using host cuticular hydrocarbons. Parasitology 137(6): 967-973.
[3] Xie, X, Z Huang, & Z Zeng (2016). Why do Varroa mites prefer nurse bees? Scientific Reports 6(1): 1-6.
[4] https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-4/
[5] Cervo, R, et al (2014). High Varroa mite abundance influences chemical profiles of worker bees and mite–host preferences. Journal of Experimental Biology 217(17): 2998-3001.
[6] Peck, D & T Seeley (2019). Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PloS one, 14(6), e0218392.
[7] DeGrandi-Hoffman, G, et al (2016) Population growth of Varroa destructor (Acari: Varroidae) in honey bee colonies is affected by the number of foragers with mites. Experimental and Applied Acarology 69(1): 21-34.
[8] Nightingale, J, et al (2008) Assessing honey bee equilibrium range and forage supply using satelite-derived phenology. In IGARSS 2008-2008 IEEE International Geoscience and Remote Sensing Symposium (Vol. 3, pp. III-763). IEEE.
I liked this article by Ernie, since it well reflected the typical learning curve of a newbee. The article originally appeared in the Summer 2023 edition of BeesCene, the journal of the BC Honey Producers’ Association, and is here included by permission.
Contents
Distance that Worker Bees will Drift 1
Effect of Distance upon Bee Drift 4
Hive-to-Hive Variation in Captured Tags 7
Validating our Methodology. 8
Mite Immigration: Results of the Stickyboard Counts. 10
Cumulative Mite Immigration. 11
Next Month. 12
Acknowledgements. 13
Citations and Notes 13
A Study on Bee and Mite Drift:
Part 4
Randy Oliver
ScientificBeekeeping.com
First published in ABJ May 2023
The results that I presented last month suggested that perhaps 5-10% of our tagged bees drifted to other colonies in the neighborhood, and (surprisingly) that drifting didn’t appear to be necessarily correlated with either the degree of mite infestation of the bees, nor the timing of collapse. So let’s move on to what else we learned.
I left off last month with a number of yet-unanswered questions. So let’s start off with one of great interest to beekeepers:
Distance that Worker Bees will Drift
Question: How far from collapsing colonies will bees (and hitchhiking mites) drift?
In order to answer this question, we had placed magnetic entrance traps on 24 Receiver hives at distances from 15 feet to three miles from the Donor hives (Figure 1).

Fig 1 The approximate layout of the Receiver hives. The Donor hives were at the center of the golden star. Drifted tag recovery is indicated in the green boxes. We recovered the vast majority of the tags from our Home yard, but also 13 tags (6% of the total) from two of the outyards.
Although we started tagging bees on the 17th of September, we didn’t recover our first tag in an outyard (a half mile distant) until nine days later (Figure 2).

Fig. 2 Bingo ― our first tag in a distant outyard! It wasn’t until nine days after we initiated tagging that we recovered the first tag from an outyard (the white dot at the tip of my thumb) — proof that bees can indeed drift over a wide neighborhood. The arrow indicates the direction to the Donor colony back home ― a half mile distant (and over two valleys). Despite placing magnetic traps on only three of the 30 hives in this outyard, we still recovered, over the course of the study, 11 tags from drifted bees (from seven different Donor colonies), indicating that substantial worker drift at this distance was “normal.”
How substantial? Since we only tagged roughly one bee out of 30 workers in each Donor hive, and placed magnetic traps on only three hives out of thirty at this yard, we can extrapolate that those 11 recovered tags suggest that a total of roughly 3000 bees drifted from the 11 Donor colonies to this yard a half mile distant!
Keep in mind that this experiment was run in an area with diverse landscapes, vegetation, and elevations (Figure 3), so we don’t know how this finding would apply to flatlands.

Fig. 3 This map shows the landscape between the Donor colonies at my place at the top, and our apiary to the south where we recovered the tags from 11 drifted bees. The road is at the bottom of a valley. We were amazed by the amount of drift to this location, not only due to the distance, but also because it involved flight over forest, two valleys, across a hayfield, and into an apiary hidden by oak forest. Why the heck would so many bees drift to strange colonies so far away?
We also recovered another two tags from three hives (out of thirty), those hives were well-hidden by trees and a fence, at a distance a full mile in the opposite direction, indicating that there was appreciable bee drift occurring even at that distance. This leads us to another question:
Effect of Distance upon Bee Drift
Question: How does the drift of bees to nearby hives compare to that to those more distant?
Figure 4 shows average tag recovery per Receiver hive by distance from the Donors.

Fig. 4 What really surprised us was how great the drift was to the hives 500 feet distant, compared to those in plain sight of the Donors at 60 feet. Not only were those Receivers located uphill, there were several buildings, trees, and other obstacles and landmarks between them and the Donors.
The above distribution of drifting bees was completely unexpected, and certainly wouldn’t be expected by chance. Take a look back at Figure 1 — for each distance from the Donors, the surface area of the landscape increases by the square of the radius [[1]]. Thus the likelihood of a drifted bee randomly landing near another hive decreases exponentially with distance.
So I ran some calculations, based upon two assumptions: (1) since there was minimal nectar flow taking place during this time period, there would be no reason to expect that a drifted bee would be more likely to fly near any particular Receiver hive location than another, and (2) the presumably false assumption (since we know that workers tend to forage as close to their hive as possible) that a drifted bee would be as likely to land a mile from its hive as it would to land more closely (which would underestimate the calculated disparity between the expected and observed values (Table 1 and Figure 5).
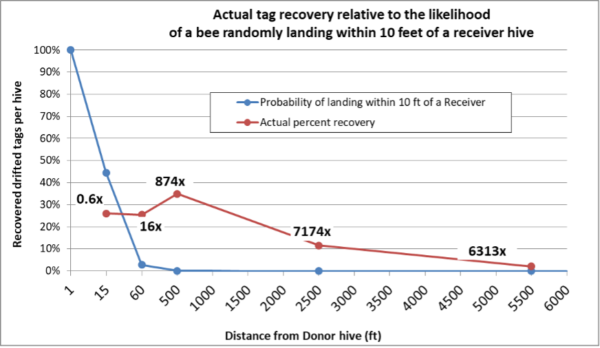
Fig. 5 The blue plot indicates the likelihood by chance of a drifted bee landing within 10 feet of a Receiver hive relative to the surface area of the landscape encompassed by that distance (the probability quickly approaches zero). The red plot is our actual percent tag recovery. The boldface numbers indicate the proportional increase over what would be expected by chance. As explained above, the fold-increase numbers are likely underestimations.
Our data clearly indicate that the drifted bees didn’t just randomly wind up in distant hives by chance — something clearly drew them to those hives.
Interpretation: The above was the most unexpected (and interesting) finding (to me): that our tagged bees drifted into more distant hives at a rate disproportionally far greater than expected by chance. Both scientific literature and field observations indicate that honey bees exhibit remarkably accurate navigation and homing ability. I can understand how a returning bee might drift to an adjacent hive, but why would the rate of drift to distant hives be so disproportionately high?
Perhaps foragers are more likely to get lost, disoriented, or confused as they get further from their homes. Could it have been because during my study — due to little nectar or pollen flow taking place — that the foragers were exploring further from their hives than they would have been had nearby forage been more abundant? One explanatory hypothesis would be that that when a bee gets disoriented, that it simply flies upwind toward the scent of the nearest hive. Or are some foragers simply unable to resist the alluring scent of another colony? This is a question crying for more research — which would be easy to do with these magnetic tags and traps.
The above discussion brings up another one of our questions:
Hive-to-hive Variation in Captured Tags
Question: Are some colonies more (or less) attractive to drifting bees (and thus mites) than others?
This subject was raised in this very journal clear back in 1932 by George King [[2]], who described the results of his study (at the University of Illinois) on the drifting of marked bees:
It was found that the amount of drifting between colonies was often over 30 percent of the total number of bees. [His test colonies were in a row, spaced 4-8 feet apart].
He noted that:
There are factors other than the distance of colonies from each other which influence drifting.
King wrote that:
Frequently one colony appears to outyield other colonies near it, and so the queen in it is selected as a suitable mother to requeen the apiary. She should be a choice queen if she is responsible for the extra showing made by her colony. However, if workers reared in neighboring colonies have drifted into her colony without an equal drift back into them, the queen cannot be credited with the results shown.
Dr. Jerry Bromenshenk (pers comm), using entrance counters, also found that some colonies gained strength due to consistently having more bees entering than exiting — indicating that drifters were continually adding to the populations of some hives in an apiary (the reverse was also true). Jay [[3]] and Pfeiffer [[4]] found that with hives placed in a row, those at the ends may pick up additional drift, but Bromenshenk found that it could also be independent of relative placement.
King’s concluding remarks were prophetic:
There are so many things to consider in beekeeping of which so little is known that more information is certainly desirable. One of the things which will bear considerable investigation is drifting.
Ninety-one years later, I couldn’t have said it better myself! Now back to our findings …
In Figure 6 we can see that some of the home yard Receivers appeared to be more (or less) attractive to drifting bees (I’m not clear whether guarding behavior made a difference, since a drifted bee only needed to make it to the entrance to be snagged by the magnetic trap).

Fig. 6 I’ve color-coded the hives by their distances from the Donor hives (the nonmagnetic capture from the Donor hives is not shown). Note that in any distance group, some colonies appeared to be more (or less) attractive to drifting bees than others (despite all hives being identically sized and colored, mostly of the same bee stock, and of roughly equal strength).
So how about my haphazard choices for the three hives to which I applied traps out of the thirty in each outyard? I have no idea as to whether they were individually attractive or unattractive to drifting bees ― so they may have over- or underestimated average drift to those yards.
The question then arises as to whether colonies that attract or allow more ingress of drifting bees also suffer more greatly from mite immigration. So let’s move on to what we found about the association between worker drift and mite immigration:
Validating our Methodology
In order to quantify mite immigration, we had to maintain Receiver colonies that were virtually free of mites (and mite reproduction). We did this by use of continual miticide treatment. We started taking formal stickyboard counts on 17 September — later than I’d hoped for, since we were already seeing substantial mite drop in our preliminary checks. This made me wonder whether our Receiver colonies were really mite free. So we performed alcohol washes on two dates to confirm (Table 2).
Despite all colonies still exhibiting very low infestation rates of the adult bees (which could have been from recently-immigrated mites), I was still curious whether R5 and R7, which exhibited consistently high sticky counts, were generating their own mites. So on 17 October I dissected 100 cells of white-eyed pupae to pre-emerging adults from R5 (Figure 7). There were zero mites. R7 was completely lacking sealed brood — no larvae were past third instar, indicating that the Carniolan colony had shut down brood rearing some time ago, only to restart a few days before inspection (so no mites could have been in the brood). These findings strongly supported that their stickyboard counts were apparently due solely or at least mainly to incoming mites.

Fig. 7 I didn’t find evidence of a single mite in the hundred pupal cells that I dissected, confirming that little or no mite reproduction was taking place in the colony with the highest daily mite drop.
Field research note: It’s important for a researcher to continually question not only their interpretation of their data, but also the validity of their methodology.
Still, I questioned whether, despite my repeated zero mite wash counts, brood dissections, and the final November rainy days check, whether our stickyboard counts truly reflected mite immigration, as opposed to surreptitious mite reproduction within the colony. So in 2019 I replicated treating receiver colonies and taking stickyboard counts from six colonies in the same yard over roughly the same time period. This time the weather was even more helpful! Take a look at Figure 8.
Fig. 8 This time there were three flight-suppressing weather events, which again confirmed that the mite drops in treated Receiver hives was due to drifting bees.
O.K., now that I’m comfortable with our methodology and data, let’s see what we found.

Mite Immigration: Results of the Stickyboard Counts
The collapsing Donor colonies were rife with mites on the workers (Figure 9).

Fig. 9 By 15 October several Donor colonies were clearly collapsing — most with plenty of marked bees still on the combs. Note the high varroa infestation rate in this photo from a collapsing colony — here you can see two workers with a mite on their back (keep in mind that most mites ride on the belly).
So let’s answer some more of our questions, starting with two that we can answer at the same time:
Cumulative Mite Immigration
Question 1: How many mites actually immigrated into our Receiver hives in late summer and fall?
Question 2: Do all colonies suffer equally from mite immigration?
We recorded semi-weekly stickyboard counts from the Receiver hives, and counted a total of nearly 3000 mites, the vast majority of which were likely immigrants. So let’s take a look at the cumulative counts (Figure 10).

Fig. 10 Receivers R5 and R6 (at roughly 60 feet from the Donors) had the highest immigration totals, whereas the adjacent R3 and R4 had the lowest ― go figure! And it’s clear that the amount of mite immigration differed greatly from hive to hive, regardless of placement. Overall, the Receiver colonies received an average of 245 apparently-immigrated mites over this time period (R5 got over 500).
Practical application: On the same property, with nine collapsing colonies, there was huge hive-to-hive variation in the number of mites that hitchhiked into Receivers up to 550 feet distant. Surprisingly, the amount of immigration wasn’t related to their proximity to the collapsing hives.
The 160 tagged bees that we know drifted to the 12 Home Receiver hives may not seem like a large number, but keep in mind that we only tagged an estimated 1/30th of the total number of workers in each donor hive (which started at average cluster sizes of greater than nine frames covered with bees), and then recovered tags from only 2.7% of those. So we can extrapolate that on average, each Home Receiver had an average of 360 Donor bees drift into it, the vast majority of which would have been untagged.
Practical application: Even with nine colonies collapsing from varroa in the Home yard, the above calculated amount of heavily-infested drifting bees could only account for an average of about 70 out of the average 245 mites that showed up on the stickyboards. This is a nagging question to me — did most of those mites come from elsewhere, or did our recovery of tagged bees not fully represent the actual degree of bee (and mite) drift from the Donors that actually took place? We may be able to answer this with a deeper analysis of our data.
Next Month
In the next installment, we’ll take a deeper look into our data, and address our remaining questions:
Question: Is mite immigration steady or episodic?
Question: How closely did mite immigration correlate with the drifting of tagged bees?
Question: Did mite immigration correlate with robbing at my location?
Acknowledgements
Thanks again to my helpers Brion and Alice Dunbar, Sandy Honigsberg, Anna Mudd, and Brooke Molina.
Citations and Notes
[1] Surface area = 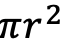
[2] King, GE (1932) Drifting bees may make production records of little value. Am. Bee J. 72: 141-142.
[3] Jay, SC (1969). Drifting honeybees in commercial apiaries v. Effect of drifting on honey production. Journal of Apicultural Research 8(1): 13-17.
[4] Pfeiffer, K & K Crailsheim (1998) Drifting of honeybees. Insectes Sociaux 45: 151-167.
Contents
The Donor Colonies 1
Tagging. 1
Results and Discussion. 2
Did the Tagged Bees Behave Normally?. 2
Progress of the Colonies. 4
Answering our Questions 4
Observations from the Donor Hives. 5
Correlation With Varroa Infestation. 6
Tag Recovery by Date. 8
Timelines of Recovery of Drifted Bees 9
Relevance to Mite Immigration. 10
Acknowledgements. 13
Citations and Notes 14
A Study on Bee Drift and Mite Immigration:
Part 3
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ April 2023
Last month I showed how we set up a field study to try to answer a number of questions about mite drift from collapsing colonies. Let’s continue.
The Donor Colonies
We had set up two mite-free Control Donor hives, and nine high-mite Donor hives intended to collapse (the tenth collapsed before we were ready). Colony strengths averaged nine frames covered with bees (range 7-13). Starting varroa mite infestation rates of the high-mite Donors (determined by alcohol wash) averaged 26 mites (range 11-55, per level half cup of bees [[1]]).
Tagging
At the start of the study on 17 September, we glued color-coded steel tags onto 50 non-flying bees in each hive, and then later tagged additional cohorts of bees (100-250 at a time) when their colony’s mite count exceeded 30, or we observed signs that collapse was imminent (loss of strength, deformed wings, or dying pupae). In some Donors that collapsed slowly, we tagged additional bees during the process. In all, we tagged a total of 6230 bees, averaging 585 per hive.
Results and Discussion
Did the Tagged Bees Behave Normally?
I showed last month how we lightly anesthetized and tagged the bees. A critical question to consider is whether the process of tagging those bees changed their behaviors. So I watched them closely afterwards (Figure 1).

Fig. 1 We tagged workers shaken from brood combs, and then behaviorally-sorted to obtain non-flying, presumably “young” bees. Checking back in the days after applying the tags, the tagged bees appeared to be behaving like “normal house bees” on the combs, as in this photo.
What was striking was that in the first days after the initial tagging, we didn’t see any tagged bees at the entrances or flying. But after around 10 days, we were surprised by seeing tagged bees suddenly appear en masse at the entrance — presumably as they shifted from nursing behavior to guarding and foraging (Figure 2).

Fig. 2 What a surprise to come out to the Donor yard one day and see the tagged bees suddenly showing themselves, exhibiting “normal” progression in behavior. It was really conspicuous and impressive! This confirmed that we were tagging cohorts of bees of similar behavioral “age.”
And then a few days later, we noticed tagged bees acting as foragers (Figure 3)

Fig. 3 I watched a single red-tagged bee show up at some pollen that I had spilled, and then apparently recruit other foragers with the same tag color. I also observed tagged bees foraging upon spilled syrup where we fill our feeder jars. All indications were that the tagged bees were behaving completely normally.
Progress of the Colonies
The Donors: The two mite-free Control Donor colonies retained their strength and health throughout the 74 days of data collection.
All the high-mite Donor colonies save one, at some point collapsed (D7 only dwindled a bit). Some began collapsing in September, but most did so during mid-October. Some colonies collapsed relatively quickly, others more slowly.
The Receivers: All Receiver colonies remained in good health. Those in the home yard with stickyboards maintained mite wash counts of zero throughout the study, other than four that had counts of 1 during the peak of bee drift on 15 October.
Answering our Questions
We had a number of questions to answer; let’s go over them one by one.
Question: What proportion of workers from collapsing colonies drift to other hives?
This is one of the primary questions that this study was designed to answer.
Observations from the Donor Hives
It quickly became clear that workers drift freely between nearby hives (the Donors were several feet apart from each other) (Figure 4).

Fig. 4 There was considerable drift of tagged bees between the Donor hives, as evidenced by bees with different tag colors showing up on the combs, as in the photo above. But since those hives did not have magnetic traps, we could not quantify the full amount of drifting taking place.
At final cleanup at the end of the study, we recovered 44 drifted tags from the bottom boards or ground in front of the Donors (2-13 tags per hive, with the drifted tags often from the most closely-adjacent colonies). That works out to 20% of all tags recovered, confirming that there’s a lot of drift between nearby hives.
Experimental limitation: Those 44 recovered tags can be considered as a gross underestimate of how many tagged bees actually drifted into nearby Donors, since the Donor hives did not have magnetic traps. Any drifted bee would most likely have later flown out to die without leaving a tag behind (as evidenced by the fact that there were very few tagged bees or dropped tags remaining in the Donor hives at the end of the study).
Previous studies have found that rates of worker bee drift between nearby hives can be up to 40% [[2], [3], [4], [5], [6]], but has been more generally been found to be in the 3-10% range. In this study we recovered drifted tags from 3.6% of our tagged bees (Table 1), but I’ll explain in a moment why that can be assumed to be an underestimate of the actual amount of drift that occurred during this study.
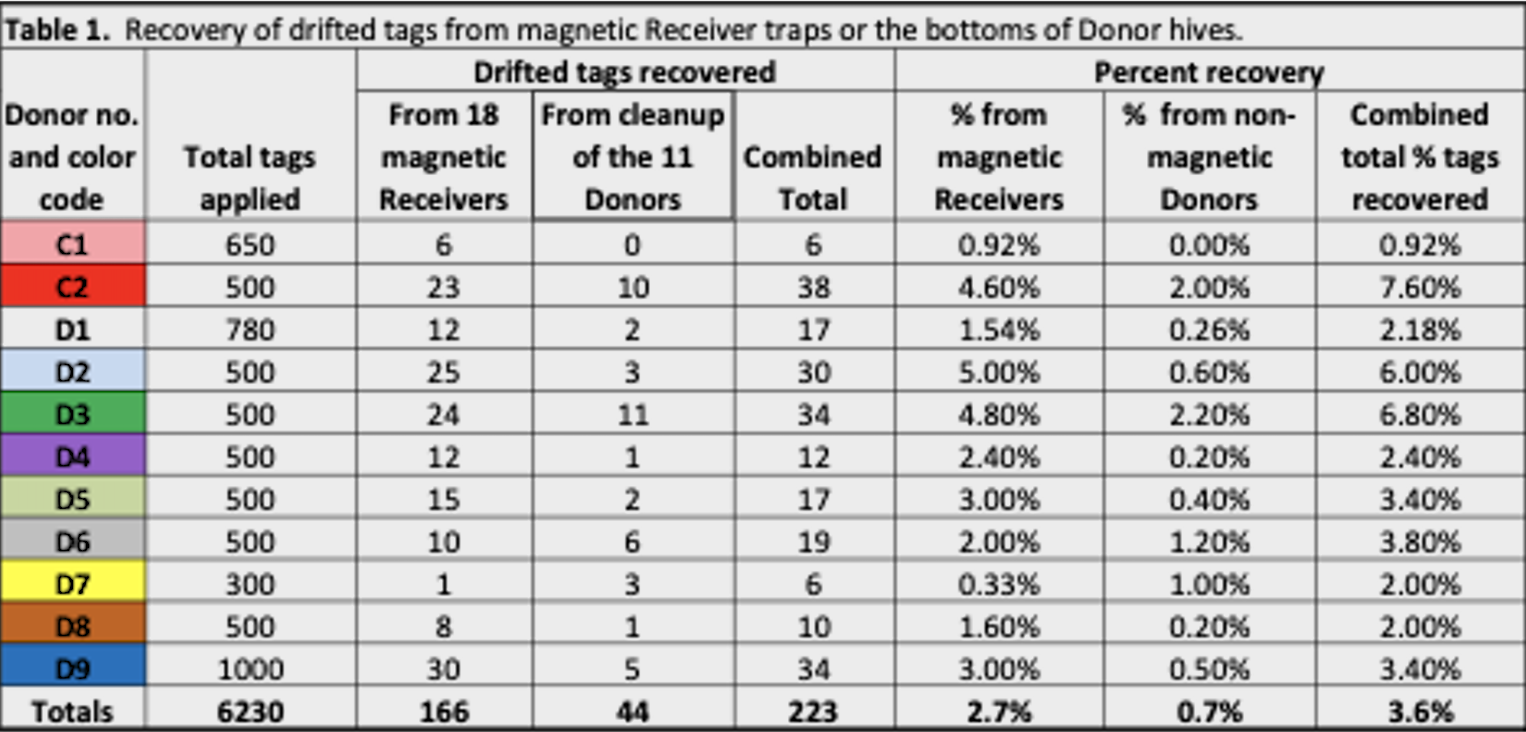
Table 1. Of the 6430 tags that we glued onto young workers, we recovered 3.6%, mostly from the magnetic traps of the Receiver hives, but also some from the bottom boards and ground in front of the Donor hives. I’ll go over this data graphically further along.
It’s an open question as to where the 6000 unrecovered tags (and their bees) wound up. The tagged bees may have drifted to colonies not involved in the study, or just eventually died in the field, as do the vast majority of workers.
We can justifiably assume that our count is an underestimation of actual drift, is since we had magnetic traps on only 3 of the 30 hives in each of our surrounding outyards, so would only expect to recover 10% of the drifted bees to those yards. Similarly, we don’t know how many of each cohort of tagged bees drifted into unknown hobbyist or feral colonies in the neighborhood.
Conclusions: Bees did indeed drift from collapsing colonies, but also from mite-free hives. Our quantified 3.6% drifted tag recovery rate can be safely assumed to be a substantial underestimate of the actual percentage of tagged bees that drifted from our Donors to other colonies.
Correlation With Varroa Infestation
Question: Does the varroa-virus complex increase the rate of bee drift into other hives?
Despite the fact that a number of parasites have been shown to affect host behavior in order to increase transmission, there’s little or no evidence to date that either varroa or DWV [[7]] induce increased drifting of honey bees [[8]].
So since this wasn’t a primary question to answer, in designing this study I included only two mite-free Control Donors, erroneously assuming that their drift rates would be similar. I could not have been more wrong! (Figure 5).
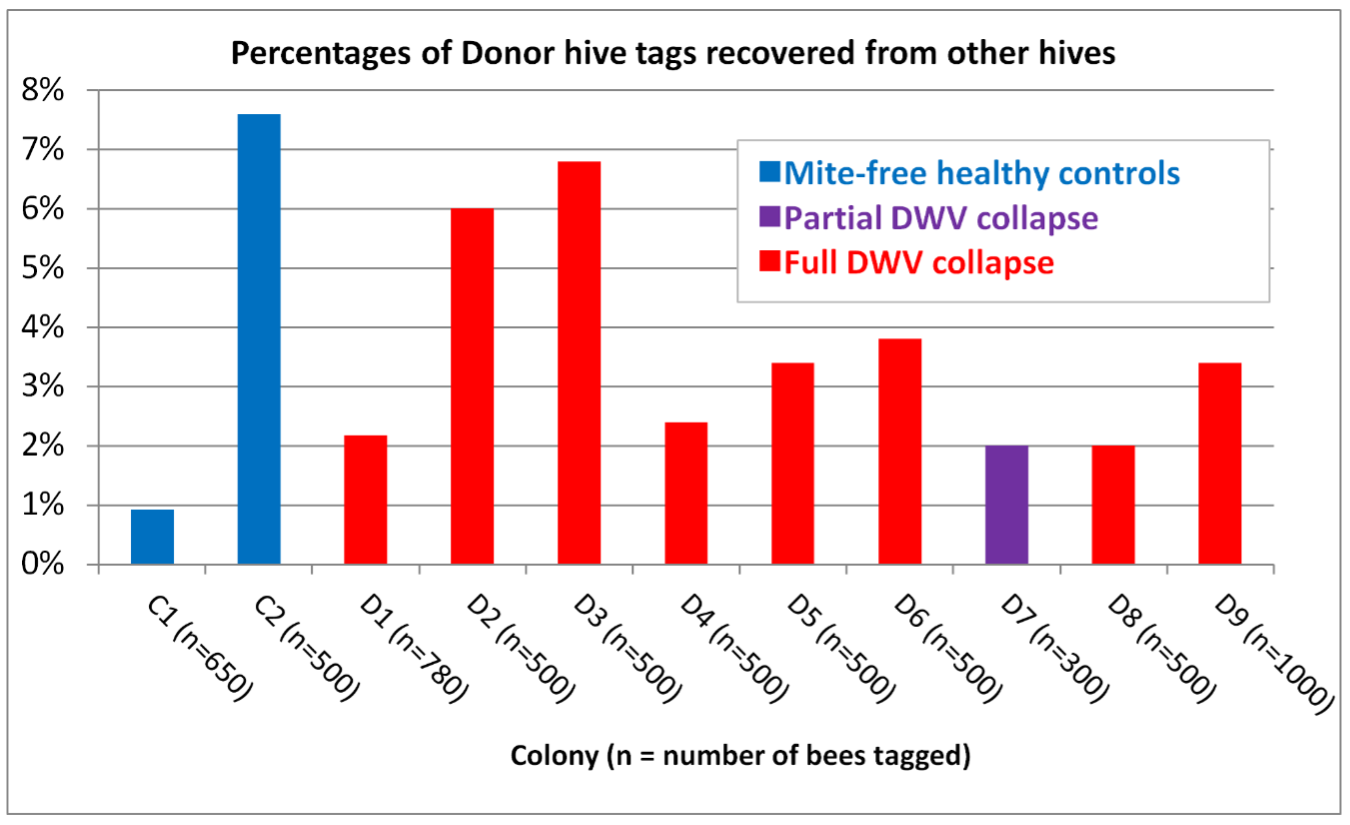
Fig. 5 It turned out that of all the Donors, the two mite-free Controls exhibited not only the lowest, but also the highest rate of drift! The rates of drift from the nine varroa-collapsing Donors all fell between that of the two mite-free Controls.
In retrospect I wish that I had run a larger number of mite-free Controls, and thank my lucky stars that I didn’t run only one, since drawing conclusions from the data of either Control alone would have been very misleading. Fortunately, these two Donor hives showed us how greatly the rate of bee drift can vary between healthy colonies.
Still curious, and since we did take periodic mite washes from the Donor colonies, I also looked to see whether there was a correlation between the intensity of the varroa infestation rate, and the degree of drifting (Figure 6).
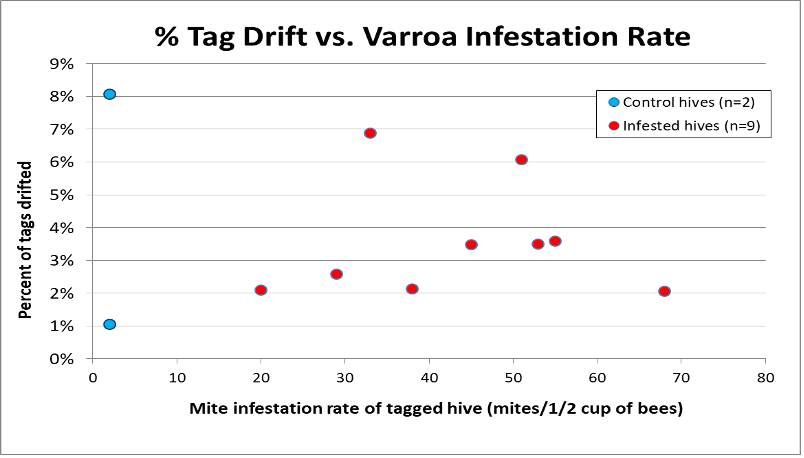
Fig. 6 I did not plan specifically to create this graph, so used mite wash data taken from our 15 October washes, or the mid-September mite counts from two Donors that had already largely collapsed by that later date. Again, there was no apparent correlation between varroa infestation rate and the rate of bee drift.
I’m not about to make any statistical claim based upon an “n” of only two hives in the Control group, but our results exemplify the sort of “ugly facts” that Thomas Huxley was referring to in his famous quote: “The great tragedy of science — the slaying of a beautiful hypothesis by an ugly fact.”
Conclusions: It would make “total sense” for the varroa-DWV complex to cause infected bees to drift to other hives. But, as found by others, our field data do not support that beguiling hypothesis.
Tag Recovery by Date
Even if the varroa-DWV complex is not the cause, that doesn’t necessarily mean that worker bees don’t tend to increase their amount of drifting as their colony collapses. Free [[9]] found that:
An individual bee is more likely to drift from a small to a large colony than vice versa.
We observed that two Donors collapsed shortly after the start of the study, whereas most of the rest collapsed in mid-October. Let’s see whether that’s reflected by tag recovery (Figure 7).
Fig. 7 The recovery of tags correlated pretty closely with the collapses of two colonies in late September, and then with the rest of the Donors in in mid-October.
The above data certainly suggest that bee drift correlates with the timing of colonies collapsing, so let’s take a closer look at our recovery of drifted tags, Donor-by-Donor.
Timelines of Recovery of Drifted Bees
My quandary when designing and executing this study
Keep in mind that this study was about mite immigration, and we’re looking at bee drift in the context that immigrated mites would need to have caught rides on either drifting or robbing bees [[10]]. From other studies, as well as my own observations, I suspected that substantial mite immigration due to drifting bees might occur from September through November in my area. That covers a 90-day timespan. But worker bees during that time of season live for an average of only about 35 days [[11], [12], [13]], so I didn’t want to mark all 500 bees at the start of the study, since many might not still be alive by the time that their colony actually collapsed.
An alternative design would have been to set up equal numbers of mite-free and high-mite Donors, and on the same day of each week, tag 50 bees in each hive, simultaneously grading each colony for strength, and then look for a correlation between loss of strength and bee drift. But since I don’t have grad students at my disposal, the logistics would have been tough. Of greater importance, there would have been smaller numbers of tagged bees in a hive at time of collapse, and my objective was to focus upon obtaining the most possible data on the drifting of workers during the time of actual collapse. That said, I strongly encourage someone to run the study described!
Did the act of tagging bees cause them to drift?
Another question arises comes from observations by a number of other researchers that bees marked shortly after emergence may exhibit high rates of “loss” within the first days after being marked. This made me curious as to whether those “losses” are an artifact of something do with the process or marking them (which would have confounded our data), or whether that early loss of tagged bees was simply due to them getting confused and drifting during their first defecation flights.
In this project, during the 5-day period between 17 and 21 September, we glued tags onto over 3000 workers, checking the magnetic traps on the Receivers on a daily basis. During this time period we recovered only a single tag the day after our initial tagging of 550 bees, and only 2 more drifted tags through the 21st. We then took the weekend off, and checked the traps on 24 September, when we recovered 17 drifted tags.
Conclusion: The above findings suggest that the manner in which we tagged bees did not initiate immediate drifting.
Relevance to Mite Immigration
I’ve confirmed that shaken non-flying “house bees” (such as we tagged) tend to exhibit a higher rate of mite infestation compared to “flyers” [[14]]. This is presumably due to the observation that nurse bees are most attractive to varroa, and thus have the highest infestation rate of any workers in the hive.
So at what age would we expect bees to tend to drift? Again returning to King’s observations in 1932:
The ages at which drifting appeared to be most prevalent were between the fifth and tenth days, which corresponds rather closely with the age at which young bees show a tendency to shift from activities within the colony to field work.
Free [[15]] followed up on this in 1958, finding that:
- Most of the bees which drift do so during their orientation flights and before they become regular foragers.
- Bees emerging in August and September drift less than those emerging earlier in the year.
- Drifting varies considerably in different circumstances, and may be extensive.
- Drones drift two to three times as frequently as workers.
And finally, Bordier [[16]], tracking 1000 tagged bees with a receiver hive placed to either side, found that 10% of them drifted to another hive, mostly when they were over 10 days of age.
We didn’t specifically set up this study to determine age of drift, but since we only tagged bees that didn’t fly off after shaking them from the combs, we can likely assume that they were young “house bees.”
Practical application: Since the issue of interest is transfer of varroa via drifting, if we’re trying to correlate mite immigration with bee drift, it’s important to know the behavioral “age” of those drifted bees. Is it mainly young workers getting lost during cleansing or orientation flights, foragers returning from their first exploratory flights, or older foragers that either got lost, disoriented, confused, or unable to resist the alluring scent of another colony?
So with the above caveats and applications explained, let’s see what we can tease out of our data. I’ve created graphs of tag application and recovery for each individual Donor colony, with the date and number of bees that we tagged in blue columns (on the left hand y axis), and the number of their tags magnetically recovered in Receiver hives in red columns (on a different scale on the right axis). The columns don’t include drifted tags later recovered from Donor hives.
Question: At what age do bees tend to drift?
We tagged bees in the mite-free Control Donor hives only in the first few days, which gives us an opportunity to get an idea of at what ages those bees drifted from those healthy colonies (Figure 8).
Fig. 8 These first two graphs are for the two mite-free Control Donor hives, in which we tagged bees only in the first few days (the blue columns). As I’ve already said, C2 then exhibited far more drifting (the red columns) than did C1 (and a greater amount shortly after tagging). Colony-to-colony variation is always the bugaboo of field research!
Interpretation: The amount of drifting of bees from the two Control Donor colonies was as different as night and day, but the overall timing of bee drift was well spread out for both colonies — indicating that the tagged bees drifted at all different ages. Surprisingly, we recovered a tag from each colony on the 70th day after first tagging, which means that even very old workers may drift.
Now let’s move to the high-mite Donors.
Question: Does bee drift correlate with the collapse of a hive?
Note: This study was designed to quantify the degree of drifting as colonies collapsed, not the correlation between collapsing and drifting. In these colonies, the correlation of drift with age or collapse may be confounded by our tagging of additional bees over time. In retrospect, if I were to run this study again, based upon the unevenness of drift from the Controls above, I might only tag bees during the first week, rather than waiting for their colonies to begin collapsing.
That said, I still find it of interest to look closely at the data. As opposed to the mite-free Controls above, all but one of the high-mite Donors did collapse. In each of those Donors, we tagged 50 bees initially, but then waited, if necessary, for signs that the colony was collapsing (decreasing strength, deformed wings, or dying pupae) to tag another 450 (or more) bees. You can tell when we thought that collapse looked imminent by the timing of the additional blue columns.
Due to the difficulty in creating a three-axis graph in Excel, I’ll provide starting and other appropriate mite wash counts in the captions. Take your time in analyzing the graphs below (and the information in the captions), as they tell us a lot about the drifting of bees during a colony’s collapse due to varroa.
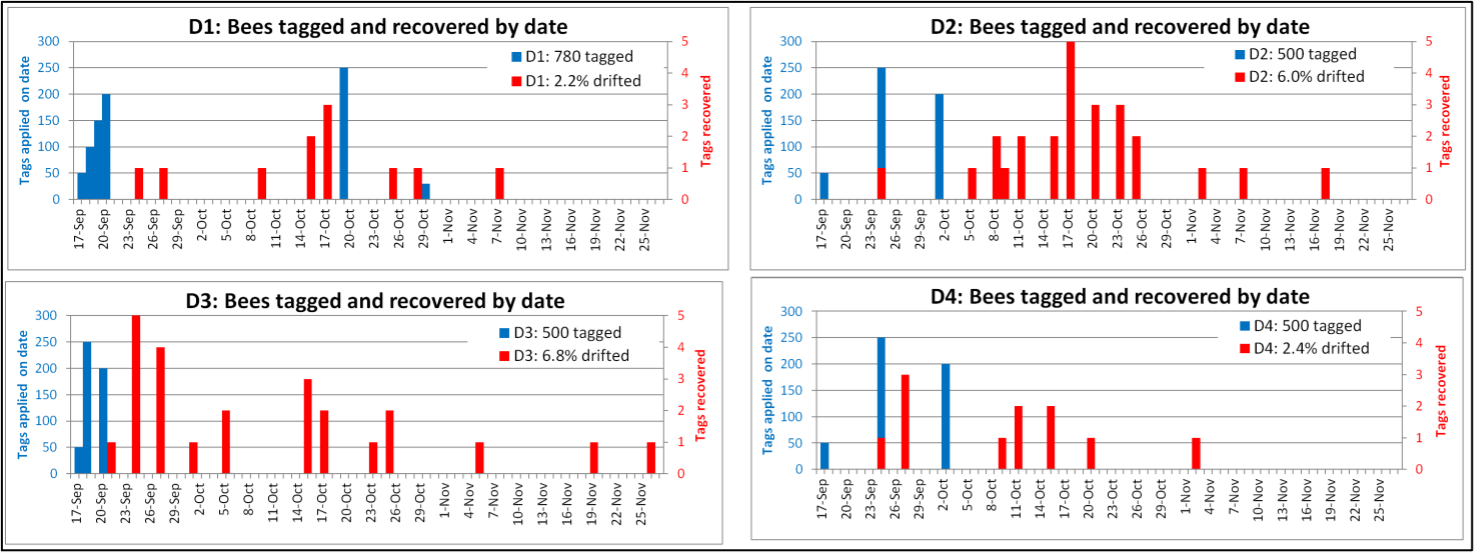
Fig. 9 Keep in mind that the scales for the blue and red columns are different. D1 started with a high mite count (30) and we expected it to collapse quickly, but it didn’t start to do so until mid-October, and then did quickly. D2 started with a relatively low mite count (19), and was still strong on 1 October, but then was collapsing quickly by 15 October, with a mite count of 51. D3 started with high mites (33), and also crashed by mid-October. Of interest, it graded at only a quarter frame of bees on 31 October, yet three of its tagged bees drifted during November (perhaps from a second drifting from another Donor?). D4 started with a low mite count (11), was still strong on 1 October with a wash count of 29, but then collapsed slowly.
Interpretation: The clumpings of the red columns for these colonies suggest that bee drift was associated with collapse, although some colonies collapsed more quickly than others.
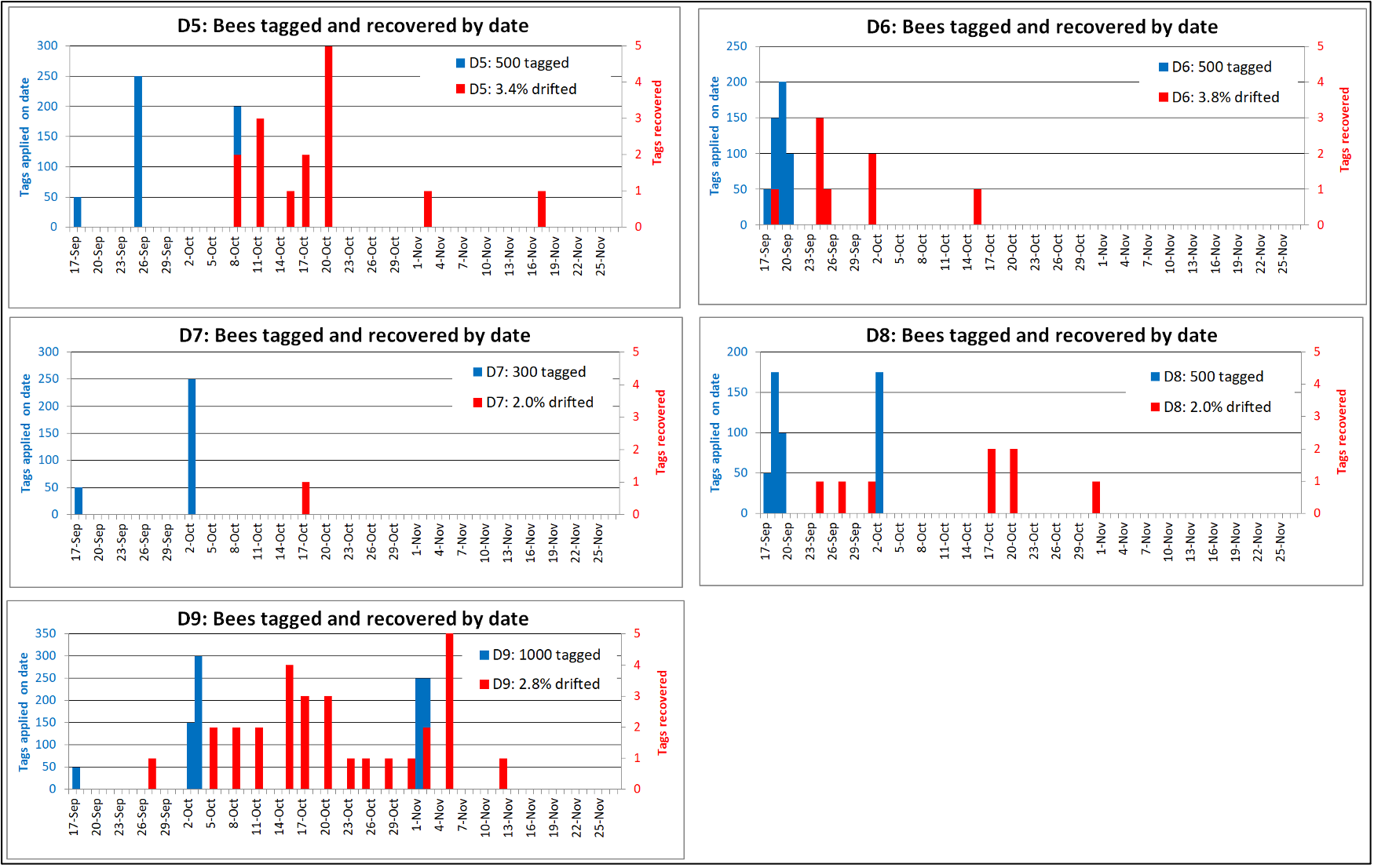
Fig. 10 Mite counts increased quickly in D5 (from 15 to 53 by 15 Oct) and it collapsed during the last half of October, whereas D6 was a strong hive with a very high starting mite count (55) that we rushed to tag as it started collapsing in September. D7 (starting count of 13) never collapsed, so we tagged only about half as many bees as for the rest of the Donors. D8 (starting count 38) collapsed fairly early, whereas D9 started strong and with a relatively low mite level (17) that increased greatly by mid-October (45) , causing signs of PMS (while it still contained 6 frames of bees). Because of its prolonged collapse (3 frames of bees remaining on 31 Oct), we tagged another 500 bees on 2 November.
Conclusion: Although our data didn’t support that mite-infested bees necessarily drift more than “normal,” it does appear that increased drift often coincides with the timing of collapse. It’s not clear whether that was just an artifact of our timing of marking, or due to bees tending to drift out of weakening colonies “with problems” into more attractive colonies.
I’ve only just begun — next month we’ll take a look at the effect of distance upon drifting bees, the “attractiveness” of some colonies to drifted bees, and then on to our data on the immigration of mites into the Receiver hives, relative to incoming drifted bees. I’ll then continue with quantifying the actual amount of mite immigration, and whether there was a correlation with robbing. As I said, this was an ambitious study, and we can learn a lot from it!
Acknowledgements
Thanks to my helpers Brooke Molina, Anna Mudd, Sandy Honigsberg, and Brion and Alice Dunbar, without whose help I could never have pulled off this study. And Dr. Norman Gary for his helpful consultation. And of course to those beekeepers whose donations enable me to pay for doing this sort of research.
Citations and Notes
[1] All mite counts in this article are for the number of mites recovered by a mechanically-agitated alcohol wash of a level half cup of bees
[2] King, GE (1932) Drifting bees may make production records of little value. Am. Bee J. 72: 141-142.
[3] Nelson, DL & SC Jay (1989). The effect of colony relocation on loss and disorientation of honeybees. Apidologie 20(3): 245-250.
[4] Jay, SC (1969). Drifting honeybees in commercial apiaries v. Effect of drifting on honey production. Journal of Apicultural Research 8(1): 13-17.
[5] Pfeiffer, K & K Crailsheim (1998). Drifting of honeybees. Insectes Sociaux 45: 151-167.
[6] Goodwin, R, et al (2006) Drift of Varroa destructor-infested worker honey bees to neighbouring colonies. Journal of Apicultural Research 45:((3) 155-156.
[7] Deformed Wing Virus.
[8] Citations in Part 1 of this series.
[9] Free, JB (1958) The drifting of honey-bees. The Journal of Agricultural Science 51(3): 294-306.
[10] Ignoring for now that from drifting drones. During the course of this study, there were very few drones in any of our colonies.
[11] Lloyd Harris, in prep.
[12] Free, J & Y Spencer‐Booth (1959). The longevity of worker honey bees (Apis mellifera). In Proceedings of the Royal Entomological Society of London. Series A, General Entomology 34 (10‐12):141-150.
[13] Sakagami, S& H Fukuda (1968) Life tables for worker honeybees. Population Ecology 10(2): 127-139.
[14] https://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-4/
[15] Free, JB (1958) op cit.
[16] Bordier, C, et al (2017) Should I stay or should I go: honeybee drifting behaviour as a function of parasitism. Apidologie 48(3): 286–297.





































































 In the photo above, the bees have accepted the queen. In the photo below, they have not, and are attempting to “ball” the queen and cook her. The bees below are behaving aggressively towards the queen, and will not move when you touch them.
In the photo above, the bees have accepted the queen. In the photo below, they have not, and are attempting to “ball” the queen and cook her. The bees below are behaving aggressively towards the queen, and will not move when you touch them. In the case above, you most likely have a loose queen remaining in the hive (or perhaps she needs another day)–no sense to release the new queen, as she’ll be killed. You need to either find the other queen, or introduce the queen into a queenless nuc.
In the case above, you most likely have a loose queen remaining in the hive (or perhaps she needs another day)–no sense to release the new queen, as she’ll be killed. You need to either find the other queen, or introduce the queen into a queenless nuc. A tip: new beekeepers often manage to allow a caged queen to fly off. If you do so, freeze, and she will often return to the cage in a few minutes. Prevent this from occurring by handling caged queens in a closed room, your car, or in your removed veil.
A tip: new beekeepers often manage to allow a caged queen to fly off. If you do so, freeze, and she will often return to the cage in a few minutes. Prevent this from occurring by handling caged queens in a closed room, your car, or in your removed veil.